Abstract
Background:
Advanced practice providers (APPs) play important roles in enrolling, educating, and caring for patients in clinical trials. However, much remains unknown about the role of APPs in managing adverse events (AEs) in early (phase I to II) clinical trials. In this study, we assessed the outpatient management of grade 3 to 4 AEs by APPs in early trials and characterized the workflow of our APP Phase I to II Fast Track (FT) Clinic.
Patients and Methods:
We retrospectively reviewed records of patients with advanced or metastatic solid tumors enrolled in phase I to II clinical trials who were seen by APPs from September 2017 to August 2018 in the APP phase I to II FT clinic in the Department of Investigational Cancer Therapeutics.
Results:
A total of 808 patients enrolled in 159 clinical trials were seen in 2,697 visits (median 3 visits per patient; range 1–28) by 10 APPs. Treatment was interrupted in 6.9% of visits, and grade 3 to 4 AEs were seen in 5.4% of visits; however, patients from 1.4% of visits were sent to the emergency center (EC) and/or admitted. Patients referred to the EC and/or admitted were more likely to have baseline hypoalbuminemia, high lactate dehydrogenase, and poor Eastern Cooperative Oncology Group performance status (i.e., ECOG > 1; p < .001). There were no associations between EC referral and gender, APP years of experience, or type of treatment.
Conclusions:
The APP Phase I to II FT Clinic has an important role in the management of AEs by APPs in early clinical trials in the outpatient setting, potentially avoiding EC visits and admissions.
The process of clinical drug development classically has to follow three phases before regulatory (US Food and Drug Administration or European Medicines Agency) approval; however, there have been exceptions in which drugs were approved for tumor location–agnostic indications in the presence of compelling targets, such as larotrectinib (Vitrakvi) for patients with NTRK fusions (Drilon et al., 2018; Joshi et al., 2020; Ricciuti et al., 2019) or pembrolizumab (Keytruda) for patients with microsatellite instability-high tumors, based on early (phase I and phase II) clinical trials (Marabelle et al., 2020).
The primary objectives of phase I clinical trials are to determine the safety and tolerability of an investigational drug or combination and to define the maximum tolerated dose (MTD) and recommended dose to be used in phase II clinical trials (Garralda et al., 2019). In contrast, the primary objective of phase II clinical trials is often to evaluate the preliminary efficacy of the treatment in a well-defined population of patients (Bui & Kummar, 2018; Garralda et al., 2019). The primary objective of phase III clinical trials is to evaluate the efficacy of the investigational drug compared with standard-of-care treatment (Garralda et al., 2019).
Patients who participate in early clinical trials usually have metastatic or locally advanced cancer and have exhausted all standard-of-care treatment options (Cassel et al., 2016). Most of these patients have been offered the option of hospice or an early clinical trial, and most tend to have significant tumor and symptom burden (Cassel et al., 2016; Kokkonen et al., 2019). Patients participating in early clinical trials may experience unknown and unexpected adverse events (AEs) from the investigational drugs, which may exacerbate preexisting comorbid conditions and require temporary treatment interruption and dose modifications. These AEs, in addition to the advanced cancer, can significantly impact patients’ quality of life (Cassel et al., 2016; Chrisoulidou et al., 2015).
The evaluation of AEs is essential in phase I clinical trials in evaluating the dose-limiting toxicities (DLTs) and determining the phase II recommended dose (RP2D). Often the first cycle is considered the DLT period, in which AEs are assessed in relation to that dose level and determine if that dose is safe and tolerable.
Unlike traditional chemotherapy side effects, AEs associated with immune checkpoint inhibitors (ICIs) such as anti–programmed cell death protein 1 (PD-1), anti–programmed cell death ligand 1 (PD-L1), or anticytotoxic T-lymphocyte associated antigen 4 (CTLA-4) antibodies can range from commonly occurring AEs such as fatigue and rash or pruritus to immune-related reactions similar to autoimmune diseases such as colitis, hepatitis, and hypothyroidism (Barber, 2019; Baxi et al., 2018; Gordon et al., 2017). These immune-related AEs (irAEs) can emerge beyond the classical DLT period defined as cycle 1. Monitoring of patients is important in determining the safety beyond the classical DLT period. Additionally, these drugs can also have less frequent AEs involving the nervous, hematologic, and urinary systems (Barber, 2019; Baxi et al., 2018; Gordon et al., 2017; Kumar et al., 2017).
Given that patients enrolled in early clinical trials may experience severe AEs between visits with their oncologist and may require weekly monitoring for safety, our institution established an advanced practice provider (APP; nurse practitioners and physician assistants)-led phase I to II fast-track (FT) clinic to address this need. Research has shown that APPs play a significant role in improving the quality of life and symptom management of patients with cancer (Alotaibi & Al Anizi, 2020). Additionally, APPs are an integral part of the clinical team, identifying eligible patients and providing education and care to patients enrolled in a clinical trial; however, much remains unknown about their role in the management of AEs in early clinical trials.
The main objectives of this study were to (1) describe the workflow of the APP Phase I to II FT Clinic, (2) assess the management of AEs by APPs in early clinical trials in the outpatient setting, and (3) assess if grade 3 to 4 AEs can be managed in the outpatient setting, thus avoiding emergency center (EC) visits and hospital admissions.
PATIENTS AND METHODS
Patients
This study included patients with advanced or metastatic solid tumors who were enrolled in phase I to II clinical trials and seen by APPs in the APP Phase I to II FT Clinic in the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center from September 2017 to August 2018. Patients seen in the FT clinic for reasons other than planned visits for safety follow-up were excluded from the current study. This study was conducted under an Institutional Review Board–approved protocol. Clinical information such as patient characteristics, type of treatments received, and the occurrence and management of grade 3 to 4 AEs (as defined by Common Terminology Criteria for Adverse Events [CTCAE] versions 4 and 5) were collected from prospectively maintained databases and/or electronic medical records. The information was transferred to an Excel spreadsheet by the department informatic technical support personnel, and missing data were entered manually by the APPs.
Patients were referred to the Clinical Center of Targeted Therapies in the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center in Houston, Texas, for evaluation for treatment options in early clinical trials. During the initial clinic visit (and after review of the patient’s medical, surgical, family, and social history; current medications; allergies; and the molecular profile of the tumor, if available), the physicians and APPs provided verbal and written education regarding the potential risks, benefits, and logistics of early clinical trials in general and about potential expected AEs and trial-related procedures and/or expectations. These were further explained by the designated clinical study coordinator or research nurse of the clinical trial. Patients who were interested in participating in a clinical trial and met the inclusion and exclusion criteria signed informed consent. Patients underwent the designated screening procedures before starting treatment, and they were seen by the primary investigators for cycle 1 treatment clearance.
APP Phase I to II FT Clinic Workflow
After patients receive the first dose of treatment, many of them are scheduled for weekly visits (e.g., days 8, 15, and 22) in the APP Phase I to II FT Clinic to assess the safety and tolerability of the treatment and identify severe adverse events that could represent dose-limiting toxicities of the investigational agent. Additionally, during the weekly patient visits, blood samples are collected to assess the absorption and distribution of the investigational drug (pharmacokinetics) and to identify possible biomarkers for response or resistance to the investigational drug(s).
Patients are seen in the APP Phase I to II FT Clinic by an APP with the help of a medical assistant. The APPs who are a part of the Phase I to II FT Clinic also work in comanaged clinics with the clinical trials’ principal investigators and have various levels of experience, ranging from 2 to 24 years. The role of an APP working in a Phase I to II FT clinic can be quite labor intensive secondary to checking the protocol regarding dose-limiting toxicities, treatment holding parameters, and prohibited medications. Therefore, prior to staffing the APP Phase I to II FT Clinic, new APPs receive 6 months of ongoing shadowing and precepting from an experienced APP preceptor and the primary investigator. Advanced practice provider competencies specific for this role do not exist; thus, general clinical trial and APP oncology competencies were utilized to train and evaluate new APPs (Calvin-Naylor et al., 2017; Coombs et al., 2020).
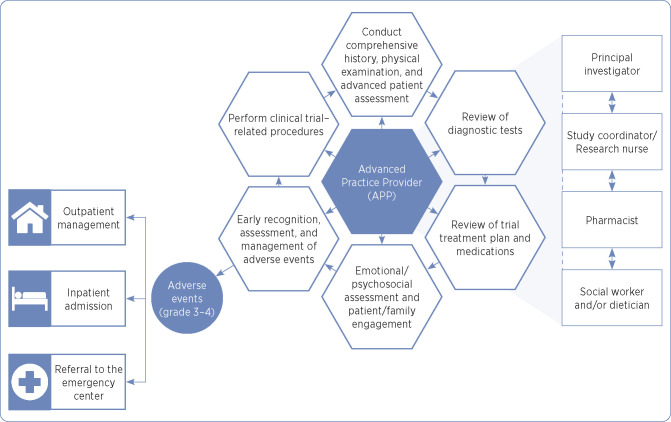
During each APP Phase I to II FT clinic visit, the APP performs a complete history and physical examination and a review of systems, assesses the patient’s Eastern Cooperative Oncology Group (ECOG) performance status (0: fully active, able to carry on all pre-disease performance without restriction; 1: restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; 2: ambulatory and capable of all self-care but unable to carry out any work activities), assesses psychosocial status, reviews or updates medication lists, reviews diagnostic and laboratory data, and identifies, grades, and manages AEs as per established national guidelines (Haanen et al., 2017; Puzanov et al., 2017; Thompson, 2018; National Comprehensive Cancer Network, 2019), institutional algorithms, and specific protocol/sponsor guidelines (Figure 1). The APP documents AEs in the electronic health record. Additionally, during each Phase I to II FT clinic visit, the APP interacts with the clinical trial’s designated clinical study coordinator or research nurse to discuss AEs, attribution of AEs, allowable medications or interventions per the clinical trial, and the need to schedule additional diagnostic testing or referral to specialists of affected organ systems.
Figure 1.
Advanced practice provider role in early phase I to II Fast Track Clinic.
Treatments and Toxicity Evaluation
We assessed patient characteristics, type of treatments received, and the occurrence of grade 3 to 4 AEs and their management. We also evaluated the EC visits and admissions 24 hours before or after visits to the APP Phase I to II FT Clinic. Cancer treatment was administered in accordance with Institutional Review Board–approved protocols, and patients received therapy under the investigators’ care.
Additionally, we assessed the baseline physical examination, CT laboratory values, and other imaging results. Based on the number of sites of metastatic disease, lactate dehydrogenase (LDH), and albumin, the Royal Marsden Hospital (RMH) prognostic score was calculated. The RMH score is a validated tool that helps predict survival of cancer patients (Garrido-Laguna et al., 2012). Patients with elevated LDH, low albumin (< 3.5 g/dL), and more than two metastatic sites (lower RMH scores) tend to have worse survival outcomes (Garrido-Laguna et al., 2012).
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics, such as age, diagnosis, gender, race, religion, ECOG performance status, type of therapy (e.g., immunotherapy, chemotherapy, targeted therapy), albumin level, LDH level, and number of metastatic sites.
Chi-squared (χ2) tests of independence were used to evaluate associations between categorical variables that included tumor type, ECOG performance status, and RMH prognostic score (range 0–3). All tests were two sided, and p values < .05 were considered statistically significant. All statistical analyses were performed with the SPSS version 26 (SPSS, Chicago, IL) software program.
RESULTS
A total of 808 patients with advanced or metastatic solid tumors were seen in 2,697 visits (median 3 visits per patient, range 1–28) by 10 APPs. Patient characteristics are summarized in Table 1. More female patients than male patients were seen in the APP Phase I to II FT Clinic. Patients were enrolled in 159 phase I to II clinical trials of targeted therapies, chemotherapies, ICIs, and other treatments. Most visits (59.7%) occurred during the dose-limiting toxicity assessment period or cycle 1, and 41% of visits were for patients receiving treatment regimens that included ICIs. The most frequent tumor types seen in the FT clinic were gastrointestinal, gynecologic, breast, sarcoma, and head and neck (Table 2).
Table 1. Patient Demographic Characteristics.
| Variable | No. (%) |
|---|---|
| Age, yr, mean (range) | 57.9 (20–88) |
| Gender | |
| Female | 437 (54) |
| Male | 371 (46) |
| Marital status | |
| Married | 653 (81) |
| Unmarried | 154 (19) |
| Race | |
| Caucasian | 595 (74) |
| African American | 76 (9) |
| Asian | 54 (7) |
| Other/Unknown | 83 (10) |
| ECOG PS | |
| 0 | 44 (5) |
| 1 | 742 (92) |
| 2 | 22 (3) |
Note. ECOG PS = Eastern Cooperative Oncology Group Performance Status.
Table 2. Cancer Types.
| Cancer type | Number of patients (N = 808) | Percentage |
|---|---|---|
| Breast | 86 | 10.6 |
| Thoracic | 61 | 7.5 |
| Head and neck | 65 | 8.0 |
| Gastrointestinal | 253 | 31.3 |
| Brain and spine | 16 | 2.0 |
| Gynecologic | 94 | 11.6 |
| Genitourinary | 59 | 7.3 |
| Sarcoma | 71 | 8.8 |
| Skin | 31 | 3.8 |
| Thyroid | 41 | 5.1 |
| Unknown primary | 8 | 1.0 |
| Other | 23 | 2.8 |
Treatment was held in 6.9% of visits, and grade 3 to 4 AEs were present in 5.4% of visits; however, only 1.4% of visits ended with the patient being sent to the EC and/or admitted for further treatment and toxicity management. Patients receiving targeted therapy were slightly more likely to be sent to the EC compared with patients receiving other treatments (5.3% vs. 2.9%, p = 0.11). However, patients who received ICIs were slightly less likely to be referred to the EC than patients who did not receive ICIs (3.6% vs. 5.1%, p = .29). No significant differences were noted for patients receiving other treatments such as chemotherapy and antibody-drug conjugates.
The majority (91%) of hematologic grade 3 to 4 AEs were managed in the APP Phase I to II FT Clinic, avoiding the need for patients to be seen in the EC or admitted. Overall, more experienced APPs were less likely to refer patients to the EC (3.2% vs. 5.3%), although this difference was not statistically significant (p = .15). Patients seen in the FT clinic and referred to the EC and/or admitted were more likely to have baseline hypoalbuminemia, high LDH, and ECOG performance status > 1 (p < .001; Table 3).
Table 3. Patient Clinical Characteristics by Emergency Center Visit Status.
| Variable | Total patients, no. (%) | EC visit, no. (%) | No EC visit, no. (%) | p a |
|---|---|---|---|---|
| Lactate dehydrogenase | .001 | |||
| Normal | 437 (62) | 11 (34) | 426 (64) | |
| Abnormal | 263 (38) | 21 (66) | 242 (36) | |
| Albumin | .004 | |||
| Normal | 727 (93) | 28 (80) | 699 (93) | |
| Abnormal | 59 (7) | 7 (7) | 52 (7) | |
| ECOG PS | < .001 | |||
| 0 | 44 (5) | 2 (6) | 42 (5) | |
| 1 | 742 (92) | 26 (72) | 716 (93) | |
| 2 | 22 (3) | 8 (22) | 14 (3) | |
| Number of metastatic sites | .3 | |||
| 1–2 | 240 (30) | 8 (22) | 232 (30) | |
| 3+ | 568 (70) | 28 (78) | 540 (70) | |
| Royal Marsden score | < .001 | |||
| 0–1 | 463 (67) | 12 (38) | 451 (68) | |
| 2–3 | 228 (33) | 20 (62) | 208 (32) |
Note. EC = emergency center; ECOG PS = Eastern Cooperative Group Performance Status.
Derived from two-sided Pearson χ² test EC visit vs. no EC visit.
There were no significant differences in RMH scores by race (p = .89). A greater proportion of female patients had high RMH scores compared with male patients (36.7% vs. 28.6%, p = .02). However, there was no significant difference in the likelihood of being referred to the EC by gender (4.3% vs. 4.6%, p = .87). Among patients with higher RMH scores, APPs with less experience were more likely to send patients to the EC than more experienced APPs, although the difference was not statistically significant (10.3% vs. 6.1%, p = .28). There was no difference by APP experience in referrals for patients with lower RMH scores.
DISCUSSION
Advanced practice providers are integral members of most academic and community oncology practices, with at least 5,350 APPs working in oncology in the United States in 2018 (Bruinooge et al., 2018). Additionally, more than 80% of oncology APPs’ time is spent in direct patient care (Bruinooge et al., 2018). Several studies have examined the role of APPs in the management of oncology clinics and have shown benefits in symptom management, chemotherapy-related AE management, and decreased rates of EC visits and hospitalizations (Handley et al., 2018; Mason et al., 2013; Ruegg, 2013; Sivendran et al., 2014). However, to our knowledge this is the first report of the role of APPs in the management of AEs of experimental oncology drugs in early clinical trials.
Our results suggest that visits in the APP Phase I to II FT Clinic are associated with low rates of EC visits and hospital admissions in patients enrolled in phase I to II trials. In fact, the majority of grade 3 to 4 hematologic toxicities were managed in the outpatient APP Phase I to II FT clinic, and only 4.5% of the patients (1.4% of all APP visits) were sent to the EC. Additionally, the investigational treatment was interrupted in only 7.3% of patient visits. These findings were similar to previous research that demonstrated that weekly APP visits were associated with low rates of hospitalizations and EC visits for patients with head and neck cancer undergoing chemotherapy (Mason et al., 2013). Therefore, using APPs in the outpatient setting to identify and manage AEs in patients enrolled in phase I to II clinical trials may assist with avoiding EC visits, frequent hospitalizations, and treatment interruptions. The identification of serious AEs is important in determining the dose-limiting toxicities and determining the right dose for further clinical studies.
Our findings suggest that APPs with less experience were more likely to send patients to the EC than more experienced APPs. One explanation for our findings is that APPs with less experience are competent; however, they may lack the confidence and clinical expertise to manage grade 3 to 4 AEs in the outpatient setting. Research is needed to determine associations between APP years of experience and expertise level, which in turn may guide interventions to improve both expertise and outcomes.
Our study results revealed that patients seen in the APP Phase I to II FT Clinic and referred to the EC and/or admitted were more likely to have higher RMH scores with baseline hypoalbuminemia, high LDH, and ECOG performance status > 1. This is consistent with previous research that found that patients with high RMH are associated with poor prognosis and greater morbidity in phase I clinical trials in oncology (Deng et al., 2018; Liu et al., 2016; Wei et al., 2018). Thus, our findings suggest such patients would benefit from close monitoring and follow-up in the APP Phase I to II FT clinic during treatment in a phase I to II clinical trial.
Often, interruption of the investigational agent because of grade 3 to 4 AEs during cycle 1 represents a DLT that is very important for determining the safety and tolerability of new experimental drugs. Through the weekly APP visits, patients who had treatment interruptions related to grade 3 to 4 AEs have avoided more serious adverse events. Furthermore, management of grade 1 to 2 AEs in the APP Phase I to II clinic might prevent future temporary unplanned interruption of cancer treatment that may negatively impact patient outcomes (Parikh et al., 2020; Yao et al., 2018).
Although our study described a rate of AEs that required care escalation, there was insufficient evidence in the literature or personal experience to judge whether the outcomes in our study were equal, inferior, or superior to other APP-led clinics. A systematic review of the role of advanced nurse practitioners (ANP) in oncology found that ANPs were able to assist patients in symptom management, stress relief, and improve quality of life, however, the review did not address the management of AEs associated with investigational cancer agents (Alotaibi & Al Anizi, 2020).
Limitations
This study had a few limitations, including a selection bias, as the patients were specifically referred for early clinical trials and were in the subset of patients deemed by their primary oncologist to be fit for investigational therapy. Additionally, because this study occurred at a single National Cancer Institute–designated comprehensive cancer center, the finding may have limited applicability in smaller community-based oncology centers. Furthermore, the patients included in this study were heterogeneous in that they were treated with a variety of investigational agents with varying mechanisms of action, which may have influenced the primary outcomes of the study.
Further research should include a study in which patients are randomly assigned to be seen weekly in the APP Phase I to II FT Clinic, every 2 weeks in the FT clinic, or only in the physician clinic for every cycle clearance (every 3 or 4 weeks) to determine whether patient outcomes differ and if there is improvement in the management of AEs for patients seen in the FT clinic. Additionally, future research should include a prospective study evaluating the FT clinic and protocol deviations, medication adherence, and patient retention.
CONCLUSION
Advanced practice providers have an important role in evaluating the safety and tolerability of investigational cancer treatments and management of possible drug-related AEs in early clinical trials. The APP Phase I to II FT Clinic is an outpatient setting that facilitates the management of possible drug-related AEs by APPs and has the potential to both identify serious grade 3 to 4 AEs and avoid EC visits and admissions. Research is needed to evaluate the benefits and clinical outcomes of patients who are enrolled in early clinical trials and seen weekly in an APP Phase I to II FT Clinic.
Footnotes
The authors have no potential conflicts of interest to disclose. This work was partially supported by the National Institutes of Health through MD Anderson Cancer Center’s Support Grant (P30 CA016672) and the NCI K12 Paul Calabresi Award CA088084.
References
- Alotaibi, T., & Al Anizi, C. A. (2020). The impact of advanced nurse practitioner (ANP) role on adult patients with cancer: A quantitative systematic review. Applied Nursing Research, 56, 151370. 10.1016/j.apnr.2020.151370 [DOI] [PubMed] [Google Scholar]
- Barber, F. D. (2019). Adverse events of oncologic immunotherapy and their management. Asia Pacific Journal of Oncology Nursing, 6(3), 212–226. 10.4103/apjon.apjon_6_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxi, S., Yang, A., Gennarelli, R. L., Khan, N., Wang, Z., Boyce, L., & Korenstein, D. (2018). Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ, 360, k793. 10.1136/bmj.k793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinooge, S. S., Pickard, T. A., Vogel, W., Hanley, A., Schenkel, C., Garrett-Mayer, E.,…Williams, S. F. (2018). Understanding the role of advanced practice providers in oncology in the United States. JCO Oncology Practice, 14(9), e518–e532. 10.1200/jop.18.00181 [DOI] [PubMed] [Google Scholar]
- Bui, N. Q., & Kummar, S. (2018). Evolution of early phase clinical trials in oncology. Journal of Molecular Medicine, 96(1), 31–38. 10.1007/s00109-017-1612-7 [DOI] [PubMed] [Google Scholar]
- Calvin-Naylor, N. A., Jones, C. T., Wartak, M. M., Blackwell, K., Davis, J. M., Divecha, R.,…Wilson, K. (2017). Education and training of clinical and translational study investigators and research coordinators: A competency-based approach. Journal of Clinical and Translational Science, 1(1), 16–25. 10.1017/cts.2016.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel, J. B., Del Fabbro, E., Arkenau, T., Higginson, I. J., Hurst, S., Jansen, L. A.,…Miller, F. G. (2016). Phase I cancer trials and palliative care: antagonism, irrelevance, or synergy? Journal of Pain and Symptom Management, 52(3), 437–445. 10.1016/j.jpainsymman.2016.02.014 [DOI] [PubMed] [Google Scholar]
- Chrisoulidou, A., Mandanas, S., Margaritidou, E., Mathiopoulou, L., Boudina, M., Georgopoulos, K., & Pazaitou-Panayiotou, K. (2015). Treatment compliance and severe adverse events limit the use of tyrosine kinase inhibitors in refractory thyroid cancer. OncoTargets and Therapy, 8, 2435–2442. 10.2147/ott.S86322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs, L. A., Noonan, K., Diane Barber, F., Mackey, H., Peterson, M. E., Turner, T., & LeFebvre, K. B. (2020). Oncology nurse practitioner competencies: Defining best practices in the oncology setting. Clinical Journal of Oncology Nursing, 24(3), 296–304. 10.1188/20.Cjon.296-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, T., Zhang, J., Meng, Y., Zhou, Y., & Li, W. (2018). Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore), 97(38), e12524. 10.1097/md.0000000000012524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drilon, A., Laetsch, T. W., Kummar, S., DuBois, S. G., Lassen, U. N., Demetri, G. D.,…Boni, V. (2018). Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. New England Journal of Medicine, 378(8), 731–739. 10.1056/nejmoa1714448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garralda, E., Dienstmann, R., Piris-Giménez, A., Braña, I., Rodon, J., & Tabernero, J. (2019). New clinical trial designs in the era of precision medicine. Molecular Oncology, 13(3), 549–557. 10.1002/1878-0261.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Laguna, I., Janku, F., Vaklavas, C., Falchook, G. S., Fu, S., Hong, D. S.,…Kurzrock, R. (2012). Validation of the Royal Marsden Hospital prognostic score in patients treated in the Phase I Clinical Trials Program at the MD Anderson Cancer Center. Cancer, 118(5), 1422–1428. 10.1002/cncr.26413 [DOI] [PubMed] [Google Scholar]
- Gordon, R., Kasler, M. K., Stasi, K., Shames, Y., Errante, M., Ciccolini, K.,…Fischer-Cartlidge, E. (2017). Checkpoint inhibitors: Common immune-related adverse events and their management. Clinical Journal of Oncology Nursing, 21(2 suppl), 45–52. 10.1188/17.Cjon.S2.45-52 [DOI] [PubMed] [Google Scholar]
- Haanen, J., Carbonnel, F., Robert, C., Kerr, K. M., Peters, S., Larkin, J., & Jordan, K. (2017). Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 28(suppl_4), iv119–iv142. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- Handley, N. R., Schuchter, L. M., & Bekelman, J. E. (2018). Best practices for reducing unplanned acute care for patients with cancer. JCO Oncology Practice, 14(5), 306–313. 10.1200/jop.17.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, S. K., Qian, K., Bisson, W. H., Watanabe-Smith, K., Huang, A., Bottomly, D.,…Tognon, C. E. (2020). Discovery and characterization of targetable NTRK point mutations in hematologic neoplasms. Blood, 135(24), 2159–2170. 10.1182/blood.2019003691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkonen, K., Tasmuth, T., Lehto, J. T., Kautiainen, H., Elme, A., Jääskeläinen, A.-S., & Saarto, T. (2019). Cancer patients’ symptom burden and health-related quality of life (HRQoL) at tertiary cancer center from 2006 to 2013: A cross-sectional study. Anticancer Research, 39(1), 271–277. 10.21873/anticanres.13107 [DOI] [PubMed] [Google Scholar]
- Kumar, V., Chaudhary, N., Garg, M., Floudas, C. S., Soni, P., & Chandra, A. B. (2017). Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Frontiers in Pharmacology, 8, 49. 10.3389/fphar.2017.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R., Cao, J., Gao, X., Zhang, J., Wang, L., Wang, B.,…Wang, Z. (2016). Overall survival of cancer patients with serum lactate dehydrogenase greater than 1000 IU/L. Tumor Biology, 37(10), 14083–14088. 10.1007/s13277-016-5228-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabelle, A., Le, D. T., Ascierto, P. A., Giacomo, A. M. D., Jesus-Acosta, A. D., Delord, J.-P.,…Diaz, Jr., L. A. (2020). Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: Results from the phase II KEYNOTE-158 study. Journal of Clinical Oncology, 38(1), 1–10. 10.1200/jco.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, H., DeRubeis, M. B., Foster, J. C., Taylor, J. M., & Worden, F. P. (2013). Outcomes evaluation of a weekly nurse practitioner-managed symptom management clinic for patients with head and neck cancer treated with chemoradiotherapy. Oncology Nursing Forum, 40(6), 581–586. 10.1188/13.Onf.40-06ap [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. (2019). NCCN Clinical Practice Guidelines in Oncology: Management of Immunotherapy-Related Toxicities. V1.2019 10.6004/jnccn.2019.0013 [DOI] [PubMed]
- Parikh, S. A., Achenbach, S. J., Call, T. G., Rabe, K. G., Ding, W., Leis, J. F.,…Shanafelt, T. D. (2020). The impact of dose modification and temporary interruption of ibrutinib on outcomes of chronic lymphocytic leukemia patients in routine clinical practice. Cancer Medicine, 9(10), 3390–3399. 10.1002/cam4.2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzanov, I., Diab, A., Abdallah, K., Bingham, C. O., 3rd, Brogdon, C., Dadu, R.,…Ernstoff, M. S. (2017). Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. Journal for Immunotherapy of Cancer, 5(1), 95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciuti, B., Genova, C., Crinò, L., Libra, M., & Leonardi, G. C. (2019). Antitumor activity of larotrectinib in tumors harboring NTRK gene fusions: A short review on the current evidence. OncoTargets and Therapy, 12, 3171–3179. 10.2147/ott.S177051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg, T. A. (2013). A nurse practitioner-led urgent care center: Meeting the needs of the patient with cancer. Clinical Journal of Oncology Nursing, 17(4), E52–E57. 10.1188/13.Cjon.E52-e57 [DOI] [PubMed] [Google Scholar]
- Sivendran, S., Holliday, R., Torre, K. D. L., & Newport, K. B. (2014). Impact of a nurse practitioner-staffed, symptom-management clinic on emergency department utilization in a large community oncology cancer institute. Journal of Clinical Oncology, 32(31_suppl), 58. 10.1200/jco.2014.32.31_suppl.58 [DOI] [Google Scholar]
- Thompson, J. A. (2018). New NCCN Guidelines: Recognition and management of immunotherapy-related toxicity. Journal of the National Comprehensive Cancer Network, 16(5S), 594–596. 10.6004/jnccn.2018.0047 [DOI] [PubMed] [Google Scholar]
- Wei, Y., Xu, H., Dai, J., Peng, J., Wang, W., Xia, L., & Zhou, F. (2018). Prognostic significance of serum lactic acid, lactate dehydrogenase, and albumin levels in patients with metastatic colorectal cancer. Biomed Research International, 2018, 1804086. 10.1155/2018/1804086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, J.-J., Jin, Y.-N., Wang, S.-Y., Zhang, F., Zhou, G.-Q., Zhang, W.-J.,…Sun, Y. (2018). The detrimental effects of radiotherapy interruption on local control after concurrent chemoradiotherapy for advanced T-stage nasopharyngeal carcinoma: An observational, prospective analysis. BMC Cancer, 18(1), 740. 10.1186/s12885-018-4495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]