Abstract
Bibliometric analysis is helpful to determine the most influential studies in a specific field. A large number of publications in anaphylaxis have been published. However, no bibliometric analysis of anaphylaxis was conducted based on our known. The aim of this study is to identify the top 100 most cited articles in anaphylaxis and analyze their bibliometric characteristics. We searched in the Web of Science core database on November 20, 2021. Articles were listed in descending order by their total citations. Hence the top 100 most cited articles in anaphylaxis were identified and analyzed. Bibliometric indicators included: year of publication, total number of citations and average citations per year (ACY), journal of publication and impact factor (IF), countries, institutes, and authors, which were analyzed by Biblioshiny. Co-occurrence was used to visualize the classification and hotspots. The top 100 most cited articles were published between 1991 and 2017. The largest number of articles was published in a single interval in 2006–2008. Total citations of the 100 articles were between 155 and 1241 and were positively correlated with the number of articles published in each 3-year interval. The top100 articles were published in 34 different journals. The Journal of Allergy and Clinical Immunology published the most (n = 41). The corresponding authors of the top100 articles were from 13 different countries, mostly in North America and Europe. Statistical analysis revealed a positive correlation between total number of citations and ACY (r = 0.670, p < 0.01) and between total number of citations and IF (r = 0.219, p < 0.05), whereas a negative correlation between ACY and length of time since publication (r = − 0.697, p < 0.01). The research focuses were classified into three clusters: (1) the epidemiology and management. (2) the risk factor and treatment. (3) the assessment and diagnosis. COVID-19 vaccines, drug allergy and management were the recent major topics. This bibliometric analysis reveals the progress and hotspots of research in anaphylaxis, which may lay a foundation for further research.
Keywords: Anaphylaxis, Bibliometric analysis, Biblioshiny
Introduction
Anaphylaxis is a serious systemic hypersensitivity reaction that is usually rapid in onset and may cause death. Severe anaphylaxis is characterized by potentially life-threatening compromise in breathing and/or the circulation and may occur without typical skin features or circulatory shock being present [1]. Recent studies have confirmed that the incidence of anaphylaxis has increased and poses a significant burden on population health and healthcare settings [2], [3]. With a better understanding of anaphylaxis, more and more articles have been published. However, the information in the database will also be more complex, making it difficult for clinicians to find most needed and valuable research.
Bibliometric analysis is the process of extracting measurable data through statistical analysis of published research studies, which can provide researchers with important messages in a specific field [4]. Citation analysis is one of bibliometric analysis methods that has been used to quantify the relative significance of a scientific article by examining the citations attributed to that paper. A thorough bibliometric analysis of the top 100 most cited articles can help the understanding of disciplinary development and future directions of a research field [5].
According to document index, some allergic diseases have been explored by bibliometric analysis, such as asthma [6], allergic rhinitis [7], and food allergy [8]. However, no bibliometric analysis of anaphylaxis was conducted based on our knowledge. Thus, this study aimed to identify the top 100 most cited articles in anaphylaxis and analyze their bibliometric characteristics.
Methods
Data sources and search strategies
Web of Science Core Database was chosen as the database to perform the literature source and bibliometric analysis for this study. The top100 most cited articles in anaphylaxis were retrieved from the database Web of Science Core Collection on November 20, 2021, with the following strategy: topic = anaphylaxis, from 1991 to 2021, no language limitation. Only article and review were included in analysis. Abstracts, editorials, proceeding papers, and book chapters articles were excluded. A total of 14,096 publications were retrieved from the Web of Science Core Database. Articles were ranked based on the total number of citations. If articles with the same total citation, recent articles were ranked higher.
Data extraction and bibliometric parameters
Research focusing on anaphylaxis or regarding anaphylaxis as a main part were included in this study. The two researchers examined the articles independently for qualify and finally reached an agreement on the list of the top 100 most cited articles. Then the top 100 list was imported into Biblioshiny and VOSviewer for bibliometric analysis.
Biblioshiny is a new advanced tool of bibliometric analyses [9, 10]. In this study, it was used it to perform a basic bibliometric analysis. To obtain more comprehensive information of the result based on co-occurrence which can visualize the research hotspots and classification, the study also constructed bibliometric maps by VOSviewer. Bibliometric indicators included year of publication, total number of citations and average citations per year (ACY), journal of publication and impact factor (IF), countries, institutes, and authors and co-occurrence network.
Statistical analysis
SPSS 19.0 was used for the statistical analysis. The Kolmogorov–Smirnov test was used to analyze the normal distribution of data. Spearman’s correlation was used to evaluate the association between total number of citations, ACY, IF, and length of time since publication. A p-value < 0.05 was accepted as statistically significant.
Ethical statement
This research did not involve intervention or data collection in animal experiments or clinical trials. Thus, approval from an ethical committee was not needed.
Results
The top 100 most cited articles are listed in Table 1, sorted in descending order according to the number of citations. In the top 100 list, 82 were articles and 18 were reviews.
Table 1.
List of the top 100 most cited articles in anaphylaxis(1991-2021)
| Rank | Article | Total Number of Citations | Average Citations per Year | Length of Time since Publication | IF |
|---|---|---|---|---|---|
| 1 | Sampson H A, Mendelson L, Rosen J P. Fatal and near-fatal anaphylactic reactions to food in children and adolescents[J]. New England Journal of Medicine, 1992, 327(6): 380–384 | 1243 | 41.43 | 30 | 91.253 |
| 2 | Bock S A, Muñoz-Furlong A, Sampson H A. Fatalities due to anaphylactic reactions to foods[J]. Journal of Allergy and Clinical Immunology, 2001, 107(1): 191–193 | 1173 | 55.86 | 21 | 10.793 |
| 3 | Chung C H, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1, 3-galactose[J]. New England journal of medicine, 2008, 358(11): 1109–1117 | 921 | 65.79 | 14 | 91.253 |
| 4 | Pumphrey R S H. Lessons for management of anaphylaxis from a study of fatal reactions[J]. Clinical and experimental allergy, 2000, 30(8): 1144–1150 | 693 | 31.50 | 22 | 5.018 |
| 5 | Brown S G A. Clinical features and severity grading of anaphylaxis[J]. Journal of Allergy and Clinical Immunology, 2004, 114(2): 371–376 | 548 | 30.44 | 18 | 10.793 |
| 6 | Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology[J]. Allergy, 2014, 69(8): 1026–1045 | 545 | 68.13 | 8 | 13.146 |
| 7 | Simons F E R, Ardusso L R F, Bilò M B, et al. World allergy organization guidelines for the assessment and management of anaphylaxis[J]. World Allergy Organization Journal, 2011, 4(2): 13–37 | 453 | 41.18 | 11 | 4.084 |
| 8 | Nelson H S, Lahr J, Rule R, et al. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract[J]. Journal of Allergy and Clinical Immunology, 1997, 99(6): 744–751 | 431 | 17.24 | 25 | 10.793 |
| 9 | Lieberman P, Nicklas R A, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update[J]. Journal of Allergy and Clinical immunology, 2010, 126(3): 477–480 | 401 | 33.42 | 12 | 10.793 |
| 10 | Commins S P, Satinover S M, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-α-1, 3-galactose[J]. Journal of Allergy and Clinical Immunology, 2009, 123(2): 426–433 | 388 | 29.85 | 13 | 10.793 |
| 11 | Sampson H A. Anaphylaxis and emergency treatment[J]. Pediatrics, 2003, 111(Supplement 3): 1601–1608 | 367 | 19.32 | 19 | 7.125 |
| 12* | Muraro A, Roberts G, Clark A, et al. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology[J]. Allergy, 2007, 62(8): 857–871 | 365 | 24.33 | 15 | 13.146 |
| 13 | Mertes P M, Laxenaire M C, Alla F. Anaphylactic and anaphylactoid reactions occurring during anesthesia in France in 1999–2000.[J]. Anesthesiology, 2003, 99(3):536 | 365 | 19.21 | 19 | 7.892 |
| 14 | Yocum M W, Butterfield J H, Klein J S, et al. Epidemiology of anaphylaxis in Olmsted County: a population-based study[J]. Journal of Allergy and Clinical Immunology, 1999, 104(2): 452–456 | 354 | 15.39 | 23 | 10.793 |
| 15 | Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis[J]. New England Journal of Medicine, 2008, 358(1): 28–35 | 346 | 24.71 | 14 | 91.253 |
| 16 | Turner P J, Gowland M H, Sharma V, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012[J]. Journal of Allergy and Clinical Immunology, 2015, 135(4): 956–963 | 337 | 48.14 | 7 | 10.793 |
| 17 | Simons F E R, Ardusso L R F, Bilò M B, et al. World Allergy Organization anaphylaxis guidelines: summary[J]. Journal of Allergy and Clinical Immunology, 2011, 127(3): 587–593 | 336 | 30.55 | 11 | 10.793 |
| 18 | Oettgen H C, Martin T R, Wynshaw-Boris A, et al. Active anaphylaxis in IgE-deficient mice[J]. Nature, 1994, 370(6488): 367–370 | 333 | 11.89 | 28 | 49.962 |
| 19 | Bernstein D I, Wanner M, Borish L, et al. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001[J]. Journal of Allergy and Clinical Immunology, 2004, 113(6): 1129–1136 | 332 | 18.44 | 18 | 10.793 |
| 20 | Li X M, Serebrisky D, Lee S Y, et al. A murine model of peanut anaphylaxis: T-and B-cell responses to a major peanut allergen mimic human responses[J]. Journal of Allergy and Clinical Immunology, 2000, 106(1): 150–158 | 329 | 14.95 | 22 | 10.793 |
| 21* | Bochner B S, Lichtenstein L M. Anaphylaxis.[J]. New England Journal of Medicine, 1991, 324(25):1785–1790 | 329 | 10.61 | 31 | 91.253 |
| 22 | Liew W K, Williamson E, Tang M L K. Anaphylaxis fatalities and admissions in Australia[J]. Journal of Allergy and Clinical Immunology, 2009, 123(2): 434–442 | 322 | 24.77 | 13 | 10.793 |
| 23 | Brockow K, Jofer C, Behrendt H, et al. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients[J]. Allergy, 2008, 63(2): 226–232 | 320 | 22.86 | 14 | 13.146 |
| 24 | Schwartz, Lawrence B. Diagnostic value of tryptase in anaphylaxis and mastocytosis.[J]. Immunology and Allergy Clinics of North America, 2006, 26(3):451–463 | 315 | 19.69 | 16 | 3.479 |
| 25 | Sampson H A, Muñoz-Furlong A, Campbell R L, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium[J]. Journal of Allergy and Clinical Immunology, 2006, 117(2): 391–397 | 314 | 19.63 | 16 | 10.793 |
| 26 | Dombrowicz D, Flamand V, Brigman K K, et al. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor α chain gene[J]. Cell, 1993, 75(5): 969–976 | 314 | 10.83 | 29 | 41.584 |
| 27 | Simons F E R, Ebisawa M, Sanchez-Borges M, et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines[J]. World Allergy Organization Journal, 2015, 8: 32 | 308 | 44.00 | 7 | 4.084 |
| 28* | Lieberman P, Camargo Jr C A, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American college of allergy, asthma and immunology epidemiology of anaphylaxis working group[J]. Annals of Allergy, Asthma and Immunology, 2006, 97(5): 596–602 | 306 | 19.13 | 16 | 6.347 |
| 29* | Kemp S F, Lockey R F. Anaphylaxis: a review of causes and mechanisms[J]. Journal of allergy and clinical immunology, 2002, 110(3): 341–348 | 301 | 15.05 | 20 | 10.793 |
| 30 | Blumchen K, Ulbricht H, Staden U, et al. Oral peanut immunotherapy in children with peanut anaphylaxis[J]. Journal of Allergy and Clinical Immunology, 2010, 126(1): 83–91 | 300 | 25.00 | 12 | 10.793 |
| 31* | Finkelman F D. Anaphylaxis: lessons from mouse models[J]. Journal of Allergy and Clinical Immunology, 2007, 120(3): 506–515 | 300 | 20.00 | 15 | 10.793 |
| 32 | Ruëff F, Przybilla B, Biló M B, et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase—a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity[J]. Journal of Allergy and Clinical Immunology, 2009, 124(5): 1047–1054 | 296 | 22.77 | 13 | 10.793 |
| 33 | Decker W W, Campbell R L, Manivannan V, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project[J]. Journal of Allergy and Clinical Immunology, 2008, 122(6): 1161–1165 | 287 | 20.50 | 14 | 10.793 |
| 34 | Miyajima I, Dombrowicz D, Martin T R, et al. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE-or IgG1-dependent passive anaphylaxis[J]. The Journal of clinical investigation, 1997, 99(5): 901–914 | 286 | 11.44 | 25 | 14.808 |
| 35 | Simons F E R. Anaphylaxis.[J]. Journal of Allergy and Clinical Immunology, 2010, 125(2):S161-S181 | 285 | 23.75 | 12 | 10.793 |
| 36 | Simons F E R, Roberts J R, Gu X, et al. Epinephrine absorption in children with a history of anaphylaxis[J]. Journal of Allergy and Clinical Immunology, 1998, 101(1): 33–37 | 273 | 11.38 | 24 | 10.793 |
| 37* | Neugut A I, Ghatak A T, Miller R L. Anaphylaxis in the United States: an investigation into its epidemiology[J]. Archives of internal medicine, 2001, 161(1): 15–21 | 272 | 12.95 | 21 | 17.333 |
| 38 | Tsujimura Y, Obata K, Mukai K, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis[J]. Immunity, 2008, 28(4): 581–589 | 268 | 19.14 | 14 | 31.745 |
| 39 | Poulos L M, Waters A M, Correll P K, et al. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993–1994 to 2004–2005[J]. Journal of Allergy and Clinical Immunology, 2007, 120(4): 878–884 | 261 | 17.40 | 15 | 10.793 |
| 40 | Grabenhenrich L B, Dölle S, Moneret-Vautrin A, et al. Anaphylaxis in children and adolescents: the European Anaphylaxis Registry[J]. Journal of Allergy and Clinical Immunology, 2016, 137(4): 1128–1137 | 259 | 43.17 | 6 | 10.793 |
| 41 | Brown A F T, McKinnon D, Chu K. Emergency department anaphylaxis: a review of 142 patients in a single year[J]. Journal of Allergy and Clinical Immunology, 2001, 108(5): 861–866 | 254 | 12.10 | 21 | 10.793 |
| 42 | Lieberman P, Nicklas R A, Randolph C, et al. Anaphylaxis—a practice parameter update 2015[J]. Annals of Allergy, Asthma and Immunology, 2015, 115(5): 341–384 | 253 | 36.14 | 7 | 6.347 |
| 43 | Bonadonna P, Perbellini O, Passalacqua G, et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels[J]. Journal of allergy and clinical immunology, 2009, 123(3): 680–686 | 251 | 19.31 | 13 | 10.793 |
| 44* | Moneret‐Vautrin D A, Morisset M, Flabbee J, et al. Epidemiology of life‐threatening and lethal anaphylaxis: a review[J]. Allergy, 2005, 60(4): 443–451 | 249 | 14.65 | 17 | 13.146 |
| 45 | Rivas M N, Burton O T, Wise P, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis[J]. Journal of Allergy and Clinical Immunology, 2013, 131(1): 201–212 | 245 | 27.22 | 9 | 10.793 |
| 46 | Laxenaire M C, Mertes P M, des Réactions Anaphylactoïdes G E. Anaphylaxis during anaesthesia. Results of a two‐year survey in France[J]. British Journal of Anaesthesia, 2001, 87(4): 549–558 | 237 | 11.29 | 21 | 9.166 |
| 47 | Laxenaire M C, Charpentier C, Feldman L. Anaphylactoid reactions to colloid plasma substitutes: incidence, risk factors, mechanisms. A French multicenter prospective study[C]Annales Francaises D'anesthesie et de Reanimation. 1994, 13(3): 301–310 | 230 | 8.21 | 28 | 1.131 |
| 48* | Hepner D L, Castells M C. Anaphylaxis during the perioperative period[J]. Anesthesia and Analgesia, 2003, 97(5): 1381–1395 | 229 | 12.05 | 19 | 5.178 |
| 49 | Mertes P M, Alla F, Tréchot P, et al. Anaphylaxis during anesthesia in France: an 8-year national survey[J]. Journal of Allergy and Clinical Immunology, 2011, 128(2): 366–373 | 225 | 20.45 | 11 | 10.793 |
| 50 | Bohlke K, Davis R L, DeStefano F, et al. Epidemiology of anaphylaxis among children and adolescents enrolled in a health maintenance organization[J]. Journal of Allergy and Clinical Immunology, 2004, 113(3): 536–542 | 223 | 12.39 | 18 | 10.793 |
| 51 | Mertes PM, Malinovsky JM, Jouffroy L, et al. Reducing the risk of anaphylaxis during anesthesia: 2011 updated guidelines for clinical Practice[J]. Journal of Investigational Allergology and Clinical Immunology. 2011;21(6):442–453 | 220 | 20.00 | 11 | 7.033 |
| 52 | Rüggeberg J U, Gold M S, Bayas J M, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data[J]. Vaccine, 2007, 25(31): 5675–5684 | 220 | 14.67 | 15 | 3.641 |
| 53 | Schwartz L B, Bradford T R, Rouse C, et al. Development of a new, more sensitive immunoassay for human tryptase: use in systemic anaphylaxis[J]. Journal of clinical immunology, 1994, 14(3): 190–204 | 220 | 7.86 | 28 | 8.317 |
| 54 | Strait R T, Morris S C, Yang M, et al. Pathways of anaphylaxis in the mouse[J]. Journal of Allergy and Clinical Immunology, 2002, 109(4): 658–668 | 219 | 10.95 | 20 | 10.793 |
| 55* | Pumphrey R. Anaphylaxis: can we tell who is at risk of a fatal reaction?[J]. Current opinion in allergy and clinical immunology, 2004, 4(4): 285–290 | 216 | 12.00 | 18 | 3.142 |
| 56 | Strait R T, Morris S C, Finkelman F D. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcγRIIb cross-linking[J]. The Journal of clinical investigation, 2006, 116(3): 833–841 | 213 | 13.31 | 16 | 14.808 |
| 57 | Kemp S F, Lockey R F, Wolf B L, et al. Anaphylaxis: a review of 266 cases[J]. Archives of internal medicine, 1995, 155(16): 1749–1754 | 213 | 7.89 | 27 | 17.333 |
| 58* | Panesar S S, Javad S, De Silva D, et al. The epidemiology of anaphylaxis in Europe: a systematic review[J]. Allergy, 2013, 68(11): 1353–1361 | 212 | 23.56 | 9 | 13.146 |
| 59 | Mangan N E, Fallon R E, Smith P, et al. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells[J]. The Journal of Immunology, 2004, 173(10): 6346–6356 | 212 | 11.78 | 18 | 5.422 |
| 60 | Laxenaire MC.Epidemiology of anesthetic anaphylactoid reactions. Fourth multicenter survey (July 1994-December 1996). Annales Francaises D'anesthesie et de Reanimation. 1999;18(7):796–809 | 212 | 9.22 | 23 | 1.131 |
| 61 | Kemp S F, Lockey R F, Simons F E R, et al. Epinephrine: the drug of choice for anaphylaxis–a statement of the World Allergy Organization[J]. World Allergy Organization Journal, 2008, 1: S18-S26 | 211 | 15.07 | 14 | 4.084 |
| 62 | Bohlke K, Davis R L, Marcy S M, et al. Risk of anaphylaxis after vaccination of children and adolescents[J]. Pediatrics, 2003, 112(4): 815–820 | 205 | 10.79 | 19 | 7.125 |
| 63 | Zabel B A, Nakae S, Zúñiga L, et al. Mast cell–expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis[J]. The Journal of experimental medicine, 2008, 205(10): 2207–2220 | 202 | 14.43 | 14 | 14.307 |
| 64 | Wood R A, Camargo Jr C A, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States[J]. Journal of Allergy and Clinical Immunology, 2014, 133(2): 461–467 | 201 | 25.13 | 8 | 10.793 |
| 65* | Simons F E R, Frew A J, Ansotegui I J, et al. Risk assessment in anaphylaxis: current and future approaches[J]. Journal of allergy and clinical immunology, 2007, 120(1): S2-S24 | 201 | 13.40 | 15 | 10.793 |
| 66 | Jerschow E, Lin R Y, Scaperotti M M, et al. Fatal anaphylaxis in the United States, 1999–2010: temporal patterns and demographic associations[J]. Journal of allergy and clinical immunology, 2014, 134(6): 1318–1328 | 196 | 24.50 | 8 | 10.793 |
| 67 | Vennekens R, Olausson J, Meissner M, et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4[J]. Nature immunology, 2007, 8(3): 312–320 | 196 | 13.07 | 15 | 25.606 |
| 68 | Yunginger J W, Nelson D R, Squillace D L, et al. Laboratory investigation of deaths due to anaphylaxis[J]. Journal of Forensic Science, 1991, 36(3): 857–865 | 191 | 6.16 | 31 | 1.832 |
| 69 | Jönsson F, Mancardi D A, Kita Y, et al. Mouse and human neutrophils induce anaphylaxis[J]. The Journal of clinical investigation, 2011, 121(4): 1484–1496 | 190 | 17.27 | 11 | 14.808 |
| 70 | Braganza S C, Acworth J P, McKinnon D R L, et al. Paediatric emergency department anaphylaxis: different patterns from adults[J]. Archives of disease in childhood, 2006, 91(2): 159–163 | 187 | 11.69 | 16 | 3.801 |
| 71 | Webb L M, Lieberman P. Anaphylaxis: a review of 601 cases[J]. Annals of Allergy, Asthma and Immunology, 2006, 97(1): 39–43 | 186 | 11.63 | 16 | 6.347 |
| 72 | Steinke J W, Platts-Mills T A E, Commins S P. The alpha-gal story: lessons learned from connecting the dots[J]. Journal of Allergy and Clinical Immunology, 2015, 135(3): 589–596 | 183 | 26.14 | 7 | 10.793 |
| 73 | Metcalfe D D, Peavy R D, Gilfillan A M. Mechanisms of mast cell signaling in anaphylaxis[J]. Journal of Allergy and Clinical Immunology, 2009, 124(4): 639–646 | 183 | 14.08 | 13 | 10.793 |
| 74* | Simons F E R, Ardusso L R F, Bilo M B, et al. 2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxis[J]. Current opinion in allergy and clinical immunology, 2012, 12(4): 389–399 | 180 | 18.00 | 10 | 3.142 |
| 75 | Cox L, Platts-Mills T A E, Finegold I, et al. American academy of allergy, asthma & immunology/American college of allergy, asthma and immunology joint task force report on omalizumab-associated anaphylaxis[J]. Journal of allergy and clinical immunology, 2007, 120(6): 1373–1377 | 179 | 11.93 | 15 | 10.793 |
| 76 | Gold M S, Sainsbury R. First aid anaphylaxis management in children who were prescribed an epinephrine autoinjector device (EpiPen)[J]. Journal of Allergy and Clinical Immunology, 2000, 106(1): 171–176 | 179 | 8.14 | 22 | 10.793 |
| 77* | Simons F E R, Ardusso L R F, Dimov V, et al. World Allergy Organization Anaphylaxis Guidelines: 2013 update of the evidence base[J]. International archives of allergy and immunology, 2013, 162(3): 193–204 | 177 | 19.67 | 9 | 2.749 |
| 78 | Dohi M, Suko M, Sugiyama H, et al. Food-dependent, exercise-induced anaphylaxis: a study on 11 Japanese cases[J]. Journal of allergy and clinical immunology, 1991, 87(1): 34–40 | 177 | 5.71 | 31 | 10.793 |
| 79 | Palosuo K, Alenius H, Varjonen E, et al. A novel wheat gliadin as a cause of exercise-induced anaphylaxis[J]. Journal of Allergy and Clinical Immunology, 1999, 103(5): 912–917 | 176 | 7.65 | 23 | 10.793 |
| 80 | Ownby D R, Tomlanovich M, Sammons N, et al. Anaphylaxis associated with latex allergy during barium enema examinations[J]. AJR. American journal of roentgenology, 1991, 156(5): 903–908 | 174 | 5.61 | 31 | 3.959 |
| 81 | Yocum, M. W., & Khan, D. A. (1994). Assessment of patients who have experienced anaphylaxis: a 3-year survey. Mayo Clinic Proceedings 69(1), 16–23 | 172 | 6.14 | 28 | 7.619 |
| 82 | Vultaggio A, Matucci A, Nencini F, et al. Anti‐infliximab IgE and non‐IgE antibodies and induction of infusion‐related severe anaphylactic reactions[J]. Allergy, 2010, 65(5): 657–661 | 170 | 14.17 | 12 | 13.146 |
| 83* | Lieberman P. Biphasic anaphylactic reactions[J]. Annals of allergy, asthma and immunology, 2005, 95(3): 217–226 | 170 | 10.00 | 17 | 6.347 |
| 84 | Pumphrey R S H, Roberts I S D. Postmortem findings after fatal anaphylactic reactions[J]. Journal of clinical pathology, 2000, 53(4): 273–276 | 170 | 7.73 | 22 | 3.411 |
| 85 | Srivastava K D, Kattan J D, Zou Z M, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy[J]. Journal of Allergy and Clinical Immunology, 2005, 115(1): 171–178 | 168 | 9.88 | 17 | 10.793 |
| 86* | Turner P J, Jerschow E, Umasunthar T, et al. Fatal anaphylaxis: mortality rate and risk factors[J]. The Journal of Allergy and Clinical Immunology: In Practice, 2017, 5(5): 1169–1178 | 167 | 33.40 | 5 | 8.861 |
| 87 | Mullins R J. Anaphylaxis: risk factors for recurrence[J]. Clinical and Experimental Allergy, 2003, 33(8): 1033–1040 | 167 | 8.79 | 19 | 5.018 |
| 88 | Helbling A, Hurni T, Mueller U R, et al. Incidence of anaphylaxis with circulatory symptoms: a study over a 3‐year period comprising 940 000 inhabitants of the Swiss Canton Bern[J]. Clinical and Experimental Allergy, 2004, 34(2): 285–290 | 166 | 9.22 | 18 | 5.018 |
| 89 | Novembre E, Cianferoni A, Bernardini R, et al. Anaphylaxis in children: clinical and allergologic features[J]. Pediatrics, 1998, 101(4): | 166 | 6.92 | 24 | 7.125 |
| 90 | Ross M P, Ferguson M, Street D, et al. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System[J]. Journal of Allergy and Clinical Immunology, 2008, 121(1): 166–171 | 165 | 11.79 | 14 | 10.793 |
| 91* | Kroigaard M, Garvey L H, Gillberg L, et al. Scandinavian Clinical Practice Guidelines on the diagnosis, management and follow‐up of anaphylaxis during anaesthesia[J]. Acta anaesthesiologica scandinavica, 2007, 51(6): 655–670 | 165 | 11.00 | 15 | 2.105 |
| 92 | Kelso J M, Jones R T, Yunginger J W. Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin[J]. Journal of allergy and clinical immunology, 1993, 91(4): 867–872 | 165 | 5.69 | 29 | 10.793 |
| 93 | Olivera A, Mizugishi K, Tikhonova A, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis[J]. Immunity, 2007, 26(3): 287–297 | 164 | 10.93 | 15 | 31.745 |
| 94 | Akin C, Scott L M, Kocabas C N, et al. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis[J]. Blood, 2007, 110(7): 2331–2333 | 164 | 10.93 | 15 | 23.629 |
| 95 | Haeberli G, Brönnimann M, Hunziker T, et al. Elevated basal serum tryptase and hymenoptera venom allergy: relation to severity of sting reactions and to safety and efficacy of venom immunotherapy[J]. Clinical and Experimental Allergy, 2003, 33(9): 1216–1220 | 164 | 8.63 | 19 | 5.018 |
| 96 | Mehl A, Wahn U, Niggemann B. Anaphylactic reactions in children–a questionnaire‐based survey in Germany[J]. Allergy, 2005, 60(11): 1440–1445 | 163 | 9.59 | 17 | 13.146 |
| 97 | Shida K, Takahashi R, Iwadate E, et al. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model[J]. Clinical and Experimental Allergy, 2002, 32(4): 563–570 | 163 | 8.15 | 20 | 5.018 |
| 98* | De Silva I L, Mehr S S, Tey D, et al. Paediatric anaphylaxis: a 5 year retrospective review[J]. Allergy, 2008, 63(8): 1071–1076 | 158 | 11.29 | 14 | 13.146 |
| 99* | Simons F E R. Anaphylaxis: recent advances in assessment and treatment[J]. Journal of Allergy and Clinical Immunology, 2009, 124(4): 625–636 | 156 | 12.00 | 13 | 10.793 |
| 100 | Shadick N A, Liang M H, Partridge A J, et al. The natural history of exercise-induced anaphylaxis: survey results from a 10-year follow-up study[J]. Journal of allergy and clinical immunology, 1999, 104(1): 123–127 | 156 | 6.78 | 23 | 10.793 |
*Review
Year of publication
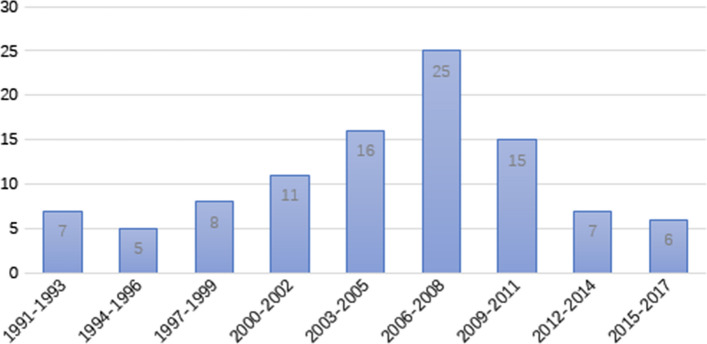
The top 100 most cited articles were published between 1991 and 2017. The number of articles published in each 3-year interval followed normal distribution (p = 0.682). The largest number of articles published in a single interval was 25, which occurred in 2006–2008.
Figure 1 shows the number of articles published in each 3-year interval.
Fig. 1.
The number of articles published in each 3 year interval
Citations
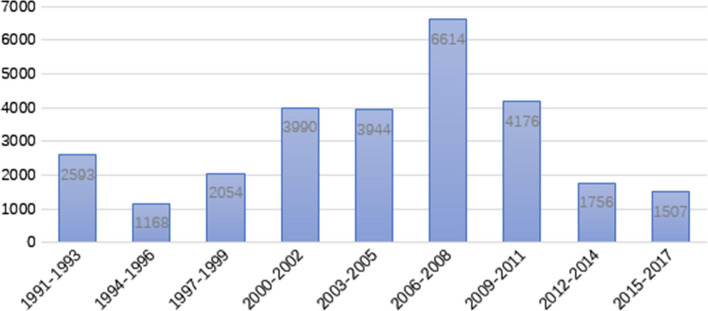
The total citations of articles in each 3-year interval are shown in Fig. 2. The total number of citations in each 3-year interval followed normal distribution (p = 0.962). The largest total number of citations in each 3-year interval was also 2006–2008. We identified a positive correlation between total citations and the number of articles published in each 3-year interval (r = 0.921, p < 0.01). Total citations of the 100 articles were between 156 and 1243, with a mean of 278.02. Top 10 most cited articles accounted for 24.44% (n = 6796) of the total citations. Average citations per year (ACY) of the top 100 articles were from 5.61 to 68.13, with a mean of 18.54. Six of the top 10 most cited articles according to total citations still ranked in the top 10 list according to average citations per year. We observed that the most cited article among the top 100 list was a study by Sampson HA with the following title: “Fatal and Near-Fatal Anaphylactic Reactions to Food in Children and Adolescents” published in New England Journal of Medicine 1992.
Fig. 2.
The number of citations in each 3 year interval
Journal of publication
Journals and their impact factor are listed in Table 2.
Table 2.
List of journals from the top 100 articles
| Journal | Number of articles | Impact factor |
|---|---|---|
| Journal of Allergy and Clinical Immunology | 41 | 10.793 |
| Allergy | 9 | 13.146 |
| Clinical and Experimental Allergy | 5 | 5.018 |
| Annals of Allergy Asthma and Immunology | 4 | 6.347 |
| New England Journal of Medicine | 4 | 91.253 |
| Journal of Clinical Investigation | 3 | 14.808 |
| Pediatrics | 3 | 7.125 |
| Annales Francaises D Anesthesie et de Reanimation | 2 | 1.131 |
| Archives of Internal Medicine | 2 | 17.333 |
| Current Opinion in Allergy and Clinical Immunology | 2 | 3.142 |
| Immunity | 2 | 31.745 |
| Acta Anaesthesiologica Scandinavica | 1 | 2.105 |
| American Journal of Roentgenology | 1 | 3.959 |
| Anesthesia and Analgesia | 1 | 5.178 |
| Anesthesiology | 1 | 7.892 |
| Annals of Emergency Medicine | 1 | 5.721 |
| Archives of Disease in Childhood | 1 | 3.801 |
| Blood | 1 | 23.629 |
| British Journal of Anaesthesia | 1 | 9.166 |
| Cell | 1 | 41.584 |
| Immunology and Allergy Clinics of North America | 1 | 3.479 |
| International Archives of Allergy and Immunology | 1 | 2.749 |
| Journal of Allergy and Clinical Immunology-In Practice | 1 | 8.861 |
| Journal of Clinical Immunology | 1 | 8.317 |
| Journal of Clinical Pathology | 1 | 3.411 |
| Journal of Experimental Medicine | 1 | 14.307 |
| Journal of Forensic Sciences | 1 | 1.832 |
| Journal of Immunology | 1 | 5.422 |
| Journal of Investigational Allergology And Clinical Immunology | 1 | 7.033 |
| Mayo Clinic Proceedings | 1 | 7.619 |
| Nature | 1 | 49.962 |
| Nature Immunology | 1 | 25.606 |
| Vaccine | 1 | 3.641 |
| World Allergy Organization Journal | 1 | 4.084 |
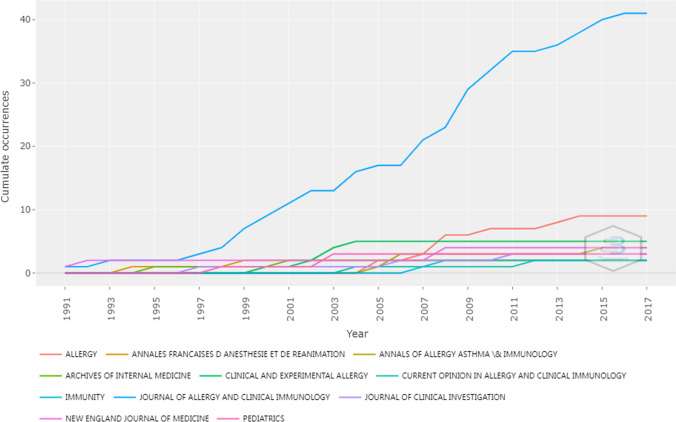
The top 100 articles were published in 34 different journals. The Journal of Allergy and Clinical Immunology published the most (n = 41), followed by Allergy (n = 9) and Clinical and Experimental Allergy (n = 5). 77% articles of top 100 were published in 11 journals. We determined that the IF of these 34 journals was from 1.131 to 91.253 (according to Clarivate Analytics 2020). There was no correlation between IF and number of publications (p > 0.05).
Figure 3 shows the dynamic changes of articles published in major journals. Since the middle 1990s, the publications of The Journal of Allergy and Clinical Immunology began to increase rapidly compared with other journals.
Fig. 3.
Dynamic changes in the number of articles in major journals
Correlation analysis between citations, ACY, IF, and publication time
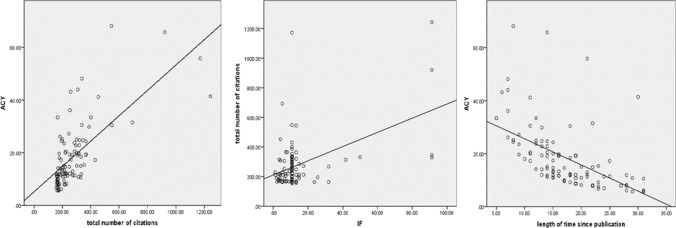
The correlation analysis for the total number of citations, ACY, IF, and length of time since publication in the top 100 list revealed a positive correlation between total number of citations and ACY (r = 0.670, p < 0.01), and between total number of citations and IF (r = 0.219, p < 0.05), whereas a negative correlation was observed between ACY and length of time since publication (r = -0.697, p < 0.01). There was no correlation between total number of citations and length of time since publication or between ACY and IF or between length of time since publication and IF (Fig. 4).
Fig. 4.
Correlation analysis between citations, ACY, IF, and publication time
Countries collaboration, affiliations, and authors
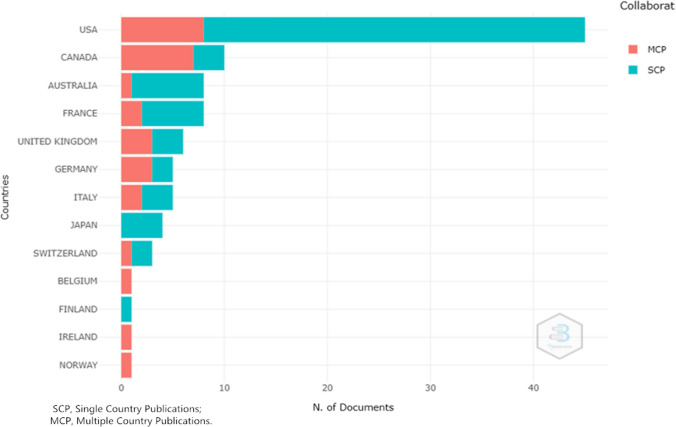
The data extracted from web of science indicated that the corresponding authors of the top 100 articles were from 13 different countries (Fig. 5). Most of the top 100 cited articles were from the USA (n = 46), followed by Canada (n = 10), Australia (n = 8), and France (n = 8).
Fig. 5.
Corresponding author’s country
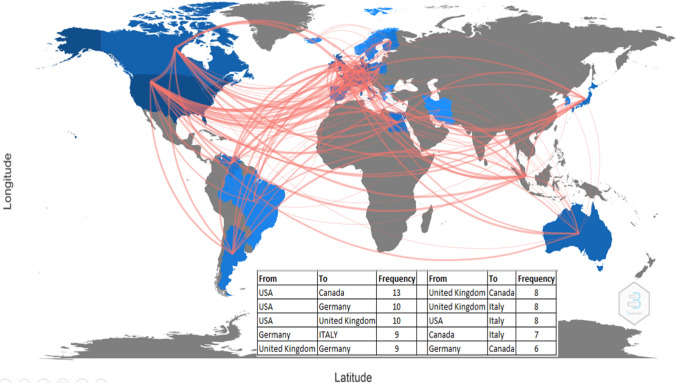
Figure 6 shows the country collaboration map worldwide generated by the Biblioshiny.
Fig. 6.
Country collaboration map
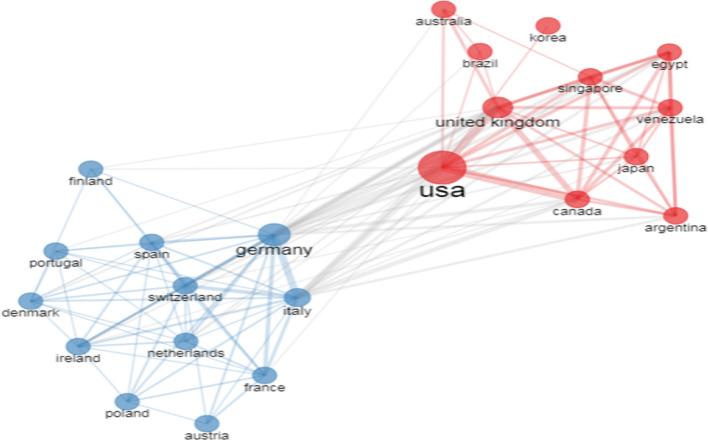
There were 219 pairs of collaborating countries worldwide, of which the top 3 were the USA and Canada with 13 collaborations, followed by the USA and Germany with 10 collaborations, as well as the USA with UK with 10 collaborations. Collaboration Network according to the Biblioshiny is shown in Fig. 7. We observed that the collaboration network had two clusters, one was the continental European countries, and the other mainly included the USA, Canada, Australia, and UK.
Fig. 7.
.Collaboration network
As to the most relevant affiliations according to Biblioshiny, University of Manitoba published the most articles (n = 21), followed by Harvard University, University of Cincinnati (n = 13), and University of Florida (n = 10) (Table 3).
Table 3.
List of the top10 affiliations in the number of articles
| Affiliations | Articles |
|---|---|
| UNIV MANITOBA | 21 |
| HARVARD UNIV | 13 |
| UNIV CINCINNATI | 13 |
| UNIV S FLORIDA | 10 |
| JOHNS HOPKINS UNIV | 8 |
| TECH UNIV MUNICH | 8 |
| UNIV EDINBURGH | 8 |
| UNIV HOSP | 8 |
| UNIV TENNESSEE | 8 |
| UNIV WASHINGTON | 8 |
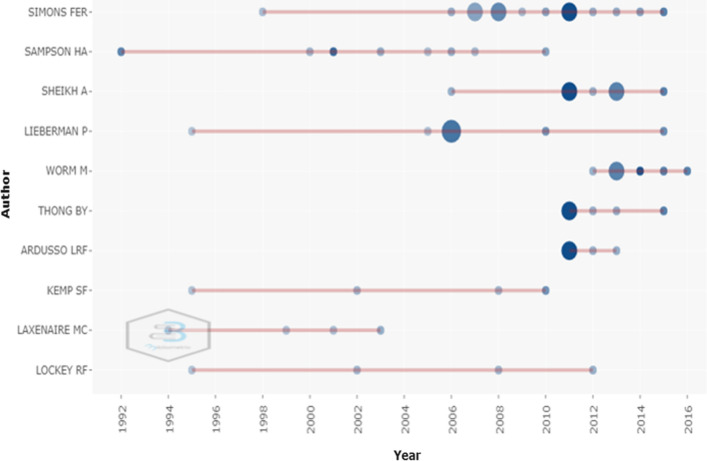
The top 10 most prolific authors demonstrated by Biblioshiny was that Simons FER from University of Manitoba produced the most top-cited articles in anaphylaxis(n = 14), followed by Sampson HA(n = 8), Sheikh A(n = 7), Lieberman P(n = 6), Worm M(n = 6), Thong BY (n = 5), Ardusso LRF(n = 4), Kemp SF(n = 4), Laxenaire MC(n = 4), Lockey RF(n = 4).
Figure 8 shows the details of the top 10 authors and their productions over time. The larger the circle, the more articles published. The deeper the color, the more citations.
Fig. 8.
Top-autors’ production over the time
The analysis of co-occurrence network
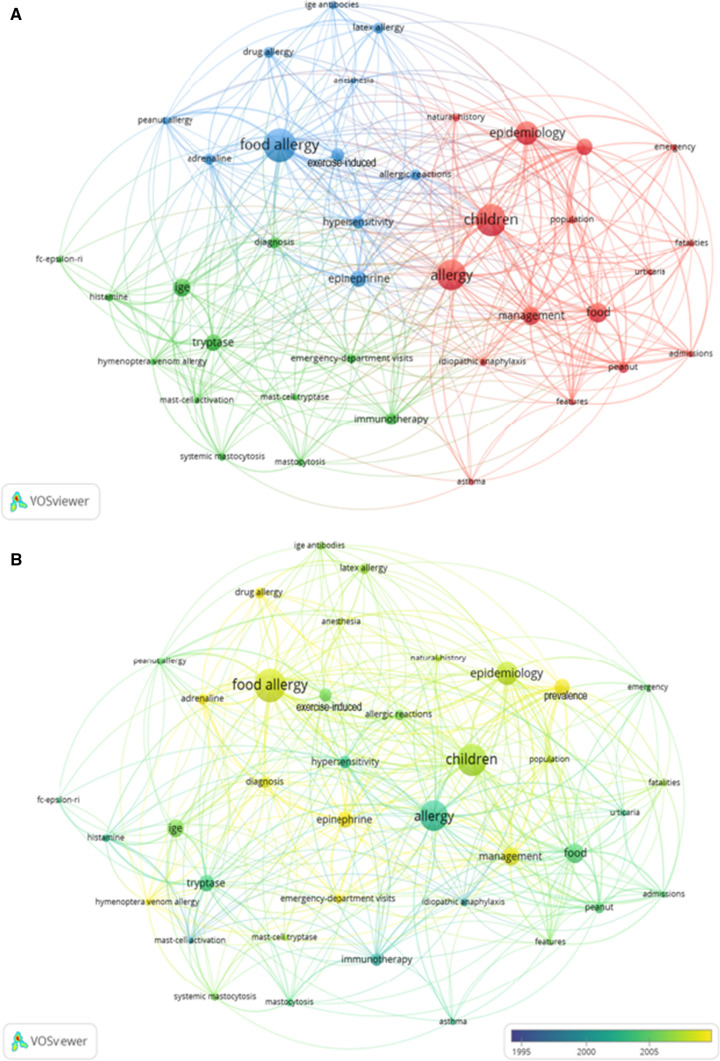
Apart from searching term “anaphylaxis,” authors keywords and keywords plus extracted from the top 100 articles were analyzed by VOSviewer (Fig. 9). According to Fig. 9A, different colors represent different clusters and the size of the ball reflects the frequency of keywords. It was observed that the research focus was classified into three clusters, marked with red, blue, and green, respectively. In addition, the color of keywords corresponds to the average publication year as shown in Fig. 9B. The recent keywords after 2005 mainly included “drug allergy,” “adrenaline,” “prevalence,” “diagnosis,” and “management.”
Fig. 9.
.Co-occurrence network
Recent articles
No articles published after 2017 were observed in the top 100 list. In order to better show the recent research hotspots, we retrieved the top 10 most cited articles published from 2018 to 2021 (Table 4). The total citations of the top 10 articles were from 58 to 151, and ACY of them were between 14.5 and 102. Nine of the top 10 most cited articles were original articles, and one was review. The correlation analysis for the total number of citations, ACY, IF, and length of time since publication in the top 10 list revealed a negative correlation between ACY and length of time since publication (r = − 0.782, p < 0.01). However, there was no correlation between the other parameters, which was different from the results of top 100. The research hotspots of the top 10 articles were COVID-19 vaccine (n = 3), pathogenesis (n = 3), perioperative anaphylaxis (n = 1), diagnosis (n = 1), and others (n = 2).
Table 4.
List of the top 10 most cited articles in anaphylaxis(2018-2021)
| Rank | Article | Total Number of Citations | Average Citations per Year | Length of Time since Publication | IF |
|---|---|---|---|---|---|
| 1 | Harper N J N, Cook T M, Garcez T, et al. Anaesthesia, surgery, and life-threatening allergic reactions: epidemiology and clinical features of perioperative anaphylaxis in the 6th National Audit Project (NAP6)[J]. British Journal of Anaesthesia, 2018, 121(1):159 | 151 | 37.75 | 4 | 9.166 |
| 2* | Mss A, Dvw B, Dbkg C, et al. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis[J]. Journal of Allergy and Clinical Immunology, 2020, 145( 4):1082–1123 | 111 | 55.5 | 2 | 10.793 |
| 3 | Gowthaman U, Chen J S, Zhang B, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE[J]. Science, 2019, 365(6456):eaaw6433 | 110 | 36.67 | 3 | 47.728 |
| 4 | CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020. MMWR-MORBIDITY AND MORTALITY WEEKLY REPORT. 2021;70(2):46–51 | 102 | 102 | 1 | 17.586 |
| 5 | Worm M, Francuzik W, Renaudin J M, et al. Factors increasing the risk for a severe reaction in anaphylaxis: An analysis of data from The European Anaphylaxis Registry[J]. Allergy, 2018 | 87 | 21.75 | 4 | 13.146 |
| 6 | Aba B, Pgwb C, Saff A R, et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach[J]. The Journal of Allergy and Clinical Immunology: In Practice, 2020 | 84 | 42 | 2 | 8.861 |
| 7 | CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine—United States, December 21, 2020-January 10, 2021.MMWR-MORBIDITY AND MORTALITY WEEKLY REPORT. 2021;70(4):125–129 | 67 | 67 | 1 | 17.586 |
| 8 | Leyva-Castillo J M, Galand C, Kam C, et al. Mechanical Skin Injury Promotes Food Anaphylaxis by Driving Intestinal Mast Cell Expansion[J]. Immunity, 2019 | 59 | 19.67 | 3 | 31.745 |
| 9 | Cardona V, Ansotegui I J, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020[J]. World Allergy Organization Journal, 2020, 13(10): 100,472 | 58 | 29 | 2 | 4.084 |
| 10 | Bahri R, Custovic A, Korosec P, et al. Mast cell activation test in the diagnosis of allergic disease and anaphylaxis[J]. Journal of Allergy and Clinical Immunology, 2018, 142(2): 485–496. e16 | 58 | 14.5 | 4 | 10.793 |
*Review
Discussion
Bibliometric analysis explores the characteristics of published articles based on specific and reliable parameters [11]. The top 100 most cited articles may be the highly recognized articles in a certain area, and analysis of these articles may quantitatively determine primary research concerns and provide information about dynamic research changes.
Among the top 100 list, the largest number of articles published in a single interval is between 2006 and 2008, which may be mainly attributed to the publication of the article in 2006 entitled “Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium” with 314 citations. Even though anaphylaxis was first described around 100 years ago, there was no universal agreement on its definition or criteria for diagnosis, which greatly hampered research into the epidemiology, pathophysiology, and management of anaphylaxis. This article provided a definition of anaphylaxis and clinical criteria for diagnosis, which became a landmark in anaphylaxis research. Subsequently, several important biomarkers were confirmed, such as platelet-activating factor [12] and tryptase [13]. In addition, owe to the technological progress and clinical research, galactose-α-1,3-galactose [14] and extensive skin disease with mastocytosis [15] were also confirmed as the important risk factors of anaphylaxis.
The number of citations generated by a paper is an indicator of the impact on the research field, but not necessarily the quality and eminence of it [16]. It would accumulate over time, so the time strongly influenced the impact assessment through citation analysis. Garfield demonstrated that older papers had more chance to be cited [17], and even the most cited papers had no citation when they were just published. Therefore, we use ACY to try to reduce this bias; however, we illustrated a positive correlation between total citations and ACY among top 100 list. It indicates that there may be a positive correlation between ACY and total citations over time.
Our analysis showed that the majority of the top 100 articles were published in The Journal of Allergy and Clinical Immunology followed by Allergy and Clinical and Experimental Allergy.
We found that there was no correlation between IF and number of articles, which indicated that IF may not represent the production of journals in top 100 list. Such findings were similar to bibliometric analyses of allergic rhinitis [7]. The correlation analysis also showed a positive correlation between total number of citations and IF, whereas a negative correlation was observed between ACY and length of time since publication. It may be explained by the inherent bias that researchers tended to choose high impact factor journals for citation and younger articles have higher ACY.
The publication of papers in different countries may reflect the influence of the country in the field of anaphylaxis to some extent. We identified the corresponding authors from 13 different countries of the top 100 most cited articles. Simons FER from University of Manitoba published the most articles, while most articles were from the USA. The European and the North America are two major research collaboration networks. Despite the relatively high prevalence in these areas, different degrees of economic growth might also explain this phenomenon.
Co-occurrence of keywords analysis, aiming to investigate the co-occurring relationships between keywords in a set of publications, can show the different research hotspots and topics [18]. According to the co-occurrence network by VOSviewer, we found that cluster 1 (red) was mainly about the epidemiology and management, cluster 2 (blue) mainly focused on the risk factor and treatment, cluster 3 (green) mainly reflected the assessment and diagnosis of anaphylaxis (Fig. 9A). According to Fig. 9B, we can see that the recent hotspots mainly on drug allergy, optimizing treatment as well as management, and improving clinical diagnosis.
Although co-occurrence analysis has shown the research hotspots in recent years, the latest research hotspots may not be shown as no articles published after 2017 in top 100 list, so we retrieved the top 10 most cited articles published from 2018 to 2021. We found that the number of articles related to COVID-19 vaccines has increased sharply (Table 4), as the COVID-19 vaccination is an essential way to control the current pandemic situation. Although anaphylaxis due to a vaccine is rare, it can lead to fear and undermine public confidence [19]; therefore, the importance of vaccine safety research is self-evident.
Although we spared no effort to eliminate potential defects in this bibliometric analysis, some limitations were inevitable. First, although Web of Science Core Database is a recommended database for clinical research and a relatively reliable source, the citations of articles may be insufficient and affect the results of bibliometric analysis. Second, the definition of anaphylaxis has been controversial for many years; therefore, when we regard the key word “anaphylaxis” as the search subject word, we may miss some relevant articles. Besides, citation ranking may not be intended to measure quality, but rather a degree of recognition. Low citation numbers may not represent low research value, and historical high citation numbers also cannot represent the longtime influence in the future. Despite these defects, the data presented here provide insight into the outline of anaphylaxis research over the past 30 years. In conclusion, this study identified the top 100 most cited articles in anaphylaxis and showed their bibliometric characteristics, which may pave the way for further study.
Acknowledgements
Not applicable
Authors' contributions
Y. Song was responsible for methodology, data analysis, writing and submission. L.S Zhang and Y.S Yang contributed the data analysis and draft writing. J.L Sun designed and supervised the study.
Funding
There is no funding for the study.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethical approval
This research did not involve intervention or data collection in animal experiments or clinical trials. Thus, approval from an ethical committee was not needed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu Song, Email: s.yu@pku.edu.cn.
Jinlyu Sun, Email: sunjinlv@pumch.cn.
References
- 1.Cardona V, Ansotegui IJ, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organiz J. 2020;13(10): [DOI] [PMC free article] [PubMed]
- 2.Pjtfa B, Decfb C, Msm D, et al. Global trends in anaphylaxis epidemiology and clinical implications[J]. J Allergy Clinical Immunol Practice, 2020; 8. [DOI] [PMC free article] [PubMed]
- 3.Sloane D, Govindarajulu U, Harrow-Mortelliti J, et al. Safety, costs, and efficacy of rapid drug desensitizations to chemotherapy and monoclonal antibodies[J]. J Allergy Clinical Immunol Practice, 2016;497–504. [DOI] [PubMed]
- 4.Valérie Durieux MA, Gevenois PA. Bibliometric indicators: quality measurements of scientific publication. Radiology. 2010;255(2):342–351. doi: 10.1148/radiol.09090626. [DOI] [PubMed] [Google Scholar]
- 5.Shi J, Du X, Cavagnaro M J, et al. A Bibliometric Analysis and Visualization of the Top-Cited Publications in Mild Traumatic Brain Injury. Front Neurol, 2021;12. [DOI] [PMC free article] [PubMed]
- 6.Qu Y, Chen Z, Hu Z, et al. The 100 most influential publications in asthma from 1960 to 2017: A bibliometric analysis[J] Respir Med. 2018;137:206–212. doi: 10.1016/j.rmed.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Wu Q, R Zheng, Wang W, et al. The top 100 most influential articles in allergic rhinitis from 1970 to 2018: A bibliometric analysis. J Int Med Res, 2019, 47(12) [DOI] [PMC free article] [PubMed]
- 8.Vanga S, Singh A, Vagadia BH, et al. Global food allergy research trend: a bibliometric analysis[J] Scientometrics. 2015;105(1):203–213. doi: 10.1007/s11192-015-1660-0. [DOI] [Google Scholar]
- 9.Aria M, Cuccurullo C. bibliometrix: An R-Tool for comprehensive science mapping analysis. J Informet. 2018;11(4):959–975. doi: 10.1016/j.joi.2017.08.007. [DOI] [Google Scholar]
- 10.Xie H, Zhang Y, Wu Z, et al. A bibliometric analysis on land degradation current status development and future directions. Land. 2020;9(1):28. doi: 10.3390/land9010028. [DOI] [Google Scholar]
- 11.Zhang Y, Chen Y. Research trends and areas of focus on the Chinese Loess Plateau: A bibliometric analysis during 1991–2018[J]. Catena, 2020; 194:
- 12.Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and anaphylaxis. New England J Med. 2008;358(1):1516–1517. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clinics North America. 2006;26(3):451–463. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brockow K, Jofer C, Behrendt H, et al. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients[J] Allergy. 2010;63(2):226–232. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 16.Cheek J, Garnham B, Quan J. What's in a Number? Issues in providing evidence of impact and quality of research(ers)[J] Qual Health Res. 2006;16(3):423–435. doi: 10.1177/1049732305285701. [DOI] [PubMed] [Google Scholar]
- 17.Garfield E. The history and meaning of the journal impact factor[J] JAMA. 2006;295(1):90. doi: 10.1001/jama.295.1.90. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Hu J, Deng S, et al. Comprehensive bibliometric analysis of the kynurenine pathway in mood disorders: focus on gut microbiota research. Front Pharmacol, 2021; 12. [DOI] [PMC free article] [PubMed]
- 19.Aba B, Pgwb C, Saff AR, et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J Allergy Clinical Immunol Practice, 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.