Abstract
Background:
Marijuana is the most-used illicit substance during pregnancy in the USA, but only two cohort studies, begun over 30 years ago, were specifically established to assess the association of pregnancy use with childhood outcomes. They found use to be associated with specific deficits in executive function at 8+ years, but did not focus on these outcomes earlier in life when intervention may be more successful. Two general purpose cohorts found increased aggression in exposed female toddlers and increased behavioural problems and tic disorders in exposed school-age children.
Objectives:
The Lifestyle and Early Achievement in Families (LEAF) study assesses the association of in utero marijuana exposure, documented prospectively by biomarker, self-report, and medical records, with executive function and aggression at age 3½-7 years.
Methods:
This ambidirectional cohort (historical cohort with continued follow-up) includes women enrolled in the Perinatal Research Repository during prenatal care at Ohio State University Wexner Medical Center and their children, recontacted 3½-7 years post-birth. Children complete 1–2 study visits including cognitive testing, behavioural observation, and maternal and teacher report of behaviour. Family and social environmental factors are assessed.
Results:
Child follow-up began in September 2016; visits continue through August 2020. There are 362 eligible children; 32% had mothers who used marijuana during pregnancy, 10% of mothers completed college, and 23% did not complete high school. Mean maternal age at study registration in pregnancy was 26.4 years, and 63% of mothers were African American. To date, 268 children have completed at least 1 study visit.
Conclusions:
The LEAF Study will document the association of prenatal marijuana exposure with development and behaviour in the current era when marijuana is more potent than when previous cohorts were studied. The results may inform policy and interventions to counsel reproductive-aged women about the risks of use during pregnancy and guide prevention and treatment of adverse effects among children.
Keywords: aggression, cannabis, child development, executive function, marijuana
1 |. BACKGROUND
Marijuana is the most commonly used illicit substance in the USA, and its use is increasing dramatically with state-level legalisation. A recent study examining the prevalence and frequency of cannabis use among pregnant women found large increases between 2002 and 2017, with reports of past month use increasing from 3.4% to 7.0%, and reports of first trimester use increasing from 5.7% to 12.1%.1 Yet, despite warnings from the American College of Obstetricians and Gynecologists to discontinue prenatal use because of “worrisome trends” and “concerns regarding impaired neurodevelopment” in offspring,2 roughly 70% of pregnant women believe using marijuana once or twice per week is associated with no or only negligible risk of harm.3 As a consequence, the USA can expect a notable increase in the number of children prenatally exposed to marijuana in the coming years.
Policy-makers, physicians, and women of reproductive age are seeking evidence from rigorous studies to inform decision making, but unfortunately, “our understanding of the long-term effects of prenatal exposure to marijuana in humans is very poor.”4 Only two major North American cohort studies were designed for this purpose: The Ottawa Prenatal Prospective Study, (OPPS), based on a middle class sample, and The Maternal Health Practices and Child Development Study (MHPCDS), based on a generally lower socio-economic status sample.5–7 Both cohorts reported that prenatal marijuana is a neuroteratogen that predicts impaired executive function (EF) and in the former study, delinquent behaviour manifesting in middle childhood, but neither study carefully evaluated EF at preschool age, when remediation is more feasible, due to the lack of robust assessment tools at the time the cohorts were evaluated. The Generation R cohort from the Netherlands reported that marijuana-exposed girls manifested increased aggressive behaviour at 18 months of age by maternal report.8 Continued follow-up of this cohort found increased cortical thickness at 6–8 years in exposed children.9 Although externalising behaviour at age 7–1010 and psychotic-like episodes at age 1011 were associated with prenatal marijuana exposure, the finding of elevated risk of these outcomes with paternal use, and with maternal prepregnancy use only, suggest that these associations might not be causal. Follow-up in early adolescence of children in the ALSPAC cohort found that in utero cannabis exposure was associated with Tourette syndrome/chronic tic disorder,12 but not with psychotic symptoms.13 The Adolescent Brain Cognitive Development (ABCD) cohort found in utero marijuana exposure to be associated with increased risk of psychosis proneness in 8.9- to 11-year-old children, but marijuana use was assessed only by retrospective report.14
Longitudinal deficits in crucial EF may help explain why prenatally exposed children are more likely to use drugs, perform poorly in school, and exhibit delinquent behaviour in adolescence.15,16 Moreover, the OPPS and MHPCDS cohorts defined exposure based solely on maternal self-report, potentially causing exposure misclassification and underestimation of effects on the child. Finally, marijuana is five times more potent today than thirty years ago17,18; thus, the effects of exposure today may be greater than observed in earlier cohorts.
2 |. OBJECTIVES
The Lifestyle and Early Achievement in Families (LEAF) study follows children of women who were recruited during pregnancy to investigate the association of maternal use of marijuana during pregnancy with their children’s EF and aggressive behaviour during ages 3½-7 years. The hypotheses of this study are twofold: we hypothesise that prenatal exposure to marijuana will be associated with deficits in EF in preschool age children and that prenatal exposure to marijuana will be associated with increased aggression in preschool and early school-age children.
3 |. STUDY POPUL ATION
The sample for the LEAF Study is drawn from women who had been recruited during pregnancy into the Ohio Perinatal Research Network Perinatal Research Repository (PRR), which began in 2010 and is ongoing. The PRR, a general purpose data and specimen repository, recruited women receiving antenatal care from several clinics at the Ohio State University Wexner Medical Center (OSUWMC), a large academic medical centre (Columbus, Ohio, USA). The PRR and its relationship to LEAF have been previously described.19 PRR inclusion criteria were age 16–50 years, ability to communicate in English, and intent to deliver at OSUWMC. Women who consented completed an intake questionnaire covering a wide variety of medical, demographic, and socio-economic domains; the questionnaire included items on use of tobacco and a single question on use19 of marijuana and other drugs of abuse to date during the current pregnancy. Women provided urine and blood samples (coinciding when possible with a clinical blood draw) at enrolment and then in each trimester for unspecified future use, and these were stored at −80°C. In addition to the intake questionnaire, women completed questionnaires assessing perceived stress,20 depressive symptoms,21 trait anxiety,22 sleep quality,23 and perceived everyday discrimination24 at enrolment and approximately once in each subsequent trimester. The mental health, sleep, and discrimination questionnaires were not administered during the first year of the study and, therefore, are available for a smaller number of women. At the conclusion of the pregnancy, the obstetrical record was abstracted to a precoded form by an obstetrical research nurse; the form included specific items about clinically noted use of various illicit drugs, including marijuana. Data from the neonate’s nursery course were also abstracted by trained personnel. The full text of the questionnaire and abstraction form items pertinent to drug use has been published.19 The PRR consent included an option to allow recontact for participation in future IRB-approved research, and approximately 75% of PRR enrolled women agreed to this.
The LEAF Study sample is drawn from women in the PRR who allowed future contact, whose children would be 42–95 months old during its anticipated course (birth dates from 2010 to early 2016) and are not known to have died. Exclusion criteria are as follows: the child is a ward of the State throughout the enrolment period, and the child has cognitive or physical impairment to a degree precluding participation in any study task. Enrolment began in 2016 and is expected to conclude in August 2020. LEAF is an ambidirectional cohort study,25 meaning that eligible children would be located, enrolled, and evaluated and then followed prospectively.
4 |. METHODS
4.1 |. Cohort location methods
The project had no contact with families between the time of delivery and recruitment for the child’s first study visit in LEAF. As a result, multiple information sources are used to locate families and to invite their participation. The PRR database included the mother’s street and email addresses, and telephone number at the time of PRR enrolment. It also contained the mother’s and child’s OSUWMC medical record numbers and the child’s NCH medical record number, if one was known, as well as the names and contact information for up to two individuals that the mother indicated would know how to reach her. These sources of information formed the basis to locate mothers and children. When the PRR information is found to be outdated, electronic medical records are referenced for updated information. Web-based search engines such as Peoplefinders and Spokeo are searched when other methods are unsuccessful. Finally, we attempt to contact the individuals listed by the mother, utilising the same web-based search engines if the information provided is out of date. Both PRR and LEAF were approved by the Institutional Review Board at NCH, and women provided separate, written informed consent to participate in each study.
Age-specific introductory letters are sent to families via email and postal mail. The letter briefly introduces the study and provides contact information for study staff. The postal letters request address correction from the Post Office for families who have moved. About 1 week later, study staff attempt phone contact with families to describe the study, assess child eligibility and for eligible children, and determine the family’s willingness to participate. Calls are made at varying times of the day on varying days of the week until the family either expresses interest in participating or declines participation. Occasionally, staff meet potentially eligible families at their clinical care appointments at NCH if they were unsuccessful in contacting the family by phone or mail.
If families agree to participate, they are scheduled to be seen at a research facility at NCH. Mother and child are evaluated in separate rooms, which are equipped with one-way mirrors and video recording capability. Families who are unable to complete the study visit at NCH are offered a home visit or a visit held at a conference room in a local public facility such as a library. If families are unable or unwilling to be seen in person, they can opt for a “surveys only” option, in which parents are mailed or emailed versions of the study instruments that they complete about themselves and their child (see Tables 1 and 2). These mailed/emailed surveys are completed under an IRB-approved waiver of consent documentation for this study.
TABLE 1.
Caregiver protocol and schedule of events
| Domain | 3.5–<4 y | 5.0–<6 y | 7.0–<8 y |
|---|---|---|---|
| Maternal reported child constructs and measures | |||
| Child healtha | Child Health Supplement from National Health Interview Survey74 | ||
| Breast feeding | Ever breast fed, age stopped (at 1st visit only) | ||
| Aggressionb | Leifer-Roberts Response Hierarchy (LRRH)56,57 Child Behavior Checklist (CBCL) 1.5–558 |
LRRH56,57; CBCL 6–1859 | |
| Syndrome scales | CBCL 1.5–558 | CBCL 6–1859 | |
| DSM-oriented scales | CBCL 1.5–558 | CBCL 6–1859 | |
| Competence scales | n/a | n/a | CBCL 6–1859 |
| Child executive functiona | Behavior Rating Inventory of Executive Function (BRIEF), Preschool version36 | BRIEF-2 (school-age version)55 | |
| Child eating behavior | n/a | Child Eating Behavior Questionnaire75 | n/a |
| Peer relationships | n/a | n/a | NIH Toolbox Positive Peer Interaction Parent Report Fixed Form (Ages 3–12)34 |
| Maternal self-reported constructs and measures | |||
| Anthropometrics | Height, weight at 1st visit (measured, three measurements taken for both height and weight) | ||
| Inhibitory control | Flanker (NIH Toolbox) at 1st visit34 | ||
| Attention | Flanker (NIH Toolbox) at 1st visit34 | ||
| Cognitive flexibility | Dimensional Change Card Sort (NIH Toolbox) at 1st visit34 | ||
| Episodic memory | Picture Sequence Memory (NIH Toolbox) at 1st visit34 | ||
| Receptive vocabulary | Picture Vocabulary (NIH Toolbox) at 1st visit34 | ||
| Reading | Oral Reading Recognition (NIH Toolbox) at 1st visit34 | ||
| Processing speed | Pattern Comparison Speed (NIH Toolbox) at 1st visit34 | ||
| Working memory | List Sorting Working Memory (NIH Toolbox) at 1st visit34 | ||
| IQ | Cognitive Fluid Composite, Cognitive Crystallized Composite, Cognitive Total Composite (NIH Toolbox) at 1st visit34 | ||
| DNA (if not already collected previously) | Buccal swab at 1st visit | ||
| Socio-demographic profile | Education, income, household composition, insurance, employment, marital status, childcare attendance; violence at home (full version at 1st visit, subset at 2nd visit) | ||
| Home environment, maternal responsivity | Home Screening Questionnaire76 | Home Screening Questionnaire76 StimQ: Preschool77,78 |
Modified MC HOME Interview (selected subscales): Encouragement of Maturity; Family Integration79 |
| Parenting practices & style | Parenting Styles and Dimensions Questionnaire (PSDQ)80 | n/a | PSDQ80 |
| Adaptive functioning | Adult Self Report (full ASR at 1st visit, subset at 2nd)81 | ||
| Syndrome Scales | Adult Self Report (full ASR at 1st visit, subset at 2nd)81 | ||
| DSM-Oriented scales | Adult Self Report (full ASR at 1st visit, subset at 2nd)81 | ||
| Substance use | Adult Self Report (full ASR at 1st visit, subset at 2nd)81 | ||
| Substance use | Drug History Questionnaire (full DHQ at 1st visit; use since last visit at 2nd visit)31 | ||
At every visit.
Component of co-primary outcomes of child’s executive function and aggressive behaviours.
TABLE 2.
Child protocol and schedule of events
| Domain | 3.5– <4 y | 5.0–<6 y | 7.0–<8 y |
|---|---|---|---|
| Directly assessed measures | |||
| Anthropometricsb | Height, weight | ||
| Inhibitory controla,b | Flanker (NIH Toolbox)34 | ||
| Attentiona,b | Flanker (NIH Toolbox)34 | ||
| Cognitive flexibilitya | Dimensional change card sort; (NIH Toolbox)34; shape school82 | Dimensional change card sort (NIH Toolbox)34 | |
| Episodic memorya,b | Picture sequence memory (NIH Toolbox)34 | ||
| Receptive vocabularyb | Picture vocabulary (NIH Toolbox)34 | ||
| Processing speeda,b | Pattern comparison speed test (NIH Toolbox)34 | ||
| Working memorya | WPPSI-IV picture memory44 | List sorting working memory (NIH Toolbox)34 | |
| IQ | Cognition early childhood composite (NIH Toolbox)34 | Cognition fluid composite; cognition early childhood composite (NIH Toolbox)34 | |
| Emotion regulationa | Toy-behind barrier45 | Disappointing gift46 | Impossible puzzle box47,48 |
| Self-reported emotion regulationa | n/a | n/a | Self-rated emotions (pictures) |
| Planninga,b | Tower of Hanoi49 | ||
| Visual Spatial Abilitya | WPPSI III block design50; Bayley puzzle51 | WPPSI III block design50 | WASI II block design52 |
| Observed aggressive behaviora,b | Bobo doll53 | Bobo after frustrating task | |
| Self-reported aggressiona | n/a | n/a | L-R RH (pictures)56,57 |
| Peer relationships | n/a | n/a | NIH Toolbox Friendship fixed form (ages 8–1734) |
| DNA, if not previously collected | Buccal swab at 1st visit | ||
Component of primary outcomes of executive function and aggressive behaviour.
At every visit.
4.2 |. Definition of exposure to marijuana and other substances of abuse
In preparation for LEAF, all archived urine samples from potentially eligible women were assayed for 11-nor-carboxy-Δ9-tetrahydrocannabinol (Δ9-THC-COOH), the primary urine THC metabolite, by gas chromatography-tandem mass spectrometry following hydrolysis.26–29 Women were considered to have used marijuana if they indicated use during pregnancy up to the date of the PRR intake questionnaire, if use was noted on the obstetrical record abstraction, or if any pregnancy urine specimen had a Δ9-THC-COOH concentration of >15 ng/mL, the concentration considered to represent active use when employing mass spectrometry.30 As a sensitivity analysis, we will ask the women retrospectively about trimester-specific use of marijuana (as a binary variable) during her pregnancy with the study child, utilising the Drug History Questionnaire,31 modified to ask trimester-specific use. The urine samples were also assayed for 16 additional substances, including several different opiates, benzoylecgonine (cocaine), and cotinine, by either liquid chromatography/mass spectrometry or liquid chromatography/tandem mass spectrometry. Assay results for these substances were reported only as positive or negative, with a cut-off of 2.5 ng/mL for positivity. We considered women to have used cocaine during pregnancy if it was noted in the intake questionnaire, the record abstraction, or a urine sample. Since many women receive opiates for pain relief during the delivery admission, we did not consider urine samples obtained two or fewer days before delivery that were positive for an opiate that would be used for short-term pain relief. Finally, since the 2.5 ng/mL cut-off for cotinine was too low to distinguish reliably between active smoking and secondhand tobacco exposure, we defined smoking during pregnancy based only on questionnaire and obstetrical chart abstraction. We have previously shown32,33 that in pregnancy cohorts not specifically focused on tobacco use, self-reported smoking is sufficiently accurate for data analysis.
4.3 |. Data collection
Eligible families complete up to two study visits at 3½, 5, or 7 years of age. Mothers complete self-administered questionnaires about themselves, their child(ren), and their home environment. If a family member other than the biological mother has legal custody of the child or if the father brings the child in for testing, that caregiver is invited to participate and complete the surveys about the child and their environment. While we use the term “parent” throughout, in some circumstances the child’s guardian might be reporting on the child. Non-custodial biological mothers are also invited to complete questionnaires about their pregnancy and to undergo cognitive testing. With the parent’s permission, for children of at least 5 years of age, the child’s teacher is contacted to complete questionnaires. To reduce information bias, study staff who have contact with participants are unaware of participants’ prospectively measured drug exposure status from the PRR.
Mothers and children undergo cognitive testing utilising the iPad-based NIH Toolbox Cognition Battery,34 which has been validated in individuals from 3 to 85 years of age. As noted in Table 1, the Toolbox is utilised to assess various aspects of maternal executive function and related cognitive characteristics. The test battery also enables determination of maternal IQ. Children complete a video-recorded series of direct assessments as detailed in Table 2. Research staff were trained in study procedures by master’s or doctoral-level developmental psychologists, and agreement was maintained by double-coding of video-recorded study visits. Videos of children’s behaviour were independently coded by pairs of research assistants, and discrepancies were resolved by discussion. Ongoing monitoring of coding reliability was conducted using Krippendorf alpha reliability coefficients (k-alpha), which account for level of measurement and random error.35 Staff received training in discovering unconscious bias through NCH, and ongoing training in working with marginalised populations; most of the staff has prior experience working with similar populations. At the conclusion of study visits, all participants were given a resource sheet and efforts were made to connect families with continued medical care and community resources as appropriate. Manuscripts emanating from the project will endeavour to describe the cohort in a respectful, non-judgmental, non-stigma-tising manner.
4.4 |. Study outcomes
Our primary outcomes are EF and aggressive behaviour of the child. EF is not a unitary entity. Rather, “the executive functions are a collection of processes that are responsible for guiding, directing and managing cognitive, emotional, and behavioural functions, particularly during active, novel problem solving.”36 EF is required for activities such as planning, organising, inhibiting inappropriate behaviours, noticing and remembering details, and managing time and space37 and has been shown to be highly predictive of academic and job success.38 In childhood, the earliest-emerging facets of EF include abilities such as inhibitory control, sustained attention, working memory, and emotion regulation, all of which undergo tremendous development between ages 3 and 5 years. Age-appropriate measures to study this very dynamic period of EF emergence have only recently been developed.39–42 While our evaluation encompasses a broad range of these functions, based on the findings of previously reported cohorts,5,43 we focus largely on planning, attention, inhibitory control, and working memory. EF and aggression are assessed by a combination of direct testing and maternal and teacher (for children in school) report using standard instruments.
Direct testing of the child utilises the NIH Toolbox,34 which includes tasks to evaluate the child’s Inhibitory Control, Attention, Cognitive Flexibility, Episodic Memory, Processing Speed and Working Memory (at ages 5 and 7). Working memory is assessed at the 3½-year-old visit with the WPPSI-IV picture memory test.44 Emotion Regulation is assessed by the Toy-behind Barrier, the Disappointing Gift, and the Impossible Puzzle Box Tasks at 3½, 5, and 7 years, respectively,45–48 and 7-year-old children self-rate their emotions during this task by pointing to predrawn pictures displaying a range of emotions. Planning ability is evaluated by the Tower of Hanoi task of age-appropriate difficulty.49 Visual-spatial ability is assessed using the WPPSI III block design task at 3½ and 5 years50 (plus the Bayley Puzzle task at 3½)51 and the WASI II Block Design task at 7 years.52 Aggressive behaviour is evaluated in the laboratory with the Bobo Doll Task at all study visits, as used by Bendersky53 to evaluate cocaine-exposed children. Immediately before this task (7-year visit only), the children complete a novel sham game designed to elicit frustration, to prime a potentially aggressive response to the Bobo Doll.
Parents will report on the child’s EF with the age-appropriate version of the Behavior Rating Inventory of Executive Function (BRIEF-P54 up to 5 years of age, BRIEF-255 for older children). If a child is in school, the teacher is invited to complete teacher versions of these instruments. The BRIEF-P is a 63-item instrument with five non-overlapping scales. The scales form a Global Executive Composite score and three overlapping indices based on the scales: the Inhibitory Self-Control Index, the Flexibility Index, and the Emergent Metacognition Index. The BRIEF-2 provides a Global Executive Composite and a Behavior Regulation Index, an Emotion Regulation Index, and a Cognitive Regulation Index. Parents report on the child’s typical behaviours in situations that might elicit aggression with the Leifer-Roberts Response Hierarchy test, which provides a series of binary forced choices for how they believe the child would react in hypothetical scenarios involving interpersonal conflict with other children.53,56,57 Seven-year-old children will, in addition, complete this instrument for themselves. Parents complete the age-appropriate Child Behavior Checklist. Teachers evaluate the child’s aggressive behaviour with peers, using the Caregiver-Teacher Report form 1.5–558 or the Teacher Report Form 6–18,59 and the Child Behavior Scale.60
In addition to the data collection noted above, data are collected on family demographics, household environment, and parenting style. A complete list of parent, child, and teacher measures, and schedule of events, is shown in Tables 1, 2, and 3, respectively. The study visits take 2–3 hours to complete, although only a subset of questionnaires and tasks are asked of the parent at the second visit. The child’s protocol includes scheduled breaks, and unscheduled breaks are allowed if the child appears to be tiring.
TABLE 3.
Teacher protocol and schedule of events
| 3.5–<4 y (V3Y) | 5.0–<6 y (V5Y) | 7.0–<8 y (V7Y) | |
|---|---|---|---|
| Aggression | n/a | Caregiver-Teacher Report Form (C-TRF) 1.5–5a 58 | Teacher Report Form (TRF) 6–18a 59 |
| Syndrome scalesa | n/a | C-TRF 1.5–5a 58 | TRF 6–18a 59 |
| DSM-oriented scalesb | n/a | C-TRF 1.5–5a 58 | TRF 6–18a 59 |
| Competence scalesc | n/a | n/a | TRF 6–18a 59 |
| Executive functiond | n/a | Behavior rating inventory of executive function preschool version (BRIEF-P)36 | Behavior rating inventory of executive function-255 (BRIEF-2) |
| Peer relationships | n/a | Child behavior scale (aggressive with peers, excluded by peers subscales)60 | Child behavior scale (aggressive with peers, excluded by peers subscales)60 |
Select subscales: Syndrome scales: Emotionally reactive, attention problems, aggressive behavior; DSM-oriented scales: Attention deficit/hyperactivity problems, oppositional defiant.
Select subscales: Syndrome scales: Social problems; attention problems; rule-breaking behavior; aggressive behavior; DSM-oriented scales: Attention deficit/hyperactivity problems, oppositional defiant problems, conduct problems.
Competence scales: Academic performance, working hard, behaving, learning, happy.
Component of co-primary outcome of executive function.
4.5 |. Primary analyses
We outline a broad strategy, but specific statistical methods will be tailored to each research question. Our co-primary outcomes are childhood EF and aggressive behaviour. Both are complex constructs with many facets. Accordingly, we are assessing many different aspects of them in different ways—maternal/teacher report, self-report, and direct observation. Given the large number of individual measures, where possible we will derive composite measures for these constructs utilising data reduction techniques such as exploratory factor analysis.61 However, we are also interested in performance on individual tasks because they generally load on specific facets of our outcomes and will examine these tasks in an exploratory manner. Our analysis will account for the non-independence of twins and siblings, as well as the mixed cross-sectional/longitudinal design. We will employ multiple imputation62 or maximum likelihood estimation for missing data,63 which allow data to be missing at random. Certain characteristics such as maternal EF might be considered confounders if they are heritable and predispose to substance use or they might be mediators if they result from substance use and impact the home environment. Our analysis will consider both possibilities. We will also consider confounding by tobacco use and mediation by factors such as preschool attendance and the home environment. We will undertake various sensitivity analyses, such as exclusion of twins and exploratory analysis of all the CBCL subscales.
5 |. PRELIMINARY RESULTS
A total of 497 pregnant women were enrolled in the PRR during the eligible time period. Of those, 381 allowed contact for future research. Three pregnancies ended at <20 weeks, leaving 378 potentially eligible pregnancies; 11 pregnancies were twin, resulting in 389 potentially eligible offspring. There were 23 stillbirths or infant deaths, reflecting the high-risk clinics from which many women were recruited. Among surviving children, three were ineligible because they were in foster care and one was ineligible because they had been diagnosed with Down syndrome. Records of three of the 362 children who were otherwise eligible did not include sufficient identifying information to allow successful location; and there were three children whose mothers had died and we lacked information on the legal guardian, leaving 356 children to be located and approached. One mother of an eligible child was located but requested that her information be purged from the PRR, so no pregnancy information is available for her, but we considered the child eligible but not followed up. A flow diagram of study enrolment is included in Figure 1.
FIGURE 1.
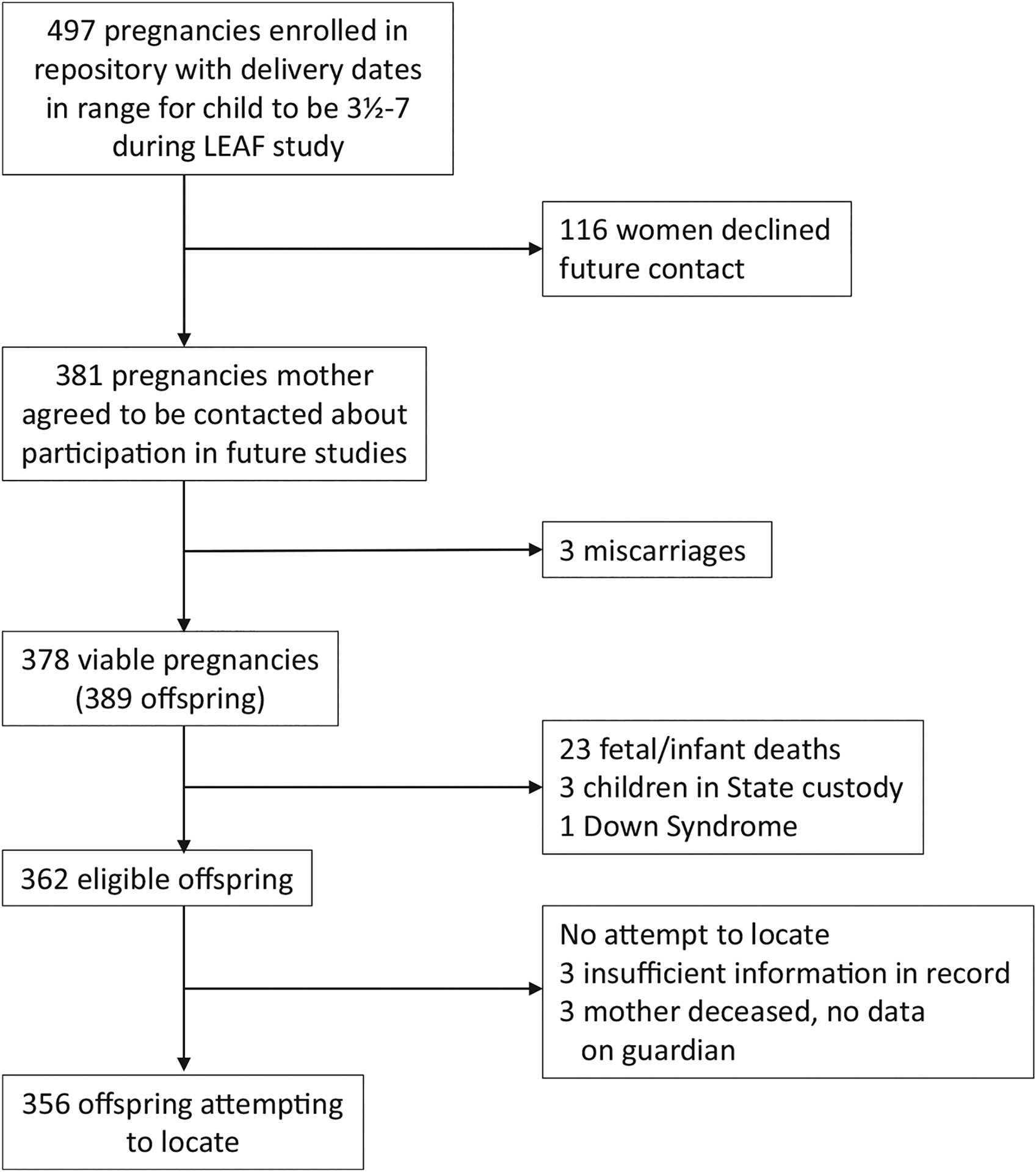
Flow diagram of study eligibility and enrolment
Tables 4 and 5 provide descriptive characteristics of mothers and newborns, respectively, based on data collected during or shortly after the pregnancy. Although we present missing characteristics as a separate category to assist understanding of the sample, in subsequent data analysis we will utilise missing data methods, such as maximum likelihood or multiple imputation, to impute these variables. Marijuana use during pregnancy was considerably more common among African American women, women with less than a college degree, women who were never married, women who smoked tobacco during the index pregnancy, women whose pregnancy was not planned, and women who used alcohol or cocaine during pregnancy. Marijuana use was slightly more common among women who had experienced homelessness or physical abuse during the past year, but not more common among women who were exposed to opiates. Parity was similar between women who did and did not use marijuana as well. Women who used marijuana entered the PRR about one week earlier than those who did not (gestational age was confirmed with sonography in 90% and 88% of women who did and did not use, respectively; records were missing in 6% of both groups, and the remainder had no evidence of having had sonography). Women who used were almost one year younger, but anthropometric values were similar between groups. Mothers who used marijuana reported slightly higher perceived stress, depressive symptoms, trait anxiety, and discrimination scores, than those who did not. Newborn gestational age was similar between mothers who did and did not use marijuana; preterm birth was common in both groups, likely because of the high-risk obstetrical clinics where many women were recruited. In contrast to gestational age, infants of mothers who used marijuana had somewhat reduced birthweights compared to those mothers who did not.
TABLE 4.
Marijuana use by maternal participant characteristics
| Maternal Characteristics | Marijuana-positivea (N = 117) | Marijuana-negativea (N = 244) |
|---|---|---|
| Race (%) | ||
| African American | 88 (75) | 140 (57) |
| Other | 29 (25) | 104 (43) |
| Education (%) | ||
| <High school | 31 (26) | 44 (18) |
| High school | 38 (32) | 80 (33) |
| Some college | 36 (31) | 70 (29) |
| College graduate | 1 (1) | 31 (13) |
| Missing | 11 (9) | 19 (8) |
| Marital status (%) | ||
| Currently married | 4 (6) | 65 (27) |
| Never married | 77 (66) | 103 (42) |
| Other | 24 (21) | 51 (21) |
| Missing | 12 (10) | 25 (10) |
| Smoked during index pregnancy (%) | ||
| No | 37 (32) | 165 (67) |
| Yes | 79 (68) | 78 (32) |
| Missing | 1 (1) | 1 (0.4) |
| Homeless within the past year (%) | ||
| No | 86 (74) | 193 (69) |
| Yes | 18 (15) | 27 (11) |
| Missing | 13 (11) | 24 (9) |
| Physical abuse within the past year (%) | ||
| No | 94 (80) | 206 (84) |
| Yes | 9 (8) | 17 (7) |
| Missing | 14 (12) | 21 (9) |
| Planned pregnancy (%) | ||
| No | 85 (73) | 160 (66) |
| Yes | 18 (15) | 59 (24) |
| Missing | 14 (12) | 25 (10) |
| Alcohol use during pregnancy (%) | ||
| No | 80 (68) | 196 (80) |
| Yes | 34 (29) | 45 (18) |
| Missing | 3 (3) | 3 (1) |
| Opiate exposure (%) | ||
| No | 111 (95) | 219 (90) |
| Yes | 6 (5) | 25 (10) |
| Cocaine exposure (%) | ||
| No | 100 (85) | 230 (95) |
| Yes | 17 (15) | 14 (6) |
| Parity (%) | ||
| 0 | 14 (12) | 30 (12) |
| ≥1 | 103 (86) | 103 (88) |
| Missing | 0 | 3 (1) |
| Marijuana-positive | Marijuana-negative | |||||
|---|---|---|---|---|---|---|
| N | Mean | (SD) | N | Mean | (SD) | |
| Gestation at entry, days | 112 | 109 | (43) | 231 | 116 | (53) |
| Height, cm | 113 | 162.3 | (7.3) | 238 | 163.3 | (7.8) |
| Prepregnant weight, kg | 106 | 74.9 | (22.3) | 220 | 79.9 | (23.5) |
| BMI, kg/m2 | 106 | 28.5 | (8.8) | 219 | 29.9 | (8.2) |
| Age, y | 116 | 25.8 | (5.1) | 243 | 26.7 | (5.4) |
| Total score, CES-Db | 79 | 15.1 | (11.0) | 183 | 14.4 | (10.7) |
| Total score, PSSb | 77 | 16.5 | (7.5) | 184 | 14.8 | (7.7) |
| Total score, STAIb | 75 | 37.6 | (10.9) | 179 | 36.4 | (10.1) |
| Total score, EDSb | 78 | 15.8 | (9.0) | 182 | 15.1 | (8.7) |
| Total score, PSQIb | 67 | 7.6 | (3.9) | 171 | 7.7 | (4.1) |
Overall positivity 117/361 (32.4%); one woman requested that her data be purged.
Multiple evaluations per pregnancy, mean is over all evaluations.
TABLE 5.
Marijuana use by neonatal characteristics
| Neonatal characteristic | Marijuana-positive (N = 117)a | Marijuana-negative(N = 224)a |
|---|---|---|
| Sex (%) | ||
| Female | 54 (46) | 111 (45) |
| Male | 56 (48) | 107 (44) |
| Indeterminate | 1 (1) | 2 (1) |
| Missing | 6 (5) | 25 (10) |
| Gestational age, wk (%) | ||
| <32 | 9 (8) | 23 (9) |
| 32–34 | 8 (7) | 8 (7) |
| 35–36 | 21 (18) | 21 (18) |
| ≥37 | 75 (64) | 150 (61) |
| Missing | 4(3) | 11 (5) |
| Birthweight, g (%) | ||
| <1500 | 11 (9) | 17 (7) |
| 1500–2499 | 25 (21) | 49 (20) |
| 2500–3999 | 71 (61) | 146 (60) |
| ≥4000 | 4 (3) | 11 (5) |
| Missing | 6 (5) | 21 (9) |
| Marijuana-positive | Marijuana-negative | |||||
|---|---|---|---|---|---|---|
| N | Mean | (SD) | N | Mean | (SD) | |
| Gestational age, d | 113 | 259 | (22) | 223 | 259 | (23) |
| Birthweight, g | 111 | 2698 | (712) | 223 | 2820 | (796) |
Overall positivity 117/361 (32.4%); one woman requested that her data be purged.
6 |. COMMENT
6.1 |. Principal findings
The LEAF Study is an ambidirectional cohort study (an historical cohort study with continued longitudinal follow-up) of mothers and their offspring in the Columbus, Ohio region, that is studying the association between maternal marijuana use during pregnancy and the subsequent development of EF and aggressive behaviour in their offspring over the preschool to early school-age years.
6.2 |. Strengths of the study
Our study design has numerous strengths: we began with a defined study population that had been recruited in a repository not focused on drug use. The general nature of the repository should diminish the incentive to misreport or to decline participation differentially by drug use. Marijuana and other drug use were assessed by questionnaire, obstetrical record review, and universal urine toxicology. Marijuana use was very common in this population and was not associated with opioid use; although cocaine use was more common among women who used marijuana than among those who did not, the vast majority of mothers who used marijuana did not use cocaine. We collected detailed, prospective information during pregnancy on stress, depressive symptoms, anxiety, discrimination, and sleep, utilising well-accepted instruments. Our neuropsychological testing incorporates valid, standardised state-of-the art methods and questionnaires, and we evaluate children’s EF at a younger age than previous cohorts did. The LEAF study builds upon the extant literature by being the first to assess maternal EF, which exhibits considerable heritability.64 Deficits in EF predispose to initiation of marijuana use,65 making maternal EF potentially a strong confounder.
6.3 |. Limitations of the data
Although our collection of data on marijuana use was comprehensive, our methods did not allow us to estimate the potency, amount, and timing of use during pregnancy nor did we record or assay for synthetic cannabinoids, although we are obtaining this information retrospectively at the time of the LEAF study evaluation. A second concern is the high occurrence of preterm birth in our study population, likely reflecting the high-risk obstetric clinics where many study women were recruited. Indeed, the occurrence of preterm birth in LEAF pregnancies is higher than that observed at OSUWMC in general, likely reflecting the clinics where much of the PRR enrolment was done.19 The high-risk nature of our population raises concerns of collider-stratification bias,66 especially if marijuana increases the risk of preterm birth. However, recent systematic reviews noted either a null association of marijuana with preterm birth,67 or that after control for concurrent tobacco use, marijuana was not associated with preterm birth,68 and marijuana use was not associated with preterm birth in our cohort,19 thereby minimising concerns of collider stratification by preterm birth. Third, our IRB approval did not allow us to collect data about potentially eligible women who were not approached or who declined to participate in the PRR and to respect the privacy of PRR women who did not allow future contact, we did not assay their urine samples. Therefore, we cannot compare marijuana use between women in the PRR and non-participants who were otherwise eligible nor can we compare use between PRR women who allowed and did not allow future contact.
Studying the association of maternal substance use during pregnancy with childhood outcomes presents numerous opportunities for unmeasured or inadequately controlled confounding by genetic, individual, or social factors that contribute to both substance use and suboptimal development, as well as concerns with immigrative selection bias if PRR participation differed jointly by marijuana use and child outcome and emigrative selection bias if loss to follow-up (or failure to participate in LEAF) differed jointly by use and child outcome. Misclassification of exposure (random or otherwise) is a concern, particularly for exposures that might be illegal and/or prompt a Child Protective Services referral. We hope to minimise these concerns by collecting detailed information on a wide range of potentially confounding factors, including maternal EF and IQ; by basing our prospective study on a general repository not focused on drug use or child development; by maintaining a high degree of follow-up (currently 74% and ongoing); by basing exposure on a multimodal assessment that included universal urine toxicology; and by not informing research staff of maternal exposure status.
Nevertheless, others have proposed alternate study designs to address these concerns. One such design is the “negative control,69” whereby paternal use during pregnancy or maternal use before pregnancy is compared to maternal use during pregnancy.10,11 A second design utilises an instrumental variable, such as Mendelian randomisation70 or public policy changes that affect marijuana use, but would not otherwise be expected to impact child development. Unfortunately, the negative control design entails assumptions that may not apply to maternal marijuana use in pregnancy.71,72 Regardless, the PRR did not inquire about maternal prepregnant marijuana use nor are there stored maternal prepregnant urine samples, analysis of which would be necessary to determine such use given the insensitivity of self-report.19,73 Although fathers in the PRR completed a brief questionnaire, it did not include marijuana use. However, paternal urine was collected for approximately 40% of pregnancies and paternal use might be investigated as a negative control in a future sensitivity analysis, should funding to analyse the urine samples be available. Instrumental variable designs can shed light on the topic, but unless the chosen instrument is extremely strongly associated with exposure, such designs generally require sample sizes much larger than we could achieve.
7 |. CONCLUSIONS
This ambidirectional cohort study of mother-child dyads seeks to investigate the association of maternal use of marijuana during pregnancy and the developing EF and aggressive behaviour of her child(ren) during their preschool years. Our long-term goal is to document the impact of prenatal marijuana exposure across development, inform marijuana policy, inform new interventions to counsel reproductive-aged women about the risks of marijuana use during pregnancy, and guide prevention and treatment of adverse effects.
Synopsis.
What’s already known
Prenatal marijuana exposure has been associated with impairments in executive function, delinquent behaviour, and substance use in late childhood and adolescence, but this evidence is based on cohorts from the 1980s–1990s when marijuana was much less potent, and effects at earlier ages were poorly understood.
Study question
The LEAF study will estimate associations between in utero exposure to marijuana as documented prospectively by biomarker, self-report, and medical records with executive function and aggressive behaviour in a prospective study of 3½- to 7-year-old children.
What this study adds
This study will extend previous findings to younger children by employing modern, neuropsychologic testing protocols, thereby offering the prospect of early detection of deficits.
ACKNOWLEDGEMENTS
We acknowledge the Ohio Perinatal Research Network Perinatal Research Repository, which formed the base population of the LEAF study.
Funding information
This research was supported by grant R01 DA042948 from the National Institute on Drug Abuse, NIH; grant #6-FY16-160 from the March of Dimes Foundation; and the National Center for Advancing Translational Sciences/National Institutes of Health (UL1TR001070). The funding sources had no input in the study design; the data collection, interpretation, or analysis; the writing of this report; or the decision to submit the article for publication.
REFERENCES
- 1.Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 2019;322:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee on Obstetric Practice. Committee Opinion No. 722: Marijuana use during pregnancy and lactation. Obstet Gynecol. 2017;130:e205–e209. [DOI] [PubMed] [Google Scholar]
- 3.Ko JY, Tong VT, Bombard JM, Hayes DK, Davy J, Perham-Hester KA. Marijuana use during and after pregnancy and association of prenatal use on birth outcomes: a population-based study. Drug Alcohol Depend. 2018;187:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried PA, Smith AM. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol. 2001;23:1–11. [DOI] [PubMed] [Google Scholar]
- 6.McLemore GL, Richardson KA. Data from three prospective longitudinal human cohorts of prenatal marijuana exposure and offspring outcomes from the fetal period through young adulthood. Data Brief. 2016;9:753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson GA, Day NL, McGauhey PJ. The impact of prenatal marijuana and cocaine use on the infant and child. Clin Obstet Gynecol. 1993;36:302–318. [DOI] [PubMed] [Google Scholar]
- 8.Huizink AC. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:45–52. [DOI] [PubMed] [Google Scholar]
- 9.El Marroun H, Tiemeier H, Franken IH, et al. Prenatal cannabis and tobacco exposure in relation to brain morphology: a prospective neuroimaging study in young children. Biol Psychiatry. 2016;79:971–979. [DOI] [PubMed] [Google Scholar]
- 10.El Marroun H, Bolhuis K, Franken IHA, et al. Preconception and prenatal cannabis use and the risk of behavioural and emotional problems in the offspring; a multi-informant prospective longitudinal study. Int J Epidemiol. 2019;48:287–296. [DOI] [PubMed] [Google Scholar]
- 11.Bolhuis K, Kushner SA, Yalniz S, et al. Maternal and paternal cannabis use during pregnancy and the risk of psychotic-like experiences in the offspring. Schizophr Res. 2018;202:322–327. [DOI] [PubMed] [Google Scholar]
- 12.Mathews CA, Scharf JM, Miller LL, Macdonald-Wallis C, Lawlor DA, Ben-Shlomo Y. Association between pre- and perinatal exposures and Tourette syndrome or chronic tic disorder in the ALSPAC cohort. Br J Psychiatry. 2014;204:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zammit S, Thomas K, Thompson A, et al. Maternal tobacco, cannabis and alcohol use during pregnancy and risk of adolescent psychotic symptoms in offspring. Br J Psychiatry. 2009;195:294–300. [DOI] [PubMed] [Google Scholar]
- 14.Fine JD, Moreau AL, Karcher NR, et al. Association of prenatal cannabis exposure with psychosis proneness among children in the adolescent brain cognitive development (ABCD) study. JAMA Psychiatry. 2019;76:762–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313–1322. [DOI] [PubMed] [Google Scholar]
- 16.Day NL, Leech SL, Goldschmidt L. The effects of prenatal marijuana exposure on delinquent behaviors are mediated by measures of neurocognitive functioning. Neurotoxicol Teratol. 2011;33:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA. New trends in cannabis potency in USA and Europe during the last decade (2008–2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5–15. [DOI] [PubMed] [Google Scholar]
- 18.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol Psychiatry. 2016;79:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klebanoff MA, Wilkins DG, Keim SA. Marijuana use during pregnancy and preterm birth: a prospective cohort study. Am J Perinatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 22.Spielberger CD, Gorsuch RL, Luschene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 23.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 24.Williams DR, Yan Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2:335–351. [DOI] [PubMed] [Google Scholar]
- 25.Klebanoff MA, Snowden JM. Historical (retrospective) cohort studies and other epidemiologic study designs in perinatal research. Am J Obstet Gynecol. 2018;219:447–450. [DOI] [PubMed] [Google Scholar]
- 26.Foltz RL, McGinnis KM, Chinn DM. Quantitative measurement of delta 9-tetrahydrocannabinol and two major metabolites in physiological specimens using capillary column gas chromatography negative ion chemical ionization mass spectrometry. Biomed Mass Spectrom. 1983;10:316–323. [DOI] [PubMed] [Google Scholar]
- 27.Huang W, Moody DE, Andrenyak DM, et al. Simultaneous determination of delta9-tetrahydrocannabinol and 11-nor-9-carboxy-delta9-tetrahydrocannabinol in human plasma by solid-phase extraction and gas chromatography-negative ion chemical ionization-mass spectrometry. J Anal Toxicol. 2001;25:531–537. [DOI] [PubMed] [Google Scholar]
- 28.Quintela O, Andrenyak DM, Hoggan AM, Crouch DJ. A validated method for the detection of Delta 9-tetrahydrocannabinol and 11-nor-9-carboxy- Delta 9-tetrahydrocannabinol in oral fluid samples by liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J Anal Toxicol. 2007;31:157–164. [DOI] [PubMed] [Google Scholar]
- 29.Wilkins D, Haughey H, Cone E, Huestis M, Foltz R, Rollins D. Quantitative analysis of THC, 11-OH-THC, and THCCOOH in human hair by negative ion chemical ionization mass spectrometry. J Anal Toxicol. 1995;19:483–491. [DOI] [PubMed] [Google Scholar]
- 30.Substance Abuse and Mental Health Services Administration. Clinical Drug Testing in Primary Care. Technical Assistance Publication (TAP) 32. HHS Publication No. (SMA) 12–4668. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. [Google Scholar]
- 31.Sobell LC, Kwan E, Sobell MB. Reliability of a drug history questionnaire (DHQ). Addict Behav. 1995;20:233–241. [DOI] [PubMed] [Google Scholar]
- 32.Klebanoff MA, Levine RJ, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentration and self-reported smoking during pregnancy. Am J Epidemiol. 1998;148:259–262. [DOI] [PubMed] [Google Scholar]
- 33.Klebanoff MA, Levine RJ, Morris CD, et al. Accuracy of self-reported cigarette smoking among pregnant women in the 1990s. Paediatr Perinat Epidemiol. 1990s;15:140–143. [DOI] [PubMed] [Google Scholar]
- 34.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80:S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data. Commun Methods and Meas. 2007;1:77–89. [Google Scholar]
- 36.Gioia GA, Espy KA, Isquith PK. BRIEF-P Behavior Rating Inventory of Executive Function- Preschool Version, Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 37.National Center for Learning Disabilities. What is Executive Function. National Center for Learning Disabilities; 2014. [cited 2014 5 September]; http://www.ncld.org/types-learning-disabilities/executive-function-disorders/what-is-executive-function [Google Scholar]
- 38.Moffitt TE, Arseneault L, Belsky D, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci U S A. 2011;108:2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlson SM. Developmentally sensitive measures of executive function in preschool children. Dev Neuropsychol. 2005;28:595–616. [DOI] [PubMed] [Google Scholar]
- 40.Kochanska G, Murray K, Jacques TY, Koenig AL, Vandegeest KA. Inhibitory control in young children and its role in emerging internalization. Child Dev. 1996;67:490–507. [PubMed] [Google Scholar]
- 41.Mahone EM, Pillion JP, Hiemenz JR. Initial development of an auditory continuous performance test for preschoolers. J Atten Disord. 2001;5:93–106. [DOI] [PubMed] [Google Scholar]
- 42.Zelazo PD, Muller U, Frye D, et al. The development of executive function in early childhood. Monogr Soc Res Child Dev. 2003;68:vii–137. [DOI] [PubMed] [Google Scholar]
- 43.Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24:309–320. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D Wechsler Preschool and Primary Scale of Intelligence—Fourth Edition (WPPSI-IV) Technical and Interpretive Manual. San Antonio, TX: Pearson; 2012. [Google Scholar]
- 45.Wu YC, Hsieh WS, Hsu CH, et al. Intervention effects on emotion regulation in preterm infants with very low birth weight: a randomize controlled trial. Res Dev Disabil. 2016;48:1–12. [DOI] [PubMed] [Google Scholar]
- 46.Saarni C An observational study of childrens attempts to monitor their expressive behavior. Child Dev. 1984;55:1504–1513. [Google Scholar]
- 47.Amsel A The role of frustrative nonreward in noncontinuous reward situations. Psychol Bull. 1958;55:102–119. [DOI] [PubMed] [Google Scholar]
- 48.Gentzler AL, Wheat AL, Palmer CA, Burwell RA. Children’s responses to cognitive challenge and links to self-reported rumination. Cogn Emot. 2013;27:305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welsh MC. Rule-guided behavior and self-monitoring on the tower of hanoi disk-transfer task. Cogn Dev. 1991;6:59–76. [Google Scholar]
- 50.Wechsler D WPPSI-3: Technical and Interpretative Manual. San Antonio, TX: Pearson; 2002. [Google Scholar]
- 51.Bayley N Bayley Scales of Infant and Toddler Development. San Antonio, TX: PsychCorp, Pearson; 2006. [Google Scholar]
- 52.Wechsler D Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II) Manual. San Antonio, TX: Pearson; 2011. [Google Scholar]
- 53.Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6:235–238. [DOI] [PubMed] [Google Scholar]
- 55.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behvaior Rating Inventory of Executive Function, Second Edition (BRIEF 2). Odessa, FL: Psychological Assessment Resources; 2016. [Google Scholar]
- 56.Reinisch JM. Prenatal exposure to synthetic progestins increases potential for aggression in humans. Science. 1981;211:1171–1173. [DOI] [PubMed] [Google Scholar]
- 57.Reinisch JM, Sanders SA. A test of sex differences in aggressive response to hypothetical conflict situations. J Pers Soc Psychol. 1986;50:1045–1049. [DOI] [PubMed] [Google Scholar]
- 58.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2013. [Google Scholar]
- 59.Achenbach TM, Rescorla AA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth and Families; 2001. [Google Scholar]
- 60.Ladd GW, Profilet SM. The child behavior scale: a teacher-report measure of young children’s aggressive, withdrawn, and prosocial behaviors. Dev Psychol. 1996;32:1008–1024. [Google Scholar]
- 61.Fabrigar LR, Wegener DT. Exploratory Factor Analysis. Oxford, UK; New York, NY: Oxford University Press; 2012. [Google Scholar]
- 62.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 63.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 64.Lee T, Mosing MA, Henry JD, et al. Genetic influences on four measures of executive functions and their covariation with general cognitive ability: the Older Australian Twins Study. Behav Genet. 2012;42:528–538. [DOI] [PubMed] [Google Scholar]
- 65.Squeglia LM, Jacobus J, Nguyen-Louie TT, Tapert SF. Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology. 2014;28:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. “Toward a clearer definition of confounding” revisited with directed acyclic graphs. Am J Epidemiol. 2012;176:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunn JK, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6:e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2016;128:713–723. [DOI] [PubMed] [Google Scholar]
- 69.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 71.Brew BK, Gong T, Williams DM, Larsson H, Almqvist C. Using fathers as a negative control exposure to test the Developmental Origins of Health and Disease Hypothesis: a case study on maternal distress and offspring asthma using Swedish register data. Scand J Public Health. 2017;45:36–40. [DOI] [PubMed] [Google Scholar]
- 72.Madley-Dowd P, Rai D, Zammit S, Heron J. Simulations and directed acyclic graphs explained why assortative mating biases the prenatal negative control design. J Clin Epidemiol. 2020;118:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young-Wolff KC, Tucker LY, Alexeeff S, et al. Trends in self-reported and biochemically tested marijuana use among pregnant females in California from 2009–2016. JAMA. 2017;318:2490–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parsons VL, Moriarity C, Jonas K, Moore TF, Davis KE, Tompkins L. Design and estimation for the national health interview survey, 2006–2015. Vital Health Stat. 2014;2:1–53. [PubMed] [Google Scholar]
- 75.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the children’s eating behaviour questionnaire. J Child Psychol Psychiatry. 2001;42:963–970. [DOI] [PubMed] [Google Scholar]
- 76.Coons CE, Thornton SM. The Home Screening Questionnaire Reference Manual. Denver, CO: JFK Child Development Center; 1981. [Google Scholar]
- 77.Dreyer BP, Mendelsohn AL, TamisLeMonda CS. Assessing the child’s cognitive home environment through parental report: reliability and validity. Early Dev Parent. 1996;5:271–287. [Google Scholar]
- 78.Mendelsohn AL, Dreyer BP, Tamis-LeMonda CS, Ahuja P. Validity of StimQ, a scale for assessing the cognitive home environment. J Dev Behav Pediatr. 1999;20:399–399. [Google Scholar]
- 79.Caldwell BM, Bradley RH. Home Observation for Measurement of the Evniornment Inventory: Manual. Tempe, AZ: Family and Human Dynamics Research Institute, Arizona State University; 2003. [Google Scholar]
- 80.Robinson CC, Mandleco B, Olsen SF, Hart CH. Authoritative, authoritarian, and permissive parenting practices - development of a new measure. Psychol Rep. 1995;77:819–830. [Google Scholar]
- 81.Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2003. [Google Scholar]
- 82.Espy KA. The shape school: assessing executive function in preschool children. Dev Neuropsychol. 1997;13:495–499. [Google Scholar]


