Abstract
BACKGROUND:
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causes COVID-19. On March 11, 2020, the WHO declared it a pandemic. SARS-CoV-2 indicates that it poses a significant threat to public health and global economy. The aim of the study was to determine (a) patient characteristics, (b) demographic characteristics, (c) comorbidities, diagnostic methods used, treatment, and outcomes, and (d) mortality rates of patients.
MATERIALS AND METHODS:
This retrospective cohort study included 352 hospitalized adult patients from Baskent University Hospital in Ankara who were confirmed cases of COVID-19 between March 2020 and March 2021. SPSS v. 14.0 was used for statistical analysis.
RESULTS:
Out of 352 patients, 55 died (males: 37, females: 18), while 297 survived (males: 162, females: 135). The most common comorbidities were hypertension (HT), diabetes mellitus (DM), coronary artery disease (CAD), cancer, Vitamin D deficiency, and chronic obstructive pulmonary disease. Comorbidities associated with mortality rate were obesity (33%) (P = 0.118), Vitamin D deficiency (28%) (P = 0.009), DM (25%) (P = 0.004), CAD (21.2%) (P = 0.142), cancer (20.9%) (P = 0.084), and HT (16.6%) (P = 0.90). Normal ward admission resulted in death in 67.3% and survival in 93.9% (P = 0.001), intensive care unit (ICU) admission resulted in death in 69.1% and survival in 18.5% (P = 0.001), and oxygen therapy was used in 80% death and survival in 39.4% (P = 0.001).
CONCLUSIONS:
Our study shows that male gender, advanced age, and presence of comorbidities in COVID 19 patients are at higher risk for severe disease, ICU admission, and death. We emphasize that morbidity and mortality can be reduced by early and comprehensive identification of risk factors and the warning systems that will meet the ICU needs of these patients.
Keywords: Comorbidities, COVID-19, demography, early identification, morbidity, mortality
Introduction
The coronavirus epidemic has become humanity, economic, and social disaster. In December 2019, a simple outbreak in Wuhan, China, sparked the current coronavirus pandemic. The pandemic was declared Public Health Emergency of International Concern in January 2020. Coronaviruses are a group of RNA viruses that cause everything from the common cold to severe acute respiratory syndrome.[1,2]
As of November 24, 2021, there are 258 million confirmed COVID-19 cases, and in just 20 months, the virus has claimed 5 million lives, and this number continues to rise. The mode of transmission of COVID-19 is a droplet and probably contact transmission.[3] Symptoms usually begin about 5 days after exposure. However, this duration is variable (range: 2–14 days).[4,5] Typical clinical symptoms in these patients include fever, dry cough, difficulty breathing, headache, and pneumonia.[5] The clinical spectrum of the disease is highly variable, ranging from asymptomatic infection to morbid conditions such as respiratory failure, acute respiratory distress syndrome (ARDS), sepsis, septic shock, and multiple organ dysfunction syndrome (MODS).[6] Most COVID-19 cases (81%) were classified as a mild or moderate disease in adults.[7]
Angiotensin-converting enzyme-2 (ACE-2) has been identified as a functional receptor for coronavirus and is highly expressed on lung epithelial cells, so the disease generally affects the respiratory system.[8] Although therapeutic procedures have changed somewhat, oxygen support of any kind, antibiotics, antiviral, and anticoagulant drugs are the basic treatment procedures.[4,5]
Although COVID-19 has been widely researched by experts, there are still some aspects about which we are unsure. The contribution of epidemiological studies in the fight against COVID-19 is undeniable because by correctly assessing certain risk factors, epidemiological studies lead to early diagnosis, and timely treatment, which exemplifies good practice.
The study's aim was to find information about (a) patient characteristics, (b) demographic considerations, (c) comorbidities, diagnostic methods used, treatment, and outcomes, and (d) patient mortality rates.
Materials and Methods
Study design and setting
In this retrospective study, 352 adult patients were enrolled. Nucleus electronic medical record system of Başkent University was used in the data collection phase. In this study, 72 parameters were determined and classified into eight different categories: demographics, comorbidities, previously used drugs, the most common findings in patients at admission, laboratory tests, radiological tests, medications, treatments administered, general complications, and mortality.
Study participants and sampling
The demographic parameters selected were age, gender, height, weight, and body mass index (BMI). The selected comorbidity parameters were hypertension (HT), diabetes mellitus (DM), coronary artery disease (CAD), asthma, chronic obstructive pulmonary disease (COPD), hyperlipidemia/hypercholesterolemia (HL/HC), pregnancy, obesity, cancer, autoimmune diseases (AI diseases), smoking, Vitamin D deficiency, previous use of ACE inhibitors (ACEI), diuretics, steroids, anticoagulants, and immunosuppressants. Steroids were not included in the criteria for immunosuppressants due to their additional anti-inflammatory effects.
The selected symptoms and signs were cough, fever, headache, arthralgia, myalgia, runny nose, throat pain, ageusia, anosmia, tinnitus, epigastric pain, dyspnea, dizziness, chest pain, nausea, changes in heart rate, blood pressure, oxygen saturation, body temperature, and consciousness.
These data were collected from the initial examination results. The selected laboratory tests were reverse transcription–polymerase chain reaction (RT-PCR), neutrophil-to-lymphocyte ratio, platelets, lymphocytes, D-dimer, and ferritin levels. Test results were compiled from the first complete blood count or other test results. The imaging tests selected were X-ray and computed tomography (CT). The ground-glass opacity (GGO) view was scanned at CT.
The treatment options selected were admission to normal ward or intensive care unit (ICU), hospitalization, oxygen therapy, noninvasive ventilation (NIV), invasive mechanical ventilation (IMV), and high-frequency jet ventilation (HFJV) and antivirals, antibiotics, anticoagulants, tocilizumab, steroids, Vitamin C, Vitamin D, and immunosuppressants.
The selected complications were pneumonia, ARDS, sepsis, septic shock, MODS, and disseminated intravascular coagulation (DIC).
Data collection tool and technique
Clinical data were collected from the COVID-19 patients who were hospitalized at Baskent University Hospital in Ankara between March 2020 and March 2021. All adult patients were diagnosed with COVID-19 according to the guidelines of Turkish Ministry of Health. Our study was reviewed after obtaining signed informed consent from the participants.
Ethical consideration
This retrospective study conformed to the guidelines of the Declaration of Helsinki, and the study was approved by the Institutional Review Board of Başkent University (KA21/240) and the Ministry of Health (https://bilimselarastirma.saglik.gov.tr/BasvuruForms3/Zeynep%20Ersoy-2021-04-05T17_26_42.xml). Official approval to collect information from the nucleus electronic medical record system was obtained from the hospital management in addition to permission from the university's research committee. Throughout the study, the patients' data remained anonymized and kept private. All information collected during the study was kept private and only used for this purpose.
Statistical analysis
To highlight the differences between the deceased (55 patients) and discharged (297 patients) populations and to shed light on the impact of risk factors on mortality, patients were divided into two groups in terms of survival status. Height, weight, BMI, and period of symptoms were excluded from the study because their number was insufficient, and their accuracy was low (mostly self-reported).
Statistical analyses were performed using the SPSS software package, version 22 for MAC (SPSS Inc., Chicago, IL) was used for statistical analysis. Descriptive statistics were used, and frequency analysis was performed. Chi-square test, and Phi, and Cramér's V test were used where appropriate. An α < 0.05 was considered statistically significant. Unfortunately, we could not calculate the alpha values for some parameters. For continuous data, the mean, maximum, and standard deviation were reported.
Results
Of the 352 patients, 55 died (male: 37, female: 18), while 297 survived (male: 162, female: 135). The mean age of the patients who died was 71.47 years, while it was 64.68 years for the patients who survived [Figure 1].
Figure 1.
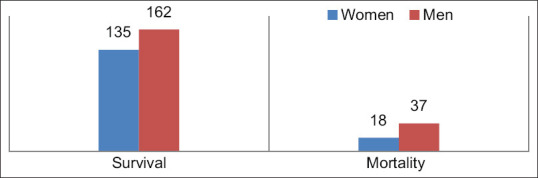
Gender differences in survival and death rates
The most common disease findings in both deceased and survivors were dyspnea (46.2% in deceased, 44.1% in survivors), fever (44.4% in deceased, 41.8% in survivors), fatigue (29.6% in deceased, 34% in survivors), and cough (33.3% in deceased, 33.3% in survivors) [Figure 2]. Other less common prehospital findings included nausea, myalgia, chest pain, headache, epigastric pain, vertigo, throat pain, arthralgia, ageusia, anosmia, and runny nose.
Figure 2.
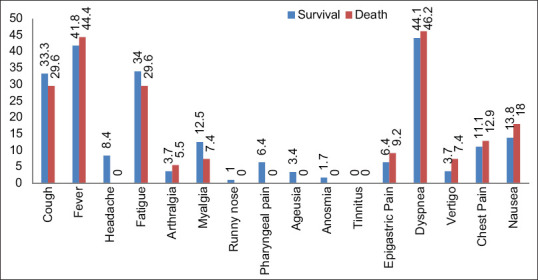
The most common prehospital symptoms patients with admitted to hospital
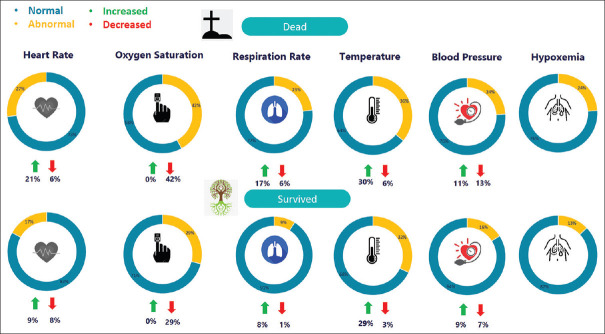
Abnormal vital signs at initial examination were decreased oxygen saturation (42% in death, 29% in survival), increased fever (30% in death, 29% in survival), tachycardia (21% in death, 9% in survival), bradycardia (6% in death, 8% in survival), tachypnea (17% in death, 8% in survival), bradypnea (6% in death, 1% in survival), HT (11% in death, 9% in survival), and hypotension (13% in death, 7% in survival) [Figure 3].
Figure 3.
The most common findings in patients at admission
The most common comorbidities were HT (58.2% deaths, 57.2% survival), DM (47.3% deaths, 29.6% survival), CAD (43.6% deaths, 30% survival), cancer (30.9% deaths, 21.5% survival), Vitamin D deficiency (29.1% deaths, 13.8% survival), and COPD (16.4% deaths, 17.8% survival) [Figure 4]. The overall mortality rate was 15.6%. Comorbidities associated with mortality rate were obesity (33%) (P = 0.118), Vitamin D deficiency (28%) (P = 0.009), DM (25%) (P = 0.004), CAD (21.2%) (P = 0.142), cancer (20.9%) (P = 0.084), and HT (16.6%) (P = 0.90) [Table 1].
Figure 4.
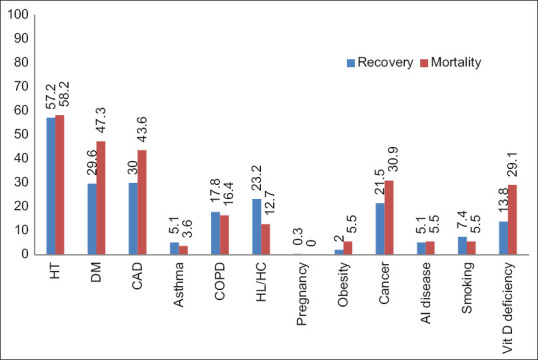
The most common comorbidities of patients
Table 1.
Comorbidities, previously used drugs, and mortality rate
| Comorbidities | Number of patients | Number of deaths | Mortality rate (%) |
|---|---|---|---|
| Obesity | 9 | 3 | 33 |
| Vitamin D deficiency | 57 | 16 | 28 |
| DM | 104 | 26 | 25 |
| CAD | 113 | 24 | 21.2 |
| Cancer | 81 | 17 | 20.9 |
| HT | 192 | 32 | 16.6 |
| COPD | 62 | 9 | 14.5 |
| Smoking | 25 | 3 | 12 |
| Asthma | 17 | 2 | 11.7 |
| HL/HC | 76 | 7 | 9.2 |
| Previously used drugs | |||
| ACEI | 17 | 1 | 5.8 |
| Diuretics | 29 | 3 | 10.3 |
| Immunosuppressants | 29 | 6 | 20.6 |
| Steroids | 14 | 0 | 0 |
| Anticoagulants | 71 | 10 | 14.08 |
HT=Hypertension, DM=Diabetes mellitus, CAD=Coronary artery disease, COPD=Chronic obstructive pulmonary disease, HL=Hyperlipidemia, HC=Hypercholesterolemia, ACEI=Angiotensin-converting enzyme inhibitor
The most common comorbidities in recovered patients were HT (57%), CAD (30%), DM (29.6%), HL/HC (23.2%), and cancer (21.5%). The most common comorbidities in deceased patients are HT (58.2%), DM (47.3%), CAD (43.6%), cancer (30.9%), and Vitamin D deficiency (29.1%).
Among the previously used drugs, immunosuppressants (11.3% for death, 7.7% for survival) (P = 0.214) were the most common. Immunosuppressants were followed by anticoagulants (18.8% death, 20.5% survival) (P = 0.815), diuretics (5.6% death, 8.8% survival) (P = 0.466), ACEI (1.8% death, 5.1% survival) (P = 0.086), and steroids (0% death, 4.7% survival) (P = 0.109) [Table 1].
Deceased patients had higher D-dimer (P = 0.009) and serum ferritin levels (P = 0.760), decreased lymphocyte count (P = 0.193), and increased neutrophil/lymphocyte ratio (P = 0.207). In general, a slightly decreased platelet count was noted (P = 0.443) [Table 2].
Table 2.
Laboratory tests
| Number of Patients | Minimum | Maximum | Mean | SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thrombocyte count (103/µL) | 297 | 55 | 24 | 26 | 766 | 422 | 219.20 | 182.32 | 107.72 | 79.90 |
| Lymphocyte count (103/µL) | 297 | 55 | 0.08 | 0.6 | 193 | 3.7 | 1.92 | 1.009 | 11.15 | 0.68 |
| Neutrophil-to-lymphocyte ratio | 297 | 55 | 0.57 | 1.29 | 55.35 | 98.43 | 7.12 | 14.17 | 7.52 | 16.36 |
| D-dimer (µg/mL) | 295 | 54 | 0.2 | 0.23 | 35.20 | 21.09 | 2.53 | 3.68 | 4.57 | 4.28 |
| Ferritin (ng/mL) | 290 | 54 | 21.1 | 38 | 50180 | 12073 | 673.14 | 1104.73 | 2978.79 | 1834.35 |
• Survival •Death. SD=Standard deviation
The RT-PCR positivity was increased in deceased patients (64%) compared with surviving patients (44%) (P = 0.001). We obtained GGO on CT in deceased patients (63.6%) compared to surviving patients (58.9%) (P = 0.605). Pathological appearance on radiograph was 81% in deceased and 63% in surviving patients, with an overall mean of 67% (P = 0.003) [Figure 5].
Figure 5.
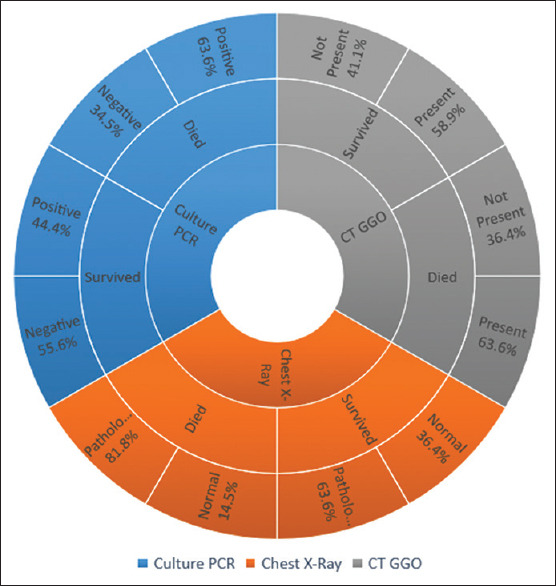
Reverse transcription-polymerase chain reaction, chest X-ray, and computed tomography-ground-glass opacity
In the normal ward, 67.3% died and 93.9% survived (P = 0.001); in the ICU, 69.1% died and 18.5% survived (P = 0.001); and oxygen therapy was used in 80% of those who died and 39.4% of those who survived (P = 0.001) [Figure 6]. Three hundred and ten patients received antibiotics (98.1% death, 85.1% survival) (P = 0.01), 303 patients received antiviral drugs (88.7% death, 85.5% survival) (P = 0.264), and 337 patients received anticoagulant therapy (100% death, 94.3% survival) (P = 0.076). Two hundred thirty-seven patients received steroid treatment (79.2% death, 65% survival) (P = 0.017) [Figure 7].
Figure 6.
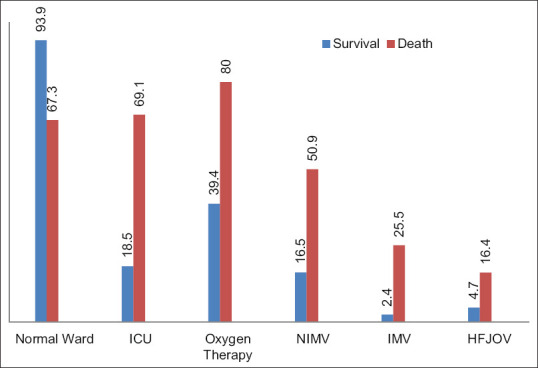
Medications and other treatments administered to patients
Figure 7.
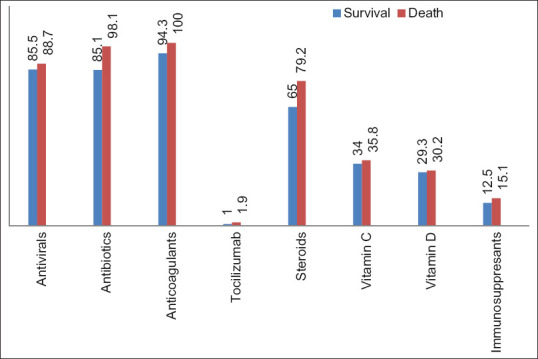
Drugs used in the treatment of patients
In our study, the need for oxygen therapy (P = 0.001), NIV (P = 0.001), IMV (P = 0.001), and HFJV (P = 0.001) was significantly higher in patients who died. Admission to ICU was significantly more in deceased patients. In our research, 14 of the 21 patients who required IMV died.
Our mortality rate (15.6%) is comparable to the recommended mortality rates (8%–21%). Regarding complications, pneumonia (P = 0.076), sepsis (P = 0.001), septic shock (P = 0.001), ARDS (P = 0.001), and MODS (P = 0.001) were the most common causes of death [Figure 8].
Figure 8.
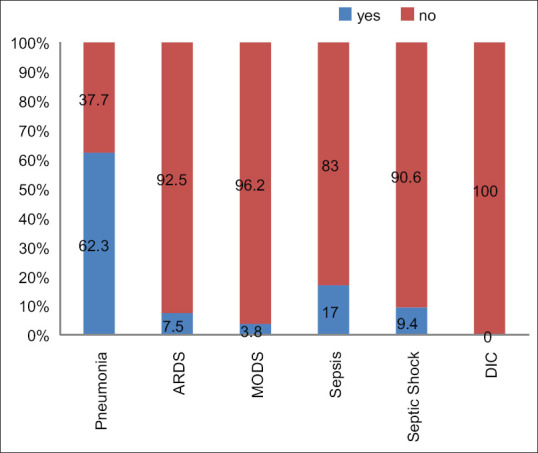
General complications and mortality of the patients
Discussion
Currently, the whole world is in the era of chaotic eco-epidemiology. Numerous devastating infectious agents are emerging and reemerging.[3] This retrospective cohort study included 354 hospitalized adult patients from Baskent University Hospital in Ankara who were confirmed cases of COVID-19 between March 2020 and March 2021. We found that older age and male gender were risk factors in deceased patients. Out of 352 patients, 55 died (male: 37, female: 18), while 297 of them survived (male: 162, female: 135). The mean age in deceased patients was 71.47 years, while in surviving patients, it was 64.68 years [Figure 1].
Bienvenu et al. pointed out that males accounted for the majority of COVID-19-related deaths. In parallel with this study, we found that older age and male gender were associated with the severity of COVID-19.[9]
We found that dyspnea, fever, fatigue, and cough were the most common findings of the disease in both deceased and surviving patients [Figure 2]. These symptoms play an important role in the early detection of the disease and thus prevent the spread of the virus.[10]
At the time of the initial examination, we determined that the patient had decreased oxygen saturation, a high temperature, tachycardia, tachypnea, hypotension, HT, and bradypnea [Figure 3].
In our study, the most common comorbidities were HT, DM, CAD, cancer, Vitamin D deficiency, HL/HC, COPD, smoking, asthma, and obesity [Figure 4]. The overall mortality rate was 15.6%. Comorbidities associated with mortality were obesity (33%), Vitamin D deficiency (28%), DM (25%), CAD (21.2%), cancer (20.9%), COPD (17.1%), and HT (16.6%) [Table 1].
As for HT, we found that although it may have a negative impact on disease progression, it is not an absolute risk factor. This is reflected in our data by the fact that the two groups (survivors and deceased) have similar values (57% for survival, 58% for death) of HT. These results are in contrast to the current literature, for example, Zhou et al.[11] found a significant difference in HT scores between survivors and nonsurvivors (48% at death, 23% at survival). Like HT, COPD can also have a negative impact on morbidity but is not an absolute risk factor for mortality, as we also found minimal differences between groups (16.4% for mortality, 17.8% for survival).
According to Kumar et al.,[12] the mortality rate was increased two fold in diabetic COVID-19 patients compared to nondiabetic patients. In our study, half of the deceased patients had DM, while only 30% of the surviving patients had DM, suggesting that it has a significant impact on disease severity, and mortality.
Furthermore, CAD is a clear risk factor for COVID-19. This is shown by both our results that 43% of deceased patients had CAD, and Barman et al.[13] show that a value of 38% was present in deceased. Although 30% of our surviving patients had CAD, this percentage was only 15% in the aforementioned study, showing us that other epidemiological factors also play an important role in disease prognosis. One of the fatal comorbidities in our study was obesity. One-third of our obese patients lost their lives, although we had only nine patients with obesity. Twenty-eight percent of our patients with Vitamin D deficiency died. Since Vitamin D deficiency is a common phenomenon in Turkey, it needs special attention. In the study of 93.1% of patients with severe COVID-19, Vitamin D insufficiency was present, which is further evidence that Vitamin D deficiency is one of the main risk factors for COVID-19.[14]
About 20.6% of patients who had received immunosuppressive drugs prior to infection with COVID-19 died. Thus, we conclude that immunosuppressive drugs increase the mortality rate of the disease, as our overall mortality rate was 15.6%, which is consistent with the literature.[15] ACEI, and diuretics, on the other hand, decrease the mortality of the disease. Diuretics and ACEI are mainly taken by HT patients. HT patients had an overall mortality rate of 16.6%. In patients who had taken diuretics and ACEI before the disease, the mortality rate decreased to 10.3% and 5.8%, respectively. Prior use of anticoagulants also reduced the mortality rate of the disease, as the mortality rate with prior use of anticoagulants was 14.1% [Table 1]. In contrast to our results, Ren et al., Rivera-Caravaca et al., and Tremblay et al. pointed out that prior use of antihypertensives and anticoagulants has no positive effect on disease prognosis.[16,17,18]
As for laboratory results, the normal value for serum D-dimer is <0.44 mg/L. In our study, patients who died from coronavirus had an average D-dimer level of 3.68 mg/L. Our results are in agreement with those of Zhou et al.[11] who also concluded that elevated D-dimer levels are associated with a worse prognosis of the disease. We observed that ferritin levels were elevated in both patients who survived (673.1 μg/L) and those who died (1104.7 μg/L). However, this increase is almost twice as high in those who died, suggesting that ferritin correlates with poor prognosis, as demonstrated by Cheng et al.[19] In addition, we found that lymphocyte levels were an indicator of disease severity [Table 2]. Patients who survived had twice as high lymphocyte levels. Illg et al.[20] showed that this may serve as a prognostic factor for COVID-19. The most common radiological finding we obtained was the presence of GGO in the CT scan, which is consistent with the study by Çinkooğlu et al.[21] However, we did not find this to be indicative of mortality as we had similar values in surviving (58.9%) and deceased (62%) patients. Long et al.[22] state that PCR positivity was increased in deceased patients (64%) compared to surviving patients (44%), which could be due to repeated testing in severe cases, which is also confirmed. As they mention, PCR can lead to false-negative results, so repeated testing should be performed to avoid misdiagnosis.[22] Therefore, we hypothesize that this fact poses a significant burden in controlling the spread of disease, as asymptomatic positive patients may not be quarantined due to negative test results [Figure 5].
In our study, the need for oxygen, NIV, IMV, and HFJV was significantly higher in deceased patients. Admission to ICU was much more common in deceased patients. In our study, out of the 21 patients who required IMV, 14 died that is 66% of those patients. This tells us two things: first, patients who survive COVID-19 usually do not need IMV and second, the need for IMV is always associated with a poor prognosis [Figure 6]. Similar results were obtained in the study by Lim et al.[23]
The prescription rate of antibiotics and antiviral drugs was 85.1%–85.5% in surviving patients and 98.1%–88.7% in deceased patients [Figure 7]. Langford et al. claimed that nonbeneficial antibiotic use was high in COVID-19 patients.[24] Our study also shows that the effectiveness of antibiotic use in reducing the mortality rate is questionable.
Mikulska et al.[25] found that early administration of tocilizumab and steroids had beneficial effects on COVID-19 pneumonia, especially in nonintubated patients. Our results suggest that tocilizumab has a positive effect on the mortality rate, but further studies are needed because our sample size was relatively small. However, according to our results, the use of steroids (79.2% in death, 65% in survival) was not helpful in reducing the mortality rate. Since we did not separate the use of tocilizumab and steroids in patients in the normal ward or in the ICU, early treatment with these drugs should be considered.
Thrombotic complications were common. Bikdeli et al.[26] pointed out that emergency physicians in particular need to be aware of coagulation disorders COVID-19 to reduce morbidity and mortality. However, we did not have a single case of DIC. The reason could be the frequent use of anticoagulants in the hospital, as almost all patients received anticoagulant therapy (100% for deaths, 94.3% for survival).
As Robba et al.[27] mentioned, COVID-19 infections have different effects on different systems. The vast majority of the patients can be classified as moderate-to-severe cases.[27] Our mortality rate (15.6%) is similar to the suggested mortality rates (8%–21%). In the literature, the rates for ARDS and MODS were 18%–61% and 20%, respectively.[28] Regarding complications, pneumonia, sepsis/septic shock, ARDS, and MODS were the most common causes of death [Figure 8].
Limitations and recommendation
Our study had some limitations. First of all, the parameters chosen were insufficient to fully understand the characteristics of the disease in patients with chronic kidney disease and cardiac problems. Screening of adolescents and children was not possible because the youngest subject was 20 years old, and the number of patients for this study was limited compared to the literature. Some complications could be reported under the heading MODS or separately.
We evaluated the Sequential Organ Failure Assessment (SOFA) and MODS scores of the patients admitted to the ICU, but we did not perform statistical analysis. This is one of our limitations.
Laboratory results and radiological images should be performed as early as possible and monitored daily. More attention should be given to COVID-19 patients as they are associated with a higher mortality rate in certain comorbidities such as obesity, DM, CAD, and cancer. As recommendation, vital signs should be closely monitored. As the mortality of the diseases is high, the patients should be thoroughly and carefully examined. If we had kept our sampling process longer, we could have achieved even more meaningful results.
Conclusions
Our study shows that male gender, advanced age, and presence of comorbidities put COVID-19 patients at higher risk of severe disease, admission to ICU, and death. Demographic characteristics of a disease could help to reduce morbidity and mortality rates, as they can determine the risk factors and give a better idea of the prognosis of the disease, allowing a more efficient application of treatment methods and reducing the burden on health systems. It is therefore important to identify these measures and act accordingly.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The project was created by NC, ZE, after the study was approved by the Local Ethics Committee at Ankara Baskent University (KA 21/240) and the Ministry of Health (https://bilimselarastirma.saglik.gov.tr/BasvuruForms3/Zeynep%20Ersoy-2021-04-05T17_26_42.xml). Patient data analyses and statistical analyses were made by YI, YST, AAG, and IMS. NC and ZE took part in the writing of the article.
References
- 1.Saji JA, Babu BP, Sebastian SR. Social influence of COVID-19: An observational study on the social impact of post-COVID-19 lockdown on everyday life in Kerala from a community perspective. J Educ Health Promot. 2020;9:360. doi: 10.4103/jehp.jehp_650_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irani M, Pakfetrat A, Mask MK. Novel coronavirus disease 2019 and perinatal outcomes. J Educ Health Promot. 2020;9:78. doi: 10.4103/jehp.jehp_189_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. 2020. Available from: 29 March 2020. https://www.who.int/news-room/commentaries/detail/modes-oftransmission-of-virus-causing-covid-19-implications-for-ipcprecaution-recommendations .
- 4.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The ıncubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med. 2020;172:577–82. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588:E6. doi: 10.1038/s41586-020-2951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulut C, Kato Y. Epidemiology of COVID-19. Turk J Med Sci. 2020;50:563–70. doi: 10.3906/sag-2004-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, McGoogan JM. Characteristics of, and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control, and Prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: Sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116:2197–206. doi: 10.1093/cvr/cvaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alimohamadi Y, Sepandi M, Taghdir M, Hosamirudsari H. Determine the most common clinical symptoms in COVID-19 patients: A systematic review and meta-analysis. J Prev Med Hyg. 2020;61:E304–12. doi: 10.15167/2421-4248/jpmh2020.61.3.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, et al. Is diabetes mellitus associated with mortality, and severity of COVID-19. A meta-analysis. Diabetes Metab Syndr. 2020;14:535–45. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barman HA, Atici A, Sahin I, Alici G, Aktas Tekin E, Baycan ÖF, et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron Artery Dis. 2020;15:10. doi: 10.1097/MCA.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karahan S, Katkat F. Impact of serum 25(OH) vitamin D level on mortality in patients with COVID-19 in Turkey. J Nutr Health Aging. 2021;25:189–96. doi: 10.1007/s12603-020-1479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myint PK, Carter B, Barlow-Pay F, Short R, Einarsson AG, Bruce E, et al. Routine use of immunosuppressants is associated with mortality in hospitalised patients with COVID-19. Ther Adv Drug Saf. 2021;12:1–17. doi: 10.1177/2042098620985690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren L, Yu S, Xu W, Overton JL, Chiamvimonvat N, Thai PN. Lack of association of antihypertensive drugs with the risk and severity of COVID-19: A meta-analysis. J Cardiol. 2021;77:482–91. doi: 10.1016/j.jjcc.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera-Caravaca JM, Núñez-Gil IJ, Vivas D, Viana-Llamas MC, Uribarri A, Becerra-Muñoz VM, et al. Clinical profile and prognosis in patients on oral anticoagulation before admission for COVID-19. Eur J Clin Invest. 2021;51:e13436. doi: 10.1111/eci.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tremblay D, van Gerwen M, Alsen M, Thibaud S, Kessler A, Venugopal S, et al. Impact of anticoagulation prior to COVID-19 infection: A propensity score-matched cohort study. Blood. 2020;136:144–7. doi: 10.1182/blood.2020006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng L, Li H, Li L, Liu C, Yan S, Chen H, et al. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Lab Anal. 2020;34:e23618. doi: 10.1002/jcla.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Illg Z, Muller G, Mueller M, Nippert J, Allen B. Analysis of absolute lymphocyte count in patients with COVID-19. Am J Emerg Med. 2021;46:16–9. doi: 10.1016/j.ajem.2021.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Çinkooğlu A, Hepdurgun C, Bayraktaroğlu S, Ceylan N, Savaş R. CT imaging features of COVID-19 pneumonia: İnitial experience from Turkey. Diagn Interv Radiol. 2020;26:308–14. doi: 10.5152/dir.2020.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, et al. Diagnosis of the coronavirus disease (COVID-19): RRT-PCR or CT.? Eur J Radiol. 2020;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim ZJ, Subramaniam A, Ponnapa Reddy M, Blecher G, Kadam U, Afroz A, et al. Case fatality rates for patients with COVID-19 requiring ınvasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med. 2021;203:54–66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langford BJ, So M, Raybardhan S, Leung V, Soucy JR, Westwood D, et al. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520–31. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikulska M, Nicolini LA, Signori A, Di Biagio A, Sepulcri C, Russo C, et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS One. 2020;15:e0237831. doi: 10.1371/journal.pone.0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bikdeli B, Talasaz AH, Rashidi F, Bakhshandeh H, Rafiee F, Rezaeifar P, et al. Intermediate-dose versus standard-dose prophylactic anticoagulation in patients with COVID-19 admitted to the Intensive Care Unit: 90-day results from the INSPIRATION R, andomized trial. Thromb Haemost. 2022;122:131–41. doi: 10.1055/a-1485-2372. [DOI] [PubMed] [Google Scholar]
- 27.Robba C, Battaglini D, Pelosi P, Rocco PR. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med. 2020;14:865–8. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Wang Q, Zhang H, Cui L, Shen F, Chen Y, et al. Viral sepsis is a complication in patients with novel corona virus disease (COVID-19) Med Drug Discov. 2020;8:100057. doi: 10.1016/j.medidd.2020.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]