Abstract
Background
Leptospirosis is an important, neglected zoonotic disease that affects people and animals in humid (sub)tropical regions. Wild canines carry the pathogen and may contaminate natural resources which may then act as a source of human infection.
Objectives
The study was designed to understand the seroprevalence of leptospirosis among domestic and wild canines in Bojnurd County, Northeast Iran.
Methods
A total of 77 serum samples, comprising 29 sera from asymptomatic wild canines [foxes (n = 25) and jackals (n = 4)] and 48 sera from asymptomatic stray dogs, was investigated. Serovars were identified and antibody titres were measured by standard microscopic agglutination test (MAT) using serial serum dilutions.
Results
Among all serum samples, 44.1% reacted positively to a Leptospira interrogans serovars. The average percentage of positive reactions was higher in stray dogs than in wild canines although this did not reach statistical significance (55.2% and 37.5%, p = 0.159). Positive reactions with L. i. Pomona, L. i. Australis and L. i. Tarasovi was detected only among jackals and foxes. Among the stray dogs, the highest number of positive sera were for L. i. Grippotyphosa (61.1%) and L. i. Canicola (50%). The highest titre detected was for L. i. canicola (1:1600) in two stray dogs and against L. i. Icterohaemorrhagiae and L. i. Pomona (1:800) in a single jackal.
Conclusions
The study revealed that leptospirosis is endemic among various canine species in the North Khorasan Province of Iran. Detailed monitoring of canines is necessary for better understanding the epidemiology of infection in our and other Iranian regions.
Keywords: fox, Iran, jackal, leptospirosis, stray dogs
In the current study, the average percentage of positive reactions among stray dogs (55.2%) was higher than wild canines (37.2%, P = 0.159). The study revealed leptospirosis is endemic among various canine species in the North Khorasan Province of Iran. Detailed monitoring of canines suggested.
1. INTRODUCTION
Leptospirosis, a globally neglected zoonotic disease causing fever for days to weeks, affects people and animals in humid (sub‐)tropical regions (Ullmann & Langoni, 2011). Leptospirosis is mild in 90% of all cases but may generate severe complications in other patients. Although the main symptoms are fever, muscle pain and headaches, the disease can also lead to serious organ failure (kidneys, liver) and haemorrhaging. The disease is spread by a large variety of both wild and domestic animals which are natural reservoirs of Leptospira spp. (Adler & de la Peña Moctezuma, 2010). A wide variety of animals host Leptospira interrogans and many of these are asymptomatic Leptospira renal carriers. They contaminate the environment by shedding bacteria in their urine and they may develop symptoms only after long incubation periods (Adler & de la Peña Moctezuma, 2010). The precise epidemiology of leptospirosis in a specific niche is defined by the close contact between the particular Leptospira serovars and their specific maintenance hosts (Fratini et al., 2020). The seroprevalence of leptospirosis in humans and different animals in Iran has been studied before (Khalili et al., 2020). Leptospirosis is endemic in the North Khorasan province, as recent studies using the microscopic agglutination test (MAT) unveiled past and present infections among both rodents (Arzamani et al., 2018) and humans (Hashemi et al., 2021). Leptospiral infection, its associated prevalence and the dominant serovars were reported as being different in canines around the world (Ab Rahman et al., 2018; Ambily et al., 2013; Aslantaş et al., 2005; Azocar‐Aedo et al., 2017; Lelu et al., 2015; Samir et al., 2015; Shi et al., 2012). Similar reports on seroprevalence have emerged from different geographic regions of Iran as well (Avizeh et al., 2009; Fahimipour et al., 2021; Jamshidi et al., 2008; Rad et al., 2004; Torkan & Momtaz, 2019). Wild and feral canines carry the pathogen and contaminate the environment including soils, surface waters, streams and rivers where the bacteria can survive for weeks to months. This acts as one of the most significant sources of infection. In the current study Leptospira spp. seroprevalence among foxes, jackals and stray dogs in Bojnurd County, Northeast of Iran, was investigated for the first time.
2. METHODS AND MATERIALS
2.1. Study population and blood sample collection
This study was conducted in the North Khorasan province in the Northeast of Iran (37.47 N and 57.33 E) (Figure 1). In a six months period, from April to September in 2020, stray dogs and wild canines were collected by the municipal animal control department and examined by veterinarians. All asymptomatic animals subjected to this study. A total of 77 blood samples were randomly selected from 29 asymptomatic wild canines [foxes (n = 25) and jackals (n = 4)] and 48 also randomly selected asymptomatic stray dogs were included in the current study. Five millilitres of blood were collected aseptically from each animal and centrifuged at 3000 rpm for 10 min. Sera were kept at –20°C in micro‐tubes. The sera were transferred to the Leptospira Research Laboratory of the Veterinary Research and Teaching Hospital at the University of Tehran for further analysis while maintaining cold chain management.
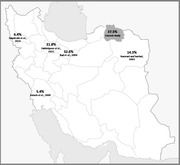
FIGURE 1.
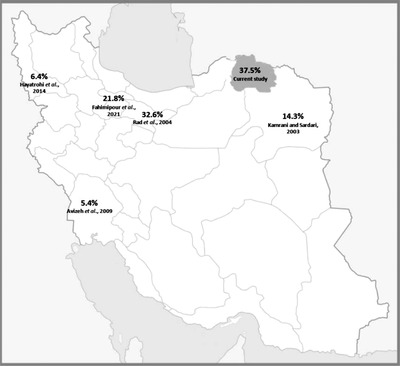
Study location and illustration of the rate of leptospiral positive reactions among canines in different geographic regions of Iran
2.2. Microscopic agglutination test (MAT)
All serum samples were subjected to MAT in duplicate to determine the exposure of individual animals to the various serovars of L. interrogans bacteria (Niloofa et al., 2015; Sakhaee, 2011; Sakhaee et al., 2010). A seven to 10 days’ culture of different serovars of L. interrogans in a liquid medium (GRA‐Sina, Sinajen, Tehran, Iran) was used as a source of cellular antigens. The density of leptospires was checked using a counting chamber (Petroff‐Hauser USA) and adjusted to 2 × 108 cell/ml. All serum samples were serially diluted in phosphate buffer solution (PBS), starting from 1 in 50 dilutions, using twofold dilution (1 in 100, 200, 400, 800 and 1600). Then, 10 μl of serum dilution was added to 10 μl of the appropriate antigen in a 96‐well plate and incubated at 30°C for 90 min. Upon completion of incubation, samples from individual wells were transferred to a microscopic slide and examined using a dark‐field microscope (Olympus B×50). One antigen control and two (positive and negative) standard serum controls were used for each 96 well plate (Sakhaee, 2011). Samples with 50% agglutination were considered positive. According to the OIE Terrestrial Manual, a titre of 1:100 diluted serum is interpreted as positive and serves international trade purposes (World Organization of Animal Health‐OIE, 2019). All samples were tested against nine leptospiral antigens (L. interrogans serovar Hardjo, L. interrogans serovar Tarasovi, L. interrogans serovar Australis, L. interrogans serovar Icterohaemorrhagiae, L. interrogans serovar Pomona, L. interrogans serovar Grippotyphosa, L. interrogans serovar Autumnalis, L. interrogans serovar Canicola and L. interrogans serovar Ballum).
2.3. Statistical analysis
Fisher exact testing was conducted for comparing the leptospiral seroprevalence among the different animals using SPSS software (version 20).
3. RESULTS
Among the 77 samples studied, 44.1% reacted positively to at least one serovar‐specific antigen preparation of L. interrogans. The rate of positive reactions was higher in stray dogs versus wild canines (55.2% and 37.5%), but this was not statistically significant (p = 0.159). The highest detected frequency was for L. interrogans serovar Grippotyphosa (n = 14) and L. interrogans serovar Canicola (n = 11) (Table 1).
TABLE 1.
Distribution of positive MAT results as measured in canine serum samples
| Type of canines | Positive/total cases (%) | Number | Serovar | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hardjo | Australis | Tarasovi | Canicola | Ictero. | Pomona | Grippo. | |||
| titre | |||||||||
| Stray dogs | 18/48 (37.5) | 7 | 100 | ||||||
| 1 | 100 | ||||||||
| 6** | 100–1600 | ||||||||
| 2 | 100 | 100 | |||||||
| 1 | 100 | 100 | 200 | ||||||
| 1 | 100 | 200 | |||||||
| Jackal/fox | 16/29 (55.2) | 1* | 400 | 800 | 800 | ||||
| 1* | 100 | 100 | |||||||
| 1* | 100 | ||||||||
| 1* | 200 | 400 | |||||||
| 3 | 100 | ||||||||
| 2 | 100 | ||||||||
| 2 | 100 | ||||||||
| 2 | 100 | ||||||||
| 1 | 200 | ||||||||
| 2 | 100 | 100 | |||||||
| Total | 48/77 (44.1) | 2 | 5 | 3 | 11 | 4 | 6 | 14 | |
*Jackal.
**Titre 200 = 3 cases, Titre 100 = 1 case and Titre 1600 = 2 cases.
Hardjo: L. interrogans serovar Hardjo, Australis: L. interrogans serovar Australis, Tarasovi: L. interrogans serovar Tarasovi, Canicola: L. interrogans serovar Canicola, Ictero: L. interrogans serovar Icterohaemorrhagiae, Pomona: L. interrogans serovar Pomona, Grippo: L. interrogans serovar Grippotyphosa.
Positive reactions with L. interrogans serovar Pomona (n = 6), L. interrogans serovar Australis (n = 5) and L. interrogans serovar Tarasovi (n = 3) were detected only among sera collected from jackals and foxes. No positive reaction against L. interrogans serovar Canicola was detected among foxes. Among the stray dogs, the highest frequency of positivity was for L. interrogans serovar Grippotyphosa (n = 11/18, 61.1%) and L. interrogans serovar Canicola (n = 9/18, 50%). Antibodies against L. interrogans serovar Hardjo (n = 2) were detected among stray dogs only (Table 1).
The highest detected titre was 1:1600 for L. interrogans serovar Canicola in two stray dogs and a titre of 1:800 against L. interrogans serovar Icterohaemorrhagiae and L. interrogans serovar Pomona in a single jackal (Table 1). Among dog samples, a positive triple reaction against L. interrogans serovar Hardjo (1:100), L. interrogans serovar Canicola (1:100) and L. interrogans serovar Grippotyphosa (1:200) was spotted. All tested jackal samples showed positive reactions. A single jackal sample revealed a response at 1:800 against L. interrogans serovar Icterohaemorrhagiae and L. interrogans serovar Pomona followed by 1:400 against L. interrogans serovar Australis (Table 1).
4. DISCUSSION
Leptospirosis is one of the most significant re‐emerging infectious diseases in Iran (Parhizgari et al., 2017). The present study investigated the prevalence of antibodies against serovar‐specific leptospiral antigens among wild canines and stray dogs using the MAT (Niloofa et al., 2015). Clinical samples were collected from animals captured in the North Khorasan province in Iran. The location of the study is in a mountainous area with 9 months of cold weather. Conditions such as these should always be considered in our type of study but the precise impact of climate is ill defined.
We report specific antibodies against various L. interrogans serovars found in jackals and foxes. The prevalence of specific antibodies against those leptospiral serovars detected among foxes in this study (55.2%) is higher than the prevalence reported in native foxes in Chile (7.7%) (Galarce et al., 2021), red foxes from Spain (47.1%) (Millán et al., 2009) and Croatia (33.8% and 31.25%) (Slavica et al., 2008; Slavica et al., 2011). The prevalence was slightly lower than recorded among red foxes from Croatia (57.6%) (Milas et al., 2006). In a recently conducted study on jackals, Nakonechnyi et al. (2019) found positive reactions against Leptospira interrogans among all nine animals that were tested.
The documented overall prevalence of 37.5% among dogs in this study is almost 2.5‐fold higher than the estimated overall prevalence of 14.6% (95% CI: 3.49–25.77) for dogs in Iran (Khalili et al., 2020). The highest recorded prevalence was from Tehran (32.6%) (Rad et al., 2004), following Alborz (21.8%) (Fahimipour et al., 2021), Khorasan Razavi (14.3%) (Kamrani & Sardari, 2003), West Azerbaijan (6.4%) (Hayatrohi et al., 2014) and Khuzestan (5.4%) (Avizeh et al., 2009). The serological prevalence reported by the current study is in the mean but broad range among those reported from other regions of the world (8% to 74.2%) (Table 2). The high prevalence of leptospiral antibodies among stray dogs conceivably results from the greater risk for leptospirosis rising from spending all of their time outdoors and in urban environments as reported for large working and hunting dogs in the United States (Adin & Cowgill, 2000; Alton et al., 2009; Birnbaum et al., 1998; Ward et al., 2002).
TABLE 2.
Prevalence and the most dominant leptospiral serovars reported from Iran and other region of world among dogs
| Study place | Reported prevalence % | Serovars | ||
|---|---|---|---|---|
| Iranian province | North Khorasan | 37.5 | L. interrogans Grippotyphosa (61.1%) and L. interrogans Canicola (50%) | Current study |
| Alborz | 21.8 | L. interrogans Canicola (33.3%), L. interrogans Icterohaemorrhagiae (25%) and L. interrogans Grippotyphosa (20.83%) | Fahimipour et al. (2021) | |
| Khuzestan | 5.4 | L. interrogans Hardjo (44.5%), L. interrogans Ballum and L. interrogans Icterohaemorrhagiae (22.2%) | Avizeh et al. (2009) | |
| Khorasan Razavi | 14.3 | L. interrogans Canicola (11.98%), L. interrogans Pomona (4.79%) and L. interrogans Hardjo (2.39%) | Kamrani and Sardari (2003) | |
| West Azerbaijan | 6.4 | L. interrogans Hardjo (44.5%), L. interrogans Icterohaemorrhagiae and Ballum (22.2%), | Hayatrohi et al. (2014) | |
| Tehran | 32.6 | L. interrogans Canicola (9%), L. interrogans Icterohaemorrhagiae (5.7%) and L. interrogans Grippotyphosa (3.7%) | Rad et al. (2004) | |
| Other countries | Thailand | 12.1 | L. interrogans Sejroe (4.4%) and L. interrogans Icterohaemorrhagiae (3.7%), | Altheimer et al. (2020) |
| India | 71.12 | L. interrogans Autumnalis (23.9%) and L. interrogans Australis (19.17%) | Ambily et al. (2013) | |
| Turkey | 43.96 | L. interrogans Bratislava (66%) and L. interrogans Canicola (21.5%) | Aslantaş et al. (2005) | |
| Sudan | 74.2 | L. interrogans Autumnalis (>70%) and L. interrogans Icterohaemorrhagiae (> 60%%) | Roqueplo et al. (2015) | |
| Gabon | 34.6 | L. interrogans Autumnalis (>30%) and L. interrogans Icterohaemorrhagiae (> 70%) | Roqueplo et al. (2015) | |
| Ivory Coast | 58.1 | L. interrogans Autumnalis (60%) and L. interrogans Grippotyphosa (40%) | Roqueplo et al. (2015) | |
| Egypt | 11.3 | L. interrogans Icterohaemorrhagiae (47.3%) and L. interrogans Canicola (52.6%) | Samir et al. (2015) | |
| Germany | 32 | L. interrogans Australis (24%), L. interrogans Grippotyphosa (20%) and L. interrogans Pomona (9%). | Mayer‐Scholl et al. (2013) | |
| Switzerland | 28.1 | L. interrogans Australis (70.5%) and L. interrogans Bratislava (69.1%) | Major et al. (2014) | |
| Spain | 25.8 | L. interrogans Icterohaemorrhagiae (19.4%) and L. interrogans Bratislava (8.5%) | López et al. (2019) | |
| Italy | 29.9 | L. interrogans Icterohaemorrhagiae (57%) and L. interrogans Bratislava (22%) | Piredda et al. (2021) | |
| Italy | 49 | L interrogans Australis (39.3%) and L. interrogans Icterohaemorrhagiae (32.1%) | Tagliabue et al. (2016) | |
| Canada | 8 | L. interrogans Autumnalis (31.3%) and L. interrogans Bratislava (15.8%) | Alton et al. (2009) | |
| Chile | 25.1 | L. interrogans Canicola (51.6 %) | Lelu et al. (2015) |
The causative agents of leptospirosis among dogs are usually the serovars L. interrogans serovar Canicola, L. interrogans serovar Icterohaemorrhagiae, L. interrogans serovar Grippotyphosa, L. interrogans serovar Pomona and L. interrogans serovar Bratislava (Klaasen & Adler, 2015). The high detection frequency for L. interrogans serovar Grippotyphosa (61.1%) and L. interrogans serovar Canicola (50%) and the lack of positivity against L. interrogans serovar Pomona among dogs in the current study is similar to the results of a study conducted by Fahimipour et al. (2019) in the Alborz province. These authors reported a high prevalence of L. interrogans serovar Canicola (33.3%), L. interrogans serovar Icterohaemorrhagiae (25%) and L. interrogans serovar Grippotyphosa (20.83%), followed by a low prevalence of L. interrogans serovar Pomona (4.1%) (Fahimipour et al., 2021). Studies conducted in other regions of Iran including Tehran (Rad et al., 2004), Khuzestan (Avizeh et al., 2009), West Azerbaijan (Hayatrohi et al., 2014) and a study in a nearby region of the current study in Khorasan Razavi (Kamrani & Sardari, 2003) illustrated positive reactions of sera with different serovars of L. interrogans (Table 2) (Figure 1). The common serovars among dogs reported here differ from those found in Asian, African, European and American countries (Table 2).
Based on serological data collected during the current study, we conclude that dogs, foxes and jackals are natural reservoirs of Leptospira in the area that we covered. Specific antibodies against L. interrogans were reported as was done among rodents in the same geographical regions before (Arzamani et al., 2018). Dogs are considered an important host for Leptospira interrogans, and having close contact with stray dogs may increase the risk of infection in humans (Lelu et al., 2015).
5. CONCLUSION
We demonstrate that leptospirosis is a significant endemic epizootic disease in the study region. More extensive investigations on the large population of wild canines is recommended for better understanding the possible transfer of infections from wild and domesticated animals to humans. First thing to better substantiate this risk would be to perform seroprevalence studies among humans from which in the end prophylactic measures may be designed.
CONFLICT OF INTEREST
The authors have no relevant financial or non‐financial interests to disclose.
FUNDING INFORMATION
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
ETHICS STATEMENT
Sample collection was performed according to the rules and regulations set by the Ethical Committee of North Khorasan University of Medical Sciences (project number: IR. NKUMS. REC1391.017.).
AUTHOR CONTRIBUTIONS
Kourosh Arzamani: Conceptualization, Methodology, Resources
Gholamreza Abdollahpour and Amir Azimian: Investigation
Hamed Ghasemzadeh‐Moghaddam: Visualization, Writing – Original Draft Preparation
Alex van Belkum: Writing – Review & Editing
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.890.
Arzamani, K. , Abdollahpour, G. , Azimian, A. , van Belkum, A. , & Ghasemzadeh‐Moghaddam, H. (2022). High‐level seroprevalence against Leptospira interrogans serovars among wild foxes, jackals and stray dogs in the North Khorasan Province, Iran. Veterinary Medicine and Science, 8, 1915–1921. 10.1002/vms3.890
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs.
REFERENCES
- Ab Rahman, M. H. A. , Hairon, S. M. , Hamat, R. A. , Jamaluddin, T. Z. M. T. , Shafei, M. N. , Idris, N. , Osman, M. , Sukeri, S. , Wahab, Z. A. , Mohammad, W. M. Z. W. , Idris, Z. , & Daud, A. (2018). Seroprevalence and distribution of leptospirosis serovars among wet market workers in northeastern Malaysia: A cross sectional study. BMC Infectious Diseases, 18(1), 569–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler, B. , & de la Peña Moctezuma, A. (2010). Leptospira and leptospirosis. Veterinary Microbiology, 140(3‐4), 287–296. [DOI] [PubMed] [Google Scholar]
- Adin, C. A. , & Cowgill, L. D. (2000). Treatment and outcome of dogs with leptospirosis: 36 cases (1990–1998). Journal of the American Veterinary Medical Association, 216(3), 371–375. [DOI] [PubMed] [Google Scholar]
- Altheimer, K. , Jongwattanapisan, P. , Luengyosluechakul, S. , Pusoonthornthum, R. , Prapasarakul, N. , Kurilung, A. , Broens, E. M. , Wagenaar, J. A. , Goris, M. G. A. , Ahmed, A. A. , Pantchev, N. , Reese, S. , & Hartmann, K. (2020). Leptospira infection and shedding in dogs in Thailand. BMC Veterinary Research, 16(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton, G. D. , Berke, O. , Reid‐Smith, R. , Ojkic, D. , & Prescott, J. F. (2009). Increase in seroprevalence of canine leptospirosis and its risk factors, Ontario 1998–2006. Canadian Journal of Veterinary Research, 73(3), 167–175. [PMC free article] [PubMed] [Google Scholar]
- Ambily, R. , Mini, M. , Joseph, S. , Krishna, S. V. , & Abhinay, G. (2013). Canine leptospirosis‐a seroprevalence study from Kerala, India. Veterinary World, 6(1), 41–42. [Google Scholar]
- Arzamani, K. , Mohammadi, Z. , Shirzadi, M. R. , Alavinia, S. M. , Jafari, B. , & Darvish, J. (2018). Faunistic study of the rodents of north Khorasan province, north east of Iran, 2011–2013. Journal of Arthropod‐Borne Diseases, 12(2), 127–134. [PMC free article] [PubMed] [Google Scholar]
- Aslantaş, Ö. , Özdemir, V. , Kiliç, S. , & Babür, C. (2005). Seroepidemiology of leptospirosis, toxoplasmosis, and leishmaniosis among dogs in Ankara, Turkey. Veterinary Parasitology, 129(3‐4), 187–191. [DOI] [PubMed] [Google Scholar]
- Avizeh, R. , Ghorbanpoor, M. , Hatami, S. , & Abdollahpor, G. (2009). Seroepidemiology of canine leptospirosis in Ahvaz, Iran. Iranian Journal of Veterinary Medicine, 2(2), 75–79. [Google Scholar]
- Azocar‐Aedo, L. , Monti, G. , & Jara, R. (2017). Similar prevalence of anti‐Leptospira antibodies in domestic dogs from urban and rural areas in southern Chile: A public health concern. Journal of Preventive Medicine and Public Health, 1, 1–5. [Google Scholar]
- Birnbaum, N. , Barr, S. , Center, S. , Schermerhorn, T. , Randolph, J. , & Simpson, K. (1998). Naturally acquired leptospirosis in 36 dogs: Serological and clinicopathological features. Journal of Small Animal Practice, 39(5), 231–236. [DOI] [PubMed] [Google Scholar]
- Fahimipour, A. , Khaki, P. , & Moradi Bidhendi, S. (2019). Seroepidemiology of leptospira infection in stray dogs by Mat during a one‐year period from rural communities of Koohsar, Alborz, Iran. In Proceedings of the 19th International Congress of Microbiology of Iran, Tehran . https://civilica.com/doc/783022
- Fahimipour, A. , Khaki, P. , & Moradi Bidhendi, S. (2021). Seroepidemiological analysis of leptospiral infection using MAT in stray dogs in Alborz, Iran. Archives of Razi Institute, 76(2), 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratini, F. , Bertelloni, F. , & Cilia, G. (2020). Leptospira infection in wild animals. Hauppauge, NY, USA: Nova Science Publisher. ISBN 978‐1‐53618‐222‐4. [Google Scholar]
- Galarce, N. , de la Fuente, S. , Escobar, B. , Dettleff, P. , Abalos, P. , Hormazábal, J. C. , Flores, R. , Sallaberry‐Pincheira, N. , & Martínez, V. (2021). Survey of zoonotic bacterial pathogens in native foxes in central Chile: First record of Brucella canis exposure. Animals, 11(7), 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi, S.‐A. , Arzamani, K. , Abdollahpour, G. , Beheshti, N. , Alavinia, M. , Azimian, A. , Neela, V. K. , van Belkum, A. , & Ghasemzadeh‐Moghaddam, H. (2021). Seroprevalence of Leptospira infection in occupational risk groups in North Khorasan province, Iran. Heliyon, 7(1), e05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatrohi, A. , Lak, A. G. , Hashempour, A. , Gholizadeh, S. S. , Abdollahpour, G. , & Zadeh, R. (2014). Survey on S eroprevalence of Leptospira serotypes in household dogs using MAT method in Urmia, Iran. Bulletin of Environment, Pharmacology, and Life Sciences, 3, 158–162. [Google Scholar]
- Jamshidi, S. , Vandyousefi, J. , Dezfoulian, O. , & Selk Ghaffari, M. (2008). Isolation of Leptospira canicola from a dog in Iran: First report. Iranian Journal of Veterinary Research, 9(3), 291–294. [Google Scholar]
- Kamrani, A. , & Sardari, K. (2003). Seroprevalence of leptospiral antibodies in stray dogs in Mashhad, Iran. In Proceedings of the World Small Animal Veterinary Association World Congress . Bangkok, Thailand. [Google Scholar]
- Khalili, M. , Sakhaee, E. , Amiri, F. B. , Safat, A. A. , Afshar, D. , & Esmaeili, S. (2020). Serological evidence of leptospirosis in Iran: A systematic review and meta‐analysis. Microbial Pathogenesis, 138, e103833. [DOI] [PubMed] [Google Scholar]
- Klaasen, H. L. E. , & Adler, B. (2015). Recent advances in canine leptospirosis: Focus on vaccine development. Veterinary Medicine: Research and Reports, 6, 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelu, M. , Muñoz‐Zanzi, C. , Higgins, B. , & Galloway, R. (2015). Seroepidemiology of leptospirosis in dogs from rural and slum communities of Los Rios Region, Chile. BMC Veterinary Research, 11(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López, M. C. , Vila, A. , Rodón, J. , & Roura, X. (2019). Leptospira seroprevalence in owned dogs from Spain. Heliyon, 5(8), e02373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major, A. , Schweighauser, A. , & Francey, T. (2014). Increasing incidence of canine leptospirosis in Switzerland. International Journal of Environmental Research and Public Health, 11(7), 7242–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer‐Scholl, A. , Luge, E. , Draeger, A. , Nöckler, K. , & Kohn, B. (2013). Distribution of Leptospira serogroups in dogs from Berlin, Germany. Vector‐Borne and Zoonotic Diseases, 13(3), 200–202. [DOI] [PubMed] [Google Scholar]
- Milas, Z. , Turk, N. , Janicki, Z. , Slavica, A. , Starešina, V. , Barbić, L. , Lojkić, M. , & Modrić, Z. (2006). Leptospiral antibodies in red foxes (Vulpes vulpes) in northwest Croatia. Veterinarski Arhiv, 76, 51–57. [Google Scholar]
- Millán, J. , Candela, M. G. , López‐Bao, J. V. , Pereira, M. , Jiménez, M. Á. , & León‐Vizcaíno, L. (2009). Leptospirosis in wild and domestic carnivores in natural areas in Andalusia, Spain. Vector‐Borne and Zoonotic Diseases, 9(5), 549–554. [DOI] [PubMed] [Google Scholar]
- Nakonechnyi, I. , Perots'ka, L. , Pyvovarova, I. , & Chornyi, V. (2019). Ecological and epizootic roles of Golden jackal, genus Canis aureus in the Northwest of Black Sea coast. Scientific Messenger of LNU of Veterinary Medicine and Biotechnologies. Series: Veterinary Sciences, 21(94), 37–43. [Google Scholar]
- Niloofa, R. , Fernando, N. , de Silva, N. L. , Karunanayake, L. , Wickramasinghe, H. , Dikmadugoda, N. , Premawansa, G. , Wickramasinghe, R. , de Silva, H. J. , Premawansa, S. , Rajapakse, S. , & Handunnetti, S. (2015). Diagnosis of leptospirosis: Comparison between microscopic agglutination test, IgM‐ELISA and IgM rapid immunochromatography test. PLoS One, 10(6), e0129236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhizgari, N. , Gouya, M. M. , & Mostafavi, E. (2017). Emerging and re‐emerging infectious diseases in Iran. Iranian Journal of Microbiology, 9(3), 122–142. [PMC free article] [PubMed] [Google Scholar]
- Piredda, I. , Ponti, M. N. , Piras, A. , Palmas, B. , Pintore, P. , Pedditzi, A. , & Chisu, V. (2021). New insights on leptospira infections in a canine population from North Sardinia, Italy: A sero‐epidemiological study. Biology, 10(6), 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad, M. , Zeynali, A. , Tabatabayi, A. , Bokaei, S. , & Vandi, Y. J. (2004). Seroprevalence and bacteriological study of canine leptospirosis in Tehran and its suburban areas. Iranian Journal of Veterinary Research, 5(2), 53–80. [Google Scholar]
- Roqueplo, C. , Marié, J.‐L. , André‐Fontaine, G. , Kodjo, A. , & Davoust, B. (2015). Serological survey of canine leptospirosis in three countries of tropical Africa: Sudan, Gabon and Ivory Coast. Comparative Immunology, Microbiology and Infectious Diseases, 38, 57–61. [DOI] [PubMed] [Google Scholar]
- Sakhaee, E. (2011). Detection of Leptospiral antibodies by microscopic agglutination test in north–east of Iran. Asian Pacific Journal of Tropical Biomedicine, 1(3), 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhaee, E. , Abdollahpour, G. , Bolourchi, M. , & Tabrizi, S. S. (2010). Comparison between microscopic agglutination test (MAT) and enzyme‐linked immunosorbent assay (ELISA) for detection of leptospiral antibodies in cattle. Comparative Clinical Pathology, 19(1), 5–9. [Google Scholar]
- Samir, A. , Soliman, R. , El‐Hariri, M. , Abdel‐Moein, K. , & Hatem, M. E. (2015). Leptospirosis in animals and human contacts in Egypt: Broad range surveillance. Revista da Sociedade Brasileira de Medicina Tropical, 48, 272–277. [DOI] [PubMed] [Google Scholar]
- Shi, D. , Liu, M. , Guo, S. , Liao, S. , Sun, M. , Liu, J. , Wang, L. , Wang, Z. , Wang, S. , Yang, D. , & Chai, T. (2012). Serological survey of canine leptospirosis in southern China. Pakistan Veterinary Journal, 32(2), 280–282. [Google Scholar]
- Slavica, A. , Cvetnić, Ž. , Milas, Z. , Janicki, Z. , Turk, N. , Konjević, D. , Toncic, J. , & Lipej, Z. (2008). Incidence of leptospiral antibodies in different game species over a 10‐year period (1996–2005) in Croatia. European Journal of Wildlife Research, 54(2), 305–311. [Google Scholar]
- Slavica, A. , Dezdek, D. , Konjevic, D. , Cvetnic, Z. , Sindicic, M. , Stanin, D. , Habus, J. , & Turk, N. (2011). Prevalence of leptospiral antibodies in the red fox (Vulpes vulpes) population of Croatia. Veterinarni Medicina, 56(4), 209–213. [Google Scholar]
- Tagliabue, S. , Figarolli, B. M. , D'Incau, M. , Foschi, G. , Gennero, M. S. , Giordani, R. , Giordani, R. , Natale, A. , Papa, P. , Ponti, N. , Scaltrito, D. , Spadari, L. , Vesco, G. , & Ruocco, L. (2016). Serological surveillance of Leptospirosis in Italy: Two‐year national data (2010–2011). Veterinaria italiana, 52, 129–138. [DOI] [PubMed] [Google Scholar]
- Torkan, S. , & Momtaz, H. (2019). Molecular detection of leptospira species serotypes in Iranian stray dogs. International Journal of Medical Laboratory, 6(2), 138–142. [Google Scholar]
- Ullmann, L. , & Langoni, H. (2011). Interactions between environment, wild animals and human leptospirosis. Journal of Venomous Animals and Toxins including Tropical Diseases, 17(2), 119–129. [Google Scholar]
- Ward, M. P. , Glickman, L. T. , & Guptill, L. F. (2002). Prevalence of and risk factors for leptospirosis among dogs in the United States and Canada: 677 cases (1970–1998). Journal of the American Veterinary Medical Association, 220(1), 53–58. [DOI] [PubMed] [Google Scholar]
- World Organization of Animal Health (OIE) (2019). Leptospirosis. In Manual of diagnostic tests and vaccines for terrestrial animals (pp. 503–516). OIE. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs.


