Abstract
Objective
This report documents a rare case of vulvar discharge associated with exogenous oestrogen exposure in a large‐breed dog.
Case description
A 4‐year‐old spayed Weimaraner bitch was presented for evaluation of inappetence and intermittent sanguineous vulvar discharge. Physical examination, vaginal cytology, haematological, and ultrasonographic findings were indicative of a uterine stump pyometra. A celiotomy was performed, and the uterine stump appeared grossly cystic and thickened. Histopathological evaluation of the removed uterine stump and ovarian pedicles revealed cystic endometrial hyperplasia and no ovarian tissue. Fifteen days after surgery, the patient presented again with a sanguineous vulvar discharge. Vaginal cytology revealed predominantly superficial cells, indicating oestrogen influence. Further questioning of the owner revealed the long‐term use of a topical oestrogen cream by a member of the household. Serial examinations were performed, and the cytology remained uniform, with predominantly superficial cells, indicating continued oestrogen influence. Progesterone and anti‐Müllerian hormone tests were negative, which made the presence of ectopic ovarian tissue unlikely. These results coupled with the history of topical oestrogen cream use in the household suggested that the patient's clinical signs were most likely due to exogenous oestrogen exposure. After the owner implemented various recommendations made to prevent the exposure, the clinical signs resolved completely.
Conclusions
This case demonstrates that although rare, exogenous oestrogen exposure can be a cause of vulvar discharge in a large‐breed spayed bitch. Therefore, regardless of the breed, exogenous oestrogen exposure must be included in the list of differential diagnoses for all clinical presentations associated with oestrogenic influence in dogs.
Keywords: canine, case report, cystic endometrial hyperplasia, oestrogen, vulvar discharge
Exogenous estrogen exposure is a rare cause of vaginal discharge in spayed bitches. The condition is more commonly reported in small‐breed dogs. This case report demonstrates that the condition may also be observed in large‐breed dogs.
1. INTRODUCTION
Vulvar discharge in spayed bitches is often associated with infections, chemical irritation, foreign bodies, anatomical defects, neoplasia, or an ovarian remnant (Lopate, 2014). Rarely, the discharge may be associated with exposure to exogenous hormones (Ganz & Wehrend, 2021; Sterman et al., 2019). Vaginal cytology is a very useful initial diagnostic step to characterize the discharge and to determine whether it is associated with oestrogenic influence (Johnson, 1991; Verstegen & Onclin, 2008). If vaginal cytology is indicative of an oestrogenic influence, the next important step is to investigate the source of oestrogen, which could be either endogenous or exogenous (Lopate, 2014; Wallace, 1991). Prolonged oestrogen exposure in dogs can result in bilateral alopecia, skin hyperpigmentation, and bone marrow suppression (Ganz & Wehrend, 2021; Sontas et al., 2009; Sterman et al., 2019). Oestrogen has also been implicated in the development of cystic endometrial hyperplasia–pyometra complex due to its role in the upregulation of endometrial progesterone receptors and cervical relaxation (Lopate, 2014; Sterman et al., 2019). Other diagnostic techniques that are helpful in cases of vulvar discharge include the measurement of serum progesterone and anti‐Müllerian hormone (AMH), ultrasonography, and exploratory laparotomy (Lopate, 2014; Verstegen & Onclin, 2008; Wallace, 1991). The measurement of AMH is especially useful for diagnosing an ovarian remnant because AMH is only produced by gonads (Turna Yilmaz et al., 2015). The management of vaginal discharge in spayed bitches depends on the underlying cause of the condition (Lopate, 2014; Verstegen & Onclin, 2008). The aim of this report is to document a rare case of vulvar discharge associated with exogenous oestrogen exposure in a spayed large‐breed bitch.
2. CLINICAL DESCRIPTION
A 4‐year‐old, 28.4 kg, spayed Weimaraner bitch was presented for evaluation of inappetence and intermittent sanguineous vulvar discharge of a few days’ duration. The owner reported that the bitch was spayed at 7 months of age simultaneous to a celiotomy performed for the removal of an intestinal foreign body. On initial presentation, the patient was bright, alert, and responsive, with elevated rectal temperature (104.2°F) and respiration rate (56 breaths per minute). Her mucous membranes were pink and slightly tacky. Physical examination indicated the presence of vulvar edema and a sanguineous vulvar discharge. Mild vaginal hyperplasia was noted on palpation. The remainder of the physical examination was unremarkable.
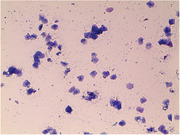
A guarded vaginal swab was collected from the cranial vagina. Vaginal cytology revealed mainly parabasal cells, occasional intermediate cells, and abundant neutrophils and red blood cells (Figure 1). Ultrasonographic findings were suggestive of an enlarged, fluid‐filled uterine stump, and the results of a complete blood count (CBC) indicated leukocytosis, neutrophilia, and monocytosis (Table 1). The serum biochemistry profile showed mild hypoalbuminemia, mildly elevated amylase, and mild hypokalaemia (Table 2). The bitch tested negative for heartworm, Lyme disease, ehrlichiosis, and anaplasmosis (Abaxis VetScan® Canine Flex4 Rapid Test).
FIGURE 1.
Vaginal smear of the bitch on initial presentation (Diff‐Quik stain, total magnification 100×)
TABLE 1.
Haematology parameters at the time of initial presentation
| Parameter | Value | Reference range |
|---|---|---|
| White blood cells (× 109/L) | 36.57 | 6.00–17.00 |
| Lymphocytes (× 109/L) | 4.68 | 1.00–4.80 |
| Monocytes (× 109/L) | 2.28 | 0.20–1.50 |
| Neutrophils (× 109/L) | 29.52 | 3.00–12.00 |
| Eosinophils (× 109/L) | 0.07 | 0.00–0.80 |
| Basophils (× 109/L) | 0.02 | 0.00–0.40 |
| Red blood cells (× 1012/L) | 6.82 | 5.50–8.50 |
| Haemoglobin (g/dL) | 15.10 | 12.00–18.00 |
| Haematocrit (%) | 53.34 | 37.00–55.00 |
| MCV (fL) | 78.00 | 60.00–77.00 |
| MCH (pg) | 22.10 | 19.50–24.50 |
| MCHC (g/dL) | 28.30 | 31.00–39.00 |
| Platelets (× 109/L) | 228.00 | 165.00–500.00 |
| MPV (fL) | 11.90 | 3.90–11.10 |
Abbreviations: MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; MPV, mean platelet volume.
TABLE 2.
Serum biochemistry parameters at the time of initial presentation
| Parameter | Value | Reference range |
|---|---|---|
| Albumin (g/dL) | 2.10 | 2.50–4.40 |
| ALP (U/L) | 70.00 | 20.00–150.00 |
| ALT (U/L) | 26.00 | 10.00–118.00 |
| Amylase (U/L) | 1409.00 | 200.00–1200.00 |
| Total bilirubin (mg/dL) | 0.40 | 0.10–0.60 |
| BUN (mg/dL) | 9.00 | 7.00–25.00 |
| Calcium (mg/dL) | 9.90 | 8.60–11.80 |
| Phosphorus (mg/dL) | 3.30 | 2.90–6.60 |
| Creatinine (mg/dL) | 0.90 | 0.30–1.40 |
| Glucose (mg/dL) | 93.00 | 60.00–110.00 |
| Sodium (mmol/L) | 152.00 | 138.00–160.00 |
| Potassium (mmol/L) | 3.60 | 3.70–5.80 |
| Total protein (g/dL) | 6.80 | 5.40–8.20 |
| Globulin (g/dL) | 4.70 | 2.30–5.20 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; BUN, blood urea nitrogen.
A uterine stump pyometra due to ovarian remnant syndrome was suspected, and an exploratory celiotomy was performed. Before surgery, the patient received acepromazine maleate (0.03 mg/kg body weight) intramuscularly (IM) and methadone (0.2 mg/kg body weight intravenously (IV) followed by induction with Propofol IV. The patient was intubated with a 9.5‐mm inner diameter endotracheal tube and maintained on a rebreathing system with isoflurane and oxygen. The patient received cefazolin (22 mg/kg, IV) at the induction of anaesthesia and was maintained on IV lactated ringer's solution throughout the procedure. Carprofen (2.2 mg/kg) was administered subcutaneously shortly before the end of the procedure for pain control.
The left ovarian pedicle had nodular structures within the tissue, but no obvious ovarian tissue; the right pedicle had no grossly discernible abnormality. The uterine stump appeared grossly cystic and thickened. The enlarged uterine stump and tissues from the left and right pedicles were submitted for histopathological evaluation (IDEXX Laboratories, Inc.). The uterine stump was reported to have a moderate haemorrhage, eosinophilic proteinaceous fluid, and sloughed endometrial cells within the uterine lumen. The endometrial glands were seen to be mildly dilated by eosinophilic proteinaceous glandular content. These findings indicated cystic endometrial hyperplasia. There was no evidence of ovarian tissue on histopathology.
The patient was discharged with cephalexin (22 mg/kg per Os every 12 h for 10 days) and carprofen (Rimadyl, 2 mg/kg Per Os every 12 h for 5 days). An Elizabethan collar was used, and instructions were provided for leash control and rest for 10 days.
Follow‐up examinations at 4‐ and 8‐days post‐operation indicated complete resolution of the vulvar discharge, normal appetite and all vital parameters within the normal limits. However, 15 days post‐operation, the patient presented again with a sanguineous vulvar discharge. Vaginal cytology revealed predominantly superficial cells (Figure 2), indicating oestrogen influence. Differential diagnoses included ectopic ovarian tissue, exogenous oestrogen exposure or an adrenal tumour. Further questioning of the owner revealed long‐term use of an oestradiol‐containing topical cream by a member of the household. The owner mentioned that the cream is applied on the arms at night, and the bitch sleeps in the same bed like that as the member applying the cream. Serial vaginal cytology was performed at 3–5 day intervals until 33 days post‐operation. The cytology remained uniform, with predominantly superficial cells, indicating continued oestrogen influence. An ovarian remnant syndrome panel consisting of progesterone and AMH (IDEXX Laboratories, Inc.) was ordered; the progesterone concentration was less than 1.0 ng/mL, and the AMH test was negative, making the presence of ovarian tissue unlikely. An adrenal panel was not conducted owing to financial constraints and the absence of any other signs of adrenal disease.
FIGURE 2.
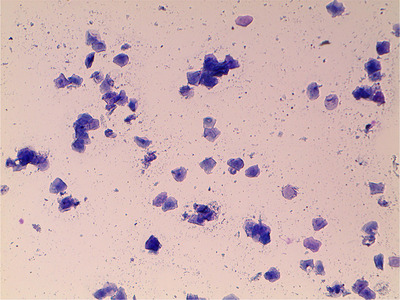
Vaginal smear of the bitch at follow‐up 15 days post celiotomy (Diff‐Quik stain, total magnification 100×)
Coupled with the history of topical oestrogen cream use in the household, the results of the diagnostic tests conducted in this case suggested that the patient's clinical signs were most likely due to exogenous oestrogen exposure. Recommendations to rehome the dog or discontinue the use of the oestrogen cream were declined. Therefore, the owner was advised to make the dog sleep in a different bed and apply the cream on the inner thigh during the daytime to decrease the exposure to the patient as much as possible. Follow‐up vaginal cytology and CBC evaluations were also recommended to monitor future oestrogen exposure and possible adverse effects on the patient's health. Although the patient was not presented again, on follow‐ups over the phone and through email until 7 months post‐surgery, the owner stated that the patient had not shown vulvar discharge or any other clinical signs associated with oestrogen exposure.
3. DISCUSSION
Although there are several anecdotal reports of vulvar discharge associated with exogenous oestrogen exposure in bitches, there is very little published information on this topic. Previously published reports included a 6‐year‐old Chihuahua with stump pyometra (Sterman et al., 2019) and a 6.5‐year‐old, 8.4 kg, crossbred bitch with recurrent signs of heat (Ganz & Wehrend, 2021). To the authors’ knowledge, this is the first report of vulvar discharge associated with exogenous oestrogen exposure in a large‐breed bitch.
At the first presentation, the bitch's clinical signs and the findings from her physical examination, vaginal cytology, ultrasonography, CBC and serum biochemistry were consistent with a stump pyometra (Lopate, 2014; Sterman et al., 2019). Pyometra in spayed bitches is typically associated with the presence of an ovarian remnant that serves as an endogenous source of oestrogen and progesterone (Hagman, 2018; Lopate, 2014). Oestrogen priming of the uterus followed by exposure to progesterone results in cystic endometrial hyperplasia and predisposition to pyometra (Noakes et al., 2001). The present case demonstrates that although rare, cystic endometrial hyperplasia and pyometra may be associated with persistent exogenous exposure to oestrogen alone. This could be attributed to prolonged relaxation of the cervix leading to ascending uterine infection and the production of bacterial toxins and inflammatory mediators (Noakes et al., 2001). The predominance of parabasal cells, neutrophils and red blood cells on vaginal cytology at initial presentation might have been a result of interference from uterine inflammation and discharge. Surgical removal of the affected uterine stump is the most effective treatment for pyometra in spayed dogs (Lopate, 2014). Follow‐up examinations at 4‐ and 8‐days after the removal of the uterine stump in the current case indicated a complete resolution of the clinical signs and restoration of all vital parameters within normal limits.
The detection of cystic endometrial hyperplasia on histopathology suggested hormonal stimulation of the uterus. Although the resected ovarian pedicles did not reveal any ovarian tissue, and the ovarian remnant in a different location was still a possibility. Other possibilities include an adrenal tumour and exogenous exposure to hormones (Verstegen & Onclin, 2008; Wallace, 1991). The predominance of superficial cells on serial vaginal cytology after the bitch presented again with the vulvar discharge was consistent with oestrogenic stimulation (Johnston et al., 2001). The history of topical oestrogen cream application by the family member and prolonged close contact of the bitch with the person suggested a high likelihood of the exogenous oestrogen exposure as a cause of the clinical presentation. A definitive diagnosis of exogenous oestrogen exposure as a cause of vulvar discharge requires the exclusion of endogenous sources of oestrogen. The nondetection of ovarian tissue on exploratory celiotomy and histopathology combined with the results of AMH and progesterone tests made the presence of an ovarian remnant unlikely in the present case (Turna Yilmaz et al., 2015; Wallace, 1991). Although the presence of an adrenal tumour could not be ruled out due to financial constraints, it would be highly unlikely due to the young age of the bitch and the absence of any other signs of adrenal disease. Therefore, it was concluded that the clinical signs observed in this case were associated with exogenous oestrogen exposure.
Anecdotally, exogenous oestrogen exposure is more common in small‐breed lap dogs, presumably due to greater physical contact with their owners and the increased chances of licking the site of application of the topical oestrogen creams. The two cases previously reported in the scientific literature were small‐breed dogs and involved topical oestrogen cream application on the forearms by the owners (Ganz & Wehrend, 2021; Sterman et al., 2019). Although a significant exogenous exposure seems less likely in a large‐breed dog, the present case demonstrates that it is possible in situations where there is a prolonged contact, such as the dog sleeping in bed with the person using a topical oestrogen cream. In such situations, the exposure can be simply limited by changing the sleeping location of the dog or the time and site of application of the oestrogen cream. This is supported by the resolution of clinical signs after the owner implemented our recommendations to change the sleeping location of the dog and alter the timing and location of the application of the cream. Other potential but relatively less feasible options for preventing exogenous oestrogen exposure include discontinuing the use of the oestrogen cream or rehoming the dog.
Persistent exposure of dogs to oestrogens, endogenous or exogenous, can result in hyperoestrogenism, often characterized by bilaterally symmetrical alopecia and/or bone marrow suppression resulting in thrombocytopenia, anaemia, leukopenia or pancytopenia (Berger et al., 2015; Ganz & Wehrend, 2021; Selk Ghaffari et al., 2009; Sterman et al., 2019). Therefore, dogs suspected to have been exposed to oestrogen should be monitored closely for these changes using sequential physical examinations and CBC evaluations.
4. CONCLUSIONS
In summary, this case demonstrates that exogenous oestrogen exposure can be a cause of vulvar discharge in a large‐breed spayed bitch. Therefore, regardless of the breed, exogenous oestrogen exposure must be included in the list of differential diagnoses for all clinical presentations associated with oestrogenic influence on dogs.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
Informed consent was obtained from the owner of the patient. No ethical approval was required as all the procedures involved were part of the routine case management at the clinic consistent with the standard of veterinary care.
AUTHOR CONTRIBUTIONS
Francesca Ivaldi: conceptualization; investigation; methodology; visualization; writing – original draft; writing – review & editing. Camille Ogdon: methodology; writing – original draft. Firdous Khan: conceptualization; investigation; methodology; resources; supervision; visualization; writing – original draft; writing – review & editing.
ACKNOWLEDGEMENTS
The authors are grateful to the staff of the St. George's University‐Small Animal Clinic for their assistance with the management of the reported clinical case. This research study did not require funding support from any intramural or extramural grant funding agency.
Ivaldi, F. , Ogdon, C. , & Khan, F. A. (2022). A rare case of vulvar discharge associated with exogenous oestrogen exposure in a spayed Weimaraner bitch. Veterinary Medicine and Science, 8, 1872–1876. 10.1002/vms3.860
Funding information
This research study did not require funding support from any intramural or extramural grant funding agency.
DATA AVAILABILITY STATEMENT
All datasets generated or analysed during this study are included in the manuscript.
REFERENCES
- Berger, D. J. , Lewis, T. P. , Schick, A. E. , Miller, R. I. , & Loeffler, D. G. (2015). Canine alopecia secondary to human topical hormone replacement therapy in six dogs. Journal of the American Animal Hospital Association, 51, 136–142. 10.5326/JAAHA-MS-6247 [DOI] [PubMed] [Google Scholar]
- Ganz, S. , & Wehrend, A. (2021). Uptake of exogenous estrogen as a differential diagnosis of ovarian‐remnant‐syndrome in a bitch: A case report. BMC Veterinary Research, 17, 225. 10.1186/s12917-021-02923-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman, R. (2018). Pyometra in small animals. The Veterinary Clinics of North America. Small Animal Practice, 48, 639–661. 10.1016/j.cvsm.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Johnson, C. A. (1991). Diagnosis and treatment of chronic vaginitis in the bitch. The Veterinary Clinics of North America. Small Animal Practice, 21, 523–531. 10.1016/s0195-5616(91)50058-9 [DOI] [PubMed] [Google Scholar]
- Johnston, S. D. , Kustritz, M. V. , & Olson, P. S. (2001). Disorders of the canine ovary. In S.D. Johnston, M. V. Kustritz, P. S. Olson (Eds.), Canine and feline theriogenology (1st ed., pp. 193–205).; Saunders. [Google Scholar]
- Lopate, C. (2014). Reproductive disorders of the spayed bitch. Clinical Theriogenology, 6, 205–217. [Google Scholar]
- Noakes, D. E. , Dhaliwal, G. K. , & England, G. C. (2001). Cystic endometrial hyperplasia/pyometra in dogs: a review of the causes and pathogenesis. Journal of Reproduction and Fertility Supplement, 57, 395–406. [PubMed] [Google Scholar]
- Selk Ghaffari, M. , Dezfoulian, O. , Aldavood, S. J. , & Masoudifard, M. (2009). Estrogen‐related alopecia due to polycystic ovaries in a terrier dog. Comparative Clinical Pathology, 18, 341–343. 10.1007/s00580-009-0815-x [DOI] [Google Scholar]
- Sontas, H. B. , Dokuzeylu, B. , Turna, O. , & Ekici, H. (2009). Estrogen‐induced myelotoxicity in dogs: A review. Canadian Veterinary Journal, 50, 1054–1058. [PMC free article] [PubMed] [Google Scholar]
- Sterman, A. A. , Mankin, K. T. , & Barton, C. L. (2019). Stump pyometra secondary to human topical estrogen hormone exposure in a spayed female Chihuahua. Journal of the American Animal Hospital Association, 55, e55604. 10.5326/JAAHA-MS-6744 [DOI] [PubMed] [Google Scholar]
- Turna Yilmaz, Ö. , Toydemir, T. S. , Kirsan, I. , Gunay Ucmak, Z. , & Caliskan Karacam, E. (2015). Anti‐Müllerian hormone as a diagnostic tool for ovarian remnant syndrome in bitches. Veterinary Research Communications, 39, 159–162. 10.1007/s11259-015-9639-0 [DOI] [PubMed] [Google Scholar]
- Verstegen, J. P. , & Onclin, K. J. (2008). Vulvovaginal hemorrhagic discharge in the dog: Caudal reproductive tract. Clinicians Brief, 12, 11–19. [Google Scholar]
- Wallace, M. S. (1991). The ovarian remnant syndrome in the bitch and queen. The Veterinary Clinics of North America. Small Animal Practice, 21, 501–507. 10.1016/s0195-5616(91)50056-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated or analysed during this study are included in the manuscript.


