Abstract
A ureterocele is a rare congenital anomaly with cystic dilation of the terminal segment of the ureter entirely within the bladder (orthotopic) or associated with ectopic ureter (ectopic). Its aetiology has not been fully clarified; however, it may involve genetic or acquired factors. Urothelial carcinoma (UC) is the most common type of canine urinary tract neoplasm, among which over 90% of cases are invasive. The non‐papillary (flat) non‐infiltrating form accounts for a very small percentage of canine UCs and is considered carcinoma in situ (CIS). The neoplastic cells of CIS remain within the ureteral mucosa and do not breach the basement membrane. UCs originating from the canine ureter are extremely rare, and no report of a ureteral UC concurrently occurring with a ureterocele has been reported. A 7‐year‐old castrated male Maltese dog weighing 3.5 kg was referred with a 2‐week history of lethargy, anorexia, pollakiuria and intermittent panting. The dog underwent open surgery for removal of bladder calculi 2 years prior, and at the time of the surgery, no other urinary system abnormalities were identified. Ultrasonographic and computed tomographic scans revealed a severely enlarged right kidney and ureter with a ureterocele on the ipsilateral side. A diagnosis of an orthotopic ureterocele causing hydronephrosis and hydroureter was established. Complete nephroureterectomy and ureterocelectomy using the marsupialisation technique were performed. The postoperative histological examination of the excised tissues showed a multifocal carcinoma in situ (non‐papillary non‐infiltrating UC) in the proximal ureter and a fluid‐filled kidney with a thin rim of fibrotic renal tissue. No neoplastic changes were observed in the ureterocele tissue. Postoperatively, the dog recovered rapidly without complications except temporary urinary incontinence, and no evidence of tumour recurrence was detected by ultrasonography performed 6 months after surgery. This case report describes the first case of a dog with an orthotopic ureterocele and ureteral UC, which occurred concurrently at the ipsilateral side of the ureter. The condition was successfully managed with a nephroureterectomy and partial ureterocelectomy.
Keywords: canine, carcinoma in situ, hydronephrosis, hydroureter, ureteral tumour, urine flow obstruction
Concurrent occurrence of orthotopic ureterocele with ureteral carcinoma in situ in a Maltese dog, resulting in urinary system disorders, and its successful surgical management.
![]()
1. BACKGROUND
A ureterocele is cystic dilation of the submucosal layer of the terminal ureter at the ureterovesical junction (UVJ) (Tanagho, 1972). A ureterocele completely within the urinary bladder is classified as orthotopic, and it is classified as ectopic if the ureteral orifice is in an abnormal position in association with an ectopic ureter (Glassberg et al., 1984). The ureteroceles are generally considered congenital anomalies; however, the embryologic origin remains uncertain (Lautzenhiser & Bjorling, 2002). Canine ureteroceles can be clinically asymptomatic unless accompanied by altered urine flow dynamics, including obstruction of the UVJ or urethra and vesicoureteral reflux (VUR) (Timberlake & Corbett, 2015). These abnormalities in urine flow can cause renal disorders, such as hydronephrosis resulting in progressive renal failure and urinary tract infection (UTI) (Abibe et al., 2020; Stiffler et al., 2002). Clinical signs include stranguria, incontinence, pollakiuria and abdominal pain (Childress et al., 2011). The aim of the management of this condition is to resolve the renal impairment associated with urinary tract obstruction, VUR, and UTI (Timberlake & Corbett, 2015). The conventional first‐line treatment for canine ureterocele is to surgically remove structural anomalies of the urinary system. Recently, the cystoscopic‐guided laser ablation technique has been applied in veterinary medicine (Anderson et al., 2020).
Canine ureteral tumours are rare, and the most common type is urothelial (transitional cell) carcinoma (UC) (Meuten & Meuten, 2017). In particular, primary ureteral neoplasms are significantly rare, considering that there have only been three case studies of canine primary ureteral UC (Cathasaigh et al., 2018; Hanika & Rebar, 1980; Kim et al., 2015). UCs can be classified based on their pattern of growth as papillary (projecting into the lumen) or non‐papillary (flat); they can also be classified based on their invasiveness (infiltrating or non‐infiltrating) and tumour grade (cellular differentiation). Papillary and infiltrating UC is the most common form of urinary system neoplasm in dogs. Non‐papillary and non‐infiltrating UC is a rare form and can be classified as carcinoma in situ (CIS) (Meuten & Meuten, 2017). This is the most well‐differentiated form of UC, in which neoplastic cells are restricted to the mucosa without breaching the basement membrane.
This report describes a canine orthotopic ureterocele associated with concurrent ureteral CIS, resulting in hydronephrosis with subsequent renal dysfunction. To the best of the authors’ knowledge, this is the first report of these two rare ureteral disorders occurring concurrently on the ipsilateral side.
2. CASE DESCRIPTION
An 8‐year‐old castrated male Maltese dog weighing 3.5 kg presented with lethargy, anorexia, pollakiuria and intermittent panting. The referring veterinarian reported that the dog underwent a bladder cystotomy to remove bladder calculi 2 years before. No other urinary system abnormalities were found on radiographic and ultrasonographic examinations. During the surgery that was performed by the referring veterinarian, the inner and outer surfaces of the bladder were visualised, and there were no suspected ureterocele lesions. Postoperatively, intermittent urinary symptoms were treated with medical therapy alone.
On initial physical examination, the dog was depressed and tachypneic (54 breaths/min) and had a body condition score of 6/9. Complete blood count and serum biochemistry tests revealed elevated white blood cell counts (16.84 K/μl; reference range, 5.05–16.76 K/μl), C‐reactive protein levels beyond the measurable range (>10 mg/dl; reference range, 0.1–1 mg/dl) and elevated levels of alkaline phosphatase (220 U/L; reference range, 23–212 U/L) and triglycerides (192 mg/dl; reference range, 10–100 mg/dl). Urine was collected by cystocentesis for urinalysis. Urinalysis revealed haematuria (5–10 erythrocytes/μl) and proteinuria (30 mg/dl). Other urinalysis parameters were within the reference ranges. On plain abdominal radiographs, right‐sided renomegaly and numerous bladder stones were identified. Abdominal ultrasonography showed a severely enlarged right kidney with a thin rim of parenchyma remaining (less than 1.2 mm of thickness), which was completely replaced by anechoic fluid (Figure 1a). On colour Doppler imaging, no vascular response was detected along the remaining renal parenchyma. The ratio between the right renal length and the aorta diameter was 13.8. The entire right ureter was tortuous, thickened and dilated. The space‐occupying cystic structure protruded inward from the right side near the trigone (Figure 1b). The intraluminal anechoic cavity of the cystic lesion appeared to connect with the right ureter. Multiple hyperechoic foci with acoustic shadows were found in the right distal ureter and bladder. For precise evaluation of the urinary tract, a CT scan was performed under general anaesthesia with the dog in ventral recumbency. The right renal length measured 7.2 times the length of the second lumbar vertebral body. The caudal vena cava was compressed by the enlarged right kidney. The right ureter was extensively elongated and dilated, leading to a stacked and plicated appearance (Figure 1c). The maximum luminal diameter of the right ureter was 13.5 mm. The cystic lesion inside the bladder was identified near the trigone (Figure 1d). The entire portion of this abnormal cystic structure was within the bladder, without prolapsing into the urethra. Based on these findings, a diagnosis of orthotopic ureterocele with right‐sided hydroureter and hydronephrosis was established, and surgical resection of these lesions was performed with the owner's consent.
FIGURE 1.
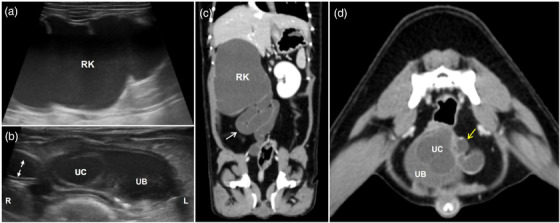
Diagnostic images at presentation. Ultrasonographic images of the right kidney (a) and the urinary bladder (b). (a) The right kidney is filled with anechoic fluid, and only a thin rim of renal parenchyma remains. (b) A cystic structure connected to the dilated right ureter (white dotted arrow line) is seen within the bladder. Postcontrast dorsal (c) and transverse (d) abdominal computed tomography scanning images. (c) The right kidney and the entire ureter (white arrow) are extensively dilated and have a stacked appearance. (d) An irregularly shaped intraluminal structure is connected to the dilated and twisted right ureter (yellow arrow). RK, right kidney; UC, ureterocele; UB, urinary bladder; R, right; L, left
For surgical resection, the dog was premedicated with cefazoline (30 mg/kg, intravenous [IV]), famotidine (1 mg/kg, IV), midazolam (0.3 mg/kg, IV), butorphanol (0.2 mg/kg, IV), and carprofen (4.4 mg/kg, subcutaneous [SC]). Anaesthesia was induced with propofol (6 mg/kg, IV). The patient was intubated, and anaesthesia was maintained with isoflurane (2%) in oxygen. A ventral midline celiotomy was performed routinely. Using a combination of blunt and sharp dissection techniques, the swollen right kidney and ureter were freed from adjacent tissues. During nephroureterectomy, the kidney and associated vessels were approached from the ventral aspect because of their large size, limiting the medial retraction of the right kidney. Both the renal artery and vein were ligated with 3‐0 polyglyconate sutures and excised. The right ureter was ligated (2‐0 polyglyconate suture) and resected at the level of the UVJ. An oval‐shaped bulging lesion near the right ureterovesical junction (UVJ) was observed on the outer bladder wall (Figure 2a). The bladder was incised along the ventral midline for cystotomy. A cystic lesion with a bilobed shape had formed on the inner bladder wall (Figure 2b). The orifices of the left and right ureters were at the normal location within the trigone (Figure 2c). No other path connected to the ureterocele was observed on the inner wall of the bladder. An incision was made on the mucosal surface of the ureterocele, and multiple uroliths within the ureterocele were retrieved (Figure 2d). Any ureterocele epithelium projecting intraluminally into the bladder was excised, and the edges of the remaining ureterocele epithelium were left unsutured. The bladder incision was closed with 3‐0 polyglyconate sutures in a single inverting layer, followed by routing closure of the abdomen. Urine and urolith samples were collected for bacterial culture tests and component analysis, respectively. The bacterial culture showed no growth, and the retrieved stones were positive for phosphate, calcium, oxalate and urate. The dog recovered well from anaesthesia, and analgesia was administered by a continuous infusion of butorphanol (0.1 mg/kg/h, IV) for 24 h after surgery, followed by oral tramadol (5 mg/kg) twice daily for 14 days. Postoperatively, the patient presented with urinary incontinence, which eased over time and completely resolved within 3 months. Ultrasonographic scans during the regular follow‐up for 6 months detected no evidence of tumour metastasis or recurrence. At 9 months of follow‐up, the dog was in good health without any urinary symptoms.
FIGURE 2.
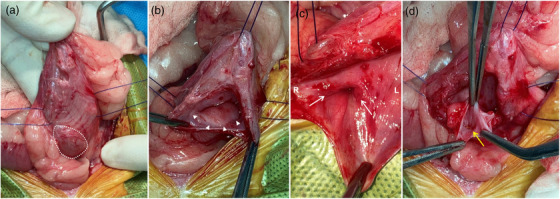
Intraoperative photographs of the urinary bladder with a ureterocele (a–d). (a) The bladder wall is swollen outward (white dotted line) at the ureterovesical junction. (b, c) The cystic structure (white arrowheads) within the bladder wall is seen, and both sides of the ureteral orifice (white arrows) are at the normal position within the trigone. (d) Several small calculi (yellow arrow) can be seen through the incision made on the mucosal surface of the ureterocele. R, right; L, left
The excised right kidney, ureter and ureterocele wall were histologically examined. Histological analysis of the right ureter was performed at three different locations: proximal, middle and distal (Figure 3a). Approximately 95% of the renal parenchyma was replaced by a massively dilated renal pelvis (Figure 3b). The remaining thin rim of renal tissue was largely composed of fibrosis (Figure 3c). Multifocally, the mucosa of the proximal ureter was replaced by neoplastic epithelial cells (Figure 3d). No neoplastic changes were found in the middle and distal parts of the ureter. The neoplastic cells have abundant amphophilic cytoplasm and large vesicular nuclei with 1 to 3 nucleoli. There was marked anisocytosis and anisokaryosis with a mitotic index of 12 per 10 high‐power fields. The proliferation of the neoplastic cells was confined to the mucosa of the ureter without infiltrating the submucosa and formed non‐papillary flat lesions. The sectional margin of the ureter at the UVJ was free of neoplastic changes. The wall of the resected ureterocele was lined by mildly hyperplastic transitional epithelium. The submucosa was fibrotic with mild submucosal inflammatory infiltration. No neoplastic changes were observed in the ureterocele specimen.
FIGURE 3.
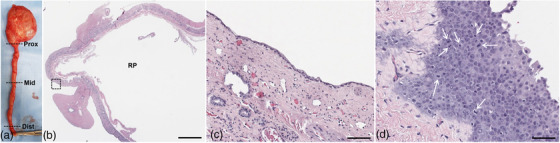
Gross photographs (a) and histopathological images of the resected kidney (b, c) and ureter (d). (a) The right kidney and ureter are massively dilated and filled with urine. The black dotted lines represent the three different locations from which the histological analyses were performed. (b) Greater than 90% of the renal parenchyma is replaced by an extensively dilated renal pelvis. Bar = 8 mm. (c) Amplified image of the area marked by the black dashed square line in Figure 3b shows that the remaining thin rim of atrophic renal tissue is largely composed of fibrotic tissue and rare renal tubules. Bar = 200 μm. (d) Histologic image obtained from the proximal part of the ureter shows piled neoplastic cells confined by the basement membrane (carcinoma in situ) with mitotic figures (white arrows). No neoplasia was observed in the middle and distal parts of the ureter. Bar = 60 μm. Prox, proximal; Mid, middle; Dist, distal; RP, renal pelvis
3. DISCUSSION AND CONCLUSIONS
Most dogs diagnosed with ureteroceles are under the age of two because of its congenital nature (Abibe et al., 2020; Anderson et al., 2020; Rogatko et al., 2019; Stiffler et al., 2002). However, since ureteroceles are usually asymptomatic (Brovida, 2014), a few dogs with ureteroceles have a late onset of symptoms and are diagnosed in old age (Rogatko et al., 2019; Stiffler et al., 2002). Several embryologic theories that have been proposed to explain this anomaly include arrested myogenesis of the distal ureter, ureteral orifice stenosis, delayed fusion of the mesonephric duct and urogenital sinus and congenital weakness of the ureteral wall (Abibe et al., 2020; Coplen & Duckett, 1995; Sutherland‐Smith et al., 2004). In human medicine, there is controversy over the aetiology of ureteroceles. Various studies have reported ureteroceles identified in adulthood and suggested that inflammation or trauma on the urinary orifice can result in stenotic orifice and consequent cystic dilation of the intramural distal ureter (Coplen & Duckett, 1995; Sumfest et al., 1995). Similarly, in veterinary medicine, there have been two reports describing the formation of ureteroceles secondary to surgical ligation of the intramural ectopic ureters (Martin et al., 1985; Rogatko et al., 2019). These reports show the possibility of the existence of acquired‐type ureteroceles in humans and dogs.
Canine ureteroceles can be categorised based on anatomical and functional classification systems (Stiffler et al., 2002). As mentioned in the introduction, the anatomical classification system classifies canine ureteroceles as orthotopic or ectopic based on the anatomical location (Glassberg et al., 1984). However, this classification system has limitations in describing concurrent renal or ureteral abnormalities and determining the prognosis. Stiffler et al. (2002) proposed a functional classification system for dogs modified from the human ureterocele grading system. In this system, the categorisation of the canine ureterocele is based on the presence of concurrent urinary tract disease, therefore enabling precise prognosis prediction. The functional classification system classifies ureteroceles without concurrent urinary tract disease as grade 1, ureteroceles with ipsilateral urinary tract disease as grade 2 and ureteroceles having coexisting bilateral hydronephrosis, hydroureter or chronic renal disease as grade 3. The dog described in this report had orthotopic ureterocele with concurrent ipsilateral hydronephrosis, hydroureter and ureteral UC. According to the functional classification criteria, this dog can be classified as grade 2. However, since the proposed classification system for dogs was not made in consideration of concurrent urinary tract neoplasms, other classification criteria are necessary to accurately classify this case.
A number of primary neoplasms are known to affect canine ureters, which include leiomyoma, leiomyosarcoma, fibroepithelial polyps, fibropapilloma mast cell tumour, spindle cell sarcoma and UC (Cathasaigh et al., 2018). However, there have been only three case reports describing a primary ureteral UC (Cathasaigh et al., 2018; Hanika & Rebar, 1980; Kim et al., 2015). The majority of urinary tract UC cases show anaplastic, papillary and infiltrative growth patterns, which can be classified as high grade (Meuten & Meuten, 2017). The dog in this case had a non‐papillary non‐infiltrative ureteral UC with moderately differentiated neoplastic cells. The tumour cells remained within the mucosa without breaching the basement membrane. Given these characteristics, the UC of this dog was classified as carcinoma in situ (CIS).
This study presents an extremely rare case in which early malignant transformation of the ureteral epithelium was concurrently present with ipsilateral ureterocele. In human medicine, nine cases of UC within the ureterocele and only one case of ureterocele with primary ureteral UC have been reported (Astigueta et al., 2016). To date, there is no report of urinary tract UC with concurrent ureterocele in veterinary medicine. Dhameja and Kailashiya (2016) suggested that cellular neoplastic transformation of the upper urinary tract urothelium can be associated with chronic irritation caused by renal stones or hydronephrosis. However, no clear causal relationship between ureteroceles and ureteral neoplasms has been identified.
Considering that the ureterocele was not observed at the time of surgery for bladder calculi and that the dog occasionally showed symptoms related to cystitis, the authors assume that chronic inflammation caused by uroliths resulted in a stenotic ureteral orifice. Furthermore, this pathological process might lead to newly formed ureteroceles or aggravate already existing undiagnosed ureteroceles. Due to a lack of evidence demonstrating these pathological associations, it could not be ascertained whether the ureterocele in this dog was congenital or acquired. However, given the late onset of clinical symptoms and a series of clinical histories of the dog, it is reasonable to consider that acquired causes took part in the occurrence of the ureterocele in this case.
The stenotic ureteral orifice caused by chronic inflammation and the dilated ureterocele pressing the intramural ureter were believed to concurrently contribute to urine flow blockage, resulting in increased pressure within the kidney and ureter. The consequent hydronephrosis and hydroureter might have contributed to the development of multifocal neoplastic changes at the proximal ureteral epithelium by inducing chronic inflammation. It is difficult to clarify the order of incidents, as the degree of ureteral luminal narrowing caused by UC was not severe and the entire length of the ureter was dilated. However, it seems unlikely that the proximal ureteral UC induced the urine flow blockage and subsequent hydronephrosis. These pathological conditions suggest the possibility of concurrent urinary tract neoplasms in dogs with ureterocele. Therefore, it is essential to conduct a thorough evaluation of the urinary system so that appropriate treatment and follow‐up management can be provided to patients with ureteroceles.
In human medicine, CIS is an accepted precursor to invasive high‐grade UCs, and the gold standard treatment for ureteral UCs is complete nephroureterectomy with bladder cuff removal because of the high rate of ureteral stump recurrence (Kalyan & Christopher, 2006; Meuten & Meuten, 2017,). Although there has been no reported case of ureteral stump recurrence in dogs, considering the extreme similarities in histopathologic characteristics between UCs in dogs and humans (Knapp & McMillian, 2007), surgical recommendations for human ureteral UCs can be translated into dogs. In the dog described here, because CIS was incidentally identified after surgery, the bladder cuff with the intramural portion of the right ureter was not removed. Therefore, the dog was closely monitored for recurrence of UC after surgery, and no evidence of metastasis or recurrence was observed on follow‐up ultrasonography.
In conclusion, this report presents the first case of a canine orthotopic ureterocele with concurrent ureteral UC and the possible causal relationship between these two different ureteral disorders. Therefore, we suggest that it is important to consider the possibility of such an association and perform thorough preoperative evaluation for the presence of concurrent urinary disorders. Additionally, further studies are necessary to develop the optimal classification system of canine ureteroceles that may be helpful in providing better treatment and determining prognosis.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
MYK and AJ did the clinical exanimation, assisted the surgery and followed up the patient after surgery. HYY was the main surgeon of this study. MYK, AJ and HHY analysed the case and drafted the manuscript. RPT preformed histologic evaluation of surgical samples. Authors have read and approved the final version of the manuscript.
ETHICS STATEMENT
This study did not require official or institutional ethical approval. The animal was handled according to high ethical standards and national legislation.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.884
ACKNOWLEDGEMENTS
The authors thank Konkuk University Animal Medical Center for providing patient data for this study.
Kim, M.‐Y. , Aerin, J. , Traslavina, R. P. , & Yoon, H.‐Y. (2022). Orthotopic ureterocele with concurrent ureteral urothelial carcinoma in a dog. Veterinary Medicine and Science, 8, 1881–1886. 10.1002/vms3.884
DATA AVAILABILITY STATEMENT
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- Abibe, R. B. , Valéria, C. , Brandão, S. , & Pereira, G. J. (2020). Symptomatic orthotopic ureterocele in a dog. Acta Scientiae Veterinariae, 48, 1–6. [Google Scholar]
- Anderson, T. , Diaz, F. L. , Caine, A. , Miller, R. , & Barnes, D. (2020). Cystoscopic‐guided laser ablation of an ectopic ureterocele in a female dog. Journal of the American Animal Hospital Association, 56, 280. [Google Scholar]
- Astigueta, J. C. , Abad‐licham, M. , Silva, E. , Alvarez, V. , Piccone, F. , Cruz, E. , & Redorta, J. P. (2016). Ureterocele urothelial carcinoma: managing a rare presentation. Ecancer, 10, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovida, C. (2014). Urogenital defects in dogs. Veterinary Focus, 24, 2–4. [Google Scholar]
- Cathasaigh, M. O. , Arenas, C. , Ortiz, A. , Hall, J. L. , & Rudorf, H. (2018). Primary ureteral urothelial (transitional cell) carcinoma in a boxer dog. Veterinary Record Case Reports, 6, 1–6. [Google Scholar]
- Childress, M. O. , Adams, L. G. , Ramos‐Vara, J. A. , Freeman, L. J. , He, S. , Constable, P. D. , & Knapp, D. W. (2011). Results of biopsy via transurethral cystoscopy and cystotomy for diagnosis of transitional cell carcinoma of the urinary bladder and urethra in dogs: 92 cases (2003‐2008). Journal of the American Veterinary Medical Association, 239, 350–356. [DOI] [PubMed] [Google Scholar]
- Coplen, D. E. , & Duckett, J. W. (1995). The modern approach to ureteroceles. Journal of Urology, 153, 166–171. [DOI] [PubMed] [Google Scholar]
- Dhameja, N. , & Kailashiya, V. (2016). A rare case of renal pelvis urothelial carcinoma in situ associated with hydronephrosis and atrophic kidney. Annals of Pathology and Laboratory Medicine, 3, 165–169. [Google Scholar]
- Glassberg, K. I. , Braren, V. , Duckett, J. W. , Jacobs, E. C. , King, L. R. , Lebowitz, R. L. , Perlmutter, A. D. , & Stephens, F. D. (1984). Suggested terminology for duplex systems, ectopic ureters and ureteroceles. Journal of Urology, 132, 1153–1154. [DOI] [PubMed] [Google Scholar]
- Hanika, C. , & Rebar, A. H. (1980). Ureteral transitional cell carcinoma in the dog. Veterinary Pathology, 17, 643–646. [DOI] [PubMed] [Google Scholar]
- Kalyan, C. L. , & Christopher, R. P. (2006). Treatment of upper tract urothelial carcinoma: A review of surgical and adjuvant therapy. Reviews in Urology, 8, 61–70. [PMC free article] [PubMed] [Google Scholar]
- Kim, S. S. , Lee, J. S. , Yun, S. K. , Kim, S. Y. , Oh, H. J. , Sohn, J. M. , Jung, S. Y. , Kim, B. E. , Ji, S. Y. , Kim, D. Y. , Kim, W. H. , Yoon, J. H. , & Choi, M. C. (2015). Primary ureteral transitional cell carcinoma in a dog. Journal of Veterinary Clinics, 32, 459–463. [Google Scholar]
- Knapp, D. W. , & McMillian, S. K. (2007). Tumors of the urinary system. In: D. M. Vail DM (Ed.). Small animal clinical oncology (4th ed., pp. 572–582). Saunders. [Google Scholar]
- Lautzenhiser, S. J. , & Bjorling, D. E. (2002). Urinary incontinence in a dog with an ectopic ureterocele. Journal of the American Animal Hospital Association, 38, 29–32. [DOI] [PubMed] [Google Scholar]
- Martin, R. A. , Harvey, H. J. , & Flanders, J. A. (1985). Bilateral ectopic ureters in a male dog: A case report. Journal of the American Animal Hospital Association, 21, 80–84. [Google Scholar]
- Meuten, D. J. , & Meuten, T. L. K . (2017). Tumors of the urinary system. In: Meuten D. J. (Ed.). Tumors in domestic animals (5th ed., pp. 632–688). Blackwell, Ames: Wiley. [Google Scholar]
- Rogatko, C. P. , Bagley, D. , Berent, A. C. , Adams, L. G. , & Weisse, C. W. (2019). Endoscopic laser‐ablation for the treatment of orthotopic and ectopic ureteroceles in dogs: 13 cases (2008‐2017). Journal of Veterinary Internal Medicine, 33, 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler, K. S. , Stevenson, M. M. A. , Mahaffey, M. B. , Howerth, E. W. , & Barsanti, J. A. (2002). Intravesical ureterocele with concurrent renal dysfunction in a dog: A case report and proposed classification system. Journal of the American Animal Hospital Association, 38, 33–39. [DOI] [PubMed] [Google Scholar]
- Sumfest, J. M. , Burns, M. W. , & Mitchell, M. E. (1995). Pseudoureterocele: Potential for misdiagnosis of an ectopic ureter as a ureterocele. British Journal of Urology, 75, 401–405. [DOI] [PubMed] [Google Scholar]
- Sutherland‐Smith, J. , Jerram, R. M. , Walker, A. M. , & Warman, C. G. A. (2004). Ectopic ureters and ureteroceles in dogs: Presentation, cause, and diagnosis. Compendium on Continuing Education for the Practising Veterinarian, 26, 303–310. [Google Scholar]
- Tanagho, E. A. (1972). Anatomy and management of ureteroceles. Journal of Urology, 107, 729–736. [DOI] [PubMed] [Google Scholar]
- Timberlake, M. D. , & Corbett, S. T. (2015). Minimally invasive techniques for management of the ureterocele and ectopic ureter: Upper tract versus lower tract approach. The Urologic Clinics of North America, 42, 61–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.


