Abstract
The incidence of ductal carcinoma in situ has increased with the rise in screening mammography; currently, ductal carcinoma in situ constitutes 20%–25% of all breast cancers, and up to half of them may become invasive. Its early detection is critical in improving the cure rate. Moreover, MRI has higher sensitivity for its detection than mammography. Herein, we report an unusual case of ductal carcinoma in situ presenting as a continuous, serpentine, linear enhancement with regional distribution on MRI.
Keywords: Ductal Carcinoma In Situ, Breast, Magnetic Resonance Imaging, Mammography, Ultrasonography
Abstract
선별 유방촬영술이 널리 사용됨에 따라 관상피내암의 빈도는 증가해왔다. 뿐만 아니라, 이의 발견에 있어서는 자기공명영상이 유방촬영술보다 더 높은 민감도를 가진다. 이에 저자들은 침윤성 유방암에 동반된 광범위한 범위의 관상피내암의 비정형적인 자기공명영상 소견을 소개하고자 한다.
INTRODUCTION
With the increasing use of screening mammography, the incidence of ductal carcinoma in situ (DCIS) has increased to the extent that it constitutes 20%–25% of all diagnosed breast cancers (1). And further, MRI is more sensitive to DCIS than mammography (2,3). It has been suggested that 30%–50% of all DCIS cases become invasive (4). Moreover, if DCIS is detected before it becomes invasive, the cure rate is 100% (4). Therefore, the detection of DCIS using imaging is important for surgical planning such as deciding whether to perform breast-conserving surgery. Reports of studies with relatively large samples revealed common MRI features of DCIS (5,6). Although MRI findings of DCIS exhibiting focal linear enhancement have been reported, those of DCIS exhibiting regionally distributed, continuous, serpentine, linear enhancement have rarely been reported. Herein, we describe the MRI features of DCIS and review the relevant literature.
CASE REPORT
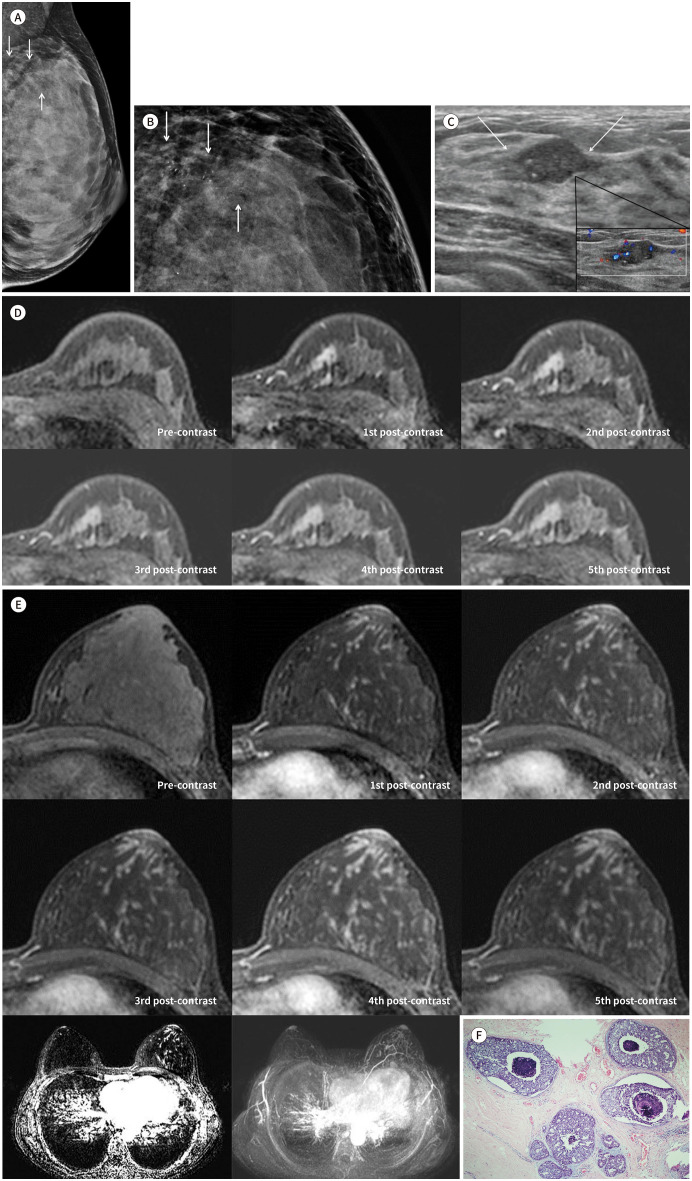
A 36-year-old female, diagnosed with invasive ductal carcinoma (IDC) of the left breast at another hospital, was referred to our hospital. She had no related symptoms such as palpable mass or nipple discharge and no personal or family history of breast cancer. On physical examination, no mass was palpable in her breast. Mammography performed at the other hospital revealed microcalcifications in the left upper breast (Fig. 1A). Magnification mammography performed in our hospital revealed fine, pleomorphic microcalcifications with regional distribution in the upper inner quadrant of the left breast (Fig. 1B). Ultrasonography (US) revealed an isoechoic mass with a diameter of 1.6 cm, indistinct margins, an irregular shape, and echogenic dots in the 11 o’clock position of the left breast. Color Doppler US revealed internal vascularities and vessels in rim of the mass (Fig. 1C). Dynamic contrast-enhanced MRI of the breast revealed a heterogeneously enhanced irregular mass with irregular margins; in terms of kinetic features, the mass exhibited initial fast and delayed washout enhancement (Fig. 1D). Moreover, unlike in the right breast, the left breast, particularly its upper portion, exhibited continuous, serpentine, linear enhancing lesions with regional distribution and visually delayed persistent or plateau enhancement (Fig. 1E). Although a computer-aided detection system was applied, this linear enhancement was not color-mapped. On a review of the mammography and US images, no corresponding lesions were detected. An accurate diagnosis of such linear enhancement was not made before surgery. Differential diagnoses of linear nonmass enhancement are high-risk lesions, such as radial scars or complex scleroing lesions, and intraductal papilloma or DCIS (7). The patient was originally scheduled to undergo left lumpectomy with sentinel lymph node biopsy, pending pathological confirmation of the IDC diagnosis using a preoperative biopsy specimen. However, on diagnosis using frozen sections, the subareolar regions of the mass were positive for DCIS, and there were multiple positive resection margins. Therefore, the surgical plan was changed to modified radical mastectomy intraoperatively. Postoperative histological examination confirmed that the main mass was IDC with micropapillary features, measured 1.3 cm × 1.0 cm in size, had no microcalcifications, and was surrounded by extensive DCIS. The IDC was positive for estrogen and progesterone receptor and equivocal for human epidermal growth factor 2. The Ki-67 score was 49%. We confirmed that the DCIS corresponded to the unusual nonmass enhancement observed on the preoperative MRIs. The DCIS lesion was extensive; measured 6.7 cm × 2.5 cm × 7.0 cm in size, with a Van Nuys Prognostic Index Score of 3, and was a mixture of cribriform-, comedo-, and solid-type carcinoma, with intraductal necrosis and microcalcifications (Fig. 1F). No chemo- or radiation therapy was performed after the surgery; at the time of writing, the patient intended to receive anti-hormone therapy.
Fig. 1. Imaging and pathologic features of ductal carcinoma in situ accompanyinig invasive ductal carcinoma in a 36-year-old female.
A. Mammography reveals microcalcifications but no mass in the left upper breast (arrows).
B. Magnification mammography reveals regional, fine pleomorphic microcalcifications (arrows).
C. Transverse ultrasonography reveals an isoechoic mass with indistinct margins, an irregular shape, and echogenic dots in the upper inner quadrant of the left breast (arrows); and color Doppler imaging reveals extensive internal and rim vascularity of the mass.
D. Axial contrast-enhanced dynamic T1-weighted imaging of the left breast reveals an irregular mass with irregular margins and heterogeneous enhancement; in terms of kinetic features, the mass exhibits initial fast and delayed washout enhancement.
E. Axial contrast-enhanced dynamic T1-weighted imaging of the left breast reveals continuous, serpentine, linear-enhancing lesions with a regional distribution (upper). Axial T1-weighted contrast-enhanced subtraction imaging (left lower) and maximum intensity projection reveal a regional continuous, serpentine, linear enhancement in the left breast (right lower).
F. Microscopic analysis of the remaining areas reveals linear-enhancing lesions on the preoperative MR images, identified as extensive, high-grade ductal carcinoma in situ, showing a mixture of cribriform- and comedo-type carcinoma with intraductal necrosis and microcalcifications (hematoxylin & eosin stain, × 40).
This study was approved by the Institutional Review Board of our institution (IRB No. 2021-02-002). Informed consent was waived because of the retrospective nature of the study.
DISCUSSION
DCIS is a noninvasive malignancy, characterized by the proliferation of malignant epithelial cells in the terminal ductal lobular unit without invading through the basement membrane (7). Menell et al. (2) reported that DCIS generally seemed to have a ductal or linear appearance on MRIs. However, other reports indicated that ductal enhancement is not common in DCIS (8,9). Viehweg et al. (9) observed that the enhancements of 48 enhancing lesions diagnosed as pure DCIS were focal (73%), ductal (17%), or diffuse (10%). Similarly, Van Goethem et al. (10) reported that ductal enhancements were observed in 8 of total 40 DCIS lesions (20%). Reports of studies with relatively large samples of DCIS cases have described common MRI features of DCIS (5,6). Concerning 79 DCIS lesions, Jansen et al. (6) reported that DCIS predominantly exhibited a nonmass morphology, with clumped or heterogeneous enhancement and segmental or linear distribution. Regarding their kinetic curves, initial fast and delayed washout enhancement was predominant. Facius et al. (5) revealed that common features of DCIS were granular or dotted enhancements with segmental distribution. They reported that only 3 of 74 DCIS cases exhibited linear or linear-branching enhancement. Regarding the kinetic curves, 37 of 74 DCIS cases exhibited initial fast and delayed plateau or washout enhancement. They observed that high-grade DCIS cases more commonly exhibited such kinetic curves than did lower-grade DCIS cases. In the present case, extensive DCIS accompanying IDC exhibited continuous, serpentine, linear enhancement with regional distribution on MRI.
In conclusion, we have reported unusual MRI features of DCIS of the breast. Radiologists should be aware that DCIS can exhibit continuous, serpentine, linear enhancement with regional distribution on MRI. Early detection of this lesion may lead to its appropriate management.
Footnotes
- Conceptualization, J.H.K.
- supervision, J.H.K.
- writing—origianl draft, K.Y.J.
- writing—review & editing, J.H.K., K.W.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding: None
References
- 1.Yamada T, Mori N, Watanabe M, Kimijima I, Okumoto T, Seiji K, et al. Radiologic-pathologic correlation of ductal carcinoma in situ. Radiographics. 2010;30:1183–1198. doi: 10.1148/rg.305095073. [DOI] [PubMed] [Google Scholar]
- 2.Menell JH, Morris EA, Dershaw DD, Abramson AF, Brogi E, Liberman L. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. Breast J. 2005;11:382–390. doi: 10.1111/j.1075-122X.2005.00121.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuhl CK, Schrading S, Bieling HB, Wardelmann E, Leutner CC, Koenig R, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485–492. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 4.Recht A, Rutgers EJ, Fentiman IS, Kurtz JM, Mansel RE, Sloane JP. The fourth EORTC DCIS Consensus meeting (Château Marquette, Heemskerk, The Netherlands, 23-24 January 1998)--conference report. Eur J Cancer. 1998;34:1664–1669. doi: 10.1016/s0959-8049(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 5.Facius M, Renz DM, Neubauer H, Böttcher J, Gajda M, Camara O, et al. Characteristics of ductal carcinoma in situ in magnetic resonance imaging. Clin Imaging. 2007;31:394–400. doi: 10.1016/j.clinimag.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Jansen SA, Newstead GM, Abe H, Shimauchi A, Schmidt RA, Karczmar GS. Pure ductal carcinoma in situ: kinetic and morphologic MR characteristics compared with mammographic appearance and nuclear grade. Radiology. 2007;245:684–691. doi: 10.1148/radiol.2453062061. [DOI] [PubMed] [Google Scholar]
- 7.Chadashvili T, Ghosh E, Fein-Zachary V, Mehta TS, Venkataraman S, Dialani V, et al. Nonmass enhancement on breast MRI: review of patterns with radiologic-pathologic correlation and discussion of management. AJR Am J Roentgenol. 2015;204:219–227. doi: 10.2214/AJR.14.12656. [DOI] [PubMed] [Google Scholar]
- 8.Mossa-Basha M, Fundaro GM, Shah BA, Ali S, Pantelic MV. Ductal carcinoma in situ of the breast: MR imaging findings with histopathologic correlation. Radiographics. 2010;30:1673–1687. doi: 10.1148/rg.306105510. [DOI] [PubMed] [Google Scholar]
- 9.Viehweg P, Lampe D, Buchmann J, Heywang-Köbrunner SH. In situ and minimally invasive breast cancer: morphologic and kinetic features on contrast-enhanced MR imaging. MAGMA. 2000;11:129–137. doi: 10.1007/BF02678476. [DOI] [PubMed] [Google Scholar]
- 10.Van Goethem M, Schelfout K, Kersschot E, Colpaert C, Weyler J, Verslegers I, et al. Comparison of MRI features of different grades of DCIS and invasive carcinoma of the breast. JBR-BTR. 2005;88:225–232. doi: 10.1016/j.clinimag.2006.01.009. [DOI] [PubMed] [Google Scholar]