Abstract
NorA, a multidrug efflux pump in Staphylococcus aureus, protects the cell from multiple drugs, including quinolones. The flqB mutation (T→G) in the 5′ untranslated region upstream of norA causes norA overexpression of 4.9-fold in cis, as measured in norA::blaZ fusions. The transcriptional initiation site of norA was unchanged in mutant and wild-type strains, but the half-life of norA mRNA was increased 4.8-fold in the flqB mutant compared to the wild-type strain. Computer-generated folding of the first 68 nucleotides of the norA transcript predicts an additional stem-loop and changes in a putative RNase III cleavage site in the flqB mutant.
Bacterial and eukaryotic cells typically contain an array of membrane transport systems involved in vital roles in maintenance of cellular homeostasis. Of these transporters, multidrug resistance (MDR) efflux systems, which confer resistance to structurally dissimilar toxic compounds, present a clinical threat, since their acquisition or increased expression may decrease susceptibility to a broad spectrum of chemotherapeutic agents, including antibiotics (19).
Staphylococcus aureus is an important pathogen and has a chromosomally encoded MDR pump, NorA. This pump protects the cell from quinolones such as norfloxacin as well as from ethidium bromide and cetrimide (15, 16). We have previously shown that the regulation of norA expression is complex and includes a two-component regulatory system, ArlS-ArlR, and specific binding of an 18-kDa protein to the norA promoter (5). Many details of the regulation of norA expression, however, remain unclear.
The flqB mutation (T→G) located upstream of the norA gene increases resistance to fluoroquinolones (16). In Staphylococcus aureus MT23142 (flqB), steady-state levels of norA mRNA are substantially greater than those in the wild-type strain ISP794 (16). It has been speculated that this mutation modifies a perfect inverted repeat sequence that may alter the binding of a regulatory protein (11), but a previous study in our laboratory showed no evidence for specific protein binding to this DNA region (5). Thus, the mechanism by which the flqB mutation increases norA expression remains unknown.
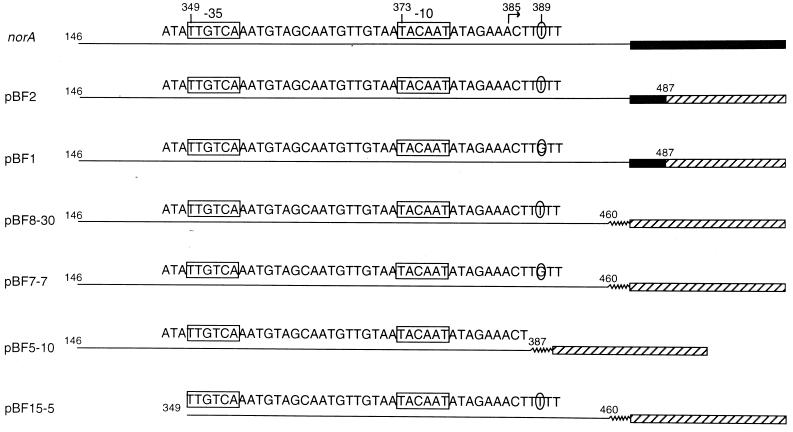
In order to understand the mechanism of the flqB mutation, we first constructed translational and transcriptional fusions of DNA sequences upstream of norA to the blaZ gene, which encodes staphylococcal β-lactamase (Fig. 1, Table 1). The promoter containing the flqB mutation (pBF1) produced increased β-lactamase activity for both the translational (4.5-fold; compare pBF1 and pBF2, Table 2) and transcriptional (4.9-fold; compare pBF8-30 and pBF7-7, Table 2) fusions. These differences could not be attributed to differences in plasmid copy number because the chloramphenicol acetyltransferase activity encoded by the plasmid-borne cat gene and measured in crude extracts of S. aureus prepared by lysis with lysostaphin (80 μg/ml for 30 min at 37°C) (5, 17) did not differ between the two constructs (data not shown). These findings confirmed the previous Northern blot analysis, indicating a higher level of norA mRNA in the flqB mutant (16) (Table 2). The flqB mutation also appears to act entirely in cis, since the β-lactamase activity of pBF8-30 in strains MT23142, containing the chromosomal flqB mutation (16), and ISP794 (wild type) did not differ (data not shown).
FIG. 1.
Maps of the 5′ segments of the norA variants examined in this study. Solid line, full-length or truncated norA 5′ UTR: wavy line, truncated bla 5′ UTR. Translated mRNA segments encoding the NorA protein (solid boxes) or the β-lactamase protein (hatched boxes) are also indicated. The numbers refer to the position of the base pairs used in the cloning to contruct the fusions (the numbering is that of Yoshida et al. [29]). The circled base is the flqB mutation. The −35 and −10 consensus sequences are boxed. The transcription initiation site is indicated by an arrow. mRNA was extracted using the RNeasy miniprep kit (Qiagen) and concentrated in a vacuum dessicator after addition of a 10% volume of ethanol, as needed.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or relevant characteristicsa | Source or reference |
|---|---|---|
| S. aureus | ||
| ISP794 | 8325 pig-131 | 24 |
| MT23142 | 8325 pig-131 flqB | 16 |
| RN4220 | 8325-4 r− | 12 |
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF) U169 deoR recA1 endA1 phoA hsdR17 (rK− mK−) supE44 λ− thi-1 gyrA96 relA1 | Gibco-BRL |
| Plasmids | ||
| pWN2018 | 10.5-kb S. aureus transcriptional promoter-probe vector, Cmr | 27 |
| pBF8-30 | Transcriptional fusion of wild-type promoter (315 bp) with blaZ gene of pWN2018 | 5 |
| pBF5-10 | Transcriptional fusion of wild-type promoter (242 bp) with blaZ gene of pWN2018 | This study |
| pBF15-5 | Transcriptional fusion of wild-type promoter (110 bp) with blaZ gene of pWN2018 | 5 |
| pBF7-7 | Transcriptional fusion of flqB promoter (315 bp) with blaZ gene of pWN2018 | This study |
| pWN1818 | 10.5-kb S. aureus translational promoter-probe vector, Cmr | 27 |
| pBF2 | Translational fusion of wild-type promoter (342 bp) with blaZ gene of pWN1818 | This study |
| pBF1 | Translational fusion of flqB promoter (342 bp) with blaZ gene of pWN1818 | This study |
Cm, chloramphenicol.
TABLE 2.
β-Lactamase activities of the different fusions of the norA promoter in S. aureus ISP794a
| Plasmid | Promoter | Fusion | β-Lactamase activityb (U/g) | Ratioc |
|---|---|---|---|---|
| pWN1818 | None | ≤2,000 | ||
| pBF2 | Wild type | Translational | 3,690 ± 240 | 1 |
| pBF1 | flqB | Translational | 16,600 ± 1,130 | 4.5 |
| pWN2018 | None | ≤2,000 | ||
| pBF8-30 | Wild type | Transcriptional | 10,200 ± 1,700 | 1 |
| pBF5-10 | Wild type truncated of 5′ mRNA | Transcriptional | 3,740 ± 680 | 0.4 |
| pBF15-5 | Wild type truncated of region upstream of the promoter | Transcriptional | 9,230 ± 510 | 0.9 |
| pBF7-7 | flqB | Transcriptional | 50,200 ± 4,610 | 4.9 |
DNA fragments (Fig. 1) were amplified by PCR, cloned into pGEM3-zf(+) (Promega), and then subcloned into the KpnI and PstI sites of pWN1818 or pWN2018 (27). The plasmid inserts were then sequenced, and the plasmids were introduced by electroporation first into S. aureus RN4220 (12) and then into strain ISP794 (24), using selection with chloramphenicol (20 μg/ml). β-Lactamase activity was measured at room temperature (10) in whole-cell cultures grown in trypticase soy broth at 37°C.
β-Lactamase activities (in units per gram of proteins) are the mean values ± standard deviations for at least three different determinations.
β-Lactamase activities were normalized to that of the wild-type promoter for both fusions.
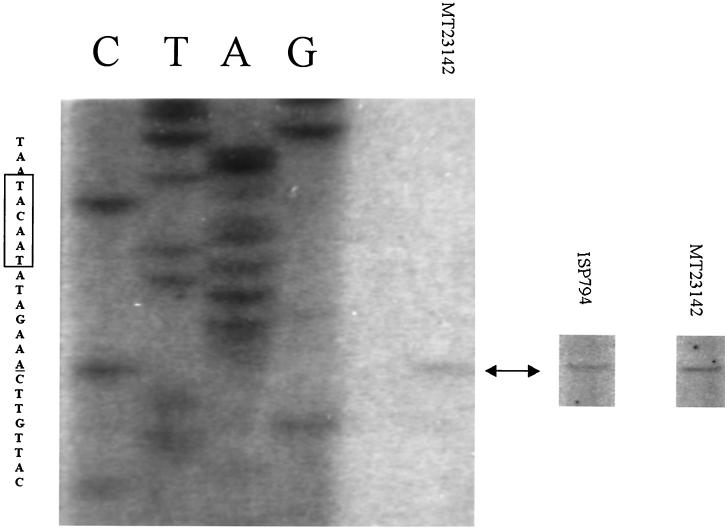
To determine if the flqB mutation resulted in a new and stronger promoter, we next determined the transcriptional start site of norA mRNA in ISP794(pBF8-30) (wild type) and MT23142(pBF7-7) (flqB mutant), using the Promega primer extension system according to the manufacturer's instructions. For both the wild-type and flqB promoters, the transcriptional start site was assigned to an A residue at a position 93 nucleotides upstream of the ATG translational start codon and 7 nucleotides downstream of the putative −10 consensus sequence (Fig. 2).
FIG. 2.
Localization of norA transcription initiation sites by primer extension analysis. Lanes A, C, G, and T represent a sequencing ladder with the same primer. The sequence shown on the left corresponds to the deduced noncoding strand (the −10 consensus sequence of the promoter is boxed, and the transcription start site is underlined). The position of the transcription initiation sites is indicated by an arrow. The weak transcription start site of ISP794 was determined using a phosphoimager and is shown on the right with that of MT23142.
This promoter assignment was confirmed by construction of two additional transcriptional fusions, one lacking the region just upstream of the promoter (plasmid pBF15-5, Fig. 1) and the other lacking the 5′ untranslated region (UTR) of norA mRNA just downstream of the transcription initiation site (plasmid pBF5-10, Fig. 1). The β-lactamase activity of strains carrying pBF15-5 and pBF5-10 was similar or decreased 2.5-fold relative to that of pBF8-30 carrying the wild-type promoter, respectively (Table 2). The β-lactamase activity of pBF5-10, however, was higher than that of the control carrying the plasmid without any promoter (Table 2), suggesting that the norA promoter was still expressed. These results are consistent with the localization of the promoter indicated in Fig. 1 and further indicate that the flqB mutation does not generate a new transcriptional start site.
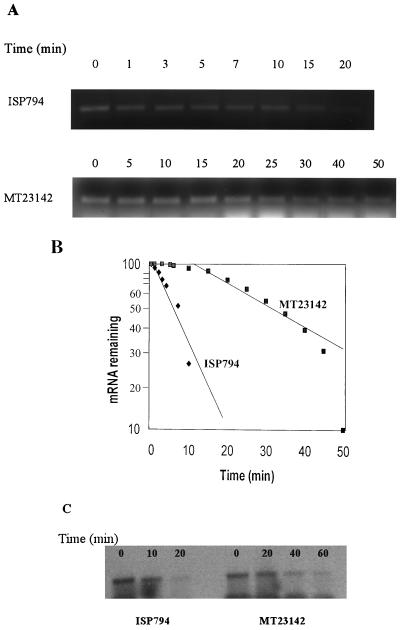
Because the flqB mutation was localized to the 5′ UTR upstream of norA, a region that has been shown in E. coli to affect mRNA stability, we measured the stability of norA mRNA by assessing its levels in ISP794 (wild type) and MT23142 (flqB) after addition of rifampin (200 μg/ml) to stop new RNA synthesis. RNA levels were determined both by reverse transcription-PCR (RT-PCR) using the SuperScript One-Step RT-PCR (Gibco-BRL) and by Northern analysis (Fig. 3). Total RNA was extracted using the SNAP total RNA kit (Invitrogen Inc.) and quantified spectrophotometrically. The RT-PCR used 16S RNA as standard RNA and norA mRNA as target RNA. Because of the low levels of norA mRNA in ISP794, 35 cycles of amplification were needed to produce a measurable signal in the linear range, and input RNA from MT23142 was adjusted to allow the same conditions to be used for reactions for both strains. Under the conditions of RT-PCR used, the levels of product DNA were found to be linearly related to the input RNA. The estimated half-life of norA mRNA was 7 min for ISP794 and 34 min for MT23142. Thus, the norA mRNA half-life was increased 4.8-fold in the flqB mutant (Fig. 3A and B), indicating that this mutation increases mRNA stability. These differences in mRNA stability were confirmed by Northern hybridization analysis using the norA gene as a probe (Fig. 3C).
FIG. 3.
Determination of norA mRNA stability. (A) Agarose gel with RT-PCR, which were performed using total cellular RNA extracted from strains ISP794 (wild type) and MT23142 (flqB mutant) after addition of rifampin (200 μg/ml) at time zero (cells grown to OD600 of 0.6) for both strains. Primers for norA mRNA (5′-AGA AAC TTT TTA CGA ATA TT-3′ and 5′-TGA CAC TGA AAA CAA AAT TA-3′) generated a 250-bp fragment. We used 0.2 μM each primer, 2 U of reverse transcriptase-Taq mix, and 4 μg of total RNA for ISP794 or 2 μg of total RNA for MT23142 for norA mRNA amplification. RT-PCR running conditions were one cycle of 45 min at 40°C, 35 cycles of 1 min at 94°C, 1 min at 40°C, and 1 min at 72°C, and one cycle of 10 min at 72°C (6, 14). (B) Semilogarithmic graph of mRNA concentration as a function of time (expressed as percent of amount at time of addition of rifampin). Densitometric analysis was performed by scanning the gel pictures and performing analysis using the public-domain National Institutes of Health Image program (version 6.1). (C) Northern hybridization of norA mRNA. Total RNA was extracted using the SNAP Total RNA kit (Invitrogen). Times indicate time after the addition of rifampin at time 0. The norA probe used was described previously (16).
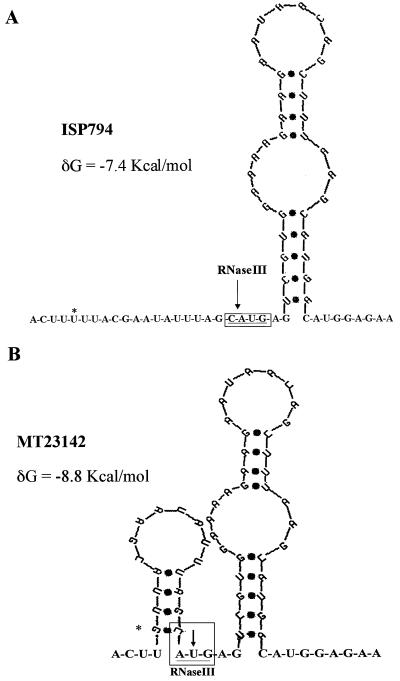
Computer-predicted folding of the region between the transcriptional start site and the Shine-Dalgarno sequence of the norA leader mRNA of strain ISP794 (wild type) showed an RNA hairpin structure in the 5′ UTR, whereas that of the flqB mutant showed an additional hairpin (Fig. 4). The flqB mutation modified a U-A pair, creating the new stem-loop, and was predicted to be more stable (−8.8 kcal) than the structure predicted in the wild type (−7.4 kcal). A putative RNase III cleavage site (CAUG) was present in the 5′ UTR (Fig. 4). Although cleavage sites for RNase III in S. aureus have not been defined, in Bacillus subtilis this sequence has been shown to be a target for RNase III (13, 20).
FIG. 4.
Computer-predicted folding of the first 68 nucleotides of the norA leader mRNA of strain ISP794 (wild-type) (A) and MT23142 (flqB) (B) between the transcriptional start site and the Shine-Dalgarno sequence. RNA secondary structure of the region was analyzed using an RNA folding program available from the Microbiology website of the University of Adelaide, Adelaide, Australia (http://www.microbiology.adelaide.edu.au). Location of the mutation (U→G) is indicated by a star. The putative RNase III cleavage site is boxed.
To estimate the effect of the flqB mutation on norA expression in Escherichia coli, we compared the MICs of ampicillin and the β-lactamase activities for E. coli strains carrying pBF8-30 (wild type) and pBF7-7 (flqB mutation); both measures were similar for the two constructs and higher (16-to 14-fold) than those for the vector plasmid alone (Table 3), indicating that the flqB mutation did not affect the efficiency of the E. coli transcriptional machinery.
TABLE 3.
Expression of β-lactamase from the norA promoter in transcriptional fusions in E. coli DH5αa
| Plasmid | MICb (μg/ml) | Ratioc | β-Lactamase activityd (u/mg) | Ratio |
|---|---|---|---|---|
| pWN2018 | 2 | 1 | 1.2 ± 0.4 | 1 |
| pBF8-30 | 32 | 16 | 17.5 ± 1.7 | 14 |
| pBF7-7 | 32 | 16 | 17.2 ± 1.5 | 14 |
Cells containing the specified plasmids were grown in LB broth and sonicated prior to determination of β-lactamase activity, which was assayed as specified in Table 2, footnotea.
MIC of ampicillin, determined on Mueller-Hinton agar.
MICs and β-lactamase activities were normalized to that of the strain carrying the vector alone.
β-Lactamase activities are expressed in units per milligram of proteins and are the means ± standard deviations of at least three determinations.
The flqB mutation resides in the 5′ UTR of the norA gene and increases norA expression in cis. Mutations in this region have been associated with increased promoter expression in the has gene of Streptococcus pyogenes (1), an increase that was postulated to be due to increased efficiency of formation of the RNA polymerase-DNA complex (23). The flqB mutation also specifically transforms the sequence TTTTT to TTGTT (Fig. 1), suggesting the possibility that the mutation interrupts reiterative transcription that is due to slippage of RNA polymerase on a homopolymeric region of nascent transcript (8). We, however, found no effect of supplemental UTP in high concentrations on the norA expression measured from the plasmid fusions (data not shown), conditions that decrease expression of other promoters (principally those involved in pyrimidine metabolism) affected by reiterative transcription (8, 26). In addition, norA expression from plasmid pBF5-10, which lacked the TTTTT sequence, was not increased, as would be predicted for a reiterative transcriptional mechanism.
Our data, however, argue, in contrast, that increased norA expression in the flqB mutant is due to increased stability of norA transcripts resulting from altered secondary structure of the 5′ UTR of norA transcripts. Although, as yet, we have no direct data to confirm the computer-predicted RNA secondary structures, they are consistent with findings in studies in E. coli in which stem-loop structures of this type in the 5′ UTR affect RNA stability (4, 22) and involve RNase E (2, 7, 9). In B. subtilis, which lacks RNase E, RNase III may be involved in RNA turnover (18, 28), suggesting that the predicted RNase III cleavage site upstream of norA that is affected by additional stem-loop in the flqB mutant may be relevant for mRNA stability in S. aureus, as has been shown in B. subtilis (13, 20, 22). Furthermore, the greater proximity of the second stem-loop to the 5′ end of the norA transcript in the flqB mutant is consistent with such structures providing greater stability to mRNAs in E. coli (21). Interestingly, although norA expressed in E. coli could be due to recognition of the same promoter as in S. aureus (25), the flqB mutation appears to have no effect on expression in E. coli, suggesting that RNA degradation pathways relating to RNase III differ in the two species (3, 20, 28).
Acknowledgments
We thank Stephen Wu for preparation of some of the RNA samples and Philip Davis and Lee Kaplan of the Massachusetts Molecular Biology Core of the Center for the Study of Inflammatory Bowel Diseases (NIH P30 DK-43351) for performing the primer extension experiments.
This work was supported by U.S. Public Health Service grant AI23988 to D.C.H. from the National Institutes of Health.
REFERENCES
- 1.Alberti S, Ashbaugh C D, Wessels M R. Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A Streptococcus. Mol Microbiol. 1998;28:343–353. doi: 10.1046/j.1365-2958.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 2.Bouvet P, Belasco J G. Control of RNase E-mediated RNA degradation by 5′-terminal base pairing in Escherichia coli. Nature. 1992;360:488–491. doi: 10.1038/360488a0. [DOI] [PubMed] [Google Scholar]
- 3.Conrad C, Rauhut R, Klug G. Different cleavage specificities of RNases III from Rhodobacter capsulatus and Escherichia coli. Nucleic Acids Res. 1998;26:4446–4453. doi: 10.1093/nar/26.19.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emory S A, Belasco J G. The ompA 5′ untranslated RNA segment functions in Escherichia coli as a growth rate-regulated mRNA stabilizer whose activity is unrelated to translational efficiency. J Bacteriol. 1990;172:4472–4481. doi: 10.1128/jb.172.8.4472-4481.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fournier B, Aras R, Hooper D C. Expression of the multidrug resistance transporter NorA from Staphylococcus aureus is modified by a two-component regulatory system. J Bacteriol. 2000;182:664–671. doi: 10.1128/jb.182.3.664-671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman W M, Walker S J, Vrana K E. Quantitative RT-PCR: pitfalls and potential. Biotechniques. 1999;26:112–125. doi: 10.2144/99261rv01. [DOI] [PubMed] [Google Scholar]
- 7.Grunberg-Manago M. Messager RNA stability and its role in control of gene expression in bacteria and phages. Annu Rev Genet. 1999;33:193–227. doi: 10.1146/annurev.genet.33.1.193. [DOI] [PubMed] [Google Scholar]
- 8.Han X, Turnbough C L., Jr Regulation of carAB expression in Escherichia coli occurs in part through UTP-sensitive reiterative transciption. J Bacteriol. 1998;180:705–713. doi: 10.1128/jb.180.3.705-713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen M J, Chen L-H, Fejzo M L S, Belasco J G. The ompA 5′ untranslated region impedes a major pathway for mRNA degradation in Escherichia coli. Mol Microbiol. 1994;12:707–716. doi: 10.1111/j.1365-2958.1994.tb01058.x. [DOI] [PubMed] [Google Scholar]
- 10.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaatz G W, Seo S M. Mechanisms of fluoroquinolone resistance in genetically related strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2733–2737. doi: 10.1128/aac.41.12.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreiswirth B N, Lofdalh S, Betley M J, O'Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature (London) 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 13.Mitra S, Bechhofer D H. Substrate specificity of an RNase III-like activity from Bacillus subtilis. J Biol Chem. 1994;269:31450–31456. [PubMed] [Google Scholar]
- 14.Murley Y M, Behari J, Griffin R, Calderwood S B. Classical and El Tor biotypes of Vibrio cholerae differ in timing of transcription of tcpPH during growth in inducing conditions. Infect Immun. 2000;68:3010–3014. doi: 10.1128/iai.68.5.3010-3014.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neyfakh A A, Borsch C M, Kaatz G W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob Agents Chemother. 1993;37:128–129. doi: 10.1128/aac.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng E Y W, Trucksis M, Hooper D C. Quinolone resistance mediated by NorA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother. 1994;38:1345–1355. doi: 10.1128/aac.38.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 18.Oguro A, Kakeshita H, Nakamura K, Yamane K, Wang W, Bechhofer D H. Bacillus subtilis RNase III cleaves both 5′- and 3′-sites of the small cytoplasmic RNA precursor. J Biol Chem. 1998;273:19542–19547. doi: 10.1074/jbc.273.31.19542. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson M, Glatz E, Rutberg B. Different processing of an mRNA species in Bacillus subtilis and Escherichia coli. J Bacteriol. 2000;182:689–695. doi: 10.1128/jb.182.3.689-695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauhut R, Klug G. mRNA degradation in bacteria. FEMS Microbiol Rev. 1999;23:353–370. doi: 10.1111/j.1574-6976.1999.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 22.Régnier P, Grunberg-Manago M. RNase III cleavages in non-coding leaders of Escherichia coli transcripts control mRNA stability and genetic expression. Biochimie. 1990;72:825–834. doi: 10.1016/0300-9084(90)90192-j. [DOI] [PubMed] [Google Scholar]
- 23.Schickor P, Metzger W, Werel W, Lederer H, Heumann H. Topography of intermediates in transcription initiation in Escherichia coli. EMBO J. 1990;9:2215–2220. doi: 10.1002/j.1460-2075.1990.tb07391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stahl M L, Pattee P A. Confirmation of protoplast fusion-derived linkages in Staphylococcus aureus by transformation with protoplast DNA. J Bacteriol. 1983;154:406–412. doi: 10.1128/jb.154.1.406-412.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Sreedharan S, Plummer K, Fisher L M. NorA plasmid resistance to fluoroquinolones: role of copy number and norA frameshift mutations. Antimicrob Agents Chemother. 1996;40:1665–1669. doi: 10.1128/aac.40.7.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu A-H T, Turnbough C L., Jr Regulation of upp expression in Escherichia coli by UTP-sensitive selection of transcriptional start sites coupled with UTP-dependent reiterative transcription. J Bacteriol. 1997;179:6665–6673. doi: 10.1128/jb.179.21.6665-6673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P-Z, Projan S, Leason K R, Novick R. Translational fusion with a secretory enzyme as an indicator. J Bacteriol. 1987;169:3082–3087. doi: 10.1128/jb.169.7.3082-3087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Bechhofer D H. Bacillus subtilis RNase III gene: cloning, function of the gene in Escherichia coli, and construction of Bacillus subtilis strains with altered rnc loci. J Bacteriol. 1997;179:7379–7389. doi: 10.1128/jb.179.23.7379-7385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]