Abstract
This study evaluated the applicability and efficacy of patient education regarding fasting recommendations to shorten fasting times in patients undergoing esophagogastroduodenoscopy (EGD). A prospective nonrandomized controlled pilot study was performed. The intervention group (IG) was educated by nurses to eat until 6 hours and drink until 2 hours before EGD. The control group (CG) received usual care. Outcomes were applicability as perceived by patients, adherence to fasting recommendations, gastric visibility, and patients' comfort. A total of 109 patients were included of whom 42 were IG patients (37%). Patients' perspectives on fasting, their experienced discomfort, professional support, and circadian rhythm influenced application of fasting recommendations. Adherence to length of fasting from foods improved with 3:14 hours (p < .001) and from liquids with 5:22 hours (p < .001) in the IG compared with the CG. Gastric visibility during EGD was better in the IG than in the CG. The IG patients experienced significant less thirst, hunger, headache, and anxiety. To successfully reduce fasting times, fasting education should include positive, individual instructions, which help patients apply the fasting recommendations within their biorhythm. Positive, concrete instructions by nurses shortened fasting times before EGD, which improved gastric visibility and reduced patient discomfort.
Prolonged fasting times appear to be a common practice and lead to discomfort in patients. Preprocedural fasting is required for esophagogastroduodenoscopy (EGD) and surgery to reduce the risk of aspiration (American Society of Anesthesiologists [ASA], 2011; Brady, Kinn, & Stuart, 2003; Smith et al., 2011). The ASA guidelines state that patients should fast at least 2 hours after ingestion of clear liquids and 6 hours after ingestion of light meals before sedation is administered. Recommendations are sometimes poorly implemented, which result in prolonged fasting (Breuer et al., 2010; Callaghan, Neale, Boger, Sampson, & Patel, 2016; Salman, Asida, & Ali, 2013). Numerous studies report median preoperative fasting times from solid foods ranging from 12 to 16 hours and from clear liquids ranging from 5 to15 hours (Abdullah Al Maqbali, 2016; Cestonaro, Madalozzo Schieferdecker, Thieme, Neto Cardoso, & Ligocki Campos, 2014; de Aguilar-Nascimento, de Almeida Dias, et al., 2014; El-Sharkawy et al., 2021; Furrer, Ganter, Klaghofer, Zollinger, & Hofer, 2006; Ingadottir et al., 2016; Khoyratty, Modi, & Ravichandran, 2010; Lamacraft et al., 2017; Tosun, Yava, & Acikel, 2015; van Noort et al., 2021; Yeniay, Tekgul, Okur, & Koroglu, 2019). Prolonged fasting decreases insulin sensitivity (Faria et al., 2009) and increases discomfort including thirst, hunger, and headache (Furrer et al., 2006; Spahn, Wessels, Grosse-Thie, & Mueller, 2009).
Fasting before EGD reduces the risk of aspiration during insertion of the scope or administration of anesthetics (American Society of Anesthesiologists Committee, 2011; Brady et al., 2003; Callaghan et al., 2016). An empty stomach as a result of fasting facilitates a clear view on the upper gastrointestinal mucosa. In an EGD the upper gastrointestinal (GI) mucosa should be free from debris and gastric residue to optimize the diagnostic yield and the treatment of benign and malignant conditions. Successful patient preparation for EGD requires the patient's thorough understanding of pre-procedural fasting instructions. Previous studies demonstrated how patient-centered instructions contribute to shortened fasting times before surgery (Hamid, 2014; Kyrtatos, Constandinou, Loizides, & Mumtaz, 2014; Power et al., 2012; Yip, Hogan, & Carey, 2021). Patient compliance was improved by saying “drink up to” instead of “allowed to drink.” (Kyrtatos et al., 2014). Available brochures and educational materials should be carefully reviewed with the patient and/or family to ensure adequate preparation. (Ausserhofer et al., 2014; Bekker, Coetzee, Klopper, & Ellis, 2015; Qureshi, Purdy, Mohani, & Neumann, 2019). In this study, we studied the applicability and efficacy of patient education on fasting recommendations among patients undergoing EGD to shorten fasting times.
Methods
Study Design
A prospective, nonrandomized, controlled pilot study was performed at the outpatient clinic of a general hospital in The Netherlands. Data were collected between November 2019 and July 2020. The study examined adherence to fasting recommendations. We educated an intervention group (IG) with concrete, evidence-based fasting instructions and used Strengthening the Reporting of Observational Studies in Epidemiology statement for transparent reporting (von Elm et al., 2014).
Study Population
The patients undergoing an EGD procedure were informed about the study by telephone. Further information was provided via a letter or email if interested. The patients were considered eligible when they were at least 18 years of age, on a regular diet, and understood the Dutch language. The patients were excluded when they had undergone bariatric surgery, had diabetes mellitus, had a proven gastroparesis, or had no swallow reflex. The patients provided written and verbal informed consent at the hospital site in a quiet and private room. This study was approved by the Medical Ethical Committee of Wageningen University, Wageningen, The Netherlands (CMO number 20/09). This study was conducted in accordance with the Declaration of Helsinki (World Medical Association, 2013).
Sample Size Calculation
We calculated the required number of patients for the IG based on findings in the control group (CG). The aim was to achieve a decrease in mean fasting time of at least 3 hours. Previous educational approaches decreased fasting time with 1.5–8.2 hours (Kyrtatos et al., 2014; Power et al., 2012) for liquids and 7.9 hours for solids (Power et al., 2012). Using α = .05 and β = .9 in the equation for continuous variables (Dell, Holleran, & Ramakrishnan, 2002), 35 patients should be included. To account for possible dropouts, we aimed to include at least 40 patients in the IG.
Fasting Instructions
Intervention Group (IG)
Within the routines to prepare for EGD, we introduced concrete, evidence-based fasting instructions. Education included the why, how, and what of the recommendations. Specifically, “to fast from solids and liquids for six and two hours” and “to eat and drink as long as permissible.” The focus of the instructions was changed to a positive, stimulating approach (Hamid, 2014; Kyrtatos et al., 2014; Power et al., 2012). Nurses informed patients about the rationale of preprocedural fasting, the consequences of prolonged fasting, and the benefits of eating and drinking as long as permissible. The patients received a timetable that contained the date and time of the EGD and the times that patients could consume their last meal and drink.
Consumption suggestions were given including meals and drinks that fitted within the Dutch eating habits such as two slices of bread with a topping of cheese, a slice of chicken filet, or jam; or low-fat yoghurt with muesli. The drinks included 200 ml of apple juice, lemonade, or tea. Patients received all information in a leaflet and an infographic.
Control Group (CG)
Routine care for patients undergoing EGD included information about the planned EGD via telephone and an information leaflet. This leaflet included instructions to fast for 6 hours from solid foods and 2 hours from clear liquids together with information about the procedure. The patients who were scheduled for an EGD in the morning were instructed to fast from midnight. The patients were allowed to have a light breakfast (i.e., sandwich or cracker) and a cup of tea before 9 a.m. if the EGD was scheduled in the afternoon. No instructions on type of meals and drinks were given.
Data Collection
Data were collected on patient characteristics, that is, gender, age, American Society of Anesthesiologists Physical Status Classification (ASA PS Classification) (Sankar, Johnson, Beattie, Tait, & Wijeysundera, 2014), history of gastric problems, indication for EGD, and length of the procedure.
Outcomes of interest were applicability of the instructions as perceived by IG patients (Bowen et al., 2009) and efficacy of the education on adherence to fasting recommendations, gastric visibility, and patients' comfort.
Applicability
Applicability was measured with patients' satisfaction with preprocedural care and patients' perceptions toward the applicability of the instructions.
Satisfaction
The Consumer Quality Index (CQ-Index) (Delnoij, Rademakers, & Groenewegen, 2010) was adapted for our setting to determine the satisfaction of patients from both groups (see Supplemental Digital Content Appendix, available at: http://links.lww.com/GNJ/A78). The patients were requested to complete the questionnaire within 6 hours after the EGD, either in the outpatient clinic or at home. The patients who did not return the questionnaire received a reminder by telephone.
Patients' Perceptions
Perceptions of IG patients toward the applicability of the instructions were investigated in a structured face-to-face interview 15 minutes before the EGD (see Supplemental Digital Content Appendix, available at: http://links.lww.com/GNJ/A79). Using open-ended questions, we investigated how patients perceived the instructions, and how they experienced it to apply them. Barriers and facilitators for adherence to the fasting recommendations were identified.
Efficacy
Adherence to Fasting Instructions
Adherence to the fasting instructions was determined by (1) the duration of fasting, (2) adequate fasting behavior, and (3) the type of last meal and drink. Duration of fasting was the time interval between the last meal or drink and start of the EGD. The times of these consumptions were based on the patients' recall within 15 minutes before the EGD. In this study, adequate fasting behavior was defined as a maximum fasting time of 8 hours for solid foods and 4 hours for clear liquids. This longer period was chosen to be able to correct for possible measurement errors and to take clinical activities into account. In both groups, type of last meal and drink were chosen by the patients themselves. Last meals included sandwiches, yoghurt, fruit, and warm meals; last drinks included lemonade, apple juice, water, tea, and coffee.
Gastric Visibility
Gastric visibility was assessed by endoscopic flush volume, gastric residual volume, the Mucosal Visibility Score (MVS), and judge ability of the mucosa. Flush volume was recorded to estimate the gastric residual volume (total suctioned volume subtracted by flush volume).
The MVS measures mucosal visibility in the lower esophagus, upper body greater curve, antrum, and fundus (Basford et al., 2016; Kuo, Sheu, Kao, Wu, & Chuang, 2002). After the procedure, the endoscopist rated the visibility of each area on a four-level scale based on pictures of each area made during introduction of the scope before flushing: (1) No adherent mucus and clear view of the mucosa; (2) a thin coating of mucus that did not obscure view of the mucosa; (3) some mucus/bubbles partially obscuring the view of the mucosa (a small mucosal lesion might be missed without flushing); and (4) heavy mucus/bubbles obscuring view of the mucosa (a small mucosal lesion could easily be missed without flushing). The MVS resulted in a total score (TMVS) between 4 and 16. The MVS has been used in other studies (Asl & Sivandzadeh, 2011; Basford et al., 2016; Chang et al., 2007; Kuo et al., 2002; Monrroy et al., 2018), and reliability of the TMVS was previously determined with Spearman's ρ (ρ= 0.79–0.83) (Kuo et al., 2002) and the weighted κ (0.901) (Monrroy et al., 2018).
Judge ability was defined as the extent to which the endoscopist was able to assess the mucosa. Judge ability of each area was rated by the endoscopist on a Numeric Rating Scale from 0 (indicating worst judge ability) to 10 (indicating perfect judge ability).
Patients' Comfort
The presence of symptoms of prolonged fasting, including nausea, vomiting, thirst, hunger, anxiety, weakness, and headache, was assessed (Asl & Sivandzadeh, 2011; Gul, Andsoy, & Ozkaya, 2018; Hydes, Yusuf, Pearl, & Trebble, 2011; Itou et al., 2012; Ko, Zhang, Telford, & Enns, 2009; Koeppe et al., 2013). The patients scored these symptoms on a 4-point Likert scale 15 minutes before the procedure. They could also add other complaints or experiences.
Data Analysis
Descriptive analyses were used for patients' characteristics and outcomes. Numbers and percentages are presented for nominal and ordinal variables. Means and standard deviations or medians with interquartile ranges are presented for continuous variables depending on normality. Differences in outcomes between study groups were evaluated using the Pearson's χ2 test, the Mann–Whitney U test, and the independent t test for nominal, ordinal, and continuous variables, respectively.
The patients' perceptions toward the instructions were analyzed using the conventional content analysis approach including open coding, categorizing, and synthesizing by defining themes (Hsieh & Shannon, 2005). Open answers were first coded and afterward discussed by two researchers. Then, codes were clustered during discussions to define categories. Themes were consequently defined on the basis of the synthesis of the codes and categories. In each step, consensus was obtained during the discussions.
Study results were analyzed on an intention-to-treat basis. Missing values were not inputed because of the explorative character of the study. A p value of less than .05 was considered significant based on two-sided tests. Statistical analyses were carried out with IBM SPSS, version 25.0 (IBM Corp, Armonk, NY).
Results
Study Sample
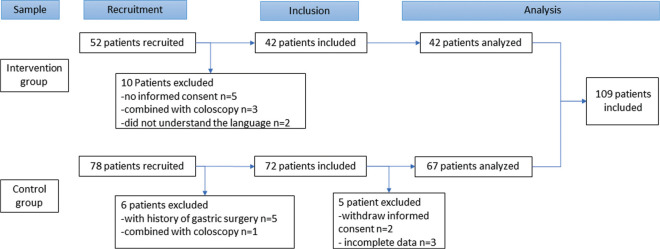
Of 130 invited patients, 18 patients (14%) were excluded: no informed consent (n = 7, 5.4%), a history of gastric surgery (n = 5, 3.8%), a colonoscopy (with oral bowel preparation) during the same procedure (n = 4, 3.1%), or not understanding the Dutch language (n = 2, 1.5%). Data of 3 patients (2.3%) were incomplete. Finally, 109 patients (84%) participated in the study, of whom 42 patients (37%) were in the IG (Figure 1). The groups were comparable in terms of age and gender distribution (Table 1). Most of the EGD procedures were performed during morning sessions (n = 91, 84%).
FIGURE 1.
Selection of patients.
TABLE 1. Patients' Characteristicsa.
| Control Group (n = 67) | Intervention Group (n = 42) | |
|---|---|---|
| Female | 42 (63) | 19 (45) |
| Age (years) | 59.6 ± 15.3 | 58.7 ± 18.6 |
| ASA PS Classificationb | ||
| I | 13 (20) | 6 (15) |
| II | 48 (73) | 32 (78) |
| III | 5 (7) | 3 (7) |
| Session | ||
| Morning | 59 (87) | 33 (79) |
| Afternoon | 9 (13) | 9 (21) |
| Indication for endoscopy | ||
| Dysphagia | 23 (34) | 14 (33) |
| Nausea | 12 (18) | 3 (7) |
| Reflux or pyrosis | 13 (19) | 9 (21) |
| Dyspepsia | 29 (43) | 11 (26) |
| Follow-up endoscopy | 9 (13) | 3 (7) |
| Other | 13 (19) | 12 (29) |
| Duration of endoscopy (minutes) | 5.8 ± 2.3 | 5.8 ± 2.3 |
| Type of last meal | ||
| Sandwich | 21 (31) | 17 (41) |
| Yoghurt | 5 (8) | 12 (29) |
| Warm meal | 28 (42) | 5 (12) |
| Fruit | 2 (3) | 3 (7) |
| Other | 11 (16) | 5 (12) |
| Type of last drink | ||
| Water | 35 (52) | 23 (55) |
| Tea | 11 (16) | 11 (26) |
| Apple juice | 1 (2) | 0 (0) |
| Lemonade | 3 (5) | 2 (5) |
| Coffee | 10 (15) | 2 (5) |
| Other | 7 (10) | 4 (10) |
Note. ASA = American Society of Anesthesiologists Physical Status Classification (ASA PS Classification).
aContinuous data are presented as mean ±SD. Categorical data are presented as n (%).
bData was missing for two patients.
Applicability
Patients' Satisfaction
Questionnaires on patients' satisfaction were returned by 45 patients (67%) from the CG and 26 patients (62%) from the IG. The majority of patients were very satisfied with mean scores of at least 8.2. The IG patients were less satisfied with the care to prepare for the EGD than CG patients. On a scale from 0 to 10, they rated this 0.56 points lower (p = .017).
Patients' Perceptions Toward the Applicability
Perceptions toward the applicability were evaluated among IG patients. The applicability of fasting education was influenced by five themes derived from the conventional content analysis: motivation, perceptions, discomfort, circadian rhythm of eating and sleeping, and professional support (Table 2).
TABLE 2. Themes, Codes, and Citations of Patients' Perceptions Toward the Applicability of the Instructions.
| Theme | Codes | Citations |
|---|---|---|
| Motivation | Contribute to science Loyal to appointments |
(110085) “I would like to support you (i.e., nurse from the study)” (110107) “I did it because of the study, without the study I would not do it” (110088) “I like to stick to the rules” (110095) “For me, a deal is a deal” |
| Perceptions | Perception toward fasting Perceptions toward the recommendations |
(110086) “For me, prolonged fasting is not a big problem, now the night was in between so it was easy” (110090) “It is clear, I had to do it before, it is obvious. Fasting belongs to these procedures” (110110) “it gave rest to be allowed to eat something. Because I always suffer from thirst due to Sjogren, it was reassuring that I could still have something” (110096) “The task was very easy, just follow two rules” |
| Discomfort | Symptoms of discomfort Physical complaints |
(110089) “I was hungry, and the advice was to eat some” (110113) “Being not allowed to drink would be difficult because the feeling of thirst, but now it was allowed to drink something” (110101) “I suffer a lot from burping, Belching, feeling nausea. Then eating during the evening is not pleasant” |
| Circadian rhythm of eating and sleeping | Eating routines Effort to fit it in eating routines Was not awake Effort to fit it in sleep routines Feeling tired/sleepy Sleep disturbances |
(110085) “I usually eat something like toast or sausage before I go to sleep, now it was yoghurt with muesli as you advised” (110096) “I never eat during the evening” (110102) “I use to have breakfast late in the morning, so the endoscopy fits optimal” (110094) “I never eat during the evening, but the advice convinced me to eat a cracker during the evening” (110114) “I was still a sleep at 7.00 AM, so when I woke up it was too late to drink” (110100) “I went to bed a bit later than normal to eat a bit later than I usually do” (110093) “I could have set an alarm, but I do not want to do that; eating late in the evening is not a problem, though” (110083) “I intended to eat at midnight, but I was tired, so I ate at 11.00 PM” (110115) “I awakened spontaneously, and then I decided to drink something” (110107) “I wake up frequently during the night, so now I went out to eat something” (110103) “I slept not good; I was awake each hour due to the procedure that was awaiting” |
| Professional support | Professional support Advice and instruction Clear instructions Social support |
(110103) “I had spoken with a nurse about it” (110104) “information about the rationale of fasting did give me more awareness; otherwise, I would not have eaten during the evening” (110098) “the clear information with examples of meals helped me” (110113) “The instructions made me more aware and made me eat something before bedtime; the advice were helpful” (110094) “husband had prepared a cup of tea” |
The theme motivation included patients' motivation to be loyal to appointments and the value to contribute to science. The patients appeared to be willing to follow rules and to be loyal to instructions. They were willing to fulfill requirements that healthcare professionals suggested them to do. Some patients were also motivated to apply the instructions because that would contribute to science. For them, this was also a reason to participate in the study. Patients may have felt the importance of applying the instructions.
The theme perceptions included patients' perceptions toward “fasting” and the recommendation “to keep eating and drinking” until 6 and 2 hours. Some patients perceived fasting as part of the instructions they were already familiar with. Fasting for was not a problem for some patients, as they could eat and drink right after the EGD. Others also believed that a longer fasting period would enhance the chance that the EGD would have good visual results, and they would not vomit afterward. Some patients mentioned that the recommendations were easy to follow. This helped provide reassurance to patients who were anxious related to fasting.
Within the theme discomfort, the patients indicated that symptoms of discomfort helped them adhere to the recommendations, for instance due to thirst. Physical complaints could either hamper or enable patients to keep eating and drinking until bedtime, such as experiencing abdominal discomfort, or always having appetite or being thirsty.
A major theme occurring from the analysis was the circadian rhythm of eating and sleeping. The patients had to fit the recommendations into their individual eating behavior during the day. This could include eating before bedtime, eating during the night, or having a late breakfast. Most patients were willing to put effort in applying the recommendations, for instance, to wake up early to eat or drink, to postpone sleeping time for a late evening snack or meal, or to prepare the meal to be eaten during the night. Some patients mentioned that they ate during the night as a result of awakening due to sleep disturbances. Other patients mentioned that the time of eating or drinking, or the time of fasting optimally fitted in their eat or sleep rhythm, which meant that both actions did not burden them. Few patients planned to eat or drink but forgot it eventually due to other activities.
Professional support was another major theme. The conversation with a nurse about the recommendations increased the urgency to apply them. For some patients, it was helpful that instructions included examples and that it was provided both on paper and by telephone. Patients became aware of the possibility to eat and drink longer. Also, support from relatives and family helped some patients comply to the instructions.
Adherence to Fasting Instructions
Fasting From Solid Foods
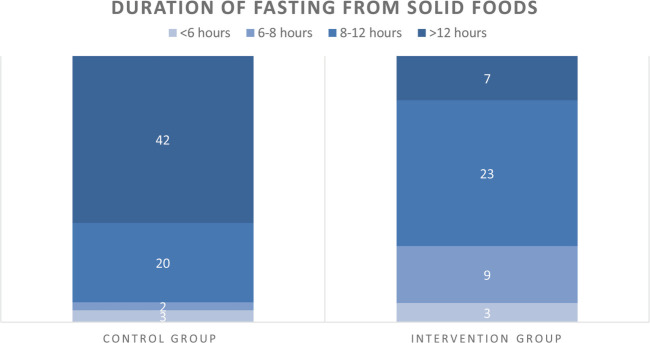
Duration of fasting from solid foods was 12:56 hours ± 3:09 hours for CG patients and 9:31 hours ± 3:01 hours for IG patients (p < .001, Table 3). Length of fasting was three times more adequate (i.e., less than 8 hours) in the IG than in the CG (n = 12 [29%] vs. n = 5 [8%], p = .003). Moreover, less patients fasted more than 12 hours in the IG compared with the CG (n = 7 [17%] vs. n = 47 [63%], p < .001) (Figure 2). Last consumptions that IG patients ate were less frequently warm meals (n = 5 [12%] vs. n = 28 [42%], p = .001) and more frequently the meal suggestions (i.e., sandwiches or yoghurt) compared with CG patients (n = 29 [70%] vs. n = 26 [38%]), p = .001).
TABLE 3. Impact of Different Fasting Instructions on Patients' Satisfaction, Fasting Times, Gastric Visibility, and Patients' Comforta.
| CG (n = 67) | IG (n = 42) | Mean Difference (95%CI) | p | |
|---|---|---|---|---|
| Patients' satisfaction | ||||
| Care to prepare for endoscopy | 8.7 ± 1.0 | 8.2 ± 1.2 | 0.56 (0.103 to 1.030) | .017 |
| Outpatient clinic | 8.7 ± 0.9 | 8.8 ± 0.9 | 0.05 (−0.481 to 0.378) | .811 |
| Overall | 8.6 ± 0.9 | 8.8 ± 0.9 | 0.06 (−0.468 to 0.356) | .786 |
| Fasting times (hh:mm) | ||||
| From solid foods | 12:56 ± 3:09 | 9:31 ± 3:01 | 3:14 (2:05 to 4:24) | <.000 |
| Morning session | 13:18 ± 2:08 | 9:48 ± 2:39 | 3:20 (2:19 to 4:20) | <.000 |
| Afternoon session | 10:34 ± 6:30 | 7:43 ± 3:39 | 2:51 (−2:32 to 8:14) | .274 |
| From clear liquids | 10:26 ± 3:26 | 5:03 ± 3:46 | 5:22 (3:57 to 6:46) | <.000 |
| Morning session | 10:35 ± 3:03 | 4:57 ± 3:42 | 5:38 (4:06 to 7:09) | <.000 |
| Afternoon session | 9:29 ± 5:29 | 5:25 ± 4:12 | 244 (−0:50 to 8:57) | .096 |
| Mucosal Visibility Score (MVS)b | ||||
| Lower esophagus | 2.2 ± 1.0 | 1.9 ± 0.9 | 0.304 (−0.085 to 0.694) | .125 |
| Corpus | 2.6 ± 1.1 | 2.0 ± 0.8 | 0.573 (0.222 to 0.925) | .002 |
| Antrum | 2.0 ± 1.0 | 1.5 ± 0.7 | 0.563 (0.198 to 0. 927) | .003 |
| Fundus | 1.9 ± 1.0 | 1.4 ± 0.7 | 0.527 (0.171 to 0.883) | .004 |
| Total MVS | 8.7 ± 3.4 | 6.8 ± 2.4 | 1.92 (0.831 to 3.013) | .001 |
| Judge ability | ||||
| Lower esophagus | 7.0 ± 2.3 | 8.4 ± 1.3 | −1.3 (−2.2 to −0.6) | <.000 |
| Corpus | 6.4 ± 2.6 | 8.2 ± 1.2 | −1.7 (-2.5 to −0.9) | <.000 |
| Antrum | 7.5 ± 2.1 | 8.8 ± 1.4 | −1.5 (−2.2 to −0.8) | <.000 |
| Fundus | 7.4 ± 2.4 | 9.1 ± 0.9 | −1.2 (−1.9 to −0.5) | .001 |
| Presence of gastric residue | 63 (96) | 35 (85) | 10.1 (−1.9 to 22.1) | .070 |
| Gastric residual volume (ml) | 58 ± 50 | 31 ± 26 | 27.0 (9.1 to 45.0) | .003 |
| Need to flush | 42 (64) | 15 (37) | 27.0 (8.4 to 45.6) | .006 |
| Flushing volume (ml) | 72 ± 44 | 48 ± 23 | 24.7 (0.9 to 48.5) | .042 |
| Symptoms of discomfort | ||||
| Nausea | 18 (27) | 10 (24) | n/a | .320 |
| Vomiting | 3 (5) | 0 (0) | n/a | .033 |
| Thirst | 43 (64) | 25 (59) | n/a | .001 |
| Hunger | 35 (52) | 19 (45) | n/a | .004 |
| Headache | 19 (28) | 6 (14) | n/a | .004 |
| Weakness | 16 (24) | 7 (17) | n/a | .077 |
| Anxiety | 28 (42) | 13 (31) | n/a | .008 |
Note. CG = control group; CI = confidence interval; IG = intervention group.
aContinuous data are presented as mean ±SD. Categorical data are presented as n (%). Values in boldface are significant.
bMVS scores ranges from 1 to 4: (1) No adherent mucus and clear view of the mucosa; (2) a thin coating of mucus that did not obscure view of the mucosa; (3) some mucus/bubbles partially obscuring view of the mucosa (a small mucosal lesion might be missed without flushing); and (4) heavy mucus/bubbles obscuring view of the mucosa (a small mucosal lesion could easily be missed without flushing). Total MVS (TMVS) are sum scores ranging from 4 to 16.
FIGURE 2.
Adherence to fasting recommendations for solid foods. Number of patients per study group who fasted for maximum 6 hours, 6–8 hours, 8–12 hours, or more than 12 hours from solid foods.
Fasting From Clear Liquids
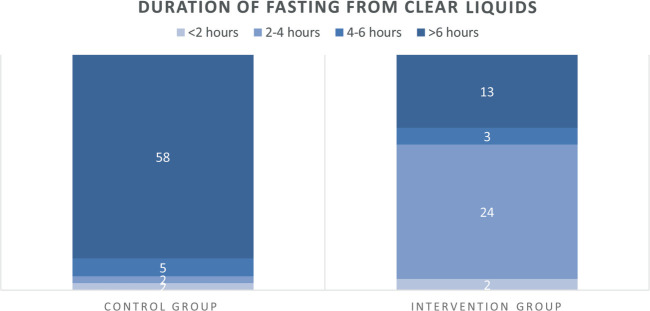
Duration of fasting from clear liquids was 10:26 hours ± 3:26 hours for CG patients and 5:03 hours ± 3:46 hours for IG patients (p < .001, Table 3). Length of fasting from clear liquids was 10 times more adequate (i.e., <4 hours) in the IG than in the CG (n = 26, 62% vs. n = 4, 6%; p < .001) (Figure 3). There were no differences in type of last drink between the groups (Table 1).
FIGURE 3.
Adherence to fasting recommendations for clear liquids. Number of patients per study group who fasted for maximum 2 hours, 2–4 hours, 4–6 hours, or more than 6 hours from clear liquids foods.
Gastric visibility
Gastric residual volume was 27.0 ml higher in CG patients than in IG patients (p = .003). Flushing during EGD occurred more often and with 24.7 ml more in CG patients than in IG patients (p = .006; p = .042; Table 3). The TMVS was 1.92 points lower in the IG than in the CG (p = .001) (Table 3). Judge ability of each area was higher in the IG than in the CG (p = .001).
Patients' Comfort
The IG patients reported significantly less (symptoms of) discomfort (Table 3), except for nausea and weakness, which were reported equally in both groups. In general, the patients most frequently reported thirst (CG: n = 43 [64%]; IG: n = 25 [59%], p = .001) and hunger (CG: n = 35 [52%]; IG: n = 19 [45%], p = .004). Vomiting before EGD happened only in three CG patients (5%, p = .033). Sixteen CG patients (24%) and seven IG patients (17%) felt weak before the EGD (p = .077) (Table 3).
Discussion
Education including the why, how, and what of fasting recommendations led to shorter fasting times in patients undergoing EGD. Applicability of the instructions was influenced by patients' motivation, their perceptions toward fasting, and the instructions, patients' discomfort, circadian rhythm of eating and sleeping, and professional support. Adequate fasting routines were applied more often for clear liquids than for solid foods. Shortened fasting times reduced discomfort and maintained gastric visibility with lower gastric residual volume and better mucosal visibility in well-informed patients.
Fasting guidelines state that patients should be encouraged to keep eating and drinking as long as Permissible (American Society of Anesthesiologists Committee, 2011). The current study demonstrated how this endorsement can be carried out in daily practice by outpatients undergoing EGD. Patients' motivation and perceptions toward both recommendations (i.e., fasting and eating and drinking as long as possible) contributed to the extent to which they were willing to apply these recommendations. Some patients in our study argued that (prolonged) fasting did not burden them, because they were used to having a late breakfast. Others endorsed the importance of fasting, or they did not see why shortened fasting times would benefit them. Patients argued that the ingestion of the last pre-EGD meal and drink should preferably fit within their individual circadian rhythm. Professional support is important to provide clear instructions on the latest time to eat and drink and examples of possible consumptions. The current study demonstrated that educating patients can prevent prolonged fasting.
Patient education belongs to fundamental nursing care enabling patients to manage their health and to make treatment decisions themselves (Ausserhofer et al., 2014; Kitson, 2018; Marcus, 2014). However, it appears to be in the top three nursing activities that remains undone (Ausserhofer et al., 2014; Bekker et al., 2015; Qureshi et al., 2019). In our study, nurses educated patients on optimal eating and drinking behavior when fasting was required. Limited evidence is available to conduct optimal education strategies regarding eating and drinking behavior (Hamid, 2014; Khoyratty et al., 2010; Kyrtatos et al., 2014; Power et al., 2012; Yip et al., 2021). In our study, Dutch-speaking patients were educated by telephone and written information. This way of education does not reach patients who are illiterate or nonnative speakers. Furthermore, information videos arise as new instruments to educate patients (Dawdy et al., 2018; Ong, Miller, Appleby, Allegretto, & Gawlinski, 2009; Veldhuijzen et al., 2021). Therefore, future studies on education strategies should address fasting combined with multimedia approaches in all languages. Furthermore, education empowers patients to participate in the care of their individual health (Marcus, 2014). Our findings confirm that active involvement of patients prevents prolonged fasting. Moreover, it indicates that patient involvement can improve adherence to guidelines. In the future, partnership of patients in preprocedural care can be achieved by addressing their individual level of knowledge and skills to apply fasting behavior (van der Scheer, Garcia, van der Laan, van der Burg, & Boenink, 2017).
To eat and drink exactly until 6 or 2 hours before the procedure may affect the sleep rhythm, especially for morning EGD sessions. Patients in this study demonstrated that drinking 2 hours before fits sufficiently in their rhythm, and a late-night snack before or during bedtime is feasible as well. It was already known that a fasting period of 6 and 2 hours before procedures is safe. This study adds the latest time that patients will likely eat and drink before procedures when they are well informed. Sleep rhythm must be taken into account when determining the final eating and drinking time for procedures that require fasting.
Our study provides knowledge on how patient-related barriers can be addressed and how patients could be instructed to acquire optimal fasting behavior. Other factors complementary to patient-related factors should be addressed as well (Cochrane et al., 2007), including organizational and healthcare staff–related factors (Carey & Hogan, 2021). Organizational barriers that affect prolonged fasting are inflexible procedure programs, planning of procedures, and organizational culture like sticking to old habits. Healthcare staff–related barriers are a lack of guideline knowhow (Abdullah Al Maqbali, 2016; Breuer et al., 2010) and lack of awareness about the impact of prolonged fasting on patients' comfort (Carey & Hogan, 2021; Carey et al., 2015). Especially, nurses have an important role in the communication of fasting instructions to patients, because they are closest to the patient (Carey et al., 2015). Moreover, keeping patients informed, comfortable, well nourished, and hydrated belongs to the fundamentals of nursing care (Dubois et al., 2017; Kitson, Conroy, Wengstrom, Profetto-McGrath, & Robertson-Malt, 2010; Kitson, 2018).
Strengths and Limitations
This study lacks randomization of patients. Randomization would have supported the effectiveness interpretation of the instructions. However, our study demonstrated efficacy meaning whether education can change fasting behavior. This feasibility approach mainly addresses how something can work, not whether it is effective. Effectiveness should be addressed in future randomized studies to determine how fasting education affects other relevant outcomes. Our patients consumed different products that might have influenced gastric visibility. Moreover, we did not correct for prokinetic medication use among the patients. The stomach empties faster from liquids than from solids (Camps, Mars, de Graaf, & Smeets, 2017). Moreover, fatty and fried foods have a longer gastric emptying time than light meals (American Society of Anesthesiologists Committee, 2011). It might have been better to standardize the consumption of food products and use of prokinetics. However, our purpose was to investigate how personal fasting instructions can be applied in real life while maintaining endoscopic quality.
Our study strengthened the growing awareness that reducing fasting times requires modern, multifaceted approaches (Carey & Hogan, 2021; van Noort et al., 2021; Yip et al., 2021). We explored how fasting recommendations can be carried out by patients in daily practice after being educated by nurses with verbal and written instructions. In our study, we intended to mediate as natural as possible enabling patients to adhere to guidelines in a suitable manner while continuing their preferences. This realistic approach strengthens the value of our study. Another strength of our study is that we evaluated the impact of shortened fasting times on gastric visibility. This outcome is relevant for endoscopic purposes. Finally, we established an adequate sample size providing sufficient power for our purposes.
Conclusion
Positive, concrete instructions on fasting and to eat and drink as long as permissible are applicable for patients undergoing EGD. Instructed patients had shorter fasting times while gastric visibility was maintained, and their physical comfort was better. Instructed patients ate and drank as long as possible by fitting it in their daily rhythm of eating and sleeping. The encouragement to keep drinking was easier to apply for most patients than to keep eating. Participation of patients is a precondition to prevent prolonged fasting and to achieve healthy fasting behavior.
Practical Implications
Patients should be involved in reducing length of fasting. Positive, concrete instructions on how patients must apply fasting before EGD will lead to more optimal fasting behavior, maintain gastric visibility and lead to improved patient comfort. Future research should validate the identified factors that influence adherence to fasting recommendations and address organizational and healthcare staff barriers to reduce prolonged fasting. Research should provide further information on the type of nutritional products that can be consumed by patients before preprocedural fasting.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Marianne Zwart, RN, Elja Limbach, RN, Lotte Peters Sengers, RN, and Karin Smit, RN, for their help with data collection.
Footnotes
This study was partially funded by a small grant from the Research Fund of the Hospital Gelderse Vallei, Ede, The Netherlands.
This study was approved by the Medical Ethical Committee of Wageningen University & Research, Wageningen, The Netherlands (CMO number 20/09). All patients provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.gastroenterologynursing.com).
Contributor Information
Harm H. J. van Noort, Email: harm.vannoort@radboudumc.nl.
Carlijn R. Lamers, Email: carlijn.lamers@wur.nl.
Hester Vermeulen, Email: hester.vermeulen@radboudumc.nl.
Getty Huisman-de Waal, Email: getty.huisman-dewaal@radboudumc.nl.
Ben J. M. Witteman, Email: ben.witteman@wur.nl.
REFERENCES
- Abdullah Al Maqbali M. (2016). Preoperative fasting for elective surgery in a regional hospital in Oman. British Journal of Nursing, 25(14), 798–802. doi:10.12968/bjon.2016.25.14.798 [DOI] [PubMed] [Google Scholar]
- American Society of Anesthesiologists Committee. (2011). Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology, 114(3), 495–511. doi:10.1097/ALN.0b013e3181fcbfd9 [DOI] [PubMed] [Google Scholar]
- Asl S. M., Sivandzadeh G. R. (2011). Efficacy of premedication with activated Dimethicone or N-acetylcysteine in improving visibility during upper endoscopy. World Journal of Gastroenterology, 17(37), 4213–4217. doi:10.3748/wjg.v17.i37.4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausserhofer D., Zander B., Busse R., Schubert M., De Geest S., Rafferty A. M., ... RN4CAST Consortium. (2014). Prevalence, patterns and predictors of nursing care left undone in European hospitals: Results from the multicountry cross-sectional RN4CAST study. BMJ Qual Saf, 23(2), 126–135. doi:10.1136/bmjqs-2013-002318 [DOI] [PubMed] [Google Scholar]
- Basford P. J., Brown J., Gadeke L., Fogg C., Haysom-Newport B., Ogollah R., Bhandari P. (2016). A randomized controlled trial of pre-procedure simethicone and N-acetylcysteine to improve mucosal visibility during gastroscopy—NICEVIS. Endosc Int Open, 4(11), E1197–E1202. doi:10.1055/s-0042-117631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker M., Coetzee S. K., Klopper H. C., Ellis S. M. (2015). Non-nursing tasks, nursing tasks left undone and job satisfaction among professional nurses in South African hospitals. Journal of Nursing Management, 23(8), 1115–1125. doi:10.1111/jonm.12261 [DOI] [PubMed] [Google Scholar]
- Bowen D. J., Kreuter M., Spring B., Cofta-Woerpel L., Linnan L., Weiner D., Fernandez M. (2009). How we design feasibility studies. American Journal of Preventive Medicine, 36(5), 452–457. doi:10.1016/j.amepre.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady M., Kinn S., Stuart P. (2003). Preoperative fasting for adults to prevent perioperative complications. Cochrane Database of Systematic Reviews (Online) (4), CD004423. doi:10.1002/14651858.CD004423 [DOI] [PubMed] [Google Scholar]
- Breuer J. P., Bosse G., Seifert S., Prochnow L., Martin J., Schleppers A., Spies C. (2010). Pre-operative fasting: A nationwide survey of German anaesthesia departments. Acta Anaesthesiologica Scandinavica, 54(3), 313–320. doi:10.1111/j.1399-6576.2009.02123.x [DOI] [PubMed] [Google Scholar]
- Callaghan J. L., Neale J. R., Boger P. C., Sampson A. P., Patel P. (2016). Variation in preparation for gastroscopy: Lessons towards safer and better outcomes. Frontline Gastroenterol, 7(3), 187–190. doi:10.1136/flgastro-2015-100647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps G., Mars M., de Graaf C., Smeets P. A. M. (2017). A tale of gastric layering and sieving: Gastric emptying of a liquid meal with water blended in or consumed separately. Physiology & Behavior, 176, 26–30. doi:10.1016/j.physbeh.2017.03.029 [DOI] [PubMed] [Google Scholar]
- Carey S. K., Conchin S., Bloomfield-Stone S. (2015). A qualitative study into the impact of fasting within a large tertiary hospital in Australia—the patients' perspective. Journal of Clinical Nursing, 24(13–14), 1946–1954. doi:10.1111/jocn.12847 [DOI] [PubMed] [Google Scholar]
- Carey S., Hogan S. (2021). Failure in systems and culture: Barriers that prevent implementation of evidence-based fasting times for patients in the acute care setting. JPEN Journal of Parenteral and Enteral Nutrition, 45(5), 933–940. doi:10.1002/jpen.1961 [DOI] [PubMed] [Google Scholar]
- Cestonaro T., Madalozzo Schieferdecker M. E., Thieme R. D., Neto Cardoso J., Ligocki Campos A. C. (2014). The reality of the surgical fasting time in the era of the ERAS protocol. Nutricion Hospitalaria, 29(2), 437–443. doi:10.3305/nh.2014.29.2.7025 [DOI] [PubMed] [Google Scholar]
- Chang C. C., Chen S. H., Lin C. P., Hsieh C. R., Lou H. Y., Suk F. M., Chen Y. F. (2007). Premedication with pronase or N-acetylcysteine improves visibility during gastroendoscopy: An endoscopist-blinded, prospective, randomized study. World Journal of Gastroenterology, 13(3), 444–447. doi:10.3748/wjg.v13.i3.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane L. J., Olson C. A., Murray S., Dupuis M., Tooman T., Hayes S. (2007). Gaps between knowing and doing: Understanding and assessing the barriers to optimal health care. Journal of Continuing Education in the Health Professions, 27(2), 94–102. doi:10.1002/chp.106 [DOI] [PubMed] [Google Scholar]
- Dawdy K., Bonin K., Russell S., Ryzynski A., Harth T., Townsend C., Szumacher E. (2018). Developing and evaluating multimedia patient education tools to better prepare prostate-cancer patients for radiotherapy treatment (randomized study). Journal of Cancer Education, 33(3), 551–556. doi:10.1007/s13187-016-1091-5 [DOI] [PubMed] [Google Scholar]
- de Aguilar-Nascimento J. E., Caporossi C., Metelo J. S., Tanajura G. H., Canevari-de-Oliveira M., da Cunha Costa R. (2014). Safe intake of an oral supplement containing carbohydrates and whey protein shortly before sedation to gastroscopy; a double blind, randomized trial. Nutricion Hospitalaria, 29(3), 681–686. doi:10.3305/nh.2014.29.3.7161 [DOI] [PubMed] [Google Scholar]
- de Aguilar-Nascimento J. E., de Almeida Dias A. L., Dock-Nascimento D. B., Correia M. I., Campos A. C., Portari-Filho P. E., Oliveira S. S. (2014). Actual preoperative fasting time in Brazilian hospitals: The BIGFAST multicenter study. Therapeutics and Clinical Risk Management, 10, 107–112. doi:10.2147/TCRM.S56255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell R. B., Holleran S., Ramakrishnan R. (2002). Sample size determination. Ilar Journal, 43(4), 207–213. doi:10.1093/ilar.43.4.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnoij D. M., Rademakers J. J., Groenewegen P. P. (2010). The Dutch consumer quality index: An example of stakeholder involvement in indicator development. BMC Health Services Research [Electronic Resource], 10, 88. doi:10.1186/1472-6963-10-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois C. A., D'Amour D., Brault I., Dallaire C., Dery J., Duhoux A., Zufferey A. (2017). Which priority indicators to use to evaluate nursing care performance? A discussion paper. Journal of Advanced Nursing, 73(12), 3154–3167. doi:10.1111/jan.13373 [DOI] [PubMed] [Google Scholar]
- El-Sharkawy A. M., Daliya P., Lewis-Lloyd C., Adiamah A., Malcolm F. L., Boyd-Carson H., ... East Midlands Surgical Academic Network. (2020). Fasting and surgery timing (FaST) audit. Clinical Nutrition, 40(3), 1405–1412. doi:10.1016/j.clnu.2020.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy A. M., Daliya P., Lewis-Lloyd C., Adiamah A., Malcolm F. L., Boyd-Carson H., ... East Midlands Surgical Academic Network. (2021). Fasting and surgery timing (FaST) audit. Clinical Nutrition, 40(3), 1405–1412. doi:10.1016/j.clnu.2020.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria M. S., de Aguilar-Nascimento J. E., Pimenta O. S., Alvarenga L. C., Jr., Dock-Nascimento D. B., Slhessarenko N. (2009). Preoperative fasting of 2 hours minimizes insulin resistance and organic response to trauma after video-cholecystectomy: A randomized, controlled, clinical trial. World Journal of Surgery, 33(6), 1158–1164. doi:10.1007/s00268-009-0010-x [DOI] [PubMed] [Google Scholar]
- Furrer L., Ganter M. T., Klaghofer R., Zollinger A., Hofer C. K. (2006). [Preoperative fasting times: patients' perspective]. Anaesthesist, 55(6), 643–649. doi:10.1007/s00101-006-0991-x [DOI] [PubMed] [Google Scholar]
- Gul A., Andsoy I. I., Ozkaya B. (2018). Preoperative fasting and patients' discomfort. Indian Journal of Surgery, 80(6), 549–553. doi:10.1007/s12262-017-1657-4 [Google Scholar]
- Hamid S. (2014). Pre-operative fasting—a patient centered approach. BMJ Quality Improvement Reports, 2(2), u605.w1252. doi:10.1136/bmjquality.u605.w1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyland D. K., Cahill N. E., Dhaliwal R. (2010). Lost in (knowledge) translation! JPEN Journal of Parenteral and Enteral Nutrition, 34(6), 610–615. doi:10.1177/0148607110361909 [DOI] [PubMed] [Google Scholar]
- Hsieh H. F., Shannon S. E. (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. doi:10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- Hydes T., Yusuf A., Pearl D. S., Trebble T. M. (2011). A survey of patients' attitudes to upper gastrointestinal endoscopy identifies the value of endoscopist-patient interactive factors. Frontline Gastroenterol, 2(4), 242–248. doi:10.1136/fg.2011.004325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingadottir B., Olafsdottir A. M., Sveinsdottir H., Asmundsdottir L. B., Asgeirsdottir L., Torp M. S., Hafsteinsdottir E. J. (2016). [Preoperative fasting: Instructions to patients and length of fasting—a prospective, descriptive survey]. Laeknabladid, 102(6), 283–288. doi:10.17992/lbl.2016.06.86 [DOI] [PubMed] [Google Scholar]
- Itou K., Fukuyama T., Sasabuchi Y., Yasuda H., Suzuki N., Hinenoya H., Suzuki T. (2012). Safety and efficacy of oral rehydration therapy until 2 h before surgery: A multicenter randomized controlled trial. Journal of Anesthesia, 26(1), 20–27. doi:10.1007/s00540-011-1261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoyratty S., Modi B. N., Ravichandran D. (2010). Preoperative starvation in elective general surgery. J Perioper Pract, 20(3), 100–102. doi:10.1177/175045891002000302 [DOI] [PubMed] [Google Scholar]
- Kitson A., Conroy T., Wengstrom Y., Profetto-McGrath J., Robertson-Malt S. (2010). Defining the fundamentals of care. International Journal of Nursing Practice, 16(4), 423–434. doi:10.1111/j.1440-172X.2010.01861.x [DOI] [PubMed] [Google Scholar]
- Kitson A. L. (2018). The fundamentals of care framework as a point-of-care nursing theory. Nursing Research, 67(2), 99–107. doi:10.1097/NNR.0000000000000271 [DOI] [PubMed] [Google Scholar]
- Ko H. H., Zhang H., Telford J. J., Enns R. (2009). Factors influencing patient satisfaction when undergoing endoscopic procedures. Gastrointestinal Endoscopy, 69(4), 883–891, quiz 891.e881. doi:10.1016/j.gie.2008.06.024 [DOI] [PubMed] [Google Scholar]
- Koeppe A. T., Lubini M., Bonadeo N. M., Moraes I., Jr., Fornari F. (2013). Comfort, safety and quality of upper gastrointestinal endoscopy after 2 hours fasting: A randomized controlled trial. BMC Gastroenterology [Electronic Resource], 13, 158. doi:10.1186/1471-230X-13-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. H., Sheu B. S., Kao A. W., Wu C. H., Chuang C. H. (2002). A defoaming agent should be used with pronase premedication to improve visibility in upper gastrointestinal endoscopy. Endoscopy, 34(7), 531–534. doi:10.1055/s-2002-33220 [DOI] [PubMed] [Google Scholar]
- Kyrtatos P. G., Constandinou N., Loizides S., Mumtaz T. (2014). Improved patient education facilitates adherence to preoperative fasting guidelines. Journal of Perioperative Practice, 24(10), 228–231. doi:10.1177/175045891402401003 [DOI] [PubMed] [Google Scholar]
- Lamacraft G., Labuschagne C., Pretorius S., Prinsloo M. C., Smit M. D., Steyn J. R. (2017). Preoperative fasting times: Prescribed and actual fasting times at Universitas Hospital Annex, Bloemfontein, South Africa. South African Medical Journal, 107(10), 910–914. doi:10.7196/SAMJ.2017.v107i10.10930 [DOI] [PubMed] [Google Scholar]
- Marcus C. (2014). Strategies for improving the quality of verbal patient and family education: A review of the literature and creation of the EDUCATE model. Health Psychology and Behavioral Medicine, 2(1), 482–495. doi:10.1080/21642850.2014.900450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monrroy H., Vargas J. I., Glasinovic E., Candia R., Azua E., Galvez C., Parra-Blanco A. (2018). Use of N-acetylcysteine plus simethicone to improve mucosal visibility during upper GI endoscopy: A double-blind, randomized controlled trial. Gastrointestinal Endoscopy, 87(4), 986–993. doi:10.1016/j.gie.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Ong J., Miller P. S., Appleby R., Allegretto R., Gawlinski A. (2009). Effect of a preoperative instructional digital video disc on patient knowledge and preparedness for engaging in postoperative care activities. Nursing Clinics of North America, 44(1), 103–115, xii. doi:10.1016/j.cnur.2008.10.014 [DOI] [PubMed] [Google Scholar]
- Power S., Kavanagh D. O., McConnell G., Cronin K., Corish C., Leonard M., Connolly J. (2012). Reducing preoperative fasting in elective adult surgical patients: A case-control study. Irish Journal of Medical Science, 181(1), 99–104. doi:10.1007/s11845-011-0765-6 [DOI] [PubMed] [Google Scholar]
- Qureshi S. M., Purdy N., Mohani A., Neumann W. P. (2019). Predicting the effect of nurse-patient ratio on nurse workload and care quality using discrete event simulation. Journal of Nursing Management, 27(5), 971–980. doi:10.1111/jonm.12757 [DOI] [PubMed] [Google Scholar]
- Salman O. H., Asida S. M., Ali H. S. (2013). Current knowledge, practice and attitude of preoperative fasting: A limited survey among Upper Egypt anesthetists. Egyptian Journal of Anaesthesia, 29(2), 125–130. doi:10.1016/j.egja.2012.10.007 [Google Scholar]
- Sankar A., Johnson S. R., Beattie W. S., Tait G., Wijeysundera D. N. (2014). Reliability of the American Society of Anesthesiologists physical status scale in clinical practice. British Journal of Anaesthesia, 113(3), 424–432. doi:10.1093/bja/aeu100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I., Kranke P., Murat I., Smith A., O'Sullivan G., Soreide E., ... European Society of Anaesthesiology. (2011). Perioperative fasting in adults and children: Guidelines from the European Society of Anaesthesiology. European Journal of Anaesthesiology, 28(8), 556–569. doi:10.1097/EJA.0b013e3283495ba1 [DOI] [PubMed] [Google Scholar]
- Spahn T. W., Wessels A., Grosse-Thie W., Mueller M. K. (2009). Assessment of pre-gastroscopy fasting period using ultrasonography. Digestive Diseases and Sciences, 54(3), 621–626. doi:10.1007/s10620-008-0394-8 [DOI] [PubMed] [Google Scholar]
- Tosun B., Yava A., Acikel C. (2015). Evaluating the effects of preoperative fasting and fluid limitation. International Journal of Nursing Practice, 21(2), 156–165. doi:10.1111/ijn.12239 [DOI] [PubMed] [Google Scholar]
- van der Scheer L., Garcia E., van der Laan A. L., van der Burg S., Boenink M. (2017). The benefits of patient involvement for translational research. Health Care Analysis, 25(3), 225–241. doi:10.1007/s10728-014-0289-0 [DOI] [PubMed] [Google Scholar]
- van Noort H. H. J., Eskes A. M., Vermeulen H., Besselink M. G., Moeling M., Ubbink D. T., Witteman B. J. M. (2021). Fasting habits over a 10-year period: An observational study on adherence to preoperative fasting and postoperative restoration of oral intake in 2 Dutch hospitals. Surgery, 170(2), 532–540. doi:10.1016/j.surg.2021.01.037 [DOI] [PubMed] [Google Scholar]
- Veldhuijzen G., Klemt-Kropp M., Terhaar Sive Droste J. S., van Balkom B., van Esch A. A. J., Drenth J. P. H. (2021). Computer-based patient education is non-inferior to nurse counselling prior to colonoscopy: A multicenter randomized controlled trial. Endoscopy, 53(3), 254–263. doi:10.1055/a-1225-8708 [DOI] [PubMed] [Google Scholar]
- von Elm E., Altman D. G., Egger M., Pocock S. J., Gotzsche P. C., Vandenbroucke J. P., Initiative S. (2014). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. International Journal of Surgery, 12(12), 1495–1499. doi:10.1016/j.ijsu.2014.07.01325046131 [Google Scholar]
- World Medical Association. (2013). World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA, 310(20), 2191–2194. doi:10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- Yeniay O., Tekgul Z. T., Okur O., Koroglu N. (2019). Unexpectedly prolonged fasting and its consequences on elderly patients undergoing spinal anesthetics. A prospective observational study 1. Acta Cirurgica Brasileira, 34(3), e201900309. doi:10.1590/s0102-865020190030000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip A., Hogan S., Carey S. (2021). Interventions aimed at reducing fasting times in acute hospital patients: A systematic literature review. Nutrition in Clinical Practice, 36(1), 133–152. doi:10.1002/ncp.10579 [DOI] [PubMed] [Google Scholar]