BACKGROUND:
Deep brain stimulation (DBS) is an effective therapy in advanced Parkinson disease (PD). Although both subthalamic nucleus (STN) and globus pallidus (GP) DBS show equivalent efficacy in PD, combined stimulation may demonstrate synergism.
OBJECTIVE:
To evaluate the clinical benefit of stimulating a combination of STN and GP DBS leads and to demonstrate biomarker discovery for adaptive DBS therapy in an observational study.
METHODS:
We performed a pilot trial (n = 3) of implanting bilateral STN and GP DBS leads, connected to a bidirectional implantable pulse generator (Medtronic Summit RC + S; NCT03815656, IDE No. G180280). Initial 1-year outcome in 3 patients included Unified PD Rating Scale on and off medications, medication dosage, Hauser diary, and recorded beta frequency spectral power.
RESULTS:
Combined DBS improved PD symptom control, allowing >80% levodopa medication reduction. There was a greater decrease in off-medication motor Unified PD Rating Scale with multiple electrodes activated (mean difference from off stimulation off medications −18.2, range −25.5 to −12.5) than either STN (−12.8, range −20.5 to 0) or GP alone (−9, range −11.5 to −4.5). Combined DBS resulted in a greater reduction of beta oscillations in STN in 5/6 hemispheres than either site alone. Adverse events occurred in 2 patients, including a small cortical hemorrhage and seizure at 24 hours postoperatively, which resolved spontaneously, and extension wire scarring requiring revision at 2 months postoperatively.
CONCLUSION:
Patients with PD preferred combined DBS stimulation in this preliminary cohort. Future studies will address efficacy of adaptive DBS as we further define biomarkers and control policy.
KEY WORDS: Parkinson disease, Subthalamic nucleus, Globus pallidus, Summit RC + S
ABBREVIATIONS:
- aDBS
daptive DBS
- BDI-II
Beck Depression Inventory-II
- CTM
communications telemetry module
- GP
globus pallidus
- GPe
globus pallidus pars externa
- Gpi
globus pallidus pars interna
- IPG
implantable pulse generator
- LEDD
levodopa equivalent daily dosing
- LFP
local field potential
- MCP
midcommissural point
- MoCA
Montreal cognitive assessment
- PD
Parkinson Disease
- PDQ-39
Parkinson Disease Questionnaire-39
- SF-36
RAND 36-Item Short Form Health Survey
- STN
subthalamic nucleus
- TUG
timed up and go
- UPDRS
Unified Parkinson Disease Rating Scale
- ZI
zona incerta.
Deep brain stimulation (DBS) is a well-established symptomatic therapy for advanced Parkinson disease (PD), improving tremor and motor fluctuations by increasing “on” time without dyskinesia.1-5 Clinical trials have demonstrated that subthalamic nucleus (STN) and globus pallidus pars interna (GPi) produce equivalent symptom relief.4,6-8 Dual GPi and STN implantation has shown a synergistic effect on symptoms and medication reduction.9 Multiple-target stimulation has been reported in complex movement disorders,10 tremor,11 and obsessive-compulsive disorder.12 Adding GPi leads may reduce dyskinesia noted with STN stimulation alone,13,14 and additional thalamic leads may help refractory tremor.15
Local field potential (LFP) biomarkers correlate with PD symptoms and therapeutic response to DBS and levodopa administration.16-18 Because STN and globus pallidus (GP) display coherent LFP oscillations in PD,19 dual leads to stimulate and sense simultaneously between these 2 structures may lead to improved biomarkers to develop adaptive DBS therapy (aDBS).20-23 Investigational, bidirectional implantable pulse generators (IPGs) are facilitating this DBS paradigm shift, including the PC + S and RC + S systems (Medtronic).
We present prospective, 1-year data from 3 patients with bilateral STN and GP leads connected to RC + S in a multiyear clinical trial (ClinicalTrials.gov No. NCT03815656) with aims of assessing clinical benefit, discovering biomarkers, and developing adaptive DBS. This is the first report of chronic sensing and stimulation from dual bilateral GP and STN DBS.
METHODS
Enrollment Criteria
This study was conducted under Duke University Institutional Review Board, Medtronic Clinical Board, and Food and Drug Administration Investigational Device Exemption approval, and subjects signed written informed consent. Inclusion criteria included diagnosis of PD, disease duration >4 years, age 75 years or younger, levodopa responsiveness with at least 30% improvement on Unified PD Rating Scale (UPDRS) part 3, and motor fluctuations or side effects (ie, dyskinesias and on/off fluctuations) despite medication optimization. Exclusion criteria included clinical diagnosis of dementia, features of atypical Parkinsonism, Montreal Cognitive Assessment (MoCA) score of less than 26, and high-risk medical comorbidities. This report focuses on the first 3 patients in this trial, who have completed 1-year outcome measurements.
Neurostimulator Placement
Stereotaxy planning was performed with 3T MRI 1-mm slice images on StealthStation (Medtronic), including T1 with contrast and fluid-attenuated inversion recovery MRI sequences. STN and GP were targeted by direct visualization on MRI. Brain trajectories were chosen to avoid sulci or vessels. After Leksell headframe was applied, intraoperative head computed tomography (CT) with Airo system was performed (Brainlab) and then merged to the MRI to determine frame coordinates. Microelectrode recording was performed to identify targets using Neuro Omega system (AlphaOmega).
After physiological identification, STN leads (Medtronic 3389) were placed with the distal 3 contacts across the span of STN and the proximal contact in zona incerta (ZI). GP leads (Medtronic 3387) were placed with the distal 2 contacts in GPi and the proximal 2 contacts in globus pallidus pars externa (GPe) after identifying the physiological border between GPe and GPi with microelectrode recordings. Placement of 2 contacts in GPe was planned to facilitate evoked potential recordings between STN and GPe.23 Test stimulation was performed for each target to assess symptomatic response and side effect thresholds. Postoperative CT was performed to verify lead placement. Leads were implanted in one surgical procedure for each patient; all leads were connected to a single Summit RC + S IPG 2 weeks later using 2 “forked” extensions to allow all 16 channels to be directed into one IPG.
DBS Programming
After IPG implantation, each subject underwent DBS programming with monopolar review of bilateral STN/ZI leads after withholding dopaminergic medications overnight. Dopaminergic medications were adjusted per neurologist discretion based on clinical response and subject feedback. Two weeks later, each subject returned for bilateral GP programming. Two weeks after that, each subject underwent dual lead programming. Additional visits were performed as needed for adjustments. Subjects were given amplitude control parameters and were instructed to toggle between the 3 stimulation groups (STN/ZI alone, GP alone, and STN + GP) for 1 to 2 weeks before switching to determine which group provided the most subjective benefit.
Clinical Assessments
Preoperative assessments included MoCA, Hauser Diary, RAND 36-Item Short Form Health Survey (SF-36), Beck Depression Inventory-II (BDI-II), levodopa equivalent daily dosing (LEDD), PD Questionnaire-39 (PDQ-39), UPDRS, and timed up and go (TUG). At 1 year postoperatively, UPDRS and TUG were performed in both the off and on medication states for each of 4 conditions: stimulation off, STN/ZI stimulation, GP stimulation, and combined STN/ZI plus GP stimulation. After subjects withheld dopaminergic medications for >12 hours, each condition was tested in a blinded, random order, with scales after 5 minutes of stimulation “wash in,” and then a new condition started after 10 minutes with no stimulation. After the 4 off-medication conditions were measured, the subjects took their morning dose of medication and trials were repeated after 30 to 60 minutes.
Biomarker Recording
LFPs were collected from the participants during research visits separate from the 1-year follow-up, with 1 participant (participant 2) off PD medications. Stimulation occurred in blocks of trials of STN, GP, or STN + GP DBS. Each trial was 60 or 300 seconds long, with a trial of DBS off for the same duration immediately preceding each DBS on trial. Variable medication status was normalized by the preceding trial without stimulation. Data were streamed to a Windows laptop running custom implementation of the Summit RC + S toolkit (Medtronic) developed in Visual Studio (Microsoft). For artifact rejection, differential recordings were performed with the 2 sensing contacts surrounding the best monopolar stimulation contact. LFPs were sampled at 500 or 1000 Hz per lead. Because participant 2 was unable to sit comfortably under sensing-compatible GPi DBS alone (because of residual tremor in the required monopolar mode), we did not collect LFP under GP DBS (Figure 1).
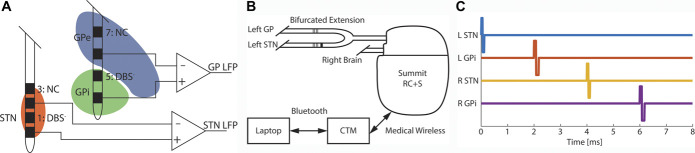
FIGURE 1.
Recording contact locations with the Summit RC + S. A, Leads were implanted such that contacts 1, 5, 9, and 13 (the second contact of each lead) were optimally situated for monopolar stimulation. Differential LFP data were recorded between contacts 0 and 2. In the GP, the lower sensing contact was in the GPi while the upper sensing contact was in GPe resulting in LFP across the regions. B, Data were transmitted from the Summit RC + S to a CTM over a short-range, proprietary link. The CTM then connected to a research laptop over Bluetooth. Conversely, control signals (eg, stimulation parameter changes) were sent from the laptop through the CTM to the IPG. C, 125-Hz STN + GPi DBS pulse timing. Pulses were delivered in each region every 8 ms with 2 ms between delivery in different regions with STN pulses leading the GPi of the same hemisphere. CTM, communications telemetry module; DBS, deep brain stimulation; GP, globus pallidus; Gpi, globus pallidus pars interna; IPG, implantable pulse generator; LFP, local field potential (differential sensing between the two contacts adjacent to the DBS electrode); NC, contact not used; STN, subthalamic nucleus.
Analysis
Primary clinical outcome was change in total UPDRS in each stimulation condition compared with off stimulation, while off medication. Secondary outcomes included change in on/off medication and stimulation TUG in each stimulation condition and change in individual subscales of the UPDRS (I, II, III, and IV), Hauser diary, SF-36, BDI-II, PDQ-39, MoCA, and LEDD at 1 year. UPDRS was rated both preoperatively and at 1 year by one unblinded in-person rater (KM) and 2 blinded video raters (BS, SM). Blinded video ratings did not include rigidity assessments. Subjects were blinded to stimulation condition to reduce bias. We present descriptive analyses of 1-year outcomes in the first 3 patients enrolled, with mean and range values. The mean change in off-medication total UPDRS score in each stimulation condition was compared with off stimulation.
LFP data were analyzed using custom scripts in MATLAB 2020b (MathWorks). Power spectral densities were calculated with the pwelch() command using the last 40 seconds of each recording. We then calculated the power in the beta band (13-30 Hz) using a windowed fast Fourier transform (1-second windowing). On starting DBS stimulation, large transients were observed in the recordings for ∼1 to 2 seconds; therefore, this period was excluded from calculations of beta power. Change in beta power was determined by subtracting median beta power during the preceding DBS off trials from that during each type of DBS. DBS off/on trial pairs were of the same duration. Significant reductions in power were determined by comparing all beta values for each type of DBS and its preceding DBS off trials, using Wilcoxon ranked-sum tests, rejecting the null hypothesis at P < .05. We treated the STN and GP of the right and left hemispheres independently and reported the response to 3 different types (STN, GPi, and STN + GPi) of DBS, resulting in a maximum of 18 possible pairings of hemisphere*DBS contact. However, because monopolar GPi stimulation was not attempted with participant 2, there were only 16 hemisphere*DBS contact pairs.
RESULTS
Lead Locations and Programming
Leads were confirmed to be in expected nuclei based on postoperative CT merged with preoperative planning MRI (Figure 2). Final lead locations for all patients in relation to midcommissural point and optimized programming settings at 1-year follow-up are detailed in Table.
FIGURE 2.
Postoperative lead localization example. Lead locations are shown in A, oblique and B, axial 3-dimensional projections from a representative subject. Reconstructions were created in lead deep brain stimulation using available Montreal Neurological Institute (MNI)-space subcortical atlases.26,27 Globus pallidus externa (blue), globus pallidus interna (green), subthalamic nucleus (orange), and red nucleus (maroon) are highlighted as volumes, and active contacts are shaded in red.
TABLE.
Lead Locations and Dual Stimulation Programming Settings at 1 Year
| Subject no. | Coordinates of deepest active contact (relative to MCP) | STN lead stim settings | GP lead stim settings | |
|---|---|---|---|---|
| STN | GP | |||
| 1 | L: X −1*0.33, Y −3.13, Z −5.76 R: 10.73, Y −2.52, Z −4.59 |
L: X −22.16, Y 5.39, Z −2.64 R: 21.62, Y 2.12, Z −3.81 |
L: C+1−, 2.3 mA, 60 µs, 125 Hz R: C+9−, 1.8 mA, 60 µs, 125 Hz |
L: C+5−, 2.5 mA, 90 µs, 125 Hz R: C+13−, 1.6 mA, 90 µs, 125 Hz |
| 2 | L: X −9.45, Y 1.91, Z −1.76 R: X 9.64, Y 0.31, Z −3.42 |
L: X 22.69, Y 3.02, Z −1.31 (not active at 1 y but later used with benefit) R: X 21.34, Y 1.15, Z −5.48 |
L: C+1−, 2.6 mA, 60 µs, 125 Hz R: C+11−, 1.1 mA, 60 µs, 125 Hz |
L: OFF R: 12−13+, 2.5 mA, 90 µs, 125 Hz |
| 3 | L: X −11.21, Y −3.34, Z −5.91 R: X 10.03, Y −3.23, Z −5.60 |
L: −22.57, Y 7.48, Z 1.09 R: X 21.56, Y 8.96, Z −2.54 |
L: C+1−, 2.5 mA, 60 µs, 125 Hz R: C+9−, 3.1 mA, 60 µs, 125 Hz |
L: C+5−, 1.5 mA, 60 µs, 125 Hz R: C+14−, 3.1 mA, 90 µs, 125 Hz |
GP, globus pallidus; MCP, midcommissural point; STN, subthalamic nucleus.
X = lateral, Y = posterior, Z = depth compared with MCP. Left STN contacts are labeled (ventral to dorsal) 0-3, right STN contacts 8-11, left GP contacts 4-7, and right GP contacts 12-15.
Participant Demographics and Stimulation Preferences
The 3 subjects enrolled from 2018 to 2019 and had 1-year assessments from 2020 to 2021. They ranged from age 55 to 65 years (mean age 60.3 years; 2 male and 1 female), and the range of disease duration was 8 to 11 years. The mean (range) blinded preoperative total UPDRS was 66.8 (55-87) off medication and was 39.2 (25-66) on medication. After programming optimization, subjects were allowed to choose between STN only, GP only, and combined stimulation. All 3 subjects preferred combined stimulation for chronic therapy based on subjective superiority for treating motor symptoms.
Primary Outcomes
Mean (range) total UPDRS at 1 year (blinded ratings with exception of rigidity) when off medication improved from 50 (39-62.5) off stimulation to 37.2 (30.5-42) STN stimulation alone, 41 (34.5-51) GP stimulation alone, and 31.8 (26.5-37) STN + GP stimulation. Greater improvements in STN + GP were seen with unblinded rigidity scores removed from mean 41 (range 33-49.5) off stimulation to 34.8 (28.5-38) STN alone, 36.7 (32.5-43) GP alone, and 30.5 (26.5-354) STN + GP. The mean difference in motor UPDRS III score from off stimulation off medication at 1 year to STN + GP was −18.2 (−25.5 to −12.5), which showed greater improvement than STN alone (−12.8; −20.5 to 0) and GP alone (−9; −11.5 to −4.5). Participant 1 developed dyskinesia with STN stimulation alone and improved with combined stimulation.
Secondary Outcomes
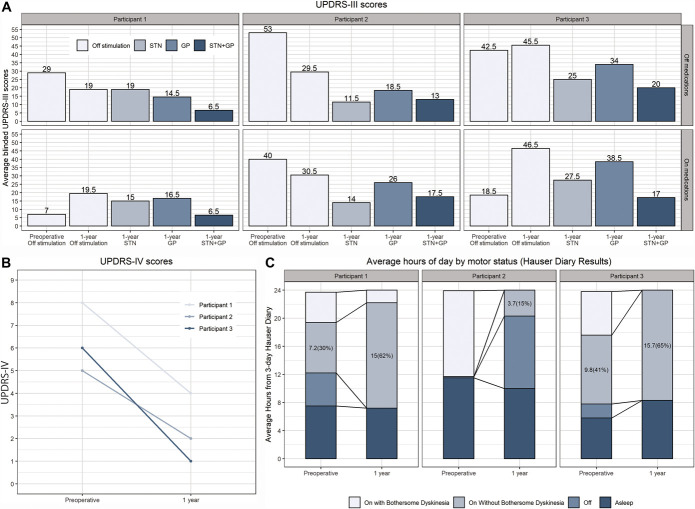
Improvement in motor scores for STN only and GP only was variable, but there was more robust improvement with combined stimulation (Figure 3A). Motor score and response to stimulation were similar in the off medication and on medication states at 1 year (Figure 3A). All 3 participants experienced >80% reduction in LEDD from a mean (range) baseline level of 835 mg (456-1450 mg) to 100 mg (0-200 mg) at 1 year. Complications of therapy (UPDRS IV) were improved in all 3 participants (Figure 3B), as was percentage of waking on time without troublesome dyskinesia (3-day Hauser diary), which improved by a mean of 5.8 hours per day [from 33% (0%-54.6%) to 71.8% (26.2%-100%)] (Figure 3C). Additional secondary outcomes included minor improvement in TUG on dual stimulation on medications (1.3 [2.8 to −0.4] seconds) compared with off stimulation and inconsistent changes in MoCA, PDQ-39, and BDI-II compared with baseline. SF-36 data also revealed inconsistent changes, with improvement in physical function, energy/fatigue, social functioning, and perceived change in health but worsening in role limitations because of physical health problems or emotional problems, emotional well-being, and bodily pain.
FIGURE 3.
Primary and secondary outcomes on line charts. A, Change in UPDRS III off and on medications at each time point and stimulation condition (at 1 year, the patients were briefly off medication for the testing). Baseline preoperative data are shown for comparison with 1-year outcomes. B, Change in UPDRS IV (complications of therapy) at 1 year compared with preoperative. C, Change in hours per day in each physical state per Hauser diary results. GP, globus pallidus; STN, subthalamic nucleus; UPDRS, Unified Parkinson Disease Rating Scale.
LFP Recordings
We recorded LFPs from the participants with either DBS off or during stimulation of the STN, GPi, or STN + GPi. We provide an example power spectral density over 40 seconds of DBS off and STN + GPi DBS (Figure 4A). We then calculated beta power (13-30 Hz) in 1-second windows. Median beta power in the STN was reduced in 15 of 16 hemisphere*DBS contact pairs while beta power in the GP was reduced in 9 of 16 hemisphere*DBS contact pairs (Figure 4B, Wilcoxon signed-rank P < .05). There was a trend for combined STN + GPi DBS to show a greater reduction in STN beta power than STN DBS alone in 5 of 6 STNs.
FIGURE 4.
Combined STN + GPi DBS reduced beta power. A, Power spectral densities of LFP of the left STN from participant 3 without DBS (black) during a 40-second period and with combined STN + GPi DBS (blue). B, Change in median 1-second beta power between DBS off and each type of DBS. Several types of DBS stimulation-evoked artifacts in the beta range caused an increase in estimated beta power. Such stimulation artifacts resulted in a >20 dB increase of beta power in participant 3's GP LFP whenever the GPi contact was used in stimulation and are therefore not displayed. DPS, deep brain stimulation; GP, globus pallidus; Gpi, globus pallidus pars interna; LFP, local field potential; STN, subthalamic nucleus
Adverse Events
Adverse events for the 3 participants included one small, delayed (24 hours) postoperative cortical hemorrhage (<1 cm diameter) at the lead entry point. This small hemorrhage was not present on intraoperative or postoperative CT scans within 12 hours but present after an isolated generalized seizure at 24 hours postoperatively. This subject was placed on levetiracetam for 3 months without seizure recurrence. A postoperative scan at 2 weeks showed resolution of the hemorrhage. Another subject developed tightness of extensions in the neck at 6 weeks postoperatively (related to the bulky, dual 40-cm forked extensions needed to connect 4 leads to a single Summit RC + S IPG), fully treated by extension revision surgery to longer (60 cm) extensions. This subject also developed postoperative, unilateral leg dyskinesia off all PD medications, which resolved after 2 weeks. There were no other study-related adverse events in these 3 participants, and these were reported to the study monitoring committee, Duke Institutional Review Board, Food and Drug Administration, and Medtronic.
DISCUSSION
This prospective study of combined STN/ZI and GP stimulation revealed improvement in motor disability scales both off and on medications compared with preoperative baseline measurements and further improvement compared with single-site STN or GP stimulation. Participants experienced dramatically reduced dopaminergic medication requirements. Two adverse events occurred, one requiring surgical correction, but with no long-term sequelae and no effect on stimulation benefit. There may be additional risk for dual-site DBS, as stated in the protocol and consent.9
Quality-of-life measures did not consistently change in this small cohort despite the motor improvement. Note that this time period spanned the COVID-19 social isolation duration, and we speculate that a dissociation between general quality-of-life measures and objective motor measures may be related to confounding medical and social issues. Although there was a significant reduction in daily levodopa, it is unclear whether this had any relation to quality-of-life measures. Levodopa was reduced to improve side effects including dyskinesia (2 participants) and fatigue (1) as well as lack of perceived need for more levodopa for a wide range of symptom treatment (all; ie, few DBS unresponsive symptoms were present with the lowered dose). We do not have enough information from this small cohort to draw conclusions about dual lead DBS and quality of life.
Dual-site LFP recordings confirm that beta frequency oscillations may provide a suitable biomarker for development of adaptive stimulation. STN beta band recordings during dual-site stimulation, in particular, showed the largest reduction. This greater beta activity reduction with dual stimulation may strengthen the use of this biomarker in development of adaptive DBS algorithms by facilitating reliable use of beta thresholds when ramping up or down stimulation. Furthermore, reductions in beta power during tremor were cited as a limitation for this biomarker 24,25; however, dual basal ganglia recordings of beta activity showed potential utility despite all 3 of the participants having measurable tremor. We continue to develop adaptive protocols with additional biomarkers, incorporating both neural and wearable data.
Limitations
This study has limitations, including most notably a small sample size which may affect generalizability of the results. Furthermore, given the goal of designing adaptive DBS, the near-complete resolution in motor fluctuations as seen on the Hauser diary and the significant reduction in medications may paradoxically limit the ability to study adaptive DBS, particularly in the context of fluctuations. aDBS carries a primary aim of titrating DBS to the amount needed based on motor fluctuations and concurrent medications. This study is strengthened by the blinding of both participants and video raters to stimulation condition on the 1-year outcome assessment and biomarker exploration across many stimulation conditions for the development of aDBS.
CONCLUSION
Dual-site DBS with a chronically implanted bidirectional device is feasible and effective for the treatment of motor symptoms of PD. Biomarker discovery from novel multiple basal ganglia chronic recordings shows promise for the development of adaptive DBS.
Acknowledgments
We would like to acknowledge considerable help and advice from Drs R. Raike and B. Isaacson at Medtronic.
Footnotes
Presented as an abstract/poster at Brain Initiative Investigators Meeting, June 15 to 17, 2021, virtual (based in Washington, DC). Sponsoring Society: NIH Brain Initiative.
Funding
This study was supported by National Institutes of Health (NIH) UH3 NS103468 and Medtronic (for providing investigational research devices under FDA IDE G180280), registered with ClinicalTrials.gov NCT03815656. Dr Cooney has received institutional (grant) support from the National Institutes of Health and the Michael J. Fox Foundation. Dr Mantri has received institutional support from the Michael J. Fox Foundation. Dr Scott receives grants/payments from Deep Brain Innovations, Inc.
Disclosures
Dr Mitchell will be serving as a site principal investigator for a Medtronic sponsored clinical trial and a trial with Deep Brain Innovations. He has done consulting for Rune Labs and Boston Scientific. Dr Cooney has received honoraria from Abbott, AbbVie, Acadia, Accorda, Amneal, Medtronic, and the Parkinson's Foundation. Dr Mantri is contracted with Grey Matter Technologies Inc and Deep Brain Innovations LLC. Dr Scott is a site Principal Investigator for clinical trials in Parkinson's disease and Huntington's disease sponsored by Biogen, Biohaven, Neurocrine, Annexon, CHDI Foundation, and Prilenia. He is on the Board of Directors of HD Reach and a member of the Safety Monitoring Board for a clinical trial in Parkinson's disease sponsored by Addex.
REFERENCES
- 1.Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013;368(7):610-622. [DOI] [PubMed] [Google Scholar]
- 2.Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010;9(6):581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odekerken VJ, Boel JA, Schmand BA, et al. GPi vs STN deep brain stimulation for Parkinson disease: three-year follow-up. Neurology. 2016;86(8):755-761. [DOI] [PubMed] [Google Scholar]
- 5.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355(9):896-908. [DOI] [PubMed] [Google Scholar]
- 6.Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. 2010;362(22):2077-2091. [DOI] [PubMed] [Google Scholar]
- 7.Okun MS, Fernandez HH, Wu SS, et al. Cognition and mood in Parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65(5):586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. 2012;79(1):55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzone P, Brown P, Dilazzaro V, et al. Bilateral implantation in globus pallidus internus and in subthalamic nucleus in Parkinson's disease. Neuromodulation. 2005;8(1):1-6. [DOI] [PubMed] [Google Scholar]
- 10.Parker T, Raghu ALB, FitzGerald JJ, Green AL, Aziz TZ. Multitarget deep brain stimulation for clinically complex movement disorders. J Neurosurg. Published online ahead of print January 3, 2020. DOI: 10.3171/2019.11.JNS192224. [DOI] [PubMed] [Google Scholar]
- 11.Oliveria SF, Rodriguez RL, Bowers D, et al. Safety and efficacy of dual-lead thalamic deep brain stimulation for patients with treatment-refractory multiple sclerosis tremor: a single-centre, randomised, single-blind, pilot trial. Lancet Neurol. 2017;16(9):691-700. [DOI] [PubMed] [Google Scholar]
- 12.Welter ML, Alves Dos Santos JF, Clair AH, et al. Deep brain stimulation of the subthalamic, accumbens, or caudate nuclei for patients with severe obsessive-compulsive disorder: a randomized crossover controlled study. Biol Psychiatry. 2020;90(10):e45-e47. [DOI] [PubMed] [Google Scholar]
- 13.Matias CM, Silva D, Machado AG, Cooper SE. “Rescue” of bilateral subthalamic stimulation by bilateral pallidal stimulation: case report. J Neurosurg. 2016;124(2):417-421. [DOI] [PubMed] [Google Scholar]
- 14.Cook RJ, Jones L, Fracchia G, et al. Globus pallidus internus deep brain stimulation as rescue therapy for refractory dyskinesias following effective subthalamic nucleus stimulation. Stereotact Funct Neurosurg. 2015;93(1):25-29. [DOI] [PubMed] [Google Scholar]
- 15.Azghadi A, Rajagopal MM, Atkinson KA, Holloway KL. Utility of GPI+VIM dual-lead deep brain stimulation for Parkinson's disease patients with significant residual tremor on medication. J Neurosurg. Published online ahead of print October 1, 2021. DOI: 10.3171/2021.4.JNS21502. [DOI] [PubMed] [Google Scholar]
- 16.Kühn AA, Kempf F, Brücke C, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci. 2008;28(24):6165-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kühn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci. 2006;23(7):1956-1960. [DOI] [PubMed] [Google Scholar]
- 18.Trager MH, Koop MM, Velisar A, et al. Subthalamic beta oscillations are attenuated after withdrawal of chronic high frequency neurostimulation in Parkinson's disease. Neurobiol Dis. 2016;96:22-30. [DOI] [PubMed] [Google Scholar]
- 19.Brown P, Mazzone P, Oliviero A, et al. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson's disease. Exp Neurol. 2004;188(2):480-490. [DOI] [PubMed] [Google Scholar]
- 20.Hoang KB, Cassar IR, Grill WM, Turner DA. Biomarkers and stimulation algorithms for adaptive brain stimulation. Front Neurosci. 2017;11:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little S, Pogosyan A, Neal S, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013;74(3):449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swann NC, de Hemptinne C, Thompson MC, et al. Adaptive deep brain stimulation for Parkinson's disease using motor cortex sensing. J Neural Eng. 2018;15(4):046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt SL, Brocker DT, Swan BD, Turner DA, Grill WM. Evoked potentials reveal neural circuits engaged by human deep brain stimulation. Brain Stimul. 2020;13(6):1706-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beudel M, Brown P. Adaptive deep brain stimulation in Parkinson's disease. Parkinsonism Relat Disord. 2016;22(suppl 1):S123-S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meidahl AC, Tinkhauser G, Herz DM, Cagnan H, Debarros J, Brown P. Adaptive deep brain stimulation for movement disorders: the long road to clinical therapy. Mov Disord. 2017;32(6):810-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ewert S, Plettig P, Li N, et al. Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. NeuroImage. 2018;170:271-282. [DOI] [PubMed] [Google Scholar]
- 27.Horn A, Kühn AA. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. NeuroImage. 2015;107:127-135. [DOI] [PubMed] [Google Scholar]