BACKGROUND:
Reports suggest that phosphatidylinositol 3-kinase pathway alterations confer increased risk of progression and poor prognosis in oligodendroglioma, IDH-mutant, and 1p/19q-codeleted molecular oligodendrogliomas (mODG). However, factors that affect prognosis in mODG have not been thoroughly studied. In addition, the benefits of adjuvant radiation and temozolomide (TMZ) in mODGs remain to be determined.
OBJECTIVE:
To evaluate the role of PIK3CA mutations in mODGs.
METHODS:
One hundred seven mODGs (2008-2019) diagnosed at 2 institutions were included. A retrospective review of clinical characteristics, molecular alterations, treatments, and outcomes was performed.
RESULTS:
The median age was 37 years, and 61 patients (57%) were male. There were 64 (60%) World Health Organization (WHO) grade 2 and 43 (40%) WHO grade 3 tumors. Eighty-two patients (77%) were stratified as high risk (age 40 years or older and/or subtotal resection per Radiation Treatment Oncology Group-9802). Gross-total resection was achieved in 47 patients (45%). Treatment strategies included observation (n = 15), TMZ (n = 11), radiation (n = 13), radiation/TMZ (n = 62), and others (n = 6). Our results show a benefit of TMZ vs observation in progression-free survival (PFS). No difference in PFS or overall survival (OS) was observed between radiation and radiation/TMZ. PIK3CA mutations were detected in 15 (14%) mODG, and shorter OS was observed in PIK3CA-mutant compared with PIK3CA wild-type mODGs (10.7 years vs 15.1 years, P = .009). WHO grade 3 tumors showed a shorter PFS, but no significant difference in OS was observed between WHO grades.
CONCLUSION:
Our findings suggest that mODGs harboring PIK3CA mutations have worse OS. Except for an advantage in PFS with TMZ treatment, adjuvant TMZ, radiation, or a combination of the two showed no significant improvement in OS.
KEY WORDS: Oligodendroglioma IDH1/IDH2-mutant, 1p/19q-codeleted, IDH1, IDH2, PIK3CA, Temozolomide, Oligodendroglioma treatment, Molecular oligodendroglioma
ABBREVIATIONS:
- CT
chemotherapy
- EOR
extent of resection
- GTR
gross total resection
- HR
hazard ratios
- IDH
isocitrate dehydrogenase
- IQR
interquartile range
- LGG
low-grade glioma
- LSI
locus specific identifier
- mODG
molecular oligodendrogliomas
- MVA
multivariable analysis
- OS
overall survival
- PCV
procarbazine, lomustine, and vincristine
- PI3K
phosphatidylinositol 3-kinase
- RCT
randomized clinical trial
- RT
radiotherapy
- RTOG
Radiation Treatment Oncology Group
- TCGA
The Cancer Genome Atlas
- TMZ
temozolomide
- WHO
World Health Organization
- WT
wild-type.
Oligodendrogliomas comprise 5.3% of gliomas.1,2 Based on the 2016 World Health Organization (WHO) fourth revised edition, the diagnosis of oligodendroglioma requires IDH1/IDH2 mutation and 1p/19q-codeletion.3 Recent studies have shown that genetic alterations can correlate with outcomes in molecularly defined gliomas (eg, CDKN2A/B loss in isocitrate dehydrogenase [IDH]-mutant astrocytomas and EGFR amplification or TERT promoter mutation in IDH wild-type astrocytomas).4,5 However, studies analyzing mutations that correlate with survival in molecular oligodendrogliomas (mODG) are limited.6,7
The phosphatidylinositol 3-kinase (PI3K) is an important regulator of cellular growth, transformation, adhesion, apoptosis, survival, and motility. PI3K activation drives various downstream pathways that regulate several cellular functions including those involved in tumor development and progression.8 Overexpression of the p110α catalytic subunit of PI3K (PIK3CA) leads to cellular transformation with concomitant phosphorylation of proteins in the AKT pathway.8 PIK3CA mutations are present in 15% to 20% of mODG.9-12 Recent studies have demonstrated that PI3K/AKT/mTOR pathway activation drives xenograft model formation, progression of disease, and poor outcomes in mODG.13,14 This pathway has also been associated with shorter survival in IDH-mutant astrocytomas.15 However, PIK3CA mutations were not an independent prognostic marker in multivariable analysis (MVA) of IDH-mutant astrocytomas despite showing a trend toward shorter overall survival (OS).5,15 Although studies suggest the possible relevance of PIK3CA mutations, the association between PIK3CA mutations and outcome in mODGs needs further evaluation.
Clinical trials have demonstrated the efficacy of procarbazine, lomustine, and vincristine (PCV) therapy in patients with mODG.16-19 In current clinical practice, patients with mODGs are frequently treated with temozolomide (TMZ), instead of PCV, based on its efficacy in high-grade astrocytomas, easiness of use, and favorable side effects profile.20 However, there is a lack of randomized clinical trials (RCTs) supporting the benefit of TMZ in mODG.
In this study, we assessed the prognostic value of PIK3CA mutations in 107 patients with mODG. Moreover, we evaluated the effects of TMZ, radiotherapy (RT), and the combination of both in the survival of patients with mODG.
METHODS
Patients and Tumor Samples
We retrospectively reviewed electronic medical records of patients with mODG diagnosed at 2 institutions between 2008 and 2019. Inclusion criteria were (1) diagnosis of oligodendroglioma with confirmed IDH1/IDH2 mutation and 1p/19q-codeletion and (2) PIK3CA mutation evaluated by sequencing (Supplementary Digital Content 1, http://links.lww.com/NEU/B511).
Age, sex, race, tumor diameter, histological diagnosis, extent of resection (EOR), risk stratification, treatment, recurrence, salvage therapy, and survival were collected using REDCap electronic data capture tools.21 Tumors were classified following the 2016 WHO Classification of Tumors of the Central Nervous System3 (Supplementary Digital Content 2, http://links.lww.com/NEU/B512). Radiographical EOR was classified as gross total resection (GTR), subtotal resection, or biopsy, as previously described.22 Risk stratification was performed according to the Radiation Treatment Oncology Group (RTOG 9802) criteria: age 40 years or older and/or subtotal resection.23,24
Recurrences and therapeutic interventions were determined by individual assessment of cases by an institutional multidisciplinary tumor board (Supplementary Digital Content 2, http://links.lww.com/NEU/B512). The primary end points were progression-free survival (PFS) and OS. This study was approved by both institutional review boards and adheres to the Strenghtening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Waiver of informed consent was granted because of the retrospective nature of this study.
Molecular Evaluation
IDH1 p.R132H status was evaluated using immunohistochemistry in some cases. IDH1/IDH2 and/or PIK3CA mutations were evaluated by sequencing (Supplementary Digital Content 2, http://links.lww.com/NEU/B512).25,26 1p/19q status was assessed by fluorescence in situ hybridization using a locus-specific identifier (LSI) 1p36 and LSI 19q13 dual-color probes from Abbott Molecular Inc, as previously described27 and obtained from electronic medical records.
Statistical Analysis
Descriptive statistics were evaluated by using the Fisher exact test/Mann–Whitney U-test. The Kaplan–Meier method was used to plot survival curves and examined by using a 2-sided log-rank test. The MVA was built using stepwise selection by combing forward and backward selection techniques to select the variables. The Akaike information criterion was used to compare the quality of the set of statistical models. The MVAs include the treatment center as a strata variable and have been corrected by the stratified Cox model. After the model selection, the final variables were used for further analysis. Age was classified using the Pignatti and RTOG 9802 cutoff criteria.23,24,28 P-values were 2-sided, and a P ≤ .05 was considered statistically significant. Statistical analyses were performed in R (v.3.5.2), EZR (1.40),29 and Prism v.8.4.2 (GraphPad). The oncoplot figure was generated using cBioPortal available tools (https://www.cbioportal.org/)9,10 (Supplementary Digital Content 2, http://links.lww.com/NEU/B512).
RESULTS
Patient Characteristics
We identified 107 mODG. The median age at diagnosis was 37 years (interquartile 30.5-47 years), 61 patients (57%) were male, and most (78%) were non-Hispanic White patients. Tumor diameter was ≥5 cm in approximately half of the patients, and 47 (45%) underwent GTR. Sixty-four (60%) and 43 cases (40%) were classified as WHO grade 2 and 3, respectively. Ninety-five tumors (88.8%) were IDH1-mutant, and 12 (11.2%) were IDH2-mutant. Forty-seven of 64 (73%) WHO grade 2 mODGs were considered high risk according to the RTOG 9802 criteria.
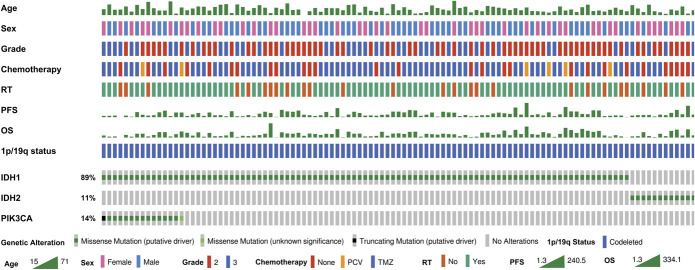
First-line therapies included observation (n = 15, 14%), TMZ alone (n = 11, 10.3%), RT alone (n = 13, 12.1%), RT/TMZ (n = 62, 58%), and others (n = 6, 5.6%). Adjuvant RT was performed in 81 (76%) patients. Forty-seven patients (44%) received ≥12 cycles of chemotherapy (CT). PIK3CA mutations were present in 15 cases (14%) (Figure 1). There were no significant differences in clinical characteristics between institutions (Table 1), except for race, in which institution 2 had a higher percentage of non-Hispanic White patients (84% vs 67%, P = .017).
FIGURE 1.
Oncoplot of oligodendroglioma, IDH-mutant, and 1p/19q-codeleted patients. IDH, isocitrate dehydrogenase; OS, overall survival; PFS, progression-free survival; PCV, procarbazine, lomustine, and vincristine; RT, radiotherapy; TMZ, temozolomide.
TABLE 1.
Demographics and Clinical Characteristics of Oligodendroglioma, IDH-Mutant, and 1p/19q-Codeleted
| Characteristic | Oligodendrogliomas (n = 107) | Institution 1 (n = 39) | Institution 2 (n = 68) | P value |
|---|---|---|---|---|
| Age at diagnosis, median (IQR) | 37 (30.5-47) | 41 (32.5-48.5) | 36 (30.0-45.0) | .244 |
| Male, N (%) | 61 (57) | 20 (51) | 41 (60) | .420 |
| Race, N (%) | .017 | |||
| Non-Hispanic White | 83 (78) | 26 (67) | 57 (84) | |
| African American | 7 (7) | 6 (15) | 1 (1) | |
| Hispanic | 9 (8) | 5 (13) | 4 (6) | |
| Asian | 2 (1) | 1 (3) | 1 (1) | |
| Others | 6 (6) | 1 (3) | 5 (7) | |
| Tumor maximum diameter ≥5 cma | 46 (50) | 14 (44) | 32 (53) | .512 |
| WHO grade 2 | 64 (60) | 23 (59) | 41 (60) | 1.000 |
| Grade 2 high risk (RTOG 9802), N (%)b | 47 (73) | 16 (70) | 31 (76) | .769 |
| First-line CT, N (%) | .183 | |||
| No CT | 28 (26) | 11 (28) | 17 (25) | |
| Temozolomide | 73 (68) | 28 (72) | 45 (66) | |
| Other CTc | 6 (6) | 0 (0) | 6 (9) | |
| First-line radiotherapy | 81 (76) | 27 (69) | 54 (79) | .251 |
| CT No. of cycles, N (%) | .971 | |||
| 0 cycles | 33 (31) | 13 (33) | 20 (29) | |
| 1-5 cycles | 13 (12) | 4 (10) | 9 (13) | |
| 6-11 cycles | 14 (13) | 5 (13) | 9 (13) | |
| ≥12 cycles | 47 (44) | 17 (44) | 30 (44) | |
| Extent of resection, N (%)d | .147 | |||
| Gross total resection | 47 (45) | 12 (38) | 33 (49) | |
| Subtotal resection | 50 (48) | 22 (59) | 28 (41) | |
| Biopsy | 8 (7) | 1 (3) | 7 (10) | |
| PIK3CA-mutant | 15 (14) | 6 (15) | 9 (13) | .778 |
CT, chemotherapy; IDH, isocitrate dehydrogenase; IQR, interquartile range; RTOG, Radiation Treatment Oncology Group; WHO, World Health Organization.
Tumor diameter was not available for 15 patients.
RTOG 9802 criteria was calculated only from grade 2 molecular oligodendrogliomas (n = 64).
Other chemotherapy included 3 patients treated with lomustine/procarbazine/vincristine and 3 patients treated with lomustine/procarbazine.
Extent of resection was not available for 4 patients.
The Stupp protocol was consistent with radiotherapy and concomitant temozolomide according to the treatment protocol previously described.
P ≤ .05 was considered as statistically significant and denoted in bold.
OS and PFS in mODG
The median follow-up (n = 107) was 80.9 months (6.7 years). At the time of this report, 61 patients (57%) showed evidence of progression with a median PFS of 79.5 months. Patients with mODG WHO grade 3 had significantly worse PFS compared with WHO grade 2 in univariable (71 vs 83 months, P = .044, log-rank test) and MVA (hazard ratios 1.91 [1.07-3.42], P = .029; Table 2). Other demographic and clinical characteristics were not associated with PFS.
TABLE 2.
Multivariable Cox Proportional Hazard Regression Models of Progression-Free Survival in Oligodendroglioma, IDH-Mutant, and 1p/19q-Codeleted (n = 107)
| Variable | HR (95% CI) | P value |
|---|---|---|
| WHO (grade III vs II) | 1.91 (1.07-3.42) | .029 |
| PIK3CA (mutant vs wild-type) | 1.74 (0.86-3.52) | .124 |
HR, hazard ratios; IDH, isocitrate dehydrogenase; WHO, World Health Organization.
P ≤ .05 was considered as statistically significant and denoted in bold.
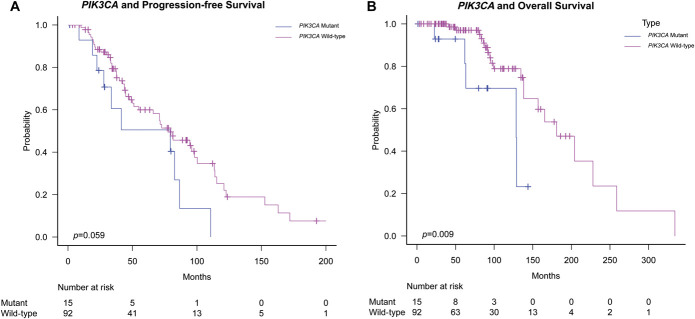
At the time of this report, 25 patients (23%) were deceased with a median OS of 165.4 months (13.8 years). PIK3CA-mutant mODG demonstrated worse OS in both univariable (128.5 vs 180.8 months, P = .009, log-rank test; Figure 2) and MVA (hazard ratios 3.59 [1.19-10.84], P = .023). Other factors, such as WHO grade, age, tumor diameter, EOR, and adjuvant therapies, did not demonstrate a statistically significant association with OS (Table 3).
FIGURE 2.
Oligodendroglioma, IDH-mutant, 1p/19q-codeleted, and outcome by PIK3CA status. A, Progression-free survival of oligodendroglioma IDH-mutant 1p/19q-codeleted by PIK3CA status in which there was no statistically significant difference (79.3 vs 79.6 months, P = .058) between PIK3CA-mutant (n = 15) and PIK3CA WT (n = 92) patients. B, OS of oligodendroglioma IDH-mutant 1p/19q-codeleted by PIK3CA status in which PIK3CA-mutant (n = 15) patients showed worse OS compared with PIK3CA WT (n = 92) patients (128.5 vs 180.8 months, P = .009). IDH, isocitrate dehydrogenase; OS, overall survival; WT, wild-type.
TABLE 3.
Multivariable Cox Proportional Hazard Regression Models of OS in Oligodendroglioma, IDH-Mutant, and 1p/19q-Codeleted (n = 107)
| Variable | HR (95% CI) | P value |
|---|---|---|
| WHO (grade III vs II) | 2.02 (0.69-5.96) | .104 |
| RTOG 9802 (high vs low risk) | 0.47 (0.19-1.17) | .104 |
| PIK3CA (mutant vs wild-type) | 3.59 (1.19-10.84) | .023 |
HR, hazard ratios; IDH, isocitrate dehydrogenase; OS, overall survival; RTOG, Radiation Therapy Oncology Group; WHO, World Health Organization.
P ≤ .05 was considered as statistically significant and denoted in bold.
PIK3CA Mutation in mODG
Evaluation of PIK3CA mutations and their biological effect showed that 15 patients (14%) harbored 19 missense mutations and 1 truncating mutation (Figure 1). Eighteen of these 20 mutations are considered “oncogenic,” and 2 are “likely oncogenic” according to Catalogue Of Somatic Mutations In Cancer (COSMIC) and OncoKB databases. In addition, 13 mutations are known to cause gain of function, and 3 are likely to cause gain of function of PIK3CA30,31 (Table S1, Supplementary Digital Content 3, http://links.lww.com/NEU/B513). There were no significant differences in clinical characteristics between PIK3CA-mutant and wild-type patients (Table S2, Supplementary Digital Content 4, http://links.lww.com/NEU/B514).
Adjuvant Treatment Strategies in mODG
Subanalysis (excluding 6 patients treated with PCV or procarbazine/lomustine) showed that patients with mODG treated with TMZ (n = 73) were more often older (P = .008), WHO grade 3 (P = .044), and more frequently received RT (P = .0002) than patients not treated with TMZ (n = 28) (Table S3, Supplementary Digital Content 5, http://links.lww.com/NEU/B515). However, subanalysis of grade 2 high-risk mODGs (n = 42, PFS P = .412, and OS P = .322) and WHO grade 2, regardless of the risk criteria (n = 59, PFS P = .513, and OS P = .171), failed to demonstrate benefit in OS or PFS after treatment with TMZ (Figure S1, Supplementary Digital Content 6, http://links.lww.com/NEU/B516).
Patients (n = 75) who received RT (excluding 6 patients treated with PCV or procarbazine/lomustine) were more likely WHO grade 3 (P = .010) and more frequently received TMZ (P = .0002) than patients not treated with RT (n = 26) (Table S4, Supplementary Digital Content 7, http://links.lww.com/NEU/B519). However, there was no survival benefit from RT (PFS P = .299, OS P = .389). Subanalyses by WHO grade or RTOG risk criteria were not performed given the relatively small sample size and events.
Additional analysis, regardless of grade or risk category, demonstrated that RT + TMZ was not superior to RT only in both OS and PFS (Figure S2, Supplementary Digital Content 8, http://links.lww.com/NEU/B521).
DISCUSSION
Key Results
We retrospectively evaluated a multi-institutional database of mODG to identify the role of PIK3CA mutation status as a prognostic marker. Our results support a deleterious effect of PIK3CA activating mutations in the survival of patients with mODGs. This finding is consistent with previous studies using the The Cancer Genome Atlas (TCGA) database and a xenograft oligodendroglial model.13,14 In agreement with the CODEL initial trial results, our study shows that TMZ does not seem to provide a significant benefit to patients with mODG.32 By contrast, evidence from multiple studies supports a benefit of PCV in combination with RT for mODGs.16-19
Interpretation and Generalization
PIK3CA Mutation in mODG
Our results show that PIK3CA-mutant mODGs have significantly worse survival than PIK3CA wild-type mODGs (Figure 2B and Table 3). PI3K/AKT/mTOR pathway alterations and PIK3CA mutations have been shown to promote malignant progression in oligodendroglioma xenograft models.13,33 A recent study using multifaceted computational assessment of risk in mODG observed that PI3K pathway activation (PIK3CA activating mutations and PIK3R1 inactivating mutations) are strongly associated with imaging and histological findings of advanced disease and correlated with poor clinical outcomes.14 However, other studies have not identified this association. For example, Tateishi et al13 did not observe a correlation between PIK3CA mutation and mODG. However, this cohort was smaller (45 patients), and only 4 patients were PIK3CA-mutant. Aoki et al15 evaluated genetic alterations and survival in 141 mODGs, but PIK3CA was only assessed as part of the RTK-PI3K-mTOR pathway, in which they included 24 other genes. Other studies evaluating survival and genetic alterations in mODG had a low incidence of PIK3CA mutations (2.1%-4.1%) and did not report an association between PIK3CA and survival.34,35 However, the study by Wijnenga et al35 only included WHO grade 2 tumors. Although previous studies have associated PIK3CA mutations with WHO grade 3 oligodendrogliomas, this was performed before the molecular definition of oligodendrogliomas.36 In our study, there was no significant association between WHO grade and PIK3CA status (Supplementary Digital Content 4, http://links.lww.com/NEU/B514). We observed that all PIK3CA-mutant patients were IDH1-mutant (Figure 1).
It has been demonstrated that pathway inhibitors (PI3K, AKT, and mTOR inhibitors) induce cytotoxic effects in PIK3CA-mutant but not in PIK3CA wild-type oligodendroglioma cells, suggesting that activating mutations in the PIK3/AKT/mTOR pathway may predict response to targeted therapies.13 Additional studies evaluating the role of PIK3CA-activating mutations in mODG are needed to validate our results.
Treatment Strategies in mODG
We did not observe therapeutic benefits of RT alone, although our sample size was small. RCTs evaluating RT against observation (delayed RT) have shown benefit in PFS but not in OS in low-grade glioma (LGG).37 Whether this is true for mODG remains to be answered, especially considering the effects of RT on quality of life (eg, detrimental effects on cognition).38,39
To date, no RCT comparing observation with CT or RT + CT for LGG or mODG has been performed. Long-term follow-up RCTs have demonstrated the substantial benefit of sequential RT and PCV compared with RT alone for anaplastic oligodendroglioma.16,17 In high-risk LGG, Buckner et al40 demonstrated that RT + PCV is superior to RT alone. This survival benefit was consistent in post hoc analysis of mODG.19 Importantly, most of the patients treated with PCV in the RCT tolerated 3 treatment cycles (6 cycles goal) because of hematological side effects. However, the benefits of PCV were evident despite shorter therapy.17,18,40 The NOA-04 RCT subanalysis demonstrated superiority of PCV compared with TMZ in anaplastic oligodendroglioma with an improved PFS (PCV 9.4 vs 4.46 years) and a trend toward improved OS (PCV not reached vs 8.09 years).41 No difference has been observed between RT and TMZ monotherapy in WHO grade 2, high-risk oligodendroglioma.42 Although a single-arm trial of RT + TMZ reported increased survival in LGG when compared with historical reports of RT alone,43 this benefit is not observed when the 5-year OS is compared with more recent studies with a similar population treated with RT alone. Moreover, it seems that the 5-year OS is lower than patients treated with RT + PCV.40 The CODEL RCT initial analysis showed that TMZ-treated patients had shorter PFS and a nonsignificant lower 3-year and 5-year OS compared with RT-treated patients.32 These RCT study arms were modified because of the benefit of RT + PCV. Currently, the phase III CODEL study (NCT00887146) is recruiting patients in 2 arms (RT + TMZ and RT + PCV). This trial will be crucial to evaluating the role of RT + TMZ in mODG.
In our study, there was no benefit of RT, TMZ, or the combination of both in OS. Despite our small sample size, the results are concordant with a recent retrospective study which showed no benefit from TMZ, RT, or their combination in WHO grade 2 mODG.20 Importantly, recent studies have demonstrated that TMZ is an independent risk factor for malignant transformation and mismatch repair pathway defects, which in turn could lead to worse survival.44,45
EOR and Outcome in mODG
Surgical resection serves multiple objectives including cytoreduction, tissue for diagnosis and molecular characterization, and symptomatic control (neurological deficit and seizure control).46 There are no RCTs evaluating the role of surgical resection in mODG. These studies are unlikely to be performed because of evidence showing survival benefits of higher resection rates and decreased residual volume in mODG.47-49 The absence of outcome benefit in mODG with GTR in our study could be explained due to the small number of patients who underwent biopsy, as our practice is to perform maximal safe resection. Other factors historically associated with outcomes, such as tumor diameter and age older than 40 years, were not associated with PFS or OS in mODG.
WHO Grade and Survival in mODG
The prognostic value of histological grading of mODG based on the WHO criteria is unclear.46 In our study, WHO grade 3 correlated with shorter PFS. However, no statistically significant association was observed between WHO grade and OS. WHO grade 3 patients were treated more frequently with adjuvant therapies, which might obscure possible differences in OS between grade 2 and 3 mODG. The lack of a significant association between WHO histological grade and the survival of patients with mODG is in agreement with the findings of recent studies.15,50 However, the lack of prognostic value of WHO histological grade in mODG needs to be confirmed by additional studies including a large number of patients with a long-term follow-up.
Limitations
The limitations of our study include its retrospective design, potential selection bias (only 41.8% of all patients with mODG underwent analysis for PIK3CA mutations; Supplementary Digital Content 1, http://links.lww.com/NEU/B511), and differences in treatment patterns among institutions. Some subgroups were small and not adequately powered to detect differences in response to therapies; therefore, statistical analysis was not performed in such subgroups. Karnofsky performance status was not available for several patients, precluding its inclusion in the statistical analysis. Our analysis failed to reveal an association between WHO histological grade, race, age, and use of TMZ and RT with OS. While a limited sample size contributes to a higher type 2 error probability and negative results should be interpreted with caution in the setting of limited statistical power, other studies have also shown similar lack of association between histological grade, TMZ and RT, and OS in mODG.15,20,32,50
CONCLUSION
Our results demonstrate that PIK3CA-mutant mODGs have worse OS. This result is in agreement with other studies (using the TCGA database and xenograft models) that have associated activation of the PI3K pathway with more aggressive growth of oligodendrogliomas. In agreement with the CODEL initial study, our results show that TMZ did not seem to improve outcomes in patients with mODG.
Footnotes
Supplemental digital content is available for this article at neurosurgery-online.com.
Presented as an abstract and poster at the Society of Neuro-Oncology 2020 virtual meeting and as an oral presentation at the United States and Canadian Academy of Pathology (USCAP) 109th Annual Meeting, February 29-March 5, 2020, Los Angeles, CA.
Funding
Research reported in this publication was partly supported by the NIH/NCI: K08CA241651 (to Dr Ballester), Sheikh Khalifa Al Nahyan Ben Zayed Institute for Personalized Cancer Therapy, the MD Anderson Cancer Center Support Grant (P30 CA016672), the University of Texas MD Anderson Cancer Center Glioblastoma Moonshot Program, Marnie Rose Foundation, and Naz Moez Sarrafzadeh Glioma Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Digital Content 1. Study patient selection flowchart.
Supplementary Digital Content 2. Methodology.
Supplementary Digital Content 3. Table S1. PIK3CA mutations in mODG and their functional consequence. Oligodendroglioma, IDH-mutant, 1p/19q-codeleted PIK3CA mutations, and biological function.
Supplementary Digital Content 4. Table S2. Demographics and clinical characteristics of oligodendroglioma, IDH-mutant, and 1p/19q-codeleted by PIK3CA status (n = 107).
Supplementary Digital Content 5. Table S3. Demographics and clinical characteristics of oligodendroglioma, IDH-mutant, and 1p/19q-codeleted by temozolomide treatment (n = 101).
Supplementary Digital Content 7. Table S4. Demographics and clinical characteristics of oligodendroglioma, IDH-mutant, and 1p/19q-codeleted by radiotherapy treatment (n = 101).
Supplementary Digital Content 6. Figure S1. Subanalysis of oligodendroglioma, IDH-mutant, 1p/19q-codeleted, and outcome by temozolomide therapy. A and B, PFS and OS of grade 2 high-risk mODG by TMZ, in which there was no statistical significance difference between TMZ-treated (n = 29) and TMZ-untreated (n = 13) patients in both PFS (54.9 vs 66.0 months, P = .412) and OS (138.2 months vs undefined, P = .322). C and D, PFS and OS of mODG WHO grade 2 regardless of the risk criteria by TMZ, in which there was no statistically significant difference between TMZ-treated (n = 38) and TMZ-untreated (n = 21) patients in both PFS (113.5 vs 72.6 months, P = .513) and OS (138.2 vs 334.1 months, P = .171). The high and low risks were determined according to the Radiation Oncology Treatment Group criteria. Patients with other treatments (n = 6) were excluded from this subanalysis. PFS, progression-free survival; OS, overall survival; mODG, molecular oligodendroglioma; TMZ, temozolomide.
Supplementary Digital Content 8. Figure S2. Radiotherapy vs radiotherapy and temozolomide in oligodendroglioma, IDH-mutant, and 1p/19q-codeleted. A and B, PFS and OS of mODG treated with RT + TMZ (n = 62) vs RT-treated (n = 13) patients, in which there was no statistically significant difference between treatments in both PFS (79.6 months vs 71 months, P = .767) and OS (165.4 months vs 138.2 months, P = .348). Patients with other treatments (n = 6) were excluded from this subanalysis. PFS, progression-free survival; OS, overall survival; mODG, molecular oligodendroglioma; RT, radiotherapy; TMZ, temozolomide.
REFERENCES
- 1.Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21(suppl 5):v1-v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Truitt G, Gittleman H, Leece R, et al. Partnership for defining the impact of 12 selected rare CNS tumors: a report from the CBTRUS and the NCI-CONNECT. J Neurooncol. 2019;144(1):53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820. [DOI] [PubMed] [Google Scholar]
- 4.Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139(3):603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appay R, Dehais C, Maurage CA, et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol. 2019;21(12):1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Thomas C, Munoz FA, et al. Polysomy is associated with poor outcome in 1p/19q codeleted oligodendroglial tumors. Neuro Oncol. 2019;21(9):1164-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94(4):455-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Lichtenberg T, Hoadley KA, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173(2):400-416e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network, Brat DJ Verhaak RGW Aldape KD et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tateishi K, Nakamura T, Juratli TA, et al. PI3K/AKT/mTOR pathway alterations promote malignant progression and xenograft formation in oligodendroglial tumors. Clin Cancer Res. 2019;25(14):4375-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halani SH, Yousefi S, Velazquez Vega J, et al. Multi-faceted computational assessment of risk and progression in oligodendroglioma implicates NOTCH and PI3K pathways. Npj Precis Oncol. 2018;2(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoki K, Nakamura H, Suzuki H, et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018;20(1):66-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Den Bent MJ, Brandes AA, Taphoorn MJB, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC Brain Tumor Group study 26951. J Clin Oncol. 2013;31(3):344-350. [DOI] [PubMed] [Google Scholar]
- 17.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32(8):783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell EH, Zhang P, Shaw EG, et al. Comprehensive genomic analysis in NRG oncology/RTOG 9802: a phase III trial of radiation versus radiation plus procarbazine, lomustine (CCNU), and vincristine in high-risk low-grade glioma. J Clin Oncol. 2020;38(29):3407-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal'a A, Coburger J, Scherer M, et al. To treat or not to treat? A retrospective multicenter assessment of survival in patients with IDH-mutant low-grade glioma based on adjuvant treatment. J Neurosurg. Published online ahead of print July 19, 2019. doi: 10.3171/2019.4.JNS183395. [DOI] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700-708. [DOI] [PubMed] [Google Scholar]
- 23.Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial—clinical article. J Neurosurg. 2008;109(5):835-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cairncross G, Cairncross G, Berkey B, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group trial 9402. J Clin Oncol. 2006;24(18):2707-2714. [DOI] [PubMed] [Google Scholar]
- 25.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dono A, Ramesh AV, Wang E, et al. The role of RB1 alteration and 4q12 amplification in IDH-WT glioblastoma. Neuro-Oncology Adv. 2021;3(1):vdab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin CA, Burger P, Morsberger L, et al. Identification of der (1; 19)(q10; p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol. 2006;65(10):988-994. [DOI] [PubMed] [Google Scholar]
- 28.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076-2084. [DOI] [PubMed] [Google Scholar]
- 29.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013;48(3):452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tate JG, Bamford S, Jubb HC, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47(D1):D941-D947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaeckle KA, Ballman K V, van den Bent M, et al. CODEL: phase III study of RT, RT + temozolomide (TMZ), or TMZ for newly-diagnosed 1p/19q codeleted oligodendroglioma. Analysis from the initial study design. Neuro Oncol. 2022;23(3):457-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakimoto H, Tanaka S, Curry WT, et al. Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clin Cancer Res. 2014;20(11):2898-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubbink HJ, Atmodimedjo PN, Kros JM, et al. Molecular classification of anaplastic oligodendroglioma using next-generation sequencing: a report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial. Neuro Oncol. 2016;18(3):388-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wijnenga MMJ, French PJ, Dubbink HJ, et al. Prognostic relevance of mutations and copy number alterations assessed with targeted next generation sequencing in IDH mutant grade II glioma. J Neurooncol. 2018;139(2):349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broderick DK, Di C, Parrett TJ, et al. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade Astrocytomas, and medulloblastomas. Cancer Res. 2004;64(15):5048-5050. [DOI] [PubMed] [Google Scholar]
- 37.Van Den Bent MJ, Afra D, De Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985-990. [DOI] [PubMed] [Google Scholar]
- 38.Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810-818. [DOI] [PubMed] [Google Scholar]
- 39.Ali FS, Hussain MR, Gutiérrez C, et al. Cognitive disability in adult patients with brain tumors. Cancer Treat Rev. 2018;65:33-40. [DOI] [PubMed] [Google Scholar]
- 40.Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wick W, Roth P, Hartmann C, et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016;18(11):1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher BJ, Pugh SL, Macdonald DR, et al. Phase 2 study of a temozolomide-based chemoradiation therapy regimen for high-risk, low-grade gliomas: long-term results of radiation therapy oncology group 0424. Int J Radiat Oncol Biol Phys. 2020;107(4):720-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oberheim Bush NA, Yu Y, Villanueva-Meyer J, et al. Temozolomide-induced hypermutation is associated with high-grade transformation, distant recurrence, and reduced survival after transformation in initially low-grade IDH-mutant diffuse gliomas. J Clin Oncol. 2020;38(15_suppl):2506-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Bent MJ, Smits M, Kros JM, Chang SM. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol. 2017;35(21):2394-2401. [DOI] [PubMed] [Google Scholar]
- 47.Harary M, Kavouridis VK, Torre M, et al. Predictors and early survival outcomes of maximal resection in WHO grade II 1p/19q-codeleted oligodendrogliomas. Neuro Oncol. 2020;22(3):369-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garton ALA, Kinslow CJ, Rae AI, et al. Extent of resection, molecular signature, and survival in 1p19q-codeleted gliomas. J Neurosurg. 2020;134(5):1357-1367. [DOI] [PubMed] [Google Scholar]
- 49.Zhu P, Du XL, Blanco AI, et al. Impact of facility type and volume in low-grade glioma outcomes. J Neurosurg. Published online ahead of print Sep 27, 2019. doi: 10.3171/2019.6.JNS19409. [DOI] [PubMed] [Google Scholar]
- 50.Iwadate Y, Matsutani T, Hara A, et al. Eighty percent survival rate at 15 years for 1p/19q co-deleted oligodendroglioma treated with upfront chemotherapy irrespective of tumor grade. J Neurooncol. 2019;141(1):205-211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Digital Content 1. Study patient selection flowchart.
Supplementary Digital Content 2. Methodology.
Supplementary Digital Content 3. Table S1. PIK3CA mutations in mODG and their functional consequence. Oligodendroglioma, IDH-mutant, 1p/19q-codeleted PIK3CA mutations, and biological function.
Supplementary Digital Content 4. Table S2. Demographics and clinical characteristics of oligodendroglioma, IDH-mutant, and 1p/19q-codeleted by PIK3CA status (n = 107).
Supplementary Digital Content 5. Table S3. Demographics and clinical characteristics of oligodendroglioma, IDH-mutant, and 1p/19q-codeleted by temozolomide treatment (n = 101).
Supplementary Digital Content 7. Table S4. Demographics and clinical characteristics of oligodendroglioma, IDH-mutant, and 1p/19q-codeleted by radiotherapy treatment (n = 101).
Supplementary Digital Content 6. Figure S1. Subanalysis of oligodendroglioma, IDH-mutant, 1p/19q-codeleted, and outcome by temozolomide therapy. A and B, PFS and OS of grade 2 high-risk mODG by TMZ, in which there was no statistical significance difference between TMZ-treated (n = 29) and TMZ-untreated (n = 13) patients in both PFS (54.9 vs 66.0 months, P = .412) and OS (138.2 months vs undefined, P = .322). C and D, PFS and OS of mODG WHO grade 2 regardless of the risk criteria by TMZ, in which there was no statistically significant difference between TMZ-treated (n = 38) and TMZ-untreated (n = 21) patients in both PFS (113.5 vs 72.6 months, P = .513) and OS (138.2 vs 334.1 months, P = .171). The high and low risks were determined according to the Radiation Oncology Treatment Group criteria. Patients with other treatments (n = 6) were excluded from this subanalysis. PFS, progression-free survival; OS, overall survival; mODG, molecular oligodendroglioma; TMZ, temozolomide.
Supplementary Digital Content 8. Figure S2. Radiotherapy vs radiotherapy and temozolomide in oligodendroglioma, IDH-mutant, and 1p/19q-codeleted. A and B, PFS and OS of mODG treated with RT + TMZ (n = 62) vs RT-treated (n = 13) patients, in which there was no statistically significant difference between treatments in both PFS (79.6 months vs 71 months, P = .767) and OS (165.4 months vs 138.2 months, P = .348). Patients with other treatments (n = 6) were excluded from this subanalysis. PFS, progression-free survival; OS, overall survival; mODG, molecular oligodendroglioma; RT, radiotherapy; TMZ, temozolomide.