BACKGROUND:
Increases in the extent of resection of both contrast-enhanced (CE) and non–contrast-enhanced (NCE) tissue are associated with substantial survival benefits in patients with isocitrate dehydrogenase wild-type glioblastoma. The fact, however, remains that these lesions exist within the framework of complex neural circuitry subserving cognition, movement, and behavior, all of which affect the ultimate survival outcome. The prognostic significance of the interplay between CE and NCE cytoreduction and neurological morbidity is poorly understood.
OBJECTIVE:
To identify a clinically homogenous population of 228 patients with newly diagnosed isocitrate dehydrogenase wild-type glioblastoma, all of whom underwent maximal safe resection of CE and NCE tissue and adjuvant chemoradiation. We then set out to delineate the competing interactions between resection of CE and NCE tissue and postoperative neurological impairment with respect to overall survival.
METHODS:
Nonparametric multivariate models of survival were generated via recursive partitioning to provide a clinically intuitive framework for the prognostication and surgical management of such patients.
RESULTS:
We demonstrated that the presence of a new postoperative neurological impairment was the key factor in predicting survival outcomes across the entire cohort. Patients older than 60 yr who suffered from at least one new impairment had the worst survival outcome regardless of extent of resection (median of 11.6 mo), whereas those who did not develop a new impairment had the best outcome (median of 28.4 mo) so long as all CE tissue was resected.
CONCLUSION:
Our data provide novel evidence for management strategies that prioritize safe and complete resection of CE tissue.
KEY WORDS: Glioma, Glioblastoma, Cognition, Neurological impairments
ABBREVIATIONS:
- CE
contrast-enhanced
- EOR
extent of resection
- GBM
glioblastoma
- HR
hazard ratio
- IDH
isocitrate dehydrogenase
- KPS
Karnofsky Performance Status
- NCE
non-CE
- RPA
recursive partitioning analysis
Glioblastoma (GBM) is characterized by its invasive nature and poor prognosis. The ability of tumor cells to integrate into the brain parenchyma and disrupt functional networks beyond the centrally necrotic tumor core is well described and makes complete surgical removal challenging. Numerous studies have demonstrated that maximal safe resection, which recently has come to mean removal of as much contrast-enhanced (CE) and non–contrast-enhanced (NCE) disease as possible, improves survival for patients with GBM.1-4 However, given clinical studies have demonstrated this survival benefit, resection margins may be pushed further into functional cortex, thereby increasing the risk of inducing permanent postoperative neurological impairments.5-8 Neurological impairments, in turn, diminish health-related quality of life and may even attenuate the relative survival benefit of cytoreduction.
Existing reports have investigated the interactive effects that postoperative neurological morbidity and extent of resection (EOR) have on survival, thereby offering suggestions for balancing this apparent trade-off.9-12 However, collectively, these studies have several key limitations that confound survival estimates including (1) aggregation of clinically heterogenous patient populations with vastly different glioma molecular subtypes based on World Health Organization grade and isocitrate dehydrogenase (IDH) status, (2) inconsistencies in the application of chemoradiation across patients, and (3) a lack of formal distinction between resection of CE and NCE disease. In this study, we address these limitations by using a homogenous population of 228 patients with IDH wild-type GBM, all of whom received adjuvant temozolomide and radiation therapy, to study the interaction between resection of CE and NCE tissue and postoperative neurological impairment on overall survival.
METHODS
Study participants were identified from a prospectively maintained registry of 761 patients with GBM who underwent resection at the University of California, San Francisco, between 1997 and 2017. Patients were included in this study if they met the following criteria: (1) newly diagnosed World Health Organization grade IV GBM on pathology, (2) wild-type for IDH 1/2 on immunohistochemistry or next-generation sequencing, (3) T1 postcontrast and T2/fluid-attenuated inversion recovery imaging taken within 24 h preoperatively and 72 h postoperatively, (4) comprehensive neurological examination documented preoperatively and 1 mo postoperatively, and (5) administration of adjuvant temozolomide and radiation therapy after cytoreductive surgery. Patients who underwent biopsy only were excluded from this study. Two hundred twenty-eight patients met the inclusion criteria and were included for final analysis (Supplemental Digital Content Figure, http://links.lww.com/NEU/A924). All patients provided written informed consent to participate in this study, which was approved by our institutional review board (IRB, 15-7500).
Measurement of EOR
Tumors were segmented using the borders of (1) contrast enhancement on T1 postcontrast imaging (ie, CE disease) and (2) hyperintense signal in expanded parenchyma on T2/fluid-attenuated inversion recovery imaging (ie, NCE disease) by 3 independent examiners blinded to all other clinical variables in line with previously established methods.13 Each grader completed an initial training period to ensure high inter-rater reliability (Supplemental Digital Content Table, http://links.lww.com/NEU/A924). EOR was then measured using previously established techniques (Supplemental Digital Content Information, Extended Methods, http://links.lww.com/NEU/A924).13,14
Statistical Analysis
Clinical variables including sex, age, and predominant lobar location and laterality of the tumor were collected at baseline. MGMT methylation status was available in only 104 patients and was therefore excluded from the analysis because of instability of imputed values in patients with missing data.13 Dichotomization of neurological outcomes was conducted by a coauthor blinded to the survival outcome.
A nonparametric, multivariate technique known as recursive partitioning analysis (RPA) was used to separate patients into clinically intuitive hierarchical groups stratified by their overall survival risk (Supplemental Digital Content Information, Extended Methods, http://links.lww.com/NEU/A924).15 Variables considered a priori for inclusion in the final multivariate RPA based on the existing literature were age, EOR of CE and NCE tissue, volume of residual CE and NCE tissue, new postoperative neurological impairment, postoperative Karnofsky Performance Status (KPS), and the change in KPS.10,13,14,16
For the purposes of model-building, univariate Cox proportional hazards analyses between each variable of interest and the survival outcome were performed and presented without corrections for multiple comparisons to prevent the removal of potentially relevant variables from the multivariate RPA.17 However, post hoc statistical tests between the final RPA groupings were corrected for multiple comparisons using the Holm–Bonferroni method.
RESULTS
Participants
Baseline clinical characteristics are summarized in Table 1. Tumors were similarly distributed across hemispheres and more likely to be found in the frontal and temporal lobes. Preoperative NCE tumor volumes were significantly higher than preoperative CE tumor volumes (P < .0001). A majority of patients presented with cognitive impairment at baseline, and slightly more than one-third of patients had aphasia and/or unilateral weakness on examination.
TABLE 1.
Baseline Clinical Characteristics
| Patients | 228 |
| Sex | |
| Female (%) | 98 (42.9) |
| Male (%) | 130 (57.0) |
| Age (yr) | |
| Mean (SD) | 59.9 (10.1) |
| Median (IQR) | 60.5 (13.4) |
| Range | 26.9-81.7 |
| Tumor laterality | |
| Left (%) | 119 (52.2) |
| Right (%) | 108 (47.4) |
| Bilateral (%) | 1 (0.4) |
| Predominant tumor location | |
| Frontal (%) | 86 (37.7) |
| Temporal (%) | 73 (32.0) |
| Parietal (%) | 46 (20.1) |
| Occipital (%) | 19 (8.3) |
| Insula (%) | 1 (0.4) |
| Thalamus | 1 |
| Cerebellum | 1 |
| Brainstem | 1 |
| Preoperative tumor volume (mL) | |
| CE disease | |
| Mean (SD) | 28.8 (26.2) |
| Median (IQR) | 20.4 (29.8) |
| Range | 0.5-172.1 |
| NCE disease | |
| Mean (SD) | 72.6 (50.0) |
| Median (IQR) | 64.4 (72.3) |
| Range | 1.2-219.4 |
| Preoperative impairment | |
| Cognitive (%) | 133 (58.3) |
| Aphasia (%) | 80 (35.1) |
| Visual (%) | 35 (15.3) |
| Hemiparesis (%) | 78 (34.2) |
| Hemiplegia (%) | 1 (0.4) |
CE, contrast-enhanced; IQR, interquartile range; NCE, non–contrast-enhanced; SD, standard deviation.
Main Results
Operative Outcomes
Outcomes of resection are summarized in Table 2. Awake craniotomy with intraoperative language mapping was performed in 19.7% of cases. The median EOR of CE tissue was significantly higher than that of NCE tissue (98.0% vs 60.0%, P < .0001). Seventy-three patients underwent total (100%) resection of CE tissue, whereas 16 patients underwent >90% EOR of NCE tissue. Nearly one-third of patients had a new neurological impairment at 1-mo postoperative follow-up. No patients developed hemiplegia. The follow-up period until death or censoring for this study ranged from 1.7 to 133.7 mo. The median overall survival across this period was 17 mo (95% CI = 15.8-20.2). Of the 228 patients included in this study, 208 died and 20 were censored by the end of the follow-up period.
TABLE 2.
Outcomes
| Awake craniotomy (%) | 45 (19.7) |
| Postoperative tumor volume (mL) | |
| CE disease | |
| Mean (SD) | 2.6 (6.7) |
| Median (IQR) | 0.4 (1.9) |
| Range | 0-57.6 |
| NCE disease | |
| Mean (SD) | 31.2 (28.1) |
| Median (IQR) | 22.2 (35.6) |
| Range | 0-146.2 |
| Extent of resection (%) | |
| CE disease | |
| Mean (SD) | 91.8 (6.7) |
| Median (IQR) | 98.0 (9.3) |
| Range | 10.0-100.0 |
| NCE disease | |
| Mean (SD) | 58.4 (21.8) |
| Median (IQR) | 60.0 (28.0) |
| Range | 0-100.0 |
| New postoperative impairment | |
| Any (%) | 67 (29.4) |
| Cognitive (%) | 31 (13.6) |
| Aphasia (%) | 13 (5.7) |
| Visual (%) | 28 (12.3) |
| Hemiparesis (%) | 15 (6.6) |
| Hemiplegia | 0 |
| Postoperative KPS | |
| Mean (SD) | 82.0 (10.3) |
| Median (IQR) | 90.0 (10.0) |
| Range | 10.0-100.0 |
| Follow-up (mo) | |
| Mean (SD) | 23.8 (19.4) |
| Median (IQR) | 17.0 (18.6) |
| Range | 1.7-133.7 |
| Median survival (mo) | 17.0 (95% CI = 15.8-20.2) |
CE, contrast-enhanced; IQR, interquartile range; NCE, non–contrast-enhanced; SD, standard deviation.
To determine whether an aggressive EOR necessitated a new postoperative neurological impairment, univariate logistic regression models were constructed. No significant associations were found between the onset of a new impairment and EOR of CE tissue (odds ratio = 1.02, 95% CI = 0.99-1.1, P = .18) or NCE tissue (odds ratio = 0.99, 95% CI = 0.98-1.00, P = .17).
Univariate Survival Models
The results of univariate Cox proportional hazards regression models for variables hypothesized a priori to be associated with overall survival are summarized in Table 3. Higher ages at diagnosis were associated with worsened survival. Modeled as continuous variables, higher extents of resection of CE and NCE tissue were both associated with improved survival. Furthermore, residual CE tumor on postoperative imaging was independently associated with worsened survival. The presence of one or more new neurological impairment(s) at 1-mo follow-up was associated with worsened survival (hazard ratio [HR] = 1.87, 95% CI = 1.38-2.53, P < .0001). New postoperative hemiparesis, in particular, had the most substantial association with survival (mean HR = 3.38, CI = 2.20-6.68, P < .0001). Cognitive and visual impairment and postoperative KPS were also independent predictors of survival; however, new postoperative aphasia and the change in KPS from baseline were not significantly associated with the outcome.
TABLE 3.
Univariate Cox Proportional Hazards Models for Variables Considered A Priori
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| Agea | 1.26 | 1.10-1.46 | .001 |
| EOR of CE tissueb | 0.91 | 0.84-0.99 | .040 |
| EOR of NCE tissueb | 0.94 | 0.88-0.99 | .040 |
| Residual CE volumec | 1.27 | 1.04-1.55 | .020 |
| Residual NCE volumed | 1.03 | .98-1.08 | .210 |
| New postoperative impairment | |||
| One or more | 1.87 | 1.38-2.53 | <.0001 |
| Cognitive | 1.51 | 1.15-2.00 | .003 |
| Aphasia | 1.12 | 0.61-2.06 | .72 |
| Visual | 1.48 | 1.08-2.04 | .016 |
| Hemiparesis | 3.83 | 2.20-6.68 | <.0001 |
| Postoperative KPSd | 0.84 | 0.73-0.97 | .0154 |
| Change in KPSd | 0.90 | 0.72-1.13 | .37 |
CE, contrast-enhanced; EOR, extent of resection; KPS, Karnofsky Performance Status; NCE, non–contrast-enhanced
Per 10-yr increase.
Per 10% increase.
Per 10-mL increase.
Per 10-point increase.
Multivariate RPA
Variables that exceeded the uncorrected statistical threshold of P < .05, including age, EOR of CE and NCE tissue, the volume of residual CE tissue, the presence of one or more new neurological impairment(s), cognitive and visual impairment, hemiparesis, and postoperative KPS, were included in the multivariate RPA. We restricted our models to the best 2 and best 3 partitions to improve clinical interpretability.
The results of RPA with the best 2 partitions and the accompanying Kaplan–Meier survival estimates based on those groupings are presented in Figure 1. The only variable that emerged from this model was the presence of a new postoperative neurological impairment at follow-up. Patients categorized in group 1 (N = 161) were identified as those without any new postoperative neurological impairments (median survival = 20.7 mo, 95% CI = 18.2-23.6), whereas those in group 2 (N = 67) had at least one impairment at follow-up (median survival = 13.2 mo, 95% CI = 12.1-15.7). The difference in survival between these groupings was statistically significant (HRGroup 2 vs 1 = 1.87, 95% CI = 1.38-2.53, P < .0001).
FIGURE 1.
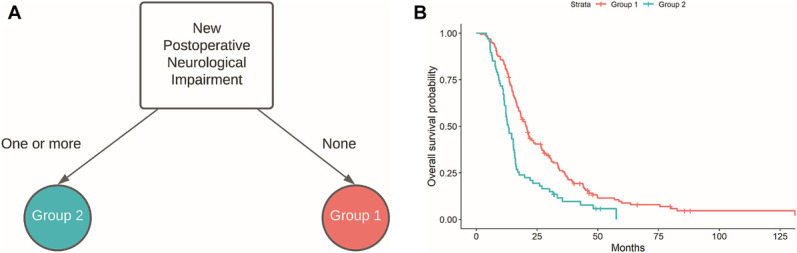
Decision tree A and Kaplan–Meier survival functions B subsequent to recursive partitioning and selection of the tree that yielded the best 2 partitions. Of all the clinical variables included in the model, new postoperative neurological impairment most efficiently stratified patients by their overall survival risk. Group 1 (N = 161) denotes patients who did not have a new neurological impairment at 1-mo postoperative follow-up (median survival = 20.7 mo, 95% CI = 18.2-23.6), whereas those in group 2 (N = 67) had at least one impairment at follow-up (median survival = 13.2 mo, 95% CI = 12.1-15.7).
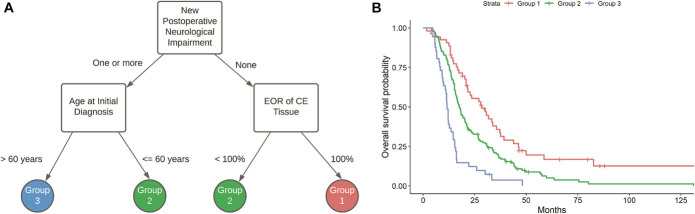
Further partitioning led to the decision tree and Kaplan–Meier survival estimates presented in Figure 2. As in the previous decision tree, the presence of a new postoperative neurological impairment informed the first split. However, in line with previous published reports, EOR of CE tissue and the patient's age at initial diagnosis emerged as significant modifiers of this effect.13 Specifically, patients in group 1 (N = 53) had (1) no new postoperative neurological impairment at follow-up and (2) underwent removal of all CE tissue, and thus had the best survival odds (median survival = 28.4 mo, 95% CI = 21.5-37.2). Patients who were categorized as intermediate risk (group 2, N = 134) met 1 of 2 criteria: they either (1) had no new neurological impairments but underwent subtotal resection of CE tissue or (2) had a new postoperative neurological impairment but were younger than 60 yr at initial diagnosis (median survival = 17.6 mo, 95% CI = 15.8-20.4). Finally, those with the worst prognosis (group 3, N = 41) experienced a new postoperative neurological impairment and were older than 60 yr at initial diagnosis, independent of EOR (median survival = 11.6 mo, 95% CI = 10.7-14.5). Post hoc Cox proportional hazards modeling with correction for multiple comparisons confirmed that each of the 3 groupings had a significantly different survival outcome: HRGroup 2 vs 1 = 1.79, 95% CI = 1.25-2.56, P = .001, HRGroup 3 vs 1 = 4.12, 95% CI = 2.62-6.47, P < .0001, and HRGroup 3 vs 2 = 2.30, 95% CI = 1.60-3.31, P < .0001. Collectively these data illustrate the impact of new neurological impairments on survival using a homogenous cohort of adult patients with newly diagnosed IDH wild-type GBM, all of whom were treated with chemoradiation.
FIGURE 2.
Decision tree A and Kaplan-Meier survival functions B subsequent to recursive partitioning and selection of the tree that yielded the best 3 partitions. Within this hierarchy, patients were separated into groups dependent on (1) whether they had a new postoperative neurological impairment at 1-mo follow-up and (2) their age and/or volumetric EOR of CE tissue. Group 1 (N = 53) denotes patients who did not have a new neurological impairment at 1-mo postoperative follow-up and underwent a complete resection of CE tissue (median survival = 28.4 mo, 95% CI = 21.5-37.2). Patients in group 2 (N = 134) either had no new impairment at follow-up but subtotal resection or had at least one impairment but were younger than 60 yr at initial diagnosis (median survival = 17.6 mo, 95% CI = 15.8-20.4). Patients in group 3 (N = 41) had at least one new impairment at follow-up and were older than 60 yr at initial diagnosis, regardless of EOR (median survival = 11.6 mo, 95% CI = 10.7-14.5). CE, contrast-enhanced; EOR, extent of resection.
DISCUSSION
Key Results
Using a homogenous population of patients with IDH wild-type GBM who (1) underwent maximal safe resection of CE and NCE tissue and (2) received adjuvant chemoradiation, we showed that new postoperative neurological impairment was the key mediator of overall survival among the entire cohort. We arrived at this conclusion after accounting for other clinical variables, such as age and KPS, that were independently associated with the survival outcome.
First, on univariate analysis, we demonstrated that even within this clinically homogenous population, there is a modest survival benefit to incremental increases in EOR of both CE and NCE tissue amounting to a 1% lower risk of death for every 1% increase in EOR. Although this finding has been replicated previously,18 we believe our study provides some of the most compelling data in support of an independent role of CE and NCE resection, given our patient population was intrinsically controlled for confounding by other life-prolonging adjuvant therapies (ie, temozolomide and radiation). We next revealed independent associations between postoperative impairment across several neurological domains (particularly motor) and an increased risk of death. Previous reports have been unable to fully appreciate this relationship, given the potential for biased referral patterns for adjuvant chemoradiation among patients with neurological deficits.9
Nevertheless, it is difficult to provide management recommendations based on these individual clinical variables because they interact in a nonlinear fashion and are often at odds with each other. In this study, we overcame this challenge by generating a multivariate hierarchical model that simultaneously provides a clinically intuitive framework for both the surgical management and prognostication of patients with IDH wild-type GBM in the post-Stupp era.
Interpretation
We demonstrated that the foremost priority of cytoreductive surgery within this population should be the safe removal of tumor-infiltrated tissue. This finding is particularly salient among patients older than 60 yr because the presence of any new postoperative neurological impairment at 1-mo follow-up, regardless of volumetric EOR, results in a worse prognosis (median survival = 11.6 mo). By contrast, we showed that patients of any age who underwent a complete resection of CE tissue without acquiring a persistent neurological impairment benefitted from a median survival of more than 28 mo. These findings not only highlight the complex interplay between neurological morbidity and mortality but also provide additional backing for the structured implementation of management approaches that both prevent neurological impairment (ie, intraoperative functional mapping) and promote postoperative recovery (ie, multidisciplinary rehabilitation).19-25 Indeed, considering 29.4% of the patients in our series had a new neurological impairment, which is comparable with the 29.6% previously reported in the literature,10 these approaches may prove to be worthwhile and cost-effective.
Interestingly, in this subpopulation of medically and surgically treated IDH wild-type GBM patients, a sharp distinction between EOR of NCE and CE tumor tissue did not emerge as separate independent variables in our multivariate statistical model. These data therefore illustrate the fact that the safe removal of CE tissue should be prioritized while minimizing postoperative neurological impairment, particularly in older patients. In addition, the effect of neurological impairment on survival may be dependent on the type of impairment, with severe motor deficits being the most harmful, implying that the a priori risk of specific impairments, given tumor size and location, should be considered and discussed with the patient when making a surgical plan. Furthermore, consistent with previous literature,16 we demonstrated that an aggressive EOR did not necessitate the onset of a new postoperative neurological impairment. These data provide further evidence that aggressive resections should be pursued whenever they can be performed safely.
In our cohort, we had one patient with bilateral (“butterfly”) GBM, an entity that is often managed with biopsy only, given the risks of surgical morbidity. However, this patient was able to undergo an 86% and 62% EOR of CE and NCE tissue, respectively, without suffering from any new neurological impairment at 1-mo follow-up, and ultimately survived for over 15 mo. Although this only represents a single case, increasing evidence suggests that maximal safe resection is also feasible in patients with bilateral GBM in part because of improvements in intraoperative neuronavigation, tractography, and passive and active cortical/subcortical mapping.26-28 Large prospective studies are necessary to explore this further.
Generalizability
Our study was retrospective and performed at a single-institution quaternary referral center where maximal safe resection of both CE and NCE tissue is the prevailing cytoreductive strategy for IDH wild-type GBM, potentially limiting the generalizability of the findings.
Limitations
First, we are unable to investigate the interaction between temporary postoperative impairments and outcomes because extensive resection of lesions that are near eloquent regions may lead to postoperative cerebral edema that transiently impair neurological function, but these deficits may not affect the patient's overall survival. Second, we did not have records of the exact dosages of radiotherapy and temozolomide that each patient included in our study received. Third, cognitive deficits were assigned based on the medical record and not formal neurocognitive or neuropsychiatric testing. Finally, given the expansive retrospective nature of our study, many patients did not undergo genomic methylation profiling, and therefore, we were unable include MGMT promoter methylation status in our multivariate survival models. Future studies with data on MGMT status may be useful to elucidate the prognostic significance of this marker in the setting of EOR and postoperative neurological function.
CONCLUSION
In a homogenous sample of IDH wild-type newly diagnosed GBM patients who went on to receive standard-of-care adjuvant temozolomide and radiotherapy, the presence of a new postoperative neurological impairment 1 mo after surgery was associated with worse survival.
Footnotes
Supplemental digital content is available for this article at www.neurosurgery-online.com.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplemental Digital Content
Supplemental Digital Content. Text, Figure, Supplemental Information. Extended methods, inter-rater reliability, and study workflow.
REFERENCES
- 1.Gritsenko PG, Atlasy N, Dieteren CEJ, et al. p120-catenin-dependent collective brain infiltration by glioma cell networks. Nat Cell Biol. 2020;22(1):97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eidel O, Burth S, Neumann JO, et al. Tumor infiltration in enhancing and non-enhancing parts of glioblastoma: a correlation with histopathology. PLoS One. 2017;12(1):e0169292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YM, Suki D, Hess K, et al. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016;124(4):977-988. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DR, Sawyer AM, Meyers CA, et al. Early measures of cognitive function predict survival in patients with newly diagnosed glioblastoma. Neuro Oncol. 2012;14(6):808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hervey-Jumper SL, Li J, Lau D, et al. Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J Neurosurg. 2015;123(2):325-339. [DOI] [PubMed] [Google Scholar]
- 7.Gogos AJ, Young JS, Morshed RA, et al. Awake glioma surgery: technical evolution and nuances. J Neurooncol. 2020;147(3):515-524. [DOI] [PubMed] [Google Scholar]
- 8.Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130(2):269-282. [DOI] [PubMed] [Google Scholar]
- 9.Gulati S, Jakola AS, Nerland US, et al. The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011;76(6):572-579. [DOI] [PubMed] [Google Scholar]
- 10.Rahman M, Abbatematteo J, Leo EKD, et al. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J Neurosurg. 2016;127(1):123-131. [DOI] [PubMed] [Google Scholar]
- 11.Jakola AS, Gulati S, Weber C, et al. Postoperative deterioration in health related quality of life as predictor for survival in patients with glioblastoma: a prospective study. PLoS One. 2011;6(12):e28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadipour Y, Kaur M, Pierscianek D, et al. Association of surgical resection, disability, and survival in patients with glioblastoma. J Neurol Surg A Cent Eur Neurosurg. 2019;80(4):262-268. [DOI] [PubMed] [Google Scholar]
- 13.Molinaro AM, Hervey-Jumper S, Morshed RA, et al. Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020;6(4):495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115-1123. [DOI] [PubMed] [Google Scholar]
- 15.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6(3):227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3-8. [DOI] [PubMed] [Google Scholar]
- 17.Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Certo F, Altieri R, Maione M, et al. FLAIRectomy in supramarginal resection of glioblastoma correlates with clinical outcome and survival analysis: a prospective, single institution, case series. Oper Neurosurg. 2021;20(2):151-163. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Jung Y, Park J, et al. Intensive rehabilitation therapy following brain tumor surgery: a pilot study of effectiveness and long-term satisfaction. Ann Rehabil Med. 2019;43(2):129-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18-27. [DOI] [PubMed] [Google Scholar]
- 21.Weyer-Jamora C, Brie MS, Luks TL, et al. Postacute cognitive rehabilitation for adult brain tumor patients. Neurosurgery. Published online February 15, 2021. doi: 10.1093/neuros/nyaa552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Witt Hamer PC, Klein M, Hervey-Jumper SL, et al. Functional outcomes and health-related quality of life following glioma surgery. Neurosurgery. 2021;88(4):720-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishna S, Kakaizada S, Almeida N, et al. Central nervous system plasticity influences language and cognitive recovery in adult glioma. Neurosurgery. 2021;89(4):539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morshed RA, Young JS, Kroliczek AA, et al. A neurosurgeon's guide to cognitive dysfunction in adult glioma. Neurosurgery. 2021;89(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morshed RA, Young JS, Lee AT, et al. Clinical pearls and methods for intraoperative awake language mapping. Neurosurgery. 2021;89(2):143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayani F, Young JS, Bonte A, et al. Safety and outcomes of resection of butterfly glioblastoma. Neurosurg Focus. 2018;44(6):E4. [DOI] [PubMed] [Google Scholar]
- 27.Burks JD, Bonney PA, Conner AK, et al. A method for safely resecting anterior butterfly gliomas: the surgical anatomy of the default mode network and the relevance of its preservation. J Neurosurg. 2016;126(6):1795-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forster MT, Behrens M, Lortz I, et al. Benefits of glioma resection in the corpus callosum. Sci Rep. 2020;10(1):16630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content. Text, Figure, Supplemental Information. Extended methods, inter-rater reliability, and study workflow.