BACKGROUND:
Hemispherectomy and its modern variants are effective surgical treatments for medically intractable unihemispheric epilepsy. Although some complications such as posthemispherectomy hydrocephalus are well documented, midline brain shift (MLBS) after hemispheric surgery has only been described anecdotally and never formally studied.
OBJECTIVE:
To assess the natural history and clinical relevance of MLBS and determine whether cerebrospinal fluid (CSF) shunting of the ipsilateral surgical cavity exacerbates MLBS posthemispheric surgery.
METHODS:
A retrospective review of consecutive pediatric patients who underwent hemispheric surgery for intractable epilepsy and at least 6 months of follow-up at UCLA between 1994 and 2018 was performed. Patients were grouped by MLBS severity, shunt placement, valve type, and valve opening pressure (VOP). MLBS was evaluated using the paired samples t-test and analysis of covariance adjusting for follow-up time and baseline postoperative MLBS.
RESULTS:
Seventy patients were analyzed, of which 23 (33%) required CSF shunt placement in the ipsilateral surgical cavity for posthemispherectomy hydrocephalus. MLBS increased between first and last follow-up for nonshunted (5.3 ± 4.9-9.7 ± 6.6 mm, P < .001) and shunted (6.6 ± 3.5-16.3 ± 9.4 mm, P < .001) patients. MLBS progression was greater in shunted patients (P = .001). Shunts with higher VOPs did not increase MLBS relative to nonshunted patients (P = .834), whereas MLBS increased with lower VOPs (P = .001). Severe MLBS was associated with debilitating headaches (P = .048).
CONCLUSION:
Patients undergoing hemispheric surgery often develop postoperative MLBS, ie, exacerbated by CSF shunting of the ipsilateral surgical cavity, specifically when using lower VOP settings. MLBS exacerbation may be related to overshunting. Severe MLBS is associated with debilitating headaches.
KEY WORDS: CSF diversion, epilepsy, hemispherectomy, intracranial pressure, midline brain shift
ABBREVIATIONS:
- ASD
antisiphon devices
- MLBS
midline brain shift
- OP
opening pressure
- PHH
posthemispherectomy hydrocephalus
- VOP
valve opening pressure.
Hemispherectomy was first described by Walter Dandy (1928) and adopted for treating intractable epilepsy after encouraging results from Kenneth McKenzie (1938) and Roland Krynauw (1950).1-4 However, undesirable posthemispherectomy sequelae including persistent intracranial bleeding and superficial cerebral hemosiderosis inspired the functional hemispherotomy, a modified approach centered on cerebral disconnections instead of resection.5-7 Despite these modifications, posthemispherectomy hydrocephalus (PHH) remains prevalent with an incidence of ∼23%, presumably because of obstruction of arachnoid granulations by hemoglobin, proteins, and inflammatory processes preventing cerebrospinal fluid (CSF) absorption.3,8-11 Placement of a CSF shunt after hemispheric surgery (ie, any surgery intended to resect and/or disconnect a cerebral hemisphere) is often necessary to treat PHH.
Some patients with intractable unihemispheric epilepsy demonstrate midline brain shift (MLBS) preoperatively because of size incongruence of the affected hemisphere, which results in lateral displacement of midline structures from the midline.12 Anecdotally, this shift progresses over time postoperatively. The evolution of radiographically identified MLBS after hemispheric surgery is largely unknown, and it is also unclear whether ipsilateral CSF shunting contributes to MLBS by exacerbating pressure imbalances between hemispheres. Furthermore, clinical consequences from MLBS remain unknown. Therefore, we reviewed 70 consecutive pediatric hemispheric surgery cases with adequate follow-up at UCLA to assess the natural history of MLBS. We hypothesized that CSF shunting of the ipsilateral surgical cavity postoperatively exacerbates MLBS and sought to evaluate its clinical significance in a large, single-center series.
METHODS
Patient Population
We retrospectively reviewed 70 patients (0-19 years old at surgery) with intractable epilepsy who underwent hemispheric surgery with at least 6 months of follow-up at UCLA Mattel Children's Hospital from 1994 to 2018. Charts were reviewed for demographics; follow-up imaging (computed tomography [CT]/MRI); date, type (anatomic/functional), and side of hemispheric surgery; underlying etiology; shunt placement; valve type; and valve opening pressure (VOP), if applicable. Histopathologic findings were grouped into 4 categories: porencephalic cyst/stroke, malformations of cortical development including hemimegalencephaly and focal cortical dysplasia, Rasmussen's encephalitis, and all other etiologies with no distinct lesion including Sturge–Weber syndrome. The UCLA Institutional Review Board approved this study and waived the need for informed consent. A retrospective analysis was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist.
Perioperative Management
Over the span of this study, 4 surgeons (A.F, G.M., Y.C., and W.P.) performed hemispheric surgeries for epilepsy with technique chosen based on surgeon preference and encompassing the evolution of techniques from more to less tissue resection. The functional hemispherotomy performed at UCLA is a variant of Rasmussen's functional hemispherectomy known as the modified lateral hemispherotomy and was used for all cases after its inception in 1997.13 All patients who underwent shunt placement were diagnosed with PHH and presented with clinical symptoms (eg, gastrointestinal upset and lethargy), radiographic evidence (eg, transependymal CSF flow and ventriculomegaly), or both. All shunts were placed ipsilaterally in the surgical cavity. We recorded clinical and radiographic indications for CSF shunting by whether they occurred in an immediate (<2 weeks) or delayed (≥2 weeks) fashion. Ten different shunt valves were used, with VOP settings and valve selected based on surgeon preference. We categorized valves between nonprogrammable fixed pressure valves with/without antisiphon devices (ASDs)—including medium pressure PS, medium pressure Medtronic, Delta, and distal slit valves—and programmable valves—including Medtronic Strata, Sophysa Polaris, and Codman Certas Plus valves. VOPs were recorded at last follow-up, and patients were dichotomized into “higher” and “lower” VOP subgroups, using the median VOP of the cohort as the dichotomization threshold. Lower VOPs were often used in infants and toddlers when there was suspicion of “low-pressure hydrocephalus” given its prevalence in younger cohorts.14
MLBS Calculation
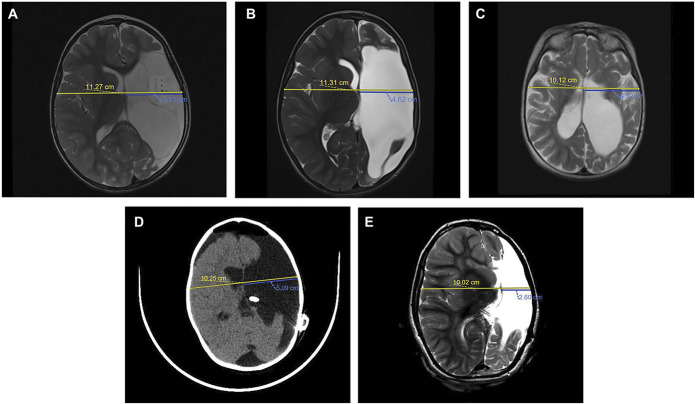
MLBS was calculated at the level of the foramen of Monro by halving the biparietal diameter and subtracting the distance from the inner table to the septum pellucidum on the side of the shift.12 A shift ≥2.0 mm was required to be considered MLBS to account for error intrinsic to measuring imaging scans. Measurements were performed and verified by 2 authors (C.A.B.M. and S.L.D.) using primarily CT studies and MRI studies if CT imaging was unavailable. Both the authors were blinded to the patients' neurological symptoms and shunt settings, if applicable. For nonshunted patients, we calculated MLBS in their first and most recent postoperative imaging (Figure 1A and 1B). For shunted patients, we calculated MLBS in their first postoperative, preshunt, first postshunt, and latest imaging (Figure 1C-1E).
FIGURE 1.
MRI and CT scans demonstrating MLBS quantification in nonshunted and shunted patients. The biparietal diameter (yellow line) is divided by 2 and subtracted from the ipsilateral distance from the inner table to the septum pellucidum (blue line). A, Preoperative axial turbo spin echo (TES) T2-weighted MRI of the pediatric patient with history of medically intractable epilepsy secondary to left perinatal middle cerebral artery stroke demonstrating a baseline MLBS of 0.51 cm. B, Postoperative axial TSE T2-weighted MRI of the patient from A at 6 months after functional hemispherectomy demonstrating a MLBS of 1.04 cm. C, Preoperative axial TSE T2-weighted MRI of the pediatric patient with history of medically refractory infantile spasm demonstrating a baseline MLBS of 0.14 cm. D, Postoperative, noncontrast enhanced axial CT of the patient from C after functional hemispherectomy and subsequent early cerebrospinal fluid shunting secondary to PHH demonstrating a MLBS of 0.04 cm. E, Surveillance axial TSE T2-weighted MRI of the patient from C and D at a 72-month follow-up with a MLBS of 2.41 cm. CSF, cerebrospinal fluid; CT, computed tomography; MLBS, midline brain shift; TSE, turbo spin echo.
Neurological Symptoms Associated With MLBS
To select for the most severe MLBS cases, patients who were 1 standard deviation above the mean MLBS at last follow-up (≥22.0 mm) were considered severe. Two groups were evaluated for neurological symptomatology: severe MLBS (≥22.0 mm) and mild MLBS (<22.0 mm). Symptoms were recorded by evaluating pre- and postoperative notes to account for baseline status and categorized as follows: motor deficits, sensory deficits, language deficits, visual deficits, cranial nerve palsies, metabolic/endocrine abnormalities, behavior changes, headaches, seizures, and other unspecified symptoms.
Statistical Analysis
Statistical analysis was conducted using RStudio (RStudio Inc., Version 1.2.1335). Continuous variables are presented using means and standard deviation, means and interquartile range, or median and range. Categorical data are presented using frequency and percentages. Mann-Whitney U and Fisher's exact tests were used to compare continuous and categorical variables, respectively. MLBS was compared between groups at specific time points using analysis of covariance, adjusting for the covariate, and confounding effects of follow-up time and baseline MLBS. The paired samples t-test was used to evaluate MLBS changes within groups. A 2-tailed P-value <.05 was the threshold for statistical significance.
RESULTS
Patients
Seventy patients who underwent hemispheric surgery for intractable epilepsy between 1994 and 2018 were assessed. The median follow-up was 3.3 years (0.5-19 years). The average age at surgery was 4.5 years, and most of the procedures were functional hemispherectomies (98%). The most common etiology was malformations of cortical development in 38 (54%) patients, followed by “others/nonlesional” in 13 (19%), Rasmussen's encephalitis in 13 (19%), and porencephalic cyst/stroke in 6 (9%).
Table 1 describes the cohort stratified by CSF shunting. Of the 70 patients, 23 (33%) underwent CSF shunt placement. Shunted patients were younger at surgery (2.5 vs 5.4 years, P = .013) and had longer follow-ups (72.1 vs 29.1 months, P = .002) than nonshunted patients. The shunted cohort also had less number of patients with Rasmussen's encephalitis (4.3% vs 25.5%, P = .048), but they were comparable across the remaining etiologies. There were no differences in sex and type or side of hemispheric surgery. Of the shunted patients, the majority (65%) received nonprogrammable valves and the remaining (35%) received programmable valves. The median time to shunting was 4 months (6 days-9.6 years). Table 2 outlines the indications for CSF diversion and are stratified by immediate vs delayed presentation. Of the 15 patients in the delayed period, 7 did not have radiographic indications, but had compelling clinical indications to prompt CSF shunting including CSF drainage from the ventriculostomy site, seizures, severe headaches, gastrointestinal upset, and lethargy.
TABLE 1.
Study Population Characteristics
| No CSF shunt (n = 47) | CSF shunt (n = 23) | P-value | |
|---|---|---|---|
| Mean age at hemispheric surgery (yr) | 5.4 (8) | 2.5 (2) | .013a |
| Sex | 1.000 | ||
| Female | 18 (38.3%) | 9 (39.1%) | |
| Male | 29 (61.7%) | 14 (60.9%) | |
| Hemispheric surgery side | .606 | ||
| Left | 29 (61.7%) | 12 (52.2%) | |
| Right | 18 (38.3%) | 11 (47.8%) | |
| Hemispheric surgery type | 1.000 | ||
| Anatomic | 2 (4.3%) | 0 (0.0%) | |
| Functional | 45 (95.7%) | 23 (100.0%) | |
| Etiology | |||
| Malformation of cortical development | 25 (53.2%) | 13 (56.5%) | 1.000 |
| Rasmussen's encephalitis | 12 (25.5%) | 1 (4.3%) | .048a |
| Porencephalic cyst/stroke | 3 (6.4%) | 3 (13.0%) | .387 |
| Others/nonlesional | 7 (14.9%) | 6 (26.1%) | .330 |
| Posthemispheric surgery MLBS (mm)b | 5.3 ± 4.9 | 6.6 ± 3.5 | .261 |
| Months from hemispheric surgery to initial postoperative imaging | 0.3 (0.0-125.9) | 0.2 (0.0-71.6) | .125 |
| Preshunt MLBS (mm) | — | 7.3 ± 3.5 | — |
| Months from hemispheric surgery to preshunt imaging | — | 4.2 (0.0-115.3) | — |
| Postshunt MLBS (mm) | — | 11.4 ± 9.5 | — |
| Months from hemispheric surgery to postshunt imaging | — | 14.2 (0.4-190.7) | — |
| Last follow-up MLBS (mm)c | 9.7 ± 6.6 | 16.3 ± 9.4 | .001a |
| Months from hemispheric surgery to last follow-up | 29.1 (6.0-167.5) | 72.1 (6.2-226.9) | .002a |
| Time-matched last follow-up MLBS (mm)c,d | 9.5 ± 7.0 | 15.4 ± 10.4 | .049a |
| Time-matched months from hemispheric surgery to last follow-upd | 37.7 (12.3-84.0) | 38.8 (17.7-77.0) | .151 |
| Seizure freedom | .114 | ||
| Yes | 34 (72.3%) | 12 (52.2%) | |
| No | 13 (27.7%) | 11 (47.8%) |
ANCOVA, analysis of covariance; MLBS, midline brain shift.
p < .05.
Comparisons based on ANCOVA controlling for time from hemispheric surgery to initial postoperative imaging.
Comparisons based on ANCOVA controlling for time from hemispheric surgery to last follow-up and initial posthemispheric surgery MLBS.
Only includes players with follow-up durations between 1.0 and 7.0 years, which represent the first and third quartile of the entire cohort.
Values are presented as mean ± standard deviation, mean (interquartile range), median (range), or number of patients (%).
TABLE 2.
Indications for CSF Shunt Placement into the Ipsilateral Surgical Cavity
| Immediate (n = 8) | Delayed (n = 15) | |
|---|---|---|
| Clinical indications | ||
| Gastrointestinal symptoms (nausea, vomiting, and by mouth difficulty) | 3 (37.5%) | 3 (20.0%) |
| Lethargy | 1 (12.5%) | 2 (13.3%) |
| CSF leak from the ventriculostomy site | 1 (12.5%) | 3 (20.0%) |
| Failure to wean off external ventricular drain | 2 (25.0%) | 0 (0.0%) |
| Subgaleal CSF collection | 1 (12.5%) | 0 (0.0%) |
| Elevated ICP | 1 (12.5%) | 0 (0.0%) |
| Seizures | 0 (0.0%) | 2 (13.3%) |
| Headaches | 0 (0.0%) | 2 (13.3%) |
| Irritability | 0 (0.0%) | 1 (6.7%) |
| Motor coordination difficulty | 0 (0.0%) | 1 (6.7%) |
| Radiographic indications | ||
| Ventriculomegaly | 3 (37.5%) | 5 (33.3%) |
| Compressive intradural CSF collection | 0 (0.0%) | 2 (13.3%) |
| Transependymal CSF flow | 0 (0.0%) | 1 (6.7%) |
CSF, cerebrospinal fluid; ICP, intracranial pressure.
Values are presented as the number of patients (%).
MLBS Increases in Patients Receiving Ipsilateral CSF Shunts
Table 1 describes MLBS at first and last follow-up stratified by shunt placement. There was no difference in MLBS at first follow-up between nonshunted and shunted patients while adjusting for time to first follow-up (5.3 ± 4.9 vs 6.6 ± 3.5 mm, P = .261). There was also no difference in time to first follow-up (P = .125), with the median of 0.2 and 0.3 months in the shunted and nonshunted cohorts, respectively.
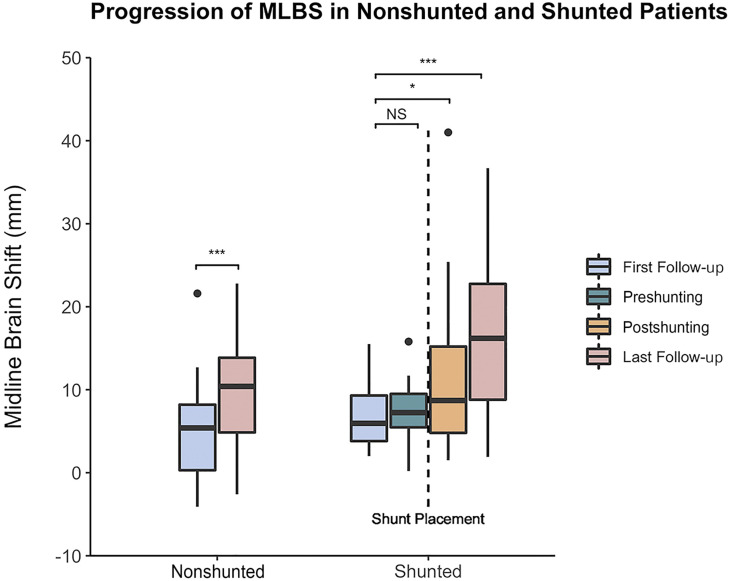
Figure 2 depicts MLBS progression across follow-ups in the nonshunted and shunted subgroups. MLBS did not significantly change before shunt placement (P = .430), with MLBS before CSF shunting being 7.3 ± 3.5 mm (Figure 2). However, MLBS significantly increased to 11.4 ± 9.5 mm after CSF shunting (P = .038). Between first and last follow-up, MLBS significantly increased by 4.4 mm for nonshunted (P < .001) and 9.9 mm for shunted patients (P < .001) (Figure 2). At last follow-up, MLBS was greater in shunted than nonshunted patients (16.3 ± 9.4 vs 9.7 ± 6.6 mm, P = .001), despite controlling for initial postoperative MLBS and time to last follow-up.
FIGURE 2.
Natural progression of MLBS in nonshunted and shunted patients. MLBS significantly increased between the first and last follow-up for nonshunted and shunted patients according to the paired samples t-test.*P < .05, **P < .01, and ***P < .001. MLBS, midline brain shift; NS, no significance.
Given the significant difference in follow-up duration between shunted and nonshunted cohorts, subgroup analysis using only patients with comparable follow-up was performed. Patients with greater than 1.0 but less than 7.0 years of follow-up, which represent the first and third quartiles of the cohort, were included in a subgroup of 12 shunted and 24 nonshunted patients. The median follow-up of the shunted (17.7-77.0 months) and nonshunted (12.3-84.0 months) patients was 38.8 and 37.7 months, respectively, and was not significantly different (P = .151). However, shunted patients still had greater MLBS than nonshunted patients (15.4 ± 10.4 vs 9.5 ± 7.0 mm, P = .049).
MLBS Increases With the Use of Lower Opening Pressure CSF Shunt Valves
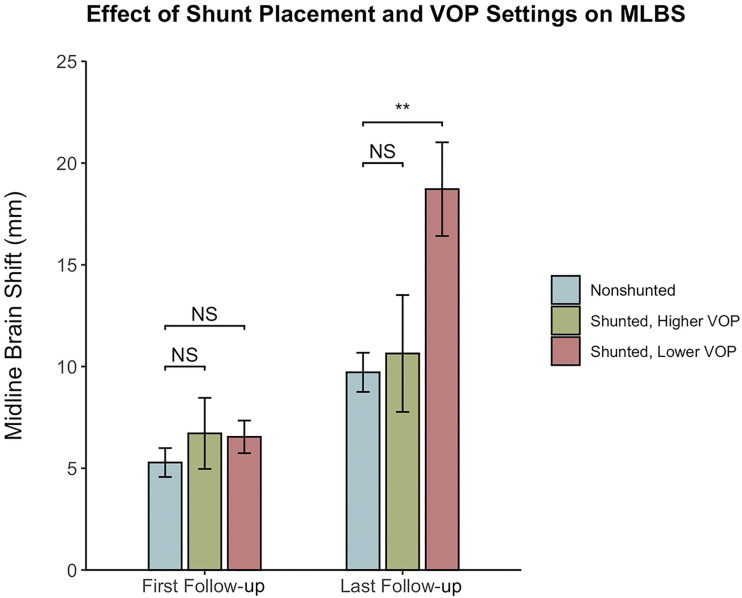
The median VOP of our cohort was 5 cmH2O, where the range was 3 cmH2O to 20 cmH2O. Patients with higher and lower VOPs were therefore defined as those with a VOP >5 and ≤5 cmH2O, respectively. The cohort was also divided based on the shunt valve type (programmable and nonprogrammable). Table 3 compares these subgroups with nonshunted patients at last follow-up. MLBS at last follow-up in nonshunted patients (9.7 ± 6.6 mm) was not different from that in patients with higher VOPs (10.6 ± 7.6 mm, P = .834) but was significantly lower than that in patients with lower VOPs (18.7 ± 9.2 mm, P = .001). Similarly, MLBS at last follow-up in nonshunted patients was not different from that in patients with programmable valves (11.2 ± 8.4 mm, P = .912), but was significantly lower than that in patients with nonprogrammable valves (18.9 ± 8.9 mm, P < .001). Figure 3 depicts MLBS progression of the VOP strata compared with nonshunted patients. Similar analyses were performed after stratifying shunted patients according to whether their shunt had an ASD. Inferential statistics was limited in the subgroup without ASDs because of small sample size (n = 4). However, patients both with and without ASDs had comparably high MLBS at last follow-up (16.5 ± 9.6 and 15.2 ± 9.4 mm, respectively), and patients with ASDs had greater MLBS than nonshunted patients (P = .011).
TABLE 3.
Subgroup Comparison of MLBS at Last Follow-Up With Nonshunted Patients
| Subgroup | MLBS at last follow-up (mm) | Difference | F-value | P-valuea |
|---|---|---|---|---|
| Higher opening pressure (n = 7) | 10.6 ± 7.6 | 0.9 | F (1, 50) = 0.0 | .834 |
| Lower opening pressure (n = 16) | 18.7 ± 9.2 | 9.0 | F (1, 58) = 12.9 | .001b |
| Programmable shunts (n = 8) | 11.2 ± 8.4 | 1.5 | F (1, 51) = 0.0 | .912 |
| Nonprogrammable shunts (n = 15) | 18.9 ± 8.9 | 9.2 | F (1, 57) = 14.8 | <.001b |
MLBS, midline brain shift.
Comparisons based on analysis of covariance controlling for time from hemispheric surgery to imaging study and initial posthemispheric surgery MLBS.
MLBS values are presented as mean ± standard deviation, and the difference is calculated relative to the nonshunted patient cohort.
p < .05.
FIGURE 3.
Comparison of MLBS in nonshunted, shunted with higher VOP, and shunted with lower VOP patients posthemispheric surgery and at last follow-up. No statistical difference was observed between nonshunted and shunted with higher VOP patients at last follow-up, whereas shunted with lower VOP patients had significantly greater MLBS than nonshunted patients according to analysis of covariance. **P < .01. MLBS, midline brain shift; NS, no significance; OP, opening pressure; VOP, valve opening pressure.
Symptoms Associated with MLBS
Fifty patients had sufficient preoperative and postoperative data available for evaluation of new neurological symptoms, which are summarized in Table 4. Seven patients (14.0%) were included in the severe group for having MLBS at last follow-up of ≥22.0 mm. Age was comparable between the 2 groups (4.7 vs 3.1 years, P = .395). Of note, the severe group underwent CSF shunting (P = .004) and experienced postoperative debilitating headaches (P = .048) more frequently than the mild cohort, and all patients with debilitating headaches and severe MLBS (n = 3) were shunted.
TABLE 4.
Clinical Symptoms Associated With Mild and Severe MLBS
| Clinical symptom | Mild (MLBS <22.0 mm, n = 43) | Severe (MLBS ≥22.0 mm, n = 7) | P-value |
|---|---|---|---|
| MLBS (mm) | 12.1 ± 5.7 | 27.3 ± 5.2 | <.001a |
| Age at hemispheric surgery (yr) | 4.7 (8) | 3.1 (4) | .395 |
| Shunt placement | .004a | ||
| Shunted | 11 (25.6%) | 6 (85.7%) | |
| Nonshunted | 32 (74.4%) | 1 (14.3%) | |
| Language deficits | 4 (9.3%%) | 3 (42.9 %) | .048a |
| Motor deficits | 43 (100.0%) | 7 (100.0%) | 1.000 |
| Sensory deficits | 6 (14.0%) | 1 (14.3%) | 1.000 |
| Visual deficits | 26 (60.5%) | 6 (85.7%) | .398 |
| Cranial nerve palsies | 27 (62.8%) | 6 (85.7%) | .398 |
| Metabolic/endocrine abnormalities | 6 (14.0%) | 1 (14.3%) | 1.000 |
| Behavior changes | 8 (18.6%) | 3 (42.9%) | .170 |
| Headaches | 4 (9.3%) | 3 (42.9%) | .048a |
| Persistent/recurrent seizures | 13 (30.2%) | 5 (71.4%) | .083 |
| Others | 5 (11.6%) | 4 (57.1%) | .015a |
MLBS, midline brain shift.
Values are presented as mean ± standard deviation, mean (interquartile range), or number of patients (%).
p < 0.05.
DISCUSSION
In this study, we evaluated the natural history of MLBS after hemispheric surgery for epilepsy and the impact of CSF shunting of the ipsilateral surgical cavity on posthemispheric surgery MLBS. We found that (1) MLBS naturally occurs toward the side of surgery postoperatively and (2) MLBS is exacerbated by CSF shunting for PHH, specifically when lower VOPs are used. Furthermore, MLBS progression because of CSF shunting with higher VOPs and programmable valves is comparable with that of nonshunted patients, thus providing indirect evidence that decreasing CSF drainage may prevent severe MLBS. We confirm that greater MLBS in shunted patients is attributed to CSF shunting using a subgroup analysis that accounts for differences in follow-up duration. Finally, we demonstrate an association between severe MLBS and debilitating headaches, providing evidence that MLBS after hemispheric surgery is clinically significant. To the best of our knowledge, this study is the first to highlight the natural history, clinical relevance, and contribution of CSF shunting to posthemispheric surgery MLBS in a large, single-center series.
The pathophysiology of PHH remains incompletely understood, but decreased CSF absorption through arachnoid granulations likely plays an important role.9,10 In our semicontemporary cohort, the rate of hydrocephalus requiring CSF shunting was 32.9%, consistent with previous studies.3,15-19 In these patients, MLBS progression and clinical symptoms, notably debilitating headaches, were common adverse outcomes, which may be mitigated by setting higher VOPs. Lower VOPs may be associated with an increased risk of CSF overdrainage and subsequent intracranial hypotension, likely contributing to severe MLBS through an exacerbated pressure gradient toward the shunted cavity.20-22 Therefore, higher VOPs may optimally balance adequate CSF shunting for PHH and MLBS prevention, as evidenced by the similarities between the higher VOP and nonshunted groups.
Programmable valves with modifiable settings as opposed to nonprogrammable valves with ASDs may also mitigate MLBS. Patients with programmable valves had comparable MLBS with nonshunted patients. Conversely, patients with nonprogrammable valves and ASDs had greater MLBS than nonshunted patients, suggesting that the ability to adjust VOP according to patient presentation may be better for preventing MLBS progression than maintenance of a constant VOP.23 This is especially important in younger patients undergoing hemispheric surgery whose CSF shunting requirements may evolve with age. In addition, CSF shunting from the contralateral hemisphere, while controversial given risks to the remaining normal brain, may reduce MLBS because of shifts in intracranial pressure away from the surgical cavity. However, further studies are necessary to assess the risk-benefit analysis of this practice.
A study from Cleveland Clinic similarly reported headaches as a postoperative concern in some patients after hemispherectomy.24 In our study, debilitating headaches may be another signal of abnormal intracranial pressure causing severe MLBS, given their association with intracranial hypotension.25 In addition, all patients with severe MLBS and debilitating headaches had CSF shunting, reinforcing the possibility that these conditions manifest from intracranial hypotension secondary to overshunting. The proportional association between MLBS severity, pressure gradients, and debilitating headaches further highlights how this demographic may benefit from conservative CSF shunting protocols.
Our cohort demonstrated no radiographic evidence, hearing impairment, or other signs to raise suspicions of superficial cerebral hemosiderosis resulting in headaches or other neurological symptoms.26,27 When comparing MLBS, follow-up duration was controlled to account for natural posthemispheric surgery MLBS progression over time. Although shunted patients were younger than nonshunted patients, univariate linear regression showed no correlation between MLBS and age, thus abrogating the need to adjust for age.
Limitations
This study has several limitations. MLBS was calculated manually and hence was subject to errors related to imaging resolution. Although subgroup analysis comparing patients with similar follow-up was performed, the timing and duration of follow-up were not standardized between shunted and nonshunted patients. Evaluating how MLBS affects adverse outcomes, rehabilitation, and seizure control and potential management strategies with programmable valves was not feasible with our available data. Given the retrospective nature of this study, evaluation of neurological symptoms is limited by the quality and thoroughness of notes documented in the patients' charts and the ability to assess these attributes in younger and/or impaired patients. Analyzing CSF shunting contralaterally to the surgical cavity and its effects on MLBS was not possible because it was performed infrequently. This study analyzes the experience of a single institution that uses the modified lateral hemispherotomy, which is well described but not adopted universally. Furthermore, hemispheric surgery techniques in this study involved a greater degree of tissue resection than purely disconnective techniques, which may result in different complication profiles, including MLBS severity and rate of hydrocephalus. Thus, these findings may not be reproducible across centers, especially when different techniques are used. Finally, despite strong evidence that shunting exacerbates MLBS, we recognize that this phenomenon is likely multifactorial and influenced by other factors such as brain compliance, turgor, and skull growth potential. Studies designed to analyze these metrics will help further characterize MLBS in this population.
CONCLUSION
MLBS occurs after hemispheric surgery and is exacerbated after CSF shunting from the ipsilateral surgical cavity. MLBS is worse with lower VOPs and nonprogrammable valves, and severe MLBS is often associated with debilitating headaches. Further studies are necessary to deduce the etiology of this phenomenon and investigate the effects of alternative strategies, such as contralateral CSF shunting and/or changes in programmable shunt and VOP settings, on MLBS severity to mitigate these undesired postoperative sequelae.
Footnotes
Portions of this work were presented in poster form at the 48th Annual Meeting of the AANS/CNS Section of Pediatric Neurological Surgery in Scottsdale, AZ, on December 7, 2019.
Funding
This study did not receive any funding or financial support. H. Westley Phillips is supported by the National Institutes of Health/National Cancer Institute grant R25 NS079198.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENT
The authors should be commended for publishing this large single-center retrospective review correlating MLBS and severe headache. Hemispheric surgery for epilepsy is among the most effective for reducing seizure burden in indicated patients; unfortunately, hydrocephalus that can result and shunting have significant consequences. This article presents level 3 evidence that MLBS toward the operated hemisphere occurs naturally, that ipsilateral shunt placement worsens this shift, and that severe MLBS is associated with intractable headaches. Still unknown is whether moving a shunt from an ipsilateral to contralateral position addresses intractable headaches in patients with severe MLBS. In my practice, this article helps support a decision to place a contralateral shunt using a medium pressure or programmable valve when a shunt becomes clinically indicated.
Jeffrey Steven Raskin
Indianapolis, Indiana, USA
REFERENCES
- 1.Bahuleyan B, Robinson S, Nair AR, Sivanandapanicker JL, Cohen AR. Anatomic hemispherectomy: historical perspective. World Neurosurg. 2013;80(3-4):396-398. [DOI] [PubMed] [Google Scholar]
- 2.Beier AD, Rutka JT. Hemispherectomy: historical review and recent technical advances. Neurosurg Focus. 2013;34(6):E11. [DOI] [PubMed] [Google Scholar]
- 3.Lew SM, Koop JI, Mueller WM, Matthews AE, Mallonee JC. Fifty consecutive hemispherectomies: outcomes, evolution of technique, complications, and lessons learned. Neurosurgery. 2014;74(2):182-194; discussion 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peacock WJ. Hemispherectomy for the treatment of intractable seizures in childhood. Neurosurg Clin N Am. 1995;6(3):549-563. [PubMed] [Google Scholar]
- 5.Oppenheimer DR, Griffith HB. Persistent intracranial bleeding as a complication of hemispherectomy. J Neurol Neurosurg Psychiatry. 1966;29(3):229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams CB. Hemispherectomy—a modification. J Neurol Neurosurg Psychiatry. 1983;46(7):617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci. 1983;10(2):71-78. [DOI] [PubMed] [Google Scholar]
- 8.Daniel RT, Lee GY, Halcrow SJ. Low-pressure hydrocephalic state complicating hemispherectomy: a case report. Epilepsia. 2002;43(5):563-565. [DOI] [PubMed] [Google Scholar]
- 9.Lew SM, Matthews AE, Hartman AL, Haranhalli N, Post-Hemispherectomy Hydrocephalus W. Posthemispherectomy hydrocephalus: results of a comprehensive, multiinstitutional review. Epilepsia. 2013;54(2):383-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phung J, Krogstad P, Mathern GW. Etiology associated with developing posthemispherectomy hydrocephalus after resection-disconnection procedures. J Neurosurg Pediatr. 2013;12(5):469-475. [DOI] [PubMed] [Google Scholar]
- 11.Strowitzki M, Kiefer M, Steudel WI. Acute hydrocephalus as a late complication of hemispherectomy. Acta Neurochir (Wien). 1994;131(3-4):253-259. [DOI] [PubMed] [Google Scholar]
- 12.Liao CC, Chen YF, Xiao F. Brain midline shift measurement and its automation: a review of techniques and algorithms. Int J Biomed Imaging. 2018;2018:4303161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemb M, Velasco TR, Parnes MS, et al. Improved outcomes in pediatric epilepsy surgery: the UCLA experience. Neurology. 2010;74(22):1768-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smalley ZS, Venable GT, Einhaus S, Klimo P, Jr. Low-pressure hydrocephalus in children: a case series and review of the literature. Neurosurgery. 2017;80(3):439-447. [DOI] [PubMed] [Google Scholar]
- 15.Cook SW, Nguyen ST, Hu B, et al. Cerebral hemispherectomy in pediatric patients with epilepsy: comparison of three techniques by pathological substrate in 115 patients. J Neurosurg. 2004;100(2 suppl pediatrics):125-141. [DOI] [PubMed] [Google Scholar]
- 16.Griessenauer CJ, Salam S, Hendrix P, et al. Hemispherectomy for treatment of refractory epilepsy in the pediatric age group: a systematic review. J Neurosurg Pediatr. 2015;15(1):34-44. [DOI] [PubMed] [Google Scholar]
- 17.Peacock WJ, Wehby-Grant MC, Shields WD, et al. Hemispherectomy for intractable seizures in children: a report of 58 cases. Childs Nerv Syst. 1996;12(7):376-384. [DOI] [PubMed] [Google Scholar]
- 18.Schusse CM, Smith K, Drees C. Outcomes after hemispherectomy in adult patients with intractable epilepsy: institutional experience and systematic review of the literature. J Neurosurg. 2018;128(3):853-861. [DOI] [PubMed] [Google Scholar]
- 19.Sood S, Ilyas M, Marupudi NI, et al. Anatomical hemispherectomy revisited-outcome, blood loss, hydrocephalus, and absence of chronic hemosiderosis. Childs Nerv Syst. 2019;35(8):1341-1349. [DOI] [PubMed] [Google Scholar]
- 20.Khan QU, Wharen RE, Grewal SS, et al. Overdrainage shunt complications in idiopathic normal-pressure hydrocephalus and lumbar puncture opening pressure. J Neurosurg. 2013;119(6):1498-1502. [DOI] [PubMed] [Google Scholar]
- 21.Gebert AF, Schulz M, Schwarz K, Thomale UW. Long-term survival rates of gravity-assisted, adjustable differential pressure valves in infants with hydrocephalus. J Neurosurg Pediatr. 2016;17(5):544-551. [DOI] [PubMed] [Google Scholar]
- 22.Bergsneider M, Yang I, Hu X, McArthur DL, Cook SW, Boscardin WJ. Relationship between valve opening pressure, body position, and intracranial pressure in normal pressure hydrocephalus: paradigm for selection of programmable valve pressure setting. Neurosurgery. 2004;55(4):851-858; discussion 858-859. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Lage JF, Ruiz-Espejo AM, Almagro MJ, Alfaro R, Felipe-Murcia M, Lopez-Guerrero AL. CSF overdrainage in shunted intracranial arachnoid cysts: a series and review. Childs Nerv Syst. 2009;25(9):1061-1069. [DOI] [PubMed] [Google Scholar]
- 24.Pandit IRA, Bingaman W. Headache frequency in children after hemispherectomy, abstract 99. Presented at 48th National Meeting of the Child Neurology Society; October 23–26, 2019; The Charlotte Convention Center, Charlotte, NC.
- 25.Agrawal D, Durity FA. Seizure as a manifestation of intracranial hypotension in a shunted patient. Pediatr Neurosurg. 2006;42(3):165-167. [DOI] [PubMed] [Google Scholar]
- 26.Lummel N, Wollenweber FA, Demaerel P, et al. Clinical spectrum, underlying etiologies and radiological characteristics of cortical superficial siderosis. J Neurol. 2015;262(6):1455-1462. [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson BE, Walton JN. Superficial haemosiderosis of the central nervous system. J Neurol Neurosurg Psychiatry 1964;27(4):332-339. [DOI] [PMC free article] [PubMed] [Google Scholar]