Abstract
A single-copy chromosomal reporter system was used to measure the intrinsic strengths and interactions between the three promoters involved in the establishment of lysogeny by coliphage 186. The maintenance lysogenic promoter pL for the immunity repressor gene cI is intrinsically ∼20-fold weaker than the lytic promoter pR. These promoters are arranged face-to-face, and transcription from pL is further weakened some 14-fold by the activity of pR. Efficient establishment of lysogeny requires the pE promoter, which lies upstream of pL and is activated by the phage CII protein to a level comparable to that of pR. Transcription of pE is less sensitive to converging pR transcription and raises cI transcription at least 55-fold. The pE promoter does not occlude pL but inhibits lytic transcription by 50%. This interference is not due to bound CII preventing elongation of the lytic transcript. The pE RNA is antisense to the anti-immune repressor gene apl, but any role of this in the establishment of lysogeny appears to be minimal.
Temperate bacteriophages provide model systems for studying developmental switches. One question of interest is how these phages achieve efficient and conditional transitions between the lytic and lysogenic alternative developmental states. Bacteriophage lambda, in response to DNA-damaging agents, leaves the lysogenic state and enters lytic development, a process termed prophage induction (7). The reverse transition, establishment of lysogeny, is conditional in lambda upon the activity of a lytic operon gene, cII (9), that responds to the physiology of the infected cell (24). Bacteriophage P2 is the prototype of the second major group of temperate bacteriophages of Escherichia coli (2, 3), and its genome shares very little DNA homology with lambda (4). P2 forms a noninducible prophage and does not require a cII-like gene in order to establish lysogeny (4). However, these traits are not shared by all P2-like phages. Bacteriophage 186 is highly related to P2 (4) yet is like lambda in being SOS inducible (28) and requiring a cII gene for efficient lysogenization (11).
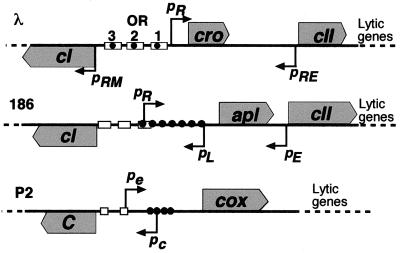
Lytic and lysogenic development are controlled in these phages by a stable switch formed by the lytic and lysogenic promoters and the first genes of the divergent lytic and lysogenic operons (Fig. 1). In lysogeny, the lysogenic or maintenance promoter (pRM/pL/pc) is active, resulting in production of the immunity repressor (CI/C), which represses the lytic promoter (pR/pe) and stimulates the lysogenic promoter. Prophage induction in lambda and 186 involves inactivation of the CI protein by activated RecA coprotease (17) or by a phage-encoded protein, Tum (10). In lytic development, the lytic promoter is active and the first gene of the lytic transcript encodes a repressor (Cro/Apl/Cox) that modulates the activity of the switch promoters (12, 15, 19). Initially, upon lytic infection of a sensitive cell, no immunity repressor is present. Establishment of lysogeny requires that adequate immunity repressor is made sufficiently quickly to block lytic development. Once present, the immunity repressor stimulates the lysogenic promoter and maintains its own production. In lambda and 186, but not P2, establishment of lysogeny requires an alternative promoter for cI (pRE/pE) that lies distal to the cro and apl genes, respectively (Fig. 1). This establishment promoter is activated by the binding of CII (13, 23). The establishment transcript extends antisense across the cro/apl gene, traverses the switch promoters and the binding sites for the switch proteins, and enters the cI gene.
FIG. 1.
Lysis-lysogeny switches of λ, 186, and P2. CI and C are immunity repressors, and Cro, Apl, and Cox are anti-immunity repressors. The diagrams are not to scale, but the relative distances between the repressor and anti-immunity repressor open reading frames for the three switches are accurate. Note the back-to-back arrangement of the lytic (pR) and lysogenic (pRM) promoters in λ, their face-to-face arrangement in 186 (pR and pL) and P2 (pe and pc), and the different locations of the binding sites for the immunity (rectangles) and anti-immunity (filled circles) repressors. pRE and pE are the CII-activated establishment promoters of λ and 186, respectively.
The activity of lambda pRE appears to promote lysogeny in a number of ways. The pRE promoter is much stronger than pRM (16), and production of CI from the pRE RNA is more efficient than from the pRM transcript, which lacks typical ribosome binding sequences (21). It has also been shown that converging transcription from pRE interferes with lytic transcription from pR (20), and it has been suggested that the pRE RNA might inhibit Cro production by an antisense mechanism (26), although no experiments have tested the latter hypothesis. In this study, we examined the roles of CII and pE in the establishment of lysogeny in 186. We did not expect differences in the efficiency of CI production from the pL and pE transcripts, since they share the 70 bases upstream of the cI start codon; however, we wished to know to what degree CII was able to increase transcription of the cI gene. In addition, we investigated the effect of the apl gene on cI transcription from pL and pE and examined the interactions between these promoters and the pR promoter.
CII increases cI transcription in the face of lytic transcription.
Given the probability that the lysogenic and establishment transcripts of cI are equally well translated, the major reason expected for the involvement of pE in the establishment of lysogeny in 186 was the enhanced transcription of cI in the face of converging transcription from the lytic promoter, which has previously been shown to reduce the activity of pL (5). In order to compare the strengths of these promoters, lacZ fusions were constructed and single copies of each were inserted into the bacterial chromosome, using the system of Simons et al. (25). To avoid the potential influence of sequence context on the promoter assay, all the comparative studies involved the same restriction fragment, carrying the three promoters under study but bearing mutations inactivating different promoters as appropriate. To inactivate the lytic promoter pR, the −35 sequence was altered from TTTACT to CTCGAG (15), whereas to inactivate the lysogenic promoter, pL the −10 sequence was altered from TAGATT to GCGCTT. The absence of active CII essentially inactivated the establishment promoter. The β-galactosidase activities obtained represent transcription across the control region and approximately the first 500 bp of the immunity repressor gene.
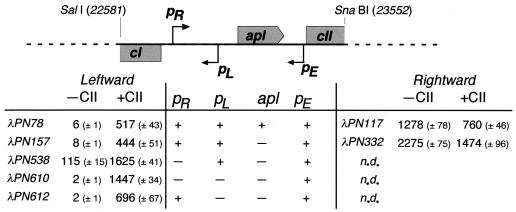
In the presence of CII, leftward transcription of cI (λPN78) increased 86-fold, from 6 to 517 U, while converging transcription from pR (λPN117) was reduced 1.7-fold, from 1,278 to 760 U (Fig. 2). This effect of CII represented a 145-fold shift in the relative strengths of cI transcription and lytic transcription.
FIG. 2.
Promoter strengths and interactions. The SalI-SnaBI restriction fragment, with the mutations and in the orientations indicated, was cloned in front of the promoterless lacZ gene of pMRR9 (13) and transferred as a single copy to the chromosome of MC1061.5 (13) using the reporter system of Simons et al. (25) as previously described (13). The β-galactosidase activity (Miller units) was determined (13) for each of these strains, transformed with either the CII expression plasmid (pPN72)(13) or its cII− derivative (pPN178 13). Values shown are the means for six individual cultures of each strain on the same day. Errors are 90% confidence limits determined by the Student t test (27). The values determined for four independent single lysogens of the same clone varied by less than 10%. Background activity (1 U) was subtracted from the activities shown and was determined by measuring the β-galactosidase activity of MC1061.5 lysogenized with λpMRR9 and transformed with either pPN72 or pPN178. Coordinates of the restriction sites refer to the numbers of base pairs from the left end of the chromosome of 186 (GenBank accession number U32222). n.d., not determined.
Apl has little or no effect on the establishment of transcription of the immunity repressor gene.
The Apl protein binds to a series of seven direct repeats that overlap the transcription start points of the lytic and lysogenic transcripts (6)(Fig. 1). Initial studies suggested an inhibitory role on transcription from pL for Apl (anti-pL)(5). Subsequently, Reed et al. (15) suggested that pR activity was more sensitive than pL to Apl and that the absence of Apl had little impact on the frequency with which infections followed the lytic or lysogenic pathway of infection (6). In the present study, we showed (Fig. 2) that Apl expressed from the single-copy reporter construct was sufficient to repress the lytic promoter twofold, from 2,275 U (λPN332) to 1,278 U (λPN117), and that Apl appeared to act in concert with CII in reducing overall lytic transcription to 760 U (λPN117). However, despite this reduction in converging transcription, the presence of Apl made little improvement in leftward transcription, which increased from 444 U (λPN157) to 517 U (λPN78), which is within the 90% confidence limits; Apl also presented no barrier to the passage of RNA polymerase from pE. These facts are in accordance with the demonstration by Dodd et al. (6) that Apl had no role in the lysis-lysogeny decision.
Promoter interactions. (i) pE and pL activities are additive.
The strength of pL alone measured 115 U (λPN538) and the base changes made at pL reduced this activity to 2 U (λPN610), essentially inactivating it (Fig. 2). The strength of pE in the absence of pL was 1,447 U (λPN610), 13-fold greater than that of pL. The sum of the individual promoter strengths (1,562 U) is similar to their combined measurement of 1,625 U (λPN538), indicating that the two transcripts neither interfered with nor assisted one another but rather were simply additive.
(ii) cI transcription in the face of pR activity.
In the presence of pR, lysogenic transcription was reduced 14-fold, from 115 U (λPN538) to 8 U (λPN157), while transcription from pE was reduced twofold, from 1,447 U (λPN610) to 696 U (λPN612). Given the additivity of transcription from pE and pL observed in the absence of pR, the expectation was that leftward transcription in the face of converging transcription from pR would also be greater for the combined activities of pE and pL than that found in the presence of pE alone. It was therefore surprising that leftward transcription fell from 696 U in the absence of pL (λPN612) to 444 U when pL was present (λPN157). It appeared as if the association of RNA polymerase with pL sensitized transcription from pE to interference by transcription from pR.
(iii) pE interference with pR activity.
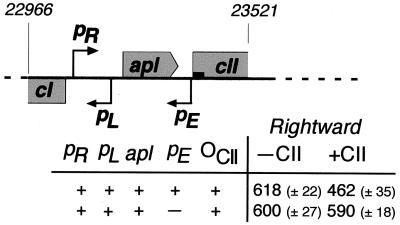
Transcription from pE reduced transcription from pR by 801 U, from 2,275 to 1,474 U (λPN332). Some of the interference of pE on pR could arise from CII bound at pE acting as a roadblock to the rightward movement of RNA polymerase from pR. To test this possibility, we used the KS11 mutant of pE (22) in which a T-to-C base-pair change in the −10 region eliminates activity of pE (<0.1% of wild type) without altering the DNA binding affinity of CII. In this experiment (Fig. 3), wild-type pE activity caused a 25% reduction in pR activity (618 to 462 U), whereas no reduction was seen with the KS11 mutant. Thus, inhibition of pR by pE is not due to CII blocking the progress of RNA polymerase but requires pE activity.
FIG. 3.
Effect of bound CII on rightward transcription. PCR products, generated from wild-type and KS11 (22) templates using primers designed to introduce EcoRI and KpnI restriction sites at the left and right ends, respectively, were inserted in front of the promoterless lacZ gene of pMRR9 (13) and transferred as single copies to the chromosome of NK7049 (25) as previously described (13). The β-galactosidase activity (Miller units) was determined for each of these strains, transformed with either pPN72 (CII+) or the parent plasmid pACYC184 (CII−). Values shown are the means for three individual cultures of each strain on the same day, and the errors are 90% confidence limits determined by the Student t test (27). The filled box at the start of the cII gene represents the CII operator (ocII), and the coordinates refer to the number of base pairs from the left end of the chromosome of 186. In our study, the β-galactosidase assays gave values consistently lower for strain NK7049 than for strain MC1061.5.
Conclusions.
We have shown that the transcription factor CII elevates 86-fold the transcription of the immunity repressor gene in the face of converging transcription from the lytic promoter, although we have as yet no evidence for the physiological relevance of the CII concentration generated in the present experiments. Furthermore, the elevation of the lysogenic transcription from pL from 8 to 115 U, when the converging transcription from pR is eliminated by mutation, confirms the proposition (5) that repression of the lytic promoter by the immunity repressor would lead to enhanced transcription of the immunity repressor gene. This suggestion not only provides a mechanism for positive feedback in the expression of the immunity repressor but also is consistent with a transient requirement for CII in the establishment of lysogeny. Thus, despite the fundamental difference in switch promoter arrangement, the role of CII in establishing the conditions for positive autoregulation of the immunity repressor is, in principle, the same for 186 and λ. A potential advantage to lysogenization, attributed to the action of the CII-activated transcription from pRE after λ infection (20), is the reduction of lytic transcription from the convergent lytic promoter pR, with the consequent reduction in the synthesis of the anti-immune repressor Cro. In 186, reduced lytic transcription was seen in the presence of active pE, but this proved of no obvious advantage to transcription from the lysogenic promoter pL. In addition, the presence of Apl displayed little impact on the level of cI transcription, so that any reduction in Apl synthesis associated with reduced lytic transcription would be of little consequence.
An outstanding goal is to identify the property differentiating P2 from its close relative 186 (4) that enables it to dispense with CII in the establishment of lysogeny. Both phages display frequencies of lysogenization on the order of 10% (2, 4) and both display a face-to-face arrangement of switch promoters, with the lytic promoter at least 100-fold stronger than the lysogenic promoter in the presence of each other and of the anti-immunity gene (1,278 versus 6 U for 186 [Fig. 2] and 38 versus 0.3 chloramphenicol transacetylase unit for P2 [19]). However, while P2 establishes a 10% frequency of lysogeny with this hundred-fold differential in promoter strengths, the use of CII by 186 to establish lysogeny brings the opposing transcripts to near equality (760 versus 517 U)(Fig. 2). The determination of promoter strengths for P2 involved the use of a plasmid reporter vector, and no controls were carried out on the possible variations in plasmid copy number for different constructs. Notwithstanding this qualification and the question of the physiological relevance of CII concentration in the present study, the contrast between 186 and P2 in relative promoter strengths in the establishment of lysogeny between P2 and 186 appears stark. Prophage inducibility is another property, along with the CII requirement, that distinguishes 186 (28) and λ (7) from P2 (2), and the potential interrelationship of prophage inducibility and the CII requirement will be explored in future studies.
Our final comments concern the 14-fold reduction of lysogenic promoter activity in the presence of an active lytic promoter. The lysogenic promoters of both P2 (19) and lambda (8) are also inhibited by the activities of their respective lytic promoters. The transcription start points of the lytic and lysogenic promoters are separated by 62 bp in 186 (5) and by 82 bp in lambda (8). In the case of P2, we predict from the sites of the presumptive −10 regions (18) about a 40-bp separation. As the switch promoters of lambda are arranged back-to-back and the RNA polymerase DNase I footprint overlaps +20 to −55 of an E. coli promoter (14), the possibility of RNA polymerase bound at one promoter precluding an RNA polymerase from binding at the second promoter was real, but the interference appears to occur at the open complex formation rather than at the binding step (8). For the face-to-face arrangement of switch promoters of 186 and P2, the probability is that RNA polymerases can occupy both promoters simultaneously. The nature of the interference for these phages has yet to be determined but presumably involves some step subsequent to the binding of the RNA polymerase to the promoter. If interference is due to an elongating RNA polymerase from the lytic promoter dislodging an RNA polymerase slow to clear the lysogenic promoter, then directionality may be important, for we did not find promoter occlusion (1) of pL by transcription from the upstream promoter pE. The nature of promoter interference is currently under study in this laboratory.
Acknowledgments
We thank Ian Dodd and Benji Callen for critical contributions.
This work was supported by a grant from the Australian Research Council to J. Barry Egan, an ARC fellowship to Keith E. Shearwin, and an Adelaide University postgraduate scholarship to Petra J. Neufing.
REFERENCES
- 1.Adhya S, Gottesman M. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell. 1982;29:939–944. doi: 10.1016/0092-8674(82)90456-1. [DOI] [PubMed] [Google Scholar]
- 2.Bertani L E, Bertani G. Genetics of P2 and related phages. Adv Genet. 1971;16:199–237. doi: 10.1016/s0065-2660(08)60359-4. [DOI] [PubMed] [Google Scholar]
- 3.Dhillon E K S, Dhillon T S, Lam Y Y, Tsang A H C. Temperate coliphages: classification and correlation with habitats. Appl Environ Microbiol. 1980;39:1046–1053. doi: 10.1128/aem.39.5.1046-1053.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodd I B, Egan J B. P2, 186 and related phages (Myoviridae) In: Webster R G, Granoff A, editors. Encyclopaedia of virology. 2nd ed. London, United Kingdom: Academic Press; 1999. pp. 1087–1094. [Google Scholar]
- 5.Dodd I B, Kalionis B, Egan J B. Control of gene expression in the temperate coliphage 186. VIII. Control of lysis and lysogeny by a transcriptional switch involving face-to-face promoters. J Mol Biol. 1990;214:27–37. doi: 10.1016/0022-2836(90)90144-B. [DOI] [PubMed] [Google Scholar]
- 6.Dodd I B, Reed M R, Egan J B. The Cro-like repressor of coliphage 186 is required for prophage excision and binds near the phage attachment site. Mol Microbiol. 1993;10:1139–1150. doi: 10.1111/j.1365-2958.1993.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldthwait D, Jacob F. Sur le mécanisme de l′induction du développement du prophage chez les bactéries lysogènes. C R Acad Sci. 1964;259:661–664. [PubMed] [Google Scholar]
- 8.Hershberger P A, de Haseth P L. RNA polymerase bound to the pR promoter of bacteriophage λ inhibits open complex formation at the divergently transcribed PRM promoter. J Mol Biol. 1991;222:479–494. doi: 10.1016/0022-2836(91)90491-n. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser A D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957;3:42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- 10.Lamont I, Brumby A M, Egan J B. UV induction of coliphage 186: prophage induction as an SOS function. Proc Natl Acad Sci USA. 1989;86:5492–5496. doi: 10.1073/pnas.86.14.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamont I, Richardson H, Carter D R, Egan J B. Genes for the establishment and maintenance of lysogeny by the temperate coliphage 186. J Bacteriol. 1993;175:5286–5288. doi: 10.1128/jb.175.16.5286-5288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer B J, Maurer R, Ptashne M. Gene regulation at the right operator (oR) of bacteriophage lambda. II. oR1, oR2, and oR3: their roles in mediating the effects of repressor and Cro. J Mol Biol. 1980;139:163–194. doi: 10.1016/0022-2836(80)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Neufing P J, Shearwin K E, Camerotto J, Egan J B. The CII protein of bacteriophage 186 establishes lysogeny by activating a promoter upstream of the lysogenic promoter. Mol Microbiol. 1996;21:751–761. doi: 10.1046/j.1365-2958.1996.351394.x. [DOI] [PubMed] [Google Scholar]
- 14.Record M T, Jr, Reznikoff W S, Craig M L, McQuade K L, Schlax P J. Escherichia coli RNA polymerase (Eς70), promoters, and the kinetics of the steps of transcription initiation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 792–821. [Google Scholar]
- 15.Reed M R, Shearwin K E, Egan J B. The dual role of the Cro-like Apl protein in prophage induction of coliphage 186. Mol Microbiol. 1997;23:669–681. doi: 10.1046/j.1365-2958.1997.2521620.x. [DOI] [PubMed] [Google Scholar]
- 16.Reichardt L F, Kaiser A D. Control of lambda repressor synthesis. Proc Natl Acad Sci USA. 1971;68:2185–2189. doi: 10.1073/pnas.68.9.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts J W, Roberts C W, Craig N L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci USA. 1978;75:4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha S, Lundqvist B, Haggard-Ljungquist E. Autoregulation of bacteriophage P2 repressor. EMBO J. 1987;6:809–814. doi: 10.1002/j.1460-2075.1987.tb04823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha S, Haggard-Ljungquist E, Nordstrom K. The Cox protein of bacteriophage P2 inhibits the formation of the repressor protein and autoregulates the early operon. EMBO J. 1987;6:3191–3199. doi: 10.1002/j.1460-2075.1987.tb02631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmeissner U, Court D, Shimatake H, Rosenberg M. Promoter for the establishment of repressor synthesis in bacteriophage λ. Proc Natl Acad Sci USA. 1980;77:3191–3195. doi: 10.1073/pnas.77.6.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shean C S, Gottesman M E. Translation of the prophage λ cI transcript. Cell. 1992;70:513–522. doi: 10.1016/0092-8674(92)90175-c. [DOI] [PubMed] [Google Scholar]
- 22.Shearwin K E, Egan J B. Establishment of lysogeny in bacteriophage 186. DNA binding and transcriptional activation by the CII protein. J Biol Chem. 2000;275:29113–29122. doi: 10.1074/jbc.M004574200. [DOI] [PubMed] [Google Scholar]
- 23.Shimatake H, Rosenberg M. Purified λ regulatory protein CII positively activates promoters for lysogenic development. Nature. 1981;292:128–132. doi: 10.1038/292128a0. [DOI] [PubMed] [Google Scholar]
- 24.Shotland Y, Shifrin A, Ziv T, Teff D, Koby S, Kobiler O, Oppenheim A B. Proteolysis of bacteriophage λ CII by Escherichia coli FtsH (HflB) J Bacteriol. 2000;182:3111–3116. doi: 10.1128/jb.182.11.3111-3116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 26.Spiegelman W G, Reichardt L F, Yaniv M, Heinemann S F, Kaiser A D, Eisen H. Bidirectional transcription and the regulation of phage λ repressor synthesis. Proc Natl Acad Sci USA. 1972;69:3156–3160. doi: 10.1073/pnas.69.11.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilks S S. Elementary statistical analysis. Princeton, N.J: Princeton University Press; 1948. [Google Scholar]
- 28.Woods W H, Egan J B. Prophage induction of noninducible coliphage 186. J Virol. 1974;14:1349–1356. doi: 10.1128/jvi.14.6.1349-1356.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]