Abstract
Background
Surgical pain management is a critical component in the success of bariatric procedures. With the opioid epidemic, there have been increased efforts to decrease opioid use. In 2019, the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program developed the BSTOP protocol, a multimodal perioperative pain management regimen to minimize opioid use. The objective of this study is to evaluate the effectiveness of the BSTOP protocol on patients’ need for opioid medications during their perioperative care.
Methods
This is a single-institution prospective cohort study on patients who underwent bariatric surgery from 10/2019 to 5/2021. Data was collected on morphine equivalent dose of opioids during different stages of inpatient and outpatient care. BSTOP was implemented on 7/2020. Primary outcomes were total inpatient and outpatient opioid use as well as hospital length of hospital stay (LOS). Gabapentin was removed from the protocol between 10/20/2020 and 12/31/2020 due to side effects; it was re-implemented on 1/1/2021 due to observed spikes in opioid use during its absence.
Results
1264 patients who had bariatric surgery between 10/2019 and 5/2021 were included in the study, with 409 patients before (pre-BSTOP) and 855 patients after BSTOP implementation. There was a 36% reduction in total inpatient opiate use and a 57% reduction in total outpatient opiate use. LOS also significantly decreased, from 1.53 to 1.28 days. 179 patients received BSTOP without gabapentin. These patients used more opioids in the post-anesthesia care unit and on the inpatient floors compared to pre-BSTOP and BSTOP with gabapentin patients. With total inpatient and outpatient opioid use, patients on BSTOP without gabapentin used fewer opioids than those pre-BSTOP. However, those on BSTOP without gabapentin used more opioids than those with gabapentin.
Conclusion
The BSTOP protocol significantly reduced inpatient and outpatient opioid use as well as LOS. Gabapentin is a crucial component of the BSTOP protocol.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-022-09646-4.
Keywords: Enhanced recovery after surgery, ERAS, BSTOP, Bariatric surgery, Obesity, Opioid
The prevalence of obesity and severe obesity have been increasing in the United States (US). The National Health and Nutrition Examination Survey (NHANES) analyzed that the age-adjusted prevalence of obesity was 42.4% and severe obesity was 9.2% in 2017–2018 [1]. Obesity is a major public health issue, as it is associated with cardiovascular disease, type 2 diabetes mellitus, hypertension, stroke, dyslipidemia, and some cancers [2]. Bariatric surgery is a medically and financially effective treatment for morbid obesity, which includes BMI ≥ 40 or BMI ≥ 35 with significant comorbidities [3, 4]. The most commonly performed bariatric procedures are the laparoscopic sleeve gastrectomy (LSG) and the laparoscopic Roux-en-Y gastric bypass (LRYGB). Over time, the eligibility and epidemiology of bariatric surgery have evolved to be more inclusive of patients with more complicated health histories, lower BMI, and those at both extremes of age [5].
As in other surgical fields, surgical pain management is a critical component in the success of bariatric procedures. However, there have been a general trend and over-reliance on using opioid medications as the primary means to curb perioperative pain. As the opioid epidemic has unfolded, increased attention has been paid to opioid prescription and opioid medication management and an interest in alternative means of pain control have resurged [6]. In the case of bariatric elective procedures, patients usually receive moderate to high doses of opioids for adequate perioperative pain control. However, there are clinical concerns of compounding obesity-related comorbidities (obstructive sleep apnea and obesity-related hypoventilation syndrome) with opioid-related complications (respiratory depression and relaxation of the upper airway dilator muscle) [7]. Furthermore, we are learning that perioperative opioid use may place patients at increased risk for persistent opiate use after discharge. In fact, patients with a pre-existing pain disorder, those on public insurance such as Medicaid, and those who had multiple surgeries in close proximity harbored the highest risk of developing an opiate use disorder [8].
Following the success of other surgical fields, bariatric surgeons have moved to adopt enhanced recovery after surgery (ERAS) protocols. These protocols focus on perioperative factors to shorten hospital stay and improve surgical outcomes, ranging from patient counseling preoperatively, minimally invasive procedures intraoperatively, multimodal analgesia, and side effects and complications prevention postoperatively [9]. A systemic review of ERAS protocols in bariatric and colorectal surgeries showed that the use of these protocols led to decreased length of hospital stay (LOS), without significant differences in overall complication and readmission rates [9, 10]. In 2019, the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) developed its own ERAS protocol, called Bariatric Surgery Targeting Opioid Prescriptions (BSTOP) [11]. It aims to decrease opioid prescriptions while improving postoperative pain management, by including multimodal methods of perioperative pain management, with minimization of opioid use, and regional anesthesia.
With a high-volume of bariatric cases (1200 cases a year) conducted by multiple surgeons on high-risk patients, our institution is nestled in a unique location to study the effects of BSTOP. We also provide services to a community that has been heavily affected by the opioid epidemic, with the highest opioid-related death rate among the five boroughs of New York City and 8th in the state [12]. In line with national and organizational efforts to reduce the overall morphine equivalent dose (MED), we sought to evaluate the safety, feasibility and effectiveness of implementing the BSTOP protocol at our high-volume academic practice. We hypothesize that the BSTOP protocol can be implemented with minimal adverse events and will lead to a drastic reduction in opiate use with adequate perioperative pain management.
Methods
Study design
This is a single-institution, retrospective cohort study for patients who underwent bariatric surgery from October 2019 to May 2021. The study was approved by Albert Einstein College of Medicine’s International Review Board (IRB) and was Health Insurance Portability and Accountability Act (HIPAA) compliant. Five board-certified minimally invasive surgeons performed all surgeries.
The baseline data was collected from October 2019 to March 2020. During the first surge of the COVID-19 pandemic in March 2020, all elective surgeries at the institution were put on hold. When elective surgeries were restarted in July 2020, the BSTOP protocol was put in place. This study includes data until May 2021.
This ERAS protocol only considered the effect of a non-opioid pain regimen. Therefore, patient preparation and education prior to the surgery and post-operative care had been kept the same. In preparation for surgery, all patients were provided with six months of nutrition and exercise recommendations focused on weight-loss. Active smokers were not eligible for bariatric surgery. During the surgery, the anesthesiologists focused on maintaining the patients’ vital signs for optimal surgical conditions. Postoperatively, both patient groups were treated symptomatically for pain, nausea, and other post-operative complications. All patients were encouraged to ambulate and put on a clear liquid diet.
Prior to BSTOP implementation, patients received patient-controlled analgesia (PCA) fentanyl or hydromorphone postoperatively until post-operative day (POD) 1. Afterwards, it was switched to an oral opioids and liquid Tylenol. Patients were also discharged with oral opioids and liquid Tylenol to take at home. They were also prescribed antiemetics.
The BSTOP protocol consists of detailed instructions for each stage of the patient’s surgical care, from the preoperative, intraoperative, postoperative, inpatient and outpatient (refer to Online Appendix for detailed protocol). Gabapentin was put on pause from 10/20/2020 due to concerns for PO intolerance with mild gastrointestinal upset and nausea when given preoperatively. However, due to increased opioid use after gabapentin cessation, gabapentin was restarted in January 2021.
Primary outcomes were total inpatient opioid use and total outpatient opioid use. Outpatient opioids used was the amount of opioids with which the patient was discharged, determined by their pain level and complications during their postoperative hospital stay. Secondary outcomes were LOS, fentanyl PCA use and hydromorphone PCA use.
Patient selection
The inclusion criteria consisted of all patients undergoing initial, revisional, or conversional intraabdominal bariatric surgical procedures. The study excluded patients taking opioids for chronic conditions in the last 10 days preceding the surgery and patients currently being treated for chronic opioid addiction. Patient demographics and health information including age, sex, American Society of Anesthesiologist (ASA) grade, body mass index (BMI), and past medical history were collected.
Surgical modalities included laparoscopy and robotic. Patients who underwent additional procedures, for example EGD, hiatal hernia repairs, and cholecystectomies were excluded from the study.
Statistical analysis
All statistical analyses were conducted using SAS. Groups were compared using independent samples T test or analysis of variance for continuous variables and χ2 test for categorical variables. Whether the patients received opioids postoperatively and upon discharge were turned into a binary variable. This was used for a multivariate logistic regression. Results were considered statistically significant if p < 0.05. All data was analyzed on an intention-to-treat basis.
Results
A total of 1264 patients were included in this retrospective analysis. Among these, 409 received bariatric surgeries prior to the implementation of the BSTOP protocol and 855 received bariatric surgeries after its implementation. The BSTOP protocol was implemented after elective surgeries were restarted at the institution, after the first COVID surge in July 2020. The patient characteristics are described in Table 1. These groups were statistically similar in terms of patient sex, BMI, and ASA. There was a statistically significant difference in terms of age, with the average age pre-BSTOP being 40.6 years and post-BSTOP being 38.1 years. In terms of the patients’ health comorbidities, there were no significant differences in the proportion of patients with hyperlipidemia, diabetes mellitus, psychiatric disorders, and pain disorders. However, there was a significant difference in the following comorbidities: hypertension (p = 0.0072), OSA (p = 0.0167), and COPD and asthma (p = 0.0058). There was no significant difference in the proportion of surgeries conducted by the five surgeons or the type of modality used. There was a significant difference in the proportion of types of procedures performed (p = 0.0031), with a percentage decrease RYGB performed (24.9 to 18.1%) and increase in sleeve gastrectomies (65.8 to 66.5%), conversions (7.6 to 12.5%), and revisions (1.7 to 2.8%) conducted.
Table 1.
Patient characteristics before and after BSTOP implementation
| Pre-BSTOP | Post-BSTOP (July 2020) | p values | |
|---|---|---|---|
| Total | 409 | 855 | |
| Sex | |||
| Female | 331 (80.9%) | 724 (84.7%) | 0.0932 |
| Male | 78 (19.1%) | 131 (15.3%) | |
| Age | |||
| Average | 40.60 | 38.07 | 0.0004 |
| BMI | |||
| Average | 44.55 (10.9%) | 44.41 (5.2%) | 0.7648 |
| Class 1 | 13 (3.2%) | 27 (3.2%) | 0.3762 |
| Class 2 | 101 (24.7%) | 182 (21.3%) | |
| Class 3 | 290 (70.9%) | 638 (74.6%) | |
| ASA | |||
| 1 | 0 (0%) | 0 (0%) | 0.5435 |
| 2 | 77 (18.8%) | 146 (17.1%) | |
| 3 | 328 (80.2%) | 703(82.2%) | |
| 4 | 4 (1.0%) | 5 (0.6%) | |
| Operating surgeon | |||
| Surgeon A | 98 (24.0%) | 215 (25.1%) | 0.2357 |
| Surgeon B | 134 (32.8%) | 307 (35.9%) | |
| Surgeon C | 38 (9.3%) | 84 (9.8%) | |
| Surgeon D | 110 (26.9%) | 212 (24.8%) | |
| Surgeon E | 29 (7.1%) | 37 (4.3%) | |
| Procedure type | |||
| Sleeve gastrectomy | 269 (65.8%) | 569 (66.5%) | 0.0031 |
| Roux-en-Y gastric bypass | 102 (24.9%) | 155 (18.1%) | |
| Conversions | 31 (7.6%) | 107 (12.5%) | |
| Revisions | 7 (1.7%) | 24 (2.8%) | |
| Modality | |||
| Laparoscopy | 380 (92.9%) | 787 (92.0%) | 0.5898 |
| Robotic | 29 (7.1%) | 68 (8.0%) | |
| Past medical history | |||
| Hypertension | 180 (44.9%) | 309 (36.1%) | 0.0072 |
| Obstructive sleep apnea | 169 (41.3%) | 294 (34.4%) | 0.0167 |
| Hyperlipidemia | 19 (4.6%) | 31 (3.6%) | 0.3842 |
| COPD/asthma | 145 (35.5%) | 238 (27.8%) | 0.0058 |
| Diabetes mellitus | 152 (37.2%) | 327 (38.2%) | 0.7107 |
| Anxiety | 60 (14.7%) | 113 (13.2%) | 0.4818 |
| Panic disorder | 1 (0.2%) | 9 (1.1%) | 0.1292 |
| Depression | 61 (14.9%) | 139 (16.3%) | 0.5405 |
| Bipolar disorder | 13 (3.2%) | 25 (2.9%) | 0.8042 |
| Psychotic disorders | 1 (0.2%) | 8 (0.9%) | 0.1715 |
| Pain disorders | 131 (32.0%) | 295 (34.5%) | 0.3841 |
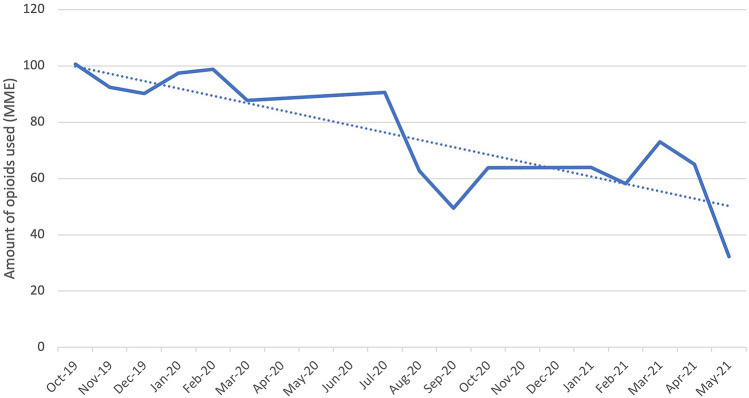
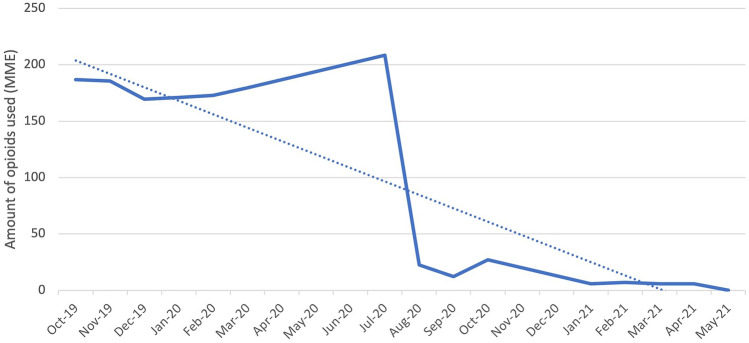
Our primary outcome of interest was the total opioids used during the patient’s hospitalization as well as upon discharge (Table 2). For this initial analysis, data collected during gabapentin removal from the BSTOP protocol was not included. There is a statistically significant decrease (p < 0.0001) in average total inpatient opioid used from 95.28 ± 37.62 MME prior to BSTOP implementation to 62.08 ± 36.68 MME after BSTOP implementation. This can be further broken down into the total amount of opioids used in the post-anesthesia care unit (PACU) and inpatient floor. There was no significant difference in the opioids used in the PACU (p = 0.7997), with pre-BSTOP average being 24.89 ± 19.55 MME and post-BSTOP average being 25.18 ± 18.52 MME. However, there was a significant decrease in total inpatient floor opioids used (p = 0.012) with pre-BSTOP average being 10.72 ± 17.11 and post-BSTOP average being 8.24 ± 14.17 (Fig. 1). Moreover, there was a significant decrease in opioids used upon discharge (p < 0.0001), with pre-BSTOP average being 177.90 ± 75.87 MME and post-BSTOP average being 43.17 ± 82.25 MME (Fig. 2). There was no significant difference between laparoscopic and robotic surgeries, in terms of the total amount of opioids used in the PACU (p = 0.8), on the inpatient floor (p = 0.4), and upon discharge (p = 0.3).
Table 2.
Comparing study outcomes before and after BSTOP implementation
| Pre-BSTOP (n = 409) | Post-BSTOP (July 2020) (n = 855) | % Difference | p values* | |
|---|---|---|---|---|
| Length of hospital stay (days) | 1.53 (SD = 0.89) | 1.28 (SD = 0.75) | 16.3 | < 0.0001 |
| Total inpatient opioids (MME) | 95.28 (SD = 37.62) | 62.08 (SD = 36.68) | 34.8 | < 0.0001 |
| Total opioids used on inpatient floor (MME) | 10.72 (SD = 17.11) | 8.24 (SD = 14.17) | 23.1 | 0.011 |
| Total opioids used in PACU* (MME) | 24.89 (SD = 19.55) | 25.18 (SD = 18.52) | − 1.2 | 0.800 |
| Total opioids used postoperatively (MME) | 35.60 (SD = 27.89) | 31.80 (SD = 26.64) | 10.7 | 0.022 |
| Total opioids used upon discharge (MME) | 177.90 (SD = 75.7) | 43.17 (SD = 84.25) | 75.7 | < 0.0001 |
*T test
Fig. 1.
Average total inpatient opioid use
Fig. 2.
Average total outpatient opioid use
Our secondary outcomes of interest included length of hospital stay, PCA fentanyl use, and PCA hydromorphone use (Tables 2, 3). There was a significant decrease in the length of hospital stay (p < 0.0001) from an average of 1.53 ± 0.89 days pre-BSTOP to an average of 1.28 ± 0.75 days post-BSTOP. Prior to BSTOP, 99.76% of patients (n = 408) used PCA fentanyl, while after BSTOP implementation 8.43% (n = 57) used PCA fentanyl. There is no correlation between PCA fentanyl use and LOS after BSTOP implementation (correlation coefficient of 0.06, p = 0.08). None of the patients received PCA hydromorphone before and after BSTOP implementation.
Table 3.
Comparing study outcomes before and after BSTOP implementation
| Pre-BSTOP (n) | Post-BSTOP (n) | Odds ratios (CI 95%) | p values* | |
|---|---|---|---|---|
| PCA fentanyl used | 408 | 57 | < 0.001 (< 0.001–0.001) | < 0.0001 |
| PCA hydromorphone used | 0 | 0 | N/A | N/A |
| Opioids used postoperatively | 379 | 569 | 0.490 (0.322–0.744) | < 0.0001 |
| Opioids used upon discharge | 395 | 94 | 0.014 (0.008–0.024) | < 0.0001 |
*χ2 test
A multivariate logistic regression showed that after controlling for patient sex, age at surgery, BMI, operating surgeon, procedure type, modality of surgery, and comorbidities including hypertension, OSA, and COPD and asthma, there was a significant decrease in provision of opioids postoperatively (OR 0.47, 95% CI 0.31–0.72). A similar multivariate logistic regression was done for opioids used after discharge, which also showed a significant reduction (OR 0.013, 95% CI 0.007–0.022).
We also conducted a sub-analysis on changes in opioid use for the 179 patients between October 20th and December 31st 2020 who underwent BSTOP but did not receive gabapentin (B-GABA) (Table 4). When an analysis of variance was conducted for pre-BSTOP, B-GABA and BSTOP with gabapentin (B + GABA), there was a significant difference in LOS (p < 0.0001), total inpatient floor opioid use (p = 0.0002), total inpatient opioid use (p < 0.0001), and total outpatient opioid use (p < 0.0001). There was no significant difference in PACU opioid use (p = 0.0812). On the inpatient floor and in the PACU, the mean total opioids given was higher in B-GABA than pre-BSTOP and B + GABA. As for the LOS and total inpatient and outpatient opioid use, the average total opioid use was greater than those of B + GABA and less than those of pre-BSTOP. When a multivariate logistic regression was done to control for patient sex, age at surgery, pre-operative BMI, operating surgeon, type of procedure, modality of surgery, and comorbidities including hypertension, OSA, and COPD and asthma, there was a significant difference between pre-BSTOP, B-GABA and B + GABA for both total inpatient and outpatient opioid use (p < 0.0001). The odds ratio comparing pre-BSTOP to B + GABA is 0.396 with 95% CI of 0.256 to 0.613. The odds ratio comparing B-GABA to B + GABA is 0.348 with 95% CI of 0.185 to 0.654.
Table 4.
Comparing study outcomes before BSTOP, BSTOP without gabapentin (B-GABA) and BSTOP with gabapentin (B + GABA)
| Pre-BSTOP | B-GABA | B + GABA | p values* | |
|---|---|---|---|---|
| Length of hospital stay (days) | 1.53 (SD = 0.89) | 1.37 (SD = 0.98) | 1.25 (SD = 0.67) | p < 0.0001 |
| Total inpatient opioids (MME) | 95.28 (SD = 37.62) | 67.27 (SD = 38.58) | 60.71 (SD = 36.06) | p < 0.0001 |
| Total opioids used on inpatient floor (MME) | 10.72 (SD = 17.11) | 11.44 (SD = 18.87) | 7.40 (SD = 12.52) | 0.0002 |
| Total opioids used in PACU (MME) | 24.89 (SD = 19.55) | 27.97 (SD = 17.18) | 24.44 (SD = 18.80) | 0.081 |
| Total opioids used postoperatively (MME) | 35.61 (SD = 27.89) | 39.41 (SD = 30.55) | 31.83 (SD = 25.30) | 0.0016 |
| Total opioids used upon discharge (MME) | 177.90 (SD = 75.70) | 111.82 (SD = 75.66) | 25.00 (SD = 76.75) | p < 0.0001 |
*T test
Discussion
Bariatric surgery is the most effective treatment for morbid obesity and its related comorbidities [13]. However, its success must be weighed against its complications and side-effects, one of them being the potential for persistent opioid use after surgery. This large, single-institution, retrospective cohort study has shown that implementation of the BSTOP protocol is very effective at decreasing total opioid usage among bariatric surgery patients. The BSTOP protocol, which was implemented at our institution in July 2020, has significantly decreased both inpatient and outpatient opioid use, PCA fentanyl use and LOS. Furthermore, the BSTOP protocol itself significantly decreased the provision of opioids on the inpatient floor and upon discharge. In other words, fewer patients who underwent bariatric surgery with the BSTOP protocol were discharged with opioids.
Substance use disorder is a rampant issue in the Bronx. In 2020, 537 Bronx residents died of drug overdose, ranking highest of the five boroughs of New York City [12]. Fentanyl was involved in 80% of the deaths; cocaine, heroin, alcohol and benzodiazepines were also other common substances [12]. Therefore, it is of no surprise that the Bronx has been particularly hard-hit by the opioid epidemic. In 2018, there were 321 opioid-overdose deaths and 1013 ED visits involving opioid overdose, with statistics increasing annually [14]. The Bronx is the 8th highest in terms of opioid-related overdose cases in New York State [15]. Compared to the rest of the state, opioid-related deaths in the Bronx disproportionately affects the Hispanic population and those aged 35- to 54-years old [16]. In addition, the Bronx has a high prevalence of depression (14.9%) and serious psychological illnesses (6.5%) [17]. These statistics are concerning, as studies show that a personal history of alcohol and drug use as well as psychiatric disorders and depression are strongly associated with developing opioid use disorder [18]. Surgeons and anesthesiologists must be cautious of opioid prescription intra- and postoperatively, as even opioid-naïve patients are vulnerable to persistent postoperative opioid use [19].
Our results show that when opioids used in the PACU were compared between pre-BSTOP and post-BSTOP, there was no significant difference in opioid use. A previous study has shown that ERAS protocol adherence for bariatric surgery pre-operatively, intraoperatively, and on the floor were above 85%, but in the PACU, was between 55 and 61% [20]. It is probable that an opioid-free intraoperative anesthetic technique does not sufficiently manage immediate postoperative pain, which requires intervention in the PACU. Currently, there is research on intraoperative use of alternative and longer-lasting forms of pain management, such as methadone and ketamine [21]. The combination of this with ERAS protocols could lead to improved postoperative pain management and recovery. Furthermore, pain management in the PACU is challenging, as patients are not fully recovered from anesthesia or sedation to express the level of pain. Therefore, emphasis has been put on investigating objective measures of pain management, such as the Behavioral Pain Scale, photoplethysmography-derived parameters, and analgesia nociception index [22].
Although unintentional, this study also showed the significant role that gabapentin plays in postoperative pain management and decreasing opioid use. When removed from the BSTOP pathway, it led to a significant increase in total opioid use on the inpatient floor and a longer average LOS. Systematic reviews investigating the effect of gabapentin on perioperative pain management has shown that it is associated with decreased opioid usage as well as a reduction in pain scores [23, 24]. In terms of its side-effects, gabapentin use has been associated with increased sedation, dizziness and visual disturbances; however, it has been correlated with fewer cases of postoperative nausea and vomiting and pruritis, which are considered common opioid-related side effects [24]. Therefore, our study confirms the critical role that gabapentin plays in ERAS protocols for improved pain management and decreased postoperative opioid use.
There are some limitations associated with our data. Firstly, there was a disruption in the study between its time course of October 2019 to May 2021.There was a pause in all elective surgeries at our institution between April 1st 2020 and June 30th 2020 due to the COVID pandemic. As the BSTOP protocol was implemented immediately after elective surgeries restarted in July 2020, there could have been confounding factors related to patients, OR personnel, and physicians returning to the hospital after a long hiatus. For example, the patient comorbidity with the greatest difference was the decrease in proportion of patients with COPD and asthma in the post-COVID cohort (35.5 to 27.8%). This could be due to a multitude of reasons, including, but not limited to, an increase in morbidity and mortality from COVID-related complications in patients with respiratory conditions and morbid obesity [25]. Furthermore, we did not collect data on surgical complications and adverse effects of opioids and other medications used. However, we used LOS as an indirect measure of these factors. Although this study has a large cohort, the study took place in the Bronx, with a predominantly low-income, high immigrant and urban population. Therefore, the results may not be generalizable to rural, high-income, and homogenous populations.
This study attempts to fill the gap in knowledge regarding the effectiveness of BSTOP in decreasing opioid use during hospitalization and upon discharge. There are various ERAS protocols used in bariatric surgery. Future studies should focus on the optimal combination of agents that should be used in such a protocol. Additionally, studies focusing on long-term follow up will be critical in understanding how these interventions affect persistent opiate use and overdose.
Conclusions
This study supports a growing body of evidence showing that implementation of ERAS protocols for bariatric patients can drastically improve patient outcomes and pain management. BSTOP led to a significant reduction in both inpatient and outpatient opioid use as well as length of hospital stay. Gabapentin plays a critical role in the BSTOP protocol and seems to potentiate other drugs to minimize opiate use.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge Ms. Marcia Markowicz in collecting the initial data for the project as well as the rest of the Minimally Invasive Surgery and Anesthesia teams for their support in this project.
Author contributions
RS: Data collection, data analysis, statistics, and manuscript writing, XP: Manuscript writing and editing, PG: Data collection, manuscript editing, VY: initial quality improvement design, protocol writing, data collection, and analysis, DA: initial quality improvement design, protocol writing, data collection, and analysis, EMA: manuscript editing, DC: manuscript editing, JK: manuscript editing, JC: conception of work and manuscript editing.
Funding
No funding to disclose.
Declarations
Disclosures
Ms. Rie Seu and Drs. Xavier Pereira, Pavel Goriacko, Vicken Yaghdjian, Daniel Appiah, Erin Moran-Atkin, Diego Camacho, Jinu Kim and Jenny Choi have no conflicts of interest or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hales CM, Caroll Margaret D, Fryar CD, Ogden CL (2020) Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief No. 360 [PubMed]
- 2.The Disease Burden Associated with Overweight and Obesity Obesity|JAMA|JAMA Network. https://jamanetwork-com.elibrary.einsteinmed.org/journals/jama/article-abstract/192030. Accessed 27 Oct 2021
- 3.Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement—PubMed. https://pubmed-ncbi-nlm-nih-gov.elibrary.einsteinmed.org/1733140/. Accessed 27 Oct 2021
- 4.Alsumali A, Eguale T, Bairdain S, Samnaliev M. Cost-effectiveness analysis of bariatric surgery for morbid obesity. Obes Surg. 2018;28(8):2203–2214. doi: 10.1007/s11695-017-3100-0. [DOI] [PubMed] [Google Scholar]
- 5.Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879–887. doi: 10.1001/jama.2020.12567. [DOI] [PubMed] [Google Scholar]
- 6.Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN. The burden of opioid-related mortality in the United States. JAMA Netw Open. 2018;1(2):e180217. doi: 10.1001/jamanetworkopen.2018.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Raaff CAL, de Vries N, van Wagensveld BA. Obstructive sleep apnea and bariatric surgical guidelines: summary and update. Curr Opin Anesthesiol. 2018;31(1):104–109. doi: 10.1097/ACO.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 8.King WC, Chen JY, Belle SH, et al. Use of prescribed opioids before and after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017;13(8):1337–1346. doi: 10.1016/j.soard.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh PM, Panwar R, Borle A, et al. Efficiency and safety effects of applying ERAS protocols to bariatric surgery: a systematic review with meta-analysis and trial sequential analysis of evidence. Obes Surg. 2017;27(2):489–501. doi: 10.1007/s11695-016-2442-3. [DOI] [PubMed] [Google Scholar]
- 10.Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD007635.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Join Bariatric Surgery Targeting Opioid Prescriptions (BSTOP). American College of Surgeons. http://www.facs.org/quality-programs/mbsaqip/news/bstop. Accessed 28 Sept 2021
- 12.NYC Office of the Chief Medical Examiner, NYC Health Department’s Bureau of Vital Statistics (2020) Overdose deaths among Bronx residents
- 13.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.New York State Department of Health (2020) Appendix: New York State Opioid Annual Report, p 74
- 15.New York State Department of Health (2020) New York State Opioid Annual Report, 2020:95
- 16.Office of Community & Population Health of Montefiore. Bronx Community Health Dashboard: Drug Use and Opioids
- 17.Office of Community & Population Health of Montefiore. Bronx Community Health Dashboard: Mental Health
- 18.Cheatle MD, Compton PA, Dhingra L, Wasser TE, O’Brien CP. Development of the revised opioid risk tool to predict opioid use disorder in patients with chronic non-malignant pain. J Pain. 2019;20(7):842–851. doi: 10.1016/j.jpain.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzman C, Harker EC, Ahmed R, et al. The association between preoperative opioid exposure and prolonged postoperative use. Ann Surg. 2021;274(5):e410–e416. doi: 10.1097/SLA.0000000000003723. [DOI] [PubMed] [Google Scholar]
- 20.Monte SV, Rafi E, Cantie S, Wohaibi E, Sanders C, Scovazzo NC. Reduction in opiate use, pain, nausea, and length of stay after implementation of a bariatric enhanced recovery after surgery protocol. Obes Surg. 2021;31(7):2896–2905. doi: 10.1007/s11695-021-05338-5. [DOI] [PubMed] [Google Scholar]
- 21.Murphy GS, Avram MJ, Greenberg SB, et al. Perioperative methadone and ketamine for postoperative pain control in spinal surgical patients: a randomized, double-blind. Placebo-controlled Trial Anesthesiol. 2021;134(5):697–708. doi: 10.1097/ALN.0000000000003743. [DOI] [PubMed] [Google Scholar]
- 22.Luo J, Min S. Postoperative pain management in the postanesthesia care unit: an update. J Pain Res. 2017;10:2687–2698. doi: 10.2147/JPR.S142889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurley RW, Cohen SP, Williams KA, Rowlingson AJ, Wu CL. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med. 2006;31(3):237–247. doi: 10.1016/j.rapm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth. 2015;114(1):10–31. doi: 10.1093/bja/aeu293. [DOI] [PubMed] [Google Scholar]
- 25.Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.