Abstract
We demonstrate that the Bacillus subtilis fosB(yndN) gene encodes a fosfomycin resistance protein. Expression of fosB requires ςW, and both fosB and sigW mutants are fosfomycin sensitive. FosB is a metallothiol transferase related to the FosA class of Mn2+-dependent glutathione transferases but with a preference for Mg2+ and l-cysteine as cofactors.
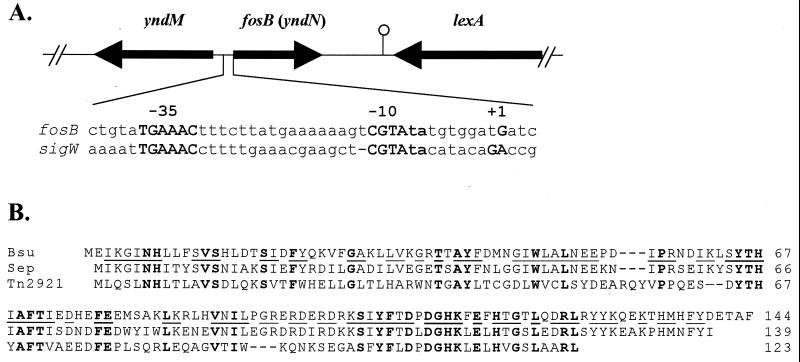
Sequencing of the Bacillus subtilis genome revealed the presence of seven new ς factors, all members of the extracytoplasmic function subfamily (12, 13). We have begun to investigate their functions by mutation of each gene and the identification of target operons (8–11). In this work, we demonstrate that yndN encodes a fosfomycin resistance (Fosr) protein that depends on ςW for expression. We have renamed yndN as fosB, based on its similarity to the fosB gene identified on a Staphylococcus epidermidis plasmid (Fig. 1B).
FIG. 1.
The fosB(yndN) gene encodes a fosfomycin resistance protein. (A) The fosB(yndN) region of the chromosome is illustrated. The fosB gene is transcribed from a ςW-dependent promoter similar in sequence to the sigW autoregulatory promoter, Pw (9). (B) Multiple sequence alignment of the 144-amino-acid B. subtilis FosB protein (Bsu) with FosB from S. epidermidis (63% identity [21]) and with FosA from Tn2921 (33% identity [14]). Residues identical in all three protein sequences are shown in bold, and those residues identical between the more closely related FosB homologs are underlined in the B. subtilis sequence.
Transcription of fosB requires ςW.
Previously, 15 ςW-dependent operons were identified by searching the genome for sequences matching the ςW autoregulatory site, Pw: TGAAAC N16 CGTA (10). Additional candidate promoters, including one for fosB (Fig. 1A), were identified with 17-bp spacer regions (10).
To confirm the role of this predicted ςW-dependent promoter, we generated a PfosB-cat-lacZ operon fusion inserted ectopically in the SPβ prophage (HB8083; Table 1) and transduced the reporter fusion into wild-type, sigW, and rsiW mutant backgrounds. Promoter activity as determined in early-stationary-phase cells yielded 18.4 Miller units of β-galactosidase in the wild-type strain (HB0052), and this was reduced to background levels (∼1 unit) in the sigW mutant (HB0023). In the rsiW (anti-ςW) mutant (HB0012), expression was elevated approximately twofold (30.5 units). This pattern is precisely that expected for a ςW-dependent promoter.
TABLE 1.
B. subtilis strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Genotype or characteristicsa | Reference, source, or derivationb |
|---|---|---|
| Strains | ||
| CU1065 | W168 trpC2 attSPβ | Lab stock |
| JH642 | W168 trpC2 pheA1 | Lab stock |
| ZB307A | W168 SPβc2Δ2::Tn917::pSK10Δ6; MLSr | 22 |
| HB4247 | JH642 sigW::kan | pKF90→JH642 |
| HB0020 | CU1065 sigW::kan | HB4247→CU1065 |
| HB0010 | CU1065 rsiW::kan | pXH51→CU1065 |
| HB0008 | CU1065 fosB::pAG4041 (Cmr) | pAG4041→CU1065 |
| HB8083 | ZB307A SPβ PfosB-cat-lacZ (MLSr Neor) | pAG3839→ZB307A |
| HB0052 | CU1065 SPβPfosB-cat-lacZ (MLSr Neor) | Transduction |
| HB0023 | HB0020 SPβ PfosB-cat-lacZ (MLSr Kanr) | Transduction |
| HB0012 | HB0010 SPβ PfosB-cat-lacZ (MLSr Kanr) | Transduction |
| HB0050 | CU1065 SPβ Pw-cat-lacZ (MLSr Neor) | 9 |
| HB0080 | CU1065::pMC82 (PxylA-fosB) | This work |
| HB0081 | HB0020::pMC82; sigW::kan (PxylA-fosB) | This work |
| HB0082 | HB0008::pMC82; fosB::cat(PxylA-fosB) | This work |
| Plasmids | ||
| pET17b | T7 RNAP driven overexpression plasmid | Novagen |
| pXT | Derivative of pDG1731; allows fusion of genes to xylose-inducible xylA promoter; integrates at thrC locus | T. Msadek |
| pJPM122 | Vector for integration of reporter fusions into SPβ (Apr Neor) | 18 |
| pJM114 | Kanamycin resistance cassette vector | 16 |
| pGEM-cat-3Zf(+) | Cloning vector | 20 |
| pDG783 | Kanamycin resistance cassette vector | 6 |
| pKF84 | Contains sigW | Lab stock |
| pKF90 | Contains sigW::kan | Construction analogous to sigW::MLS (reference 9) |
| pXH50 | pGEM-cat-3Zf(+) carrying rsiW | Lab stock |
| pXH51 | rsiW::kan in pGEM-cat-3Zf(+) | Lab stock |
| pAG4041 | pGEM-cat-3Zf(+) carrying internal fragment (330 bp) of fosB (PCR primers 275, 276) | This work |
| pAG3839 | pJPM122 carrying PfosB (PCR from 273, 274) | This work |
| pMC82 | pXT carrying fosB (PCR from 470, 309) | This work |
| pMC50 | pET17b with fosB (PCR from 308, 309) | This work |
| Oligonucleotidesc | ||
| 275 | CGGAATTCAGTTTCGCATTTGGATACA | fosB (internal; F) |
| 276 | CGTCTAGAAGCCTGTCTTGAAGGGTT | fosB (internal; R) |
| 273 | GCGAAGCTTCCGTTTTTGTTTACACTGGTA | fosB promoter F |
| 274 | GCGGATCCTAGCAAGTGATTGATTCCTTTTA | fosB promoter R |
| 470 | CGCGGATCCATTCATAATGGTCATGTT | fosB F |
| 309 | CCGGAATTCTGGTTGTGCTATCAAA | fosB R |
| 370 | CCCTTTACCAAAAGCTTTGCACC | fosB (for primer extension; R) |
| 308 | CTAGTCTAGACAGTCCGTTTTTGTT | fosB (for overproduction; F) |
MLS, macrolides-lincosamides-streptogramin B; Neo, neomycin; Kan, Kanamycin, Ap, ampicillin; Cm, chloramphenicol.
Arrows indicate transformation with either plasmid or chromosomal DNA as indicated.
Introduced restriction sites used for cloning are underlined. F, forward; R, reverse (relative to fosB).
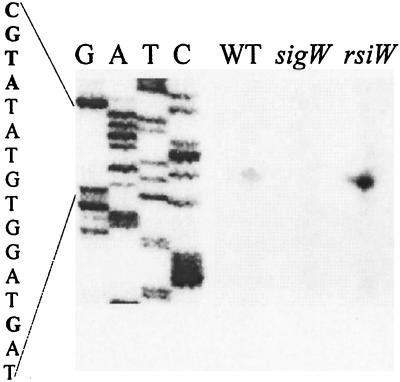
We used reverse transcriptase primer extension mapping to identify the transcriptional start site for fosB as a G residue 10 bases downstream from the −10 region CGTA motif (Fig. 2). There were no other start sites visible in the primer extension experiment, which is consistent with the idea that ςW is largely, if not exclusively, responsible for fosB transcription. The fosB gene is apparently monocistronic, as it is flanked on either side by genes transcribed from the complementary strand of the genome (Fig. 1A).
FIG. 2.
Expression of fosB depends on ςW. Primer extension analysis of the fosB transcription start site is shown. RNA was isolated from logarithmically growing cells in Luria-Bertani medium of strains CU1065 (wild type), HB0020 (sigW mutant), and HB0010 (rsiW mutant). Equal amounts (100 μg) of total RNA were annealed with radiolabeled oligonucleotide no. 370 prior to analysis by reverse transcriptase primer extension. The transcription start site is the G residue shown in bold. G,A,T, and C are the sequencing ladder obtained with the same primer.
fosB and sigW mutants are sensitive to fosfomycin.
Both fosB (HB0008) and sigW (HB0020) mutants are fosfomycin sensitive: an MIC of 50 μg/ml for the mutants compared to 800 μg/ml for the wild type in liquid culture. Similarly, the sigW and fosB mutants have a much greater zone of growth inhibition in disk diffusion assays (∼25-mm zone for wild type versus >50 mm for the mutants). The fosB and sigW mutant strains did not display altered sensitivity to several other antibiotics, including vancomycin, cephalosporin C, penicillin G, d-cycloserine, tunicamycin, nisin, and bacitracin. Induction of fosB from a xylose-inducible promoter completely restores Fosr to either the sigW mutant (HB0081) or, as expected, to the fosB mutant (HB0082). Thus, fosB is the only ςW-dependent gene required for wild-type levels of Fosr.
Expression of FosB confers fosfomycin resistance to Escherichia coli.
For mechanistic studies, we overproduced FosB in E. coli. Transformation of E. coli BL21/DE3(pLysS) with pMC50 leads to high fosfomycin resistance, even under noninducing conditions (MIC > 20 mg/ml, as judged using commercial antibiotic disks [Becton Dickinson, Cockeysville, Md.]). This is comparable to the Fosr imparted by a similar FosA overexpression plasmid (MIC > 30 mg/ml). This suggests that an appropriate thiol cofactor for FosB is present in E. coli. To compare the relative efficacy of FosA and FosB in protecting E. coli against fosfomycin, the MIC determinations were repeated using plates containing 20 mM glucose-6-phosphate (an inducer of fosfomycin uptake). Under these conditions, FosA still supported high-level fosfomycin resistance (MIC, >30 mg/ml), while resistance of the strain expressing FosB was dramatically reduced (MIC, ∼1.25 mg/ml). This difference may relate to the lower catalytic efficiency of FosB compared to FosA (see below).
FosB: an Mg2+-dependent cysteine thiol transferase.
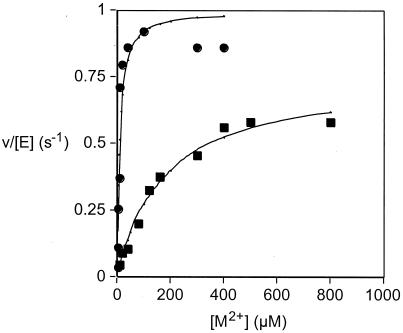
FosB was purified using modifications of the FosA procedure (3). Gel filtration chromatography indicated that FosB is a dimer in either the presence or absence of divalent metal ions. The metal ion selectivity of FosB was examined and found to be Ni2+ ∼ Mg2+ > Mn2+ > Fe2+ > Cu2+ > Ca2+ ∼ Co2+ > Zn2+ when screened with a fixed (0.5 mM) concentration of metal. Activation was almost 10-fold greater with Ni2+ and Mg2+ (Fig. 3) than with Mn2+. Although Ni2+ activates the enzyme at lower concentrations, the activation constant for Mg2+ (200 μM) is well below the prevailing Mg2+ concentration of about 1 mM. Therefore, we suggest that Mg2+ is the physiologically relevant metal. In contrast to FosA (2), FosB does not require a monovalent cation for optimal activity.
FIG. 3.
Activation of FosB with Ni2+ and Mg2+ using l-cysteine as the thiol substrate. The experimental data for Ni2+ (●) and Mg2+ (▪) were fit to the quadratic form of a simple binding isotherm to obtain the activation constant for the metal (KactM) and the maximum rate constant (kmaxM) for activation under the conditions of the assay. The solid lines are fits of the experimental data for the two metals, with KactNi = 9 ± 3 μM, kmaxNi = 1.0 ± 0.1 s−1, KactMg = 180 ± 30 μM, and kmaxMg = 0.75 ± 0.04 s−1. For comparison, the results for Mn2+ under the same conditions were KactMn = 3.5 ± 0.9 μM and kmaxMn = 0.11 ± 0.01 s−1.
FosA functions as a glutathione transferase (3). Since B. subtilis, like many gram-positive bacteria, lacks detectable levels of glutathione (5, 15), it seems likely that FosB must use a different thiol. To identify the FosB thiol cofactor, the rate of appearance of conjugates of fosfomycin with various thiols was determined as described previously (3, 4). l-Cysteine and coenzyme A sulfhydryl (CoASH) are two abundant thiols in gram-positive bacteria (5, 15). Of the two, only l-cysteine supports a modest enzyme activity (Table 2). The product of the FosB-catalyzed addition of l-cysteine to fosfomycin, examined by heteronuclear multiple-bond correlation nuclear magnetic resonance spectroscopy, is (1R,2S)-1-(S-l-cysteinyl)-2-hydroxypropylphosphonate (data not shown), identical to the product produced by FosA with l-cysteine (4). Extended incubations of FosB, fosfomycin, and various divalent metal ions indicated that no degradation of the antibiotic occurred in the absence of a thiol substrate.
TABLE 2.
Steady-state kinetic parameters of the thiol substrate and metal-ion selectivity of FosB
| Thiol substrate | Metal ion | kcat (s−1) | kcat/Kmthiol (M−1 s−1) | Kmthiol (mM) |
|---|---|---|---|---|
| l-Cys | Mg2+ | 6.3 ± 0.3 | 180 ± 20 | 35 ± 3 |
| GSH | Mg2+ | 0.027 ± 0.002 | 1.8 ± 0.2 | 15 ± 2 |
| CoASH | Mg2+ | —a | 0.40 ± 0.03 | >50 |
| l-Cys | Ni2+ | 7.8 ± 0.5 | 190 ± 30 | 41 ± 3 |
| GSH | Ni2+ | 0.066 ± 0.005 | 9.1 ± 1.7 | 7 ± 1 |
| l-Cys | Mn2+ | —a | 6.9 ± 0.2 | >200 |
| GSH | Mn2+ | —a | 0.093 ± 0.001 | >50 |
| CoASH | Mn2+ | —a | 0.0009 ± 0.0001 | >100 |
A linear dependence on the thiol substrate concentration was observed, precluding a determination of kcat. Therefore, a conservative lower limit for Kmthiol was estimated from the substrate concentration range used.
Although with glutathione the catalytic efficiency of FosB is significantly less than that of FosA (kcat/Km = [1.7 ± 0.3] × 105 M−1 s−1), they are about equally active with l-cysteine (kcat/Km = 410 ± 40 M−1 s−1). These rate constants are still at least 106 to 108 greater than those reported for the spontaneous reactions (2). The optimal catalytic efficiency of FosB (with l-cysteine) is far lower than FosA efficiency (with glutathione), which may reflect an intrinsic difference in catalytic efficiency or could indicate that a physiologically relevant cofactor for FosB has not yet been identified. To verify that l-cysteine is the thiol donor in vivo, it will be necessary to characterize the product of fosfomycin inactivation from intact cells.
Summary.
Fosfomycin is a clinically important, broad-spectrum antibiotic that irreversibly inactivates MurA, which catalyzes the first committed step in peptidoglycan biosynthesis (17). Resistance arises predominantly via mutations in the chromosomally encoded transport pathways (7) or by resistance genes found on transmissible plasmids. At least two classes of plasmid-borne Fosr determinants have been described (reviewed in reference 19). The best characterized, fosA, encodes a Mn2+-dependent glutathione transferase (1–3). A related resistance gene, fosB, is from an S. epidermidis plasmid. FosB is 38% identical to FosA, suggesting a similar mechanism of action (21), as confirmed in this study. Indeed, the plasmid-borne fosA resistance determinants may have arisen from chromosomal genes, like fosB, that serve a defensive role within soil microorganisms such as B. subtilis.
There are several significant mechanistic differences between FosA and FosB. FosB, unlike FosA, does not function efficiently with glutathione and instead appears to use l-cysteine as the physiological thiol donor. Analyses of S. epidermidis FosB have also indicated that glutathione is not involved in detoxification (R. Asano and J. Davies, personal communication). A second mechanistic difference is that FosB uses Mg2+ rather than Mn2+ as metal cofactor. The final notable difference between FosA and FosB is catalytic efficiency. FosA exhibits a very high catalytic efficiency (kcat/Km), perhaps in response to selection pressures imposed by the clinical use of fosfomycin.
The assignment of fosB to the ςW regulon further validates the “consensus search” approach for defining alternative ς factor regulons (9–11), and it supports the emerging picture of ςW as a regulator of a broad, “antibiosis” regulon involved in both the production of, and defense against, antimicrobial compounds.
Acknowledgments
We thank Xuejun Huang, Ahmed Gaballa, and Kurt Fredrick for construction of plasmids and strains used in this work and T. Msadek for providing plasmid pXT.
This work was supported by grants from the National Institutes of Health to J.D.H. (GM47446) and R.N.A. (AI42756).
REFERENCES
- 1.Arca P, Hardisson C, Suarez J E. Purification of a glutathione S-transferase that mediates fosfomycin resistance in bacteria. Antimicrob Agents Chemother. 1990;34:844–848. doi: 10.1128/aac.34.5.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernat B A, Laughlin L T, Armstrong R N. Elucidation of a monovalent cation dependence and characterization of the divalent cation binding site of the fosfomycin resistance protein (FosA) Biochemistry. 1999;38:7462–7469. doi: 10.1021/bi990391y. [DOI] [PubMed] [Google Scholar]
- 3.Bernat B A, Laughlin L T, Armstrong R N. Fosfomycin resistance protein (FosA) is a manganese metalloglutathione transferase related to glyoxalase I and the extradiol dioxygenases. Biochemistry. 1997;36:3050–3055. doi: 10.1021/bi963172a. [DOI] [PubMed] [Google Scholar]
- 4.Bernat B A, Laughlin L T, Armstrong R N. Regiochemical and stereochemical course of the reaction catalyzed by the fosfomycin resistance protein, FosA. J Org Chem. 1998;6:3778–3780. [Google Scholar]
- 5.Fahey R C, Brown W C, Adams W B, Worsham M B. Occurrence of glutathione in bacteria. J Bacteriol. 1978;133:1126–1129. doi: 10.1128/jb.133.3.1126-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guérout-Fleury A-M, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 7.Horii T, Kimura T, Sato K, Shibayama K, Ohta M. Emergence of fosfomycin-resistant isolates of Shiga-like toxin-producing Escherichia coli O26. Antimicrob Agents Chemother. 1999;43:789–793. doi: 10.1128/aac.43.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X, Decatur A, Sorokin A, Helmann J D. The Bacillus subtilis ςX protein is an extracytoplasmic function sigma factor contributing to the survival of high temperature stress. J Bacteriol. 1997;179:2915–2921. doi: 10.1128/jb.179.9.2915-2921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Fredrick K L, Helmann J D. Promoter recognition by Bacillus subtilis ςW: autoregulation and partial overlap with the ςX regulon. J Bacteriol. 1998;180:3765–3770. doi: 10.1128/jb.180.15.3765-3770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Gaballa A, Cao M, Helmann J D. Identification of target promoters for the Bacillus subtilis extracytoplasmic function ς factor, ςW. Mol Microbiol. 1999;31:361–371. doi: 10.1046/j.1365-2958.1999.01180.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Helmann J D. Identification of target promoters for the Bacillus subtilis ςX factor using a consensus-directed search. J Mol Biol. 1998;279:165–173. doi: 10.1006/jmbi.1998.1765. [DOI] [PubMed] [Google Scholar]
- 12.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial ς factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Missiakas D, Raina S. The extracytoplasmic function sigma factors: role and regulation. Mol Microbiol. 1998;28:1059–1066. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 14.Navas J, Leon J, Arroyo M, Garcia Lobo J M. Nucleotide sequence and intracellular location of the product of the fosfomycin resistance gene from transposon Tn2921. Antimicrob Agents Chemother. 1990;34:2016–2018. doi: 10.1128/aac.34.10.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton G L, Arnold K, Price M S, Sherrill C, Delcardayre S B, Aharonowitz Y, Cohen G, Davies J, Fahey R C, Davis C. Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J Bacteriol. 1996;178:1990–1995. doi: 10.1128/jb.178.7.1990-1995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perego M. Integrational vectors for genetic manipulation in Bacillus subtilis. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 615–624. [Google Scholar]
- 17.Skarzynski T, Mistry A, Wonacott A, Hutchinson S E, Kelly V A, Duncan K. Structure of UDP-N-acetylglucosamine enolpyruvyl transferase, an enzyme essential for the synthesis of bacterial peptidoglycan, complexed with substrate UDP-N-acetylglucosamine and the drug fosfomycin. Structure. 1996;4:1465–1474. doi: 10.1016/s0969-2126(96)00153-0. [DOI] [PubMed] [Google Scholar]
- 18.Slack F J, Mueller J P, Sonenshein A L. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993;175:4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suarez J E, Mendoza M C. Plasmid-encoded fosfomycin resistance. Antimicrob Agents Chemother. 1991;35:791–795. doi: 10.1128/aac.35.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youngman P. Use of transposons and integrational vectors for mutagenesis and construction of gene fusions in Bacillus species. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley and Sons, Ltd.; 1990. pp. 221–266. [Google Scholar]
- 21.Zilhao R, Courvalin P. Nucleotide sequence of the fosB gene conferring fosfomycin resistance in Staphylococcus epidermidis. FEMS Microbiol Lett. 1990;56:267–272. doi: 10.1016/s0378-1097(05)80052-7. [DOI] [PubMed] [Google Scholar]
- 22.Zuber P, Losick R. Role of AbrB and SpoOA- and SpoOB-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]