Abstract
Background
We have followed the COVID-19 clinical trial research agenda from the beginning using the COVID-evidence.org platform. Now, two years after the COVID-19 pandemic started, our aim was to re-examine this research agenda with the latest data to provide a global perspective on the research landscape with a focus on Germany.
Methods
We reviewed and updated previously published data on the COVID-19 clinical research agenda as of 28February 2022 focusing on randomized trials. We used the COVID-evidence.org platform including registry entries from ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform as well as publications from the Living OVerview of Evidence platform for COVID-19 (L·OVE).
Results
Two years on from the pandemic outbreak, there were 4,673 registered trials. The majority of these trials have remained small with a median of 120 planned participants (IQR 60-320). In the first hundred days of the pandemic most of them (50 %) had been registered in China. More than two years later, the five countries with the most registered trials (alone or within a framework of international collaborations) were the USA (825 trials; 18 %), Iran (619 trials; 13 %), India (566 trials; 12 %), China (353 trials; 8 %), and Spain (309 trials; 7 %). Only 119 trials were reported to have a study site in Germany (2.5 % of the registered trials). Of the 4,673 trials registered, 15 % (694 trials) had published their results by February 2022. The clinical research agenda has been marked by both successes, such as the large RECOVERY trial providing evidence on 10 treatments for COVID-19 including over 45,000 patients as of February 2022, and failures: worldwide only 57 randomized trials have been registered over two years that aimed to assess non-pharmaceutical interventions (e.g., face mask policies and lockdown measures) to prevent COVID-19, and only 11 of them had published results informing decisions that have an impact on the life of billions of people worldwide.
Conclusions
The COVID-19 clinical research agenda has highlighted the substantial effort of the research community but also the challenges of the clinical research ecosystem. Most importantly, it has shed light on the ability to circumvent traditional barriers and to make trials more useful even under extraordinary conditions. The time to learn our lessons and apply them is now, and the time to demonstrate how we have improved the system is before the next pandemic.
Keywords: COVID-19, Clinical research, Non-pharmaceutical intervention, Germany
Zusammenfassung
Hintergrund
Wir haben die COVID-19-Forschungsagenda für klinische Studien von Anfang an über die Plattform COVID-evidence.org verfolgt. Jetzt, zwei Jahre nach Beginn der COVID-19-Pandemie, haben wir diese Forschungsagenda mit den neuesten Daten erneut untersuchen wollen, um einen Überblick über die gesamte Forschungslandschaft mit einem Fokus auf Deutschland zu geben.
Methoden
Wir haben die zuvor veröffentlichten Daten zur klinischen COVID-19-Forschungsagenda mit Stand vom 28. Februar 2022 untersucht und aktualisiert, mit einem Fokus auf randomisierte Studien. Wir nutzten die Plattform COVID-evidence.org einschließlich der Registereinträge von ClinicalTrials.gov und der WHO International Clinical Trials Registry Platform sowie Publikationen der Living Overview of Evidence Plattform für COVID-19 (L-OVE).
Ergebnisse
Zwei Jahre nach Ausbruch der Pandemie waren 4.673 Studien registriert. Die Mehrzahl der Studien war nach wie vor klein mit einem Median von 120 geplanten Teilnehmern (IQR 60-320). In den ersten 100 Tagen der Pandemie wurden die meisten Studien (50 %) in China registriert. Mehr als zwei Jahre später waren die USA (825 Studien; 18 %), Iran (619 Studien; 13 %), Indien (566 Studien; 12 %), China (353 Studien; 8 %) und Spanien (309 Studien; 7 %) die fünf Länder mit den meisten registrierten Studien (allein oder im Rahmen internationaler Kooperationen). Nur für 119 Studien wurde ein Studienstandort in Deutschland berichtet (2,5 % der registrierten Studien). Von den 4.673 registrierten Studien hatten 15% (694 Studien) im Februar 2022 Ergebnisse veröffentlicht. Die klinische Forschungsagenda ist geprägt von Erfolgen, wie z. B. der sehr großen RECOVERY-Studie, die im Februar 2022 über 45.000 Patienten eingeschlossen hatte und Evidenz zu 10 Behandlungen für COVID-19 lieferte, aber auch von Misserfolgen: In den letzten zwei Jahren wurden weltweit nur 57 randomisierte Studien zur Bewertung nichtpharmazeutischer Maßnahmen (z. B. Interventionen mit Masken oder Lockdown-Maßnahmen) zur Vorbeugung von COVID-19 registriert, und nur von 11 dieser Studien wurden Ergebnisse veröffentlicht, die Entscheidungen stützen können, welche das Leben von Milliarden Menschen weltweit beeinflussen.
Schlussfolgerungen
Die COVID-19-Agenda für die klinische Forschung hat die beeindruckenden Anstrengungen der Forschergemeinschaft, aber auch die Herausforderungen des klinischen Forschungssystems deutlich gemacht. Vor allem aber hat sie gezeigt, dass es möglich ist, traditionelle Hürden zu umgehen und Studien auch unter außergewöhnlichen Bedingungen nützlicher zu machen. Die Zeit, die Lehren daraus zu ziehen und sie anzuwenden, ist jetzt, und die Zeit, zu zeigen, wie wir das System verbessert haben, ist vor der nächsten Pandemie.
Schlüsselwörter: COVID-19, Klinische Forschung, Nicht-pharmazeutische Intervention, Deutschland
Introduction
Never before has biomedical research received so much attention as with the COVID 19 pandemic. COVID-19 has affected virtually all fields of science and widely dominated health sciences in the first two years of the pandemic [1]. Initially, basic research and preclinical studies played a key role, with great sucess: rapidly the understanding of the virus grew, diagnostic tests were established, and development of vaccines began [2]. The biomedical research focus then shifted to the evaluation of therapeutic interventions.
Randomized clinical trials aim to assess the benefits and harms of health interventions, making them essential for optimal decisions about the implementation of care and health interventions and underscoring their important role in clinical research and pandemic management. Yet, clinical research is often not decision-oriented and patient-centered, and therefore not as useful as it should be [3]. The COVID-19 clinical research agenda has seen some great success stories of highly pragmatic platform trials such as the RECOVERY trial which was the spearhead of providing decision-oriented and patient-centered evidence. Conversely, the global clinical research system utterly failed to inform decisions on non-pharmaceutical interventions to prevent COVID-19 (for example, testing and lockdown measures although they had such an unprecedented impact on human life [4].
We have followed the COVID-19 clinical trial research agenda from the beginning using the COVID-evidence.org platform [4], [5], [6], [7], [8], [9], [10], smiliarly to other projects [11], [12]. Now, more than two years into the COVID-19 pandemic, we looked again at this research agenda with the latest data to provide a global perspective on the research landscape, highlight the contribution of Germany to the research agenda, and focus on select areas of lights and shadow that have emerged over time.
Methods and data sources
We reviewed and updated previously published data on the COVID-19 clinical research agenda in the early days of the pandemic [5], during the first year globally [6] and with a focus on Germany [7] and on non-pharmaceutical interventions (NPIs) [4], focusing on randomized trials. To give a full picture of the entire COVID-19 research agenda of the past two years, we used the COVID-evidence.org platform (as of February 28, 2022) [9], [10], [13]. COVID-evidence is a living database that covers registry entries and publications of worldwide planned, ongoing, and completed randomized clinical trials (RCTs) on any intervention to treat or prevent SARS-CoV-2-infections (for methodological details see protocol [10]). Data sources are international trial registries (ClinicalTrials.gov; WHO International Clinical Trials Registry Platform) and the Living OVerview of Evidence platform for COVID-19 (L·OVE) which as been shown to be a reliable alternative to searching traditional literature databases [14].
The early days
It did not take long until the first RCTs for COVID-19 have been planned and registered. There were 11 RCTs registered (all Chinese) in the first 30 days after the World Health Organization (WHO) China Country Office was notified of cases of pneumonia of unknown cause on December 31, 2019. Five hundred and sixteen RCTs were planned or registered in the first 100 days, until April 9, 2020 [5]. They aimed to include over 350,000 participants worldwide. The early RCTs were predominantly small (median sample size 144; interquartile range [IQR] 70-334) and had a remarkable focus on some highly dominating treatments combined with a very wide spectrum of intervention types.
Almost all trials explored treatments (89%; 457 of 516), and most where pharmaceutical (91%; 468 of 516). The two most frequently investigated pharmacological classes were antivirals (e.g., lopinavir/ritonavir) and antimalarials (e.g., hydroxychloroquine).
Astonishingly, a single drug, hydroxychloroquine, was studied in almost 100 mostly small RCTs in the first 100 days. Even more remarkable was that most of these small trials have been registered although at that time a mega-trial planning with over 5,000 participants had already been registered. An efficient clinical research agenda would have required that research resources be allocated elsewhere once a mega-trial was registered. Of note, only 8% (40 of 516) of all RCTs were mega-trials with over 5,000 participants, among which were the now well-known REMAP-CAP [15] and RECOVERY [16] trials.
This great interest in a single substance was contrasted by little interest in investigating preventive measures, although widely employed in highly diverse ways and forms, impacting billions of persons across the world. Only 3 RCTs on non-pharmaceutical interventions (NPIs) for prevention were planned at that time, aiming to investigate facial masks (NCT04296643, ChiCTR2000030317, NCT04337541).
These 516 trials were conducted in 38 countries alone, or in the context of international collaborations, but more than 70% came from China (50%; n = 257), the United States (10%; n = 53), France (5%; n = 29) or Spain (4%; n = 21) – only 4% were international collaborations (n = 21). Of all 516 RCTs initiated worldwide in the first 100 days, only 12 mentioned a German site (2%), of which 6 were conducted only in Germany (1%) and 6 in the context of international collaborations (1%) [5].
After one year
Throughout the first year of the pandemic, the number of trials steadily increased [6]. After the initial dominance of trials conducted in Asia (mostly China), the number of trials registered in the rest of the world increased rapidly as the pandemic spread through March 2020. There were 2,814 registered randomized trials worldwide as of February 16, 2021. Most were still small with only 18% planning to recruit >500 participants and only 3% planning to recruit >5000 participants. A small proportion of 6% of trials linked to registry entries provided published results, allowing to inform decisions promptly. While this is indeed a small proportion – it does represent a remarkable absolute number of 171 trials with results published as of February 16, 2021 [6].
In Germany, 65 trials assessing interventions to treat or prevent COVID-19 were nationally and internationally registered during 2020 according to a recent analysis of all studies having planned to recruit participants in Germany [7]. This work showed that the majority of the trials were planned in collaboration with researchers from other countries (59%), were industry-funded (54%), assessed treatments (86%), and included hospitalized patients (68%). Overall, these 65 trials planned to recruit 187,179 participants worldwide, and 20,696 of them in Germany; and the trials were as small as the vast majority of studies worldwide, with an average number of planned participants per trial of 106 (IQR 40 to 345). As of May 21, 2021, 17 trials provided published results. A survey on the recruitment accrual in all 65 studies from Germany indicated that this was a clear challenge, as from the planned German participants, an estimated number of 13% were recruited (median 15 per trial [IQR 0 to 44]); and only 3 trials (nearly) reached the targeted sample size [7]. We closer looked at this part of the global research landscape, as this was surprising for many, given the long-standing tradition of biomedical research in Germany [29], the large pharmaceutical industry sector, country size and economic power.
After two years
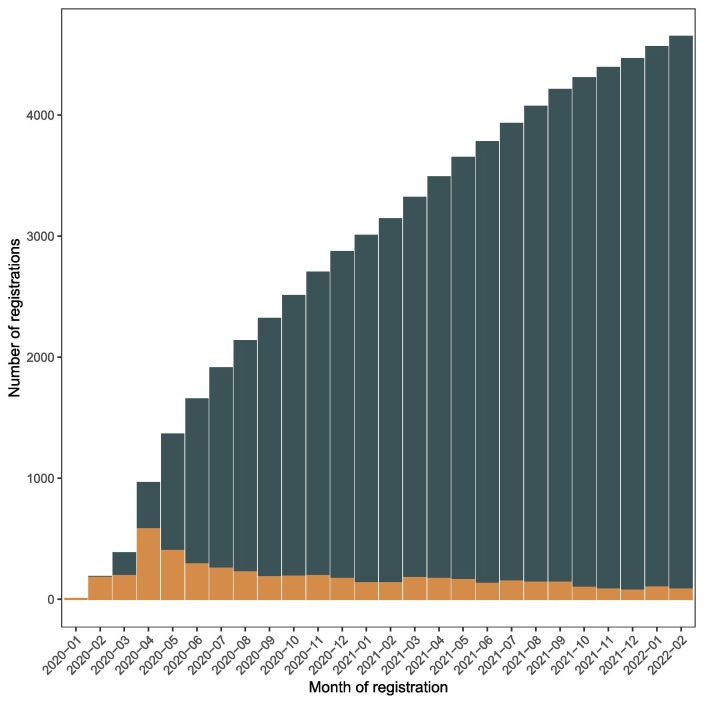
One year later, then two years after the start of the pandemic, the clinical research trial output has increased more than 1.5-fold with 4,673 registered trials as of February 28, 2022. After a peak of registrations in April 2020 (with 582 RCTs registered in a month), the number of new registrations drastically decreased but reached a steady level with a median of 135 trials registered each month (IQR 98-148) from the beginning of 2021 (Figure 1 ). The majority of trials continued to be small with a median of 120 planned participants (IQR 60-320) and 17% included > 500 participants and only 2% (144 trials) > 5000 participants.
Figure 1.
Number of COVID-19 trial registrations over time. Cumulative registration (dark blue) and number of registrations per month (orange). Of note, 22 RCTs were registereg before January 1st 2020, before the pandemic started but were then adapted to assess COVID-19 related interventions or to include COVID-19 patients.
An example of the COVID-19 research agenda’s inefficiency is the registration of additional 24 trials of hydroxychloroquine in 2021, planning to include over 8,000 participants (Table 1 ). These trials either described hydroxychloroquine as an experimental drug, or as standard of care in their registry entry, although there was published evidence from a meta-analysis of 28 RCTs available in early 2021 indicating that this controversial drug was associated with increased mortality [17].
Table 1.
Overview of RCTs assessing therapies explored in RECOVERY (based on 4,673 RCTs registered as of February 28, 2022).
| Intervention | RECOVERY results release date | N of trials (% of all trials) | N of all trials registered Before/After RECOVERY results released | N of trials with a German part Before/after RECOVERY |
Planned sample size all trials§
|
Planned sample size with a Geman part
|
|---|---|---|---|---|---|---|
| Hydroxychloroquine | 5 Jun 2020 | 340 (7.3%) |
259/80* | 6/0 |
|
|
| Dexamethasone | 16 Jun 2020 | 86 (1.8%) |
20/66 | none |
|
- |
| Lopinavir-Ritonavir | 29 Jun 2020 | 105 (2.2%) |
81/24 | 1/0 |
|
|
| Azithromycin | 14 Dec 2020 | 100 (2.1%) |
90/10 | none |
|
- |
| Convalescent plasma | 15 Jan 2021 | 100 (2.1%) |
92/8 | 6/0 |
|
|
| Tocilizumab | 11 Feb 2021 | 75 (1.6%) |
64/11 | 3/0 |
|
|
| Colchicine | 5 May 2021 | 62 (1.3%) |
44/18 | none |
|
|
| Aspirin | 8 Jun 2021 | 17 (0.4%) |
16/1 | 1/0 |
|
|
| Regeneron's monoclonal antibody combination | 16 Jun 2021 | 11 (0.2%) |
5/6 | none |
|
- |
| Baricitinib | 3 Mar 2022 | 21 (0.4%) |
21/0 | 2/0 |
|
|
Abbreviations: CI: Confidence Intervals; IQR: Interquartile range.
27 RCTs had missing information on the intervention being assessed.
Missing registration date for 1 RCT for hydroxychloroquine.
Missing planned sample size for 60 RCTs for hydroxychloroquine; 5 for vaccine; 4 for lopinavir-ritonavir; 11 for azithromycin; 2 for tocilizumab; 1 for dexamethasone; 2 aspirin; 2 colchicine; 2 for convalescent plasma; and 4 for chloroquine.
The share of countries
The share of countries in global trial research has changed over time, with some countries increasingly initiating a large share of RCTs.
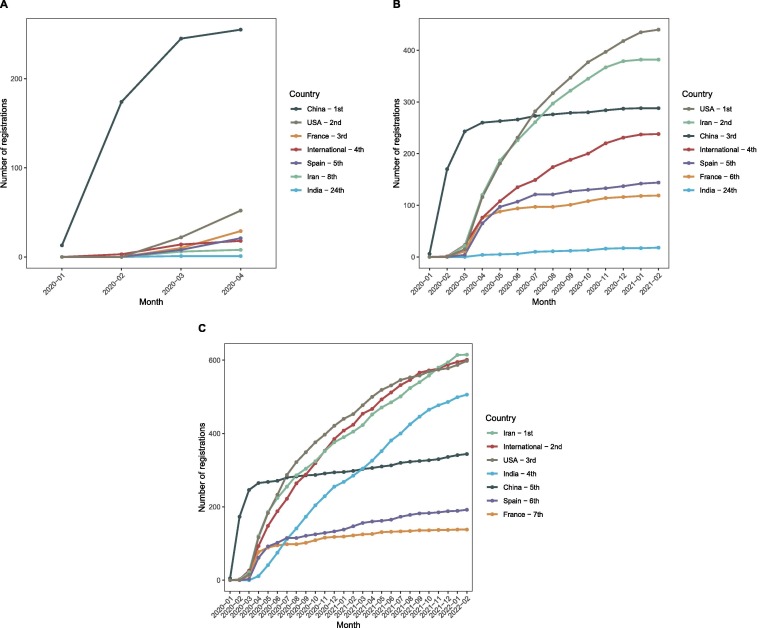
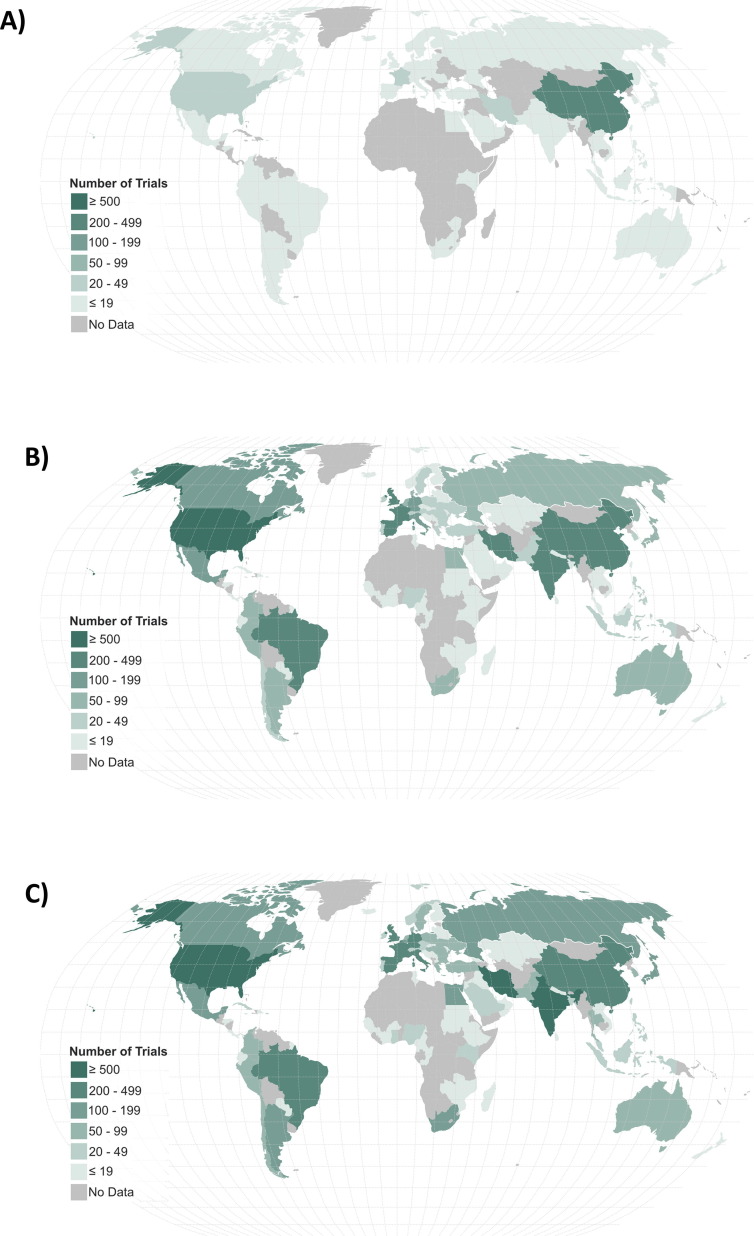
The 516 randomized studies registered in the early days were conducted (or at least planned to be conducted) in 38 countries or in the context of international collaborations, but more than 70% of the trials came from China , the United States, France or Spain. Over time, Asia continued to lead with 40% of the registered trials globally after two years (n = 1,857; considering only the national trials; Table 2 ), but China’s initial high share declined to 7% of all trials after two years (n = 343). New countries have emerged among the 5 with the highest number of registrations such as Iran (13%; n = 615) and India (11%; n = 506) (Figure 2 and Table 2). Two other countries have shown substantial increase in their clincial trial research contribution compared with the 100 days clinical research agenda: Brazil from 7 to 136 (3%) and Egypt from 1 to 88 (2%) national trials registered (Figure 3 ). Of note, although international trials with sites in multiple countries represented only 4% in the early days, this effort quickly increased to 13% of all registered trials (Figure 2 and Table 2).
Table 2.
30 highest-ranked global clinical research contributions for COVID-19 trials (conducted in a single country nationally and conducted in multiple countries internationally; based on 4,673 RCTs registered as of February 28, 2022).
|
National contribution only (n = 4,673) |
National and international contribution (n = 4,673) |
|||
|---|---|---|---|---|
| Rank | Countries | N (%) | Countries | N (%) |
| 1 | Iran | 615 (13.2%) | USA | 825 (17.7%) |
| 2 | International | 611 (13.1%) | Iran | 619 (13.2%) |
| 3 | USA | 605 (12.9%) | India | 566 (12.1%) |
| 4 | India | 506 (10.8%) | China | 353 (7.6%) |
| 5 | China | 344 (7.4%) | Spain | 309 (6.6%) |
| 6 | Spain | 193 (4.1%) | Brazil | 273 (5.8%) |
| 7 | France | 139 (3%) | United Kingdom | 211 (4.5%) |
| 8 | Brazil | 136 (2.9%) | France | 209 (4.5%) |
| 9 | Egypt | 88 (1.9%) | Mexico | 159 (3.4%) |
| 10 | Italy | 82 (1.8%) | Italy | 148 (3.2%) |
| 11 | Japan | 77 (1.6%) | Canada | 134 (2.9%) |
| 12 | Canada | 73 (1.6%) | Germany | 119 (2.5%) |
| 13 | Germany | 61 (1.3%) | Japan | 117 (2.5%) |
| 14 | Mexico | 57 (1.2%) | Argentina | 101 (2.2%) |
| 15 | Turkey | 54 (1.2%) | Russia | 98 (2.1%) |
| 16 | Australia | 53 (1.1%) | Egypt | 97 (2.1%) |
| 17 | Thailand | 49 (1%) | Belgium | 83 (1.8%) |
| 18 | Russia | 47 (1%) | Australia | 79 (1.7%) |
| 19 | Denmark | 41 (0.9%) | Turkey | 78 (1.7%) |
| 20 | Netherlands | 41 (0.9%) | Colombia | 73 (1.6%) |
| 21 | Pakistan | 37 (0.8%) | Netherlands | 71 (1.5%) |
| 22 | Belgium | 34 (0.7%) | Denmark | 68 (1.5%) |
| 23 | Argentina | 29 (0.6%) | Thailand | 66 (1.4%) |
| 24 | Cuba | 28 (0.6%) | Peru | 64 (1.4%) |
| 25 | Indonesia | 28 (0.6%) | Poland | 64 (1.4%) |
| 26 | Bangladesh | 25 (0.5%) | South Korea | 56 (1.2%) |
| 27 | Israel | 24 (0.5%) | Ukraine | 50 (1.1%) |
| 28 | Sweden | 24 (0.5%) | Pakistan | 49 (1%) |
| 29 | Colombia | 22 (0.5%) | Israel | 45 (1%) |
| 30 | Poland | 18 (0.4%) | Indonesia | 43 (0.9%) |
Of note, 241 registered trials did not report the countries of conduct.
Figure 2.
Evolution of the 5 highest ranked contributors in number of COVID-19 trial registrations during A) the early days (100 days), B) after one year, and C) after two years.
Figure 3.
Evolution of COVID-19 trial registrations throughout the world during A) the early days (100 days), B) after one year, and C) after two years.
In the same period, in Germany a total of 61 trials were planned as national trials (Table 2) and 57 as international trials, so that there were overall 119 trials with a reported study site in Germany.
Dissemination of the evidence
The proportion of the 4,673 trials globally (including both, national and international projects) that provided published results which may provide evidence to inform decisions remaind small with 15%; however, the absolute number of 694 trials with results available either as publication, preprint or posted on the registry is still remarkable. Conversely, the proportion of the trials with published results is higher in Germany, with 26% (31 of 119). Here it is interesting to note that 42% (24 of 58) of the international trials that planned to include sites in Germany have been published, but only 11% (7 of 61) of the trials fully conducted in Germany delivered published results over 2 years. Overall, two years after the pandemic started, there are 31 clinical trials from Germany (alone or among other countries) with published results to treat or prevent COVID-19.
A story of success: The RECOVERY trial
The large, highly pragmatic (i.e. decision-oriented [18]) RECOVERY trial from the United Kingdom is clearly the very success story of clinical research during this pandemic since the results provided evidence directly informing clinical decisions on the treatment of millions of patients. RECOVERY received ethical approval within 9 days, recruited 10,000 patients within 6 weeks and provided the first treatment to reduce mortality within 3 months of its conceptualization [16], [19]. As of March 2022, over 47,000 patients had been recruited in nearly 200 sites across six countries providing results for ten randomized treatment evaluations and revealing four effective COVID-19 treatments (Table 1) [20]. Of note, beyond successfully finding treatments that work, RECOVERY has improved care of millions of patients by revealing that many promising treatments do not work or even do harm, thus protecting patients from unneccessary or harmful interventions. This may even be the most important contribution to the clinical research agenda, and a characteristic of excellent clinical research that has often been, and still is, undervalued (for example, by still using misleading labels for some excellent research as “negative trial”) [21].
This pandemic strikingly illustrated a phenomenon constantly observed in medical history, that many treatments were promising in theory, often widely used by experts with the best intentions, and sometimes supported by large-scale observational evidence, yet shown to be ineffective or even harmful in subsequent randomized trials [22].
RECOVERY has finally buried the traditional myth that randomized trials have to take a long time, need to have complex designs, and do not provide real-world evidence. Although high quality observational real-world data are of utmost importance to monitor the health of populations and development of the pandemic, the therapeutic breakthroughs for COVID-19 have not been generated by non-randomized, observational “real-world” evidence. While these approaches have been touted as critical cutting-edge technologies to guide treatment and support drug approval, and could even replace clinical trials, their track record in COVID-19 has been disappointing [6]. Neither hydroxychloroquine nor convalescent plasma – to only name two – have been able to demonstrate the benefits claimed by several very large observational studies [23], [24]. Without the well-conducted randomized clinical trials, millions of COVID-19 patients could have continued to be exposed to ineffective or even harmful treatments.
RECOVERY is a platform trial that compares multiple intervention arms using a factorial design where patients can receive more than one experimental treatment. The pragmatic features of the study design, with broad eligibility criteria reduced to the essentials (i.e. hospitalization, confirmed or suspected SARS-CoV-2 infection, and no specific contraindication to participation), have clearly fostered recruitment [18]. This is in contrast to the exclusion of patients of older age or with comorbidities, which would be hard to justify in trials that seek to inform real-world decisions in these populations for whom COVID-19 treatments are most needed.
An essential aspect of RECOVERY was its direct integration into patient care, with most centers being non-academic hospitals [25]. The primary endpoint, all-cause mortality at 28 days, was collected online and further information was gathered via national health registries using routinely collected data [26].
Overall, the potential of learning from COVID-19 to transform clinical research to make it more useful and patient-centered is high by establishing hundreds of platform trials for all relevant conditions as the very normal approach to evaluate treatment choices, by promoting rapid and most efficient data sharing processes, and by having a culture of clinical research as the standard of care. However, future research is needed to address the methodological challenges with this rather novel design [27].
A story of failure: non-parmaceutical interventions (NPIs)
For over two years now, in March 2022, the life of billions of people worldwide has been affected by drastic measures aiming to improve health by preventing infections with COVID-19. This included quarantine and isolation, the closing of schools, workplaces, shops, clubs, bars and restaurants, travel restrictions, the prohibition to leave their own home, meet friends and relatives even in the last days of their lives. Diagnostic interventions in numerous forms of testing regimens are part of daily life for many. Protective equipment such as surgical or N95 masks, thus far used only in healthcare settings or for specific professions, have been rolled-out to be used by everybody, in some places even by toddlers. Numerous strategies target various populations, some everybody, some only vulnerable persons. Even after almost two years, parts of the German population were forbidden to leave their homes at night if they had certain health characteristics (immune status), and some hospitals and nursing homes were closed for visitors and relatives of inpatients and residents.
This, of course, has massive ethical implications that require careful consideration of the benefits and harms of these measures. Whether or not someone is affected by such a measure usually seems to depend more on where they live and the regulations chosen by local decision-makers than on the individual’s risk profiles, population health determinants or clear, evidence-based criteria. For example, while in one part of a country like Germany, school children had to wear masks in class or were regularly tested, in other parts of the very same country this was at the same time not required and politicians heavily debate such regulations [28], [29]. This is a status of political equipoise, that may call for randomized assessments.
Many of these so-called non-pharmacological interventions (NPIs) are more or less supported by mechanistic assumptions, physical principles, preclinical and animal experiments. However, all novel pharmacological interventions that undergo clinical evaluation also are based on sound assumptions, theoretical principles and preclinical evaluation and animal experiments. And yet almost all of these candidate treatments, estimated 90%, fail to show sufficient benefit and safety to get approval [30]. Would it be reasonable to assume that NPIs are more frequently effective than the carefully developed pharmaceutical products seeking market accesss? As for drug treatments, the established method to show benefits and harms for non-pharmaceutical interventions is the randomized trial.
Continous monitoring of all NPI trials shows that as of February 28, 2022, 57 randomized trials were registered worldwide to assess how to effectively prevent COVID-19 with social or behavioural interventions, devices, or any other NPI [8], [13]. These trials were predominantly set up in adult community populations in North America and Europe assessing testing regimens or protective equipment (i.e., masks). Only some of them were planned to investigate strategies to mitigate the COVID-19 spread in nursing homes, schools, or universities. Randomized trials assessing the benefits and harms of masking children for many hours per day are lacking. The status of the NPI trials shows that only around one quarter of the trials (26%) is completed and half of them (47%) are still ongoing.
For the completed NPI trials, we are aware of only 11 trials with published results (Table 3 ). None of the trials came from Germany, highlighting that the German contribution to generate high-quality evidence supporting political decisions on the benefits and harms of NPIs is minimal. It cannot be ruled out that randomized trials from Germany have remained unregistered, but to our knowledge they have not appeared publicly in the scientific debate as it would be expected from important evidence.
Table 3.
Overview of the 11 randomized assessments of non-pharmaceutical interventions to prevent from COVID-19 with published results (based on 4,675 RCTs registered as of February 28, 2022).
| Registry ID | Intervention(s) | Comparator(s) | Setting, country | N individuals / clusters | Start to end date | Results |
|---|---|---|---|---|---|---|
| Trial acronym | ||||||
| Reference | ||||||
|
NCT04620798 [38] NA |
Immediate provision of COVID-19 antibody test results in addition to assessment of engagement with COVID-19 prevention behaviors following testing of university students | Delayed provision of COVID-19 antibody test results in addition to assessment of engagement with COVID-19 prevention behaviors following testing of university students | University, USA | 1,076 / NA | 2020-09 to 2020-11 | No differences in numbers of serovonverted participants (from seronegative to seropositive) between the study groups (secondary outcome). |
| ChiCTR2000030317 [39] NA |
Fully closed negative-pressure gastroscope isolation mask | No mask | Hospital, China | 320 / NA | 2020-02 to 2020-04 | No COVID-19 cases were recorded during follow-up in both study groups (secondary outcome). |
|
NCT04337541 [40] DANMASK-19 |
Surgical face mask recommendation | No recommendation | Community, Denmark | 6,024 / NA | 2020-04 to 2020-06 | No differences in SARS-CoV-2 infections between the study groups (primary outcome). |
|
NCT04406909 [41] TRAiN |
Access to indoor fitness center applying physical distancing and enhanced hand and surface hygiene | No access | Community, Norway | 3,764 / NA | 2020-05 to 2020-06 | No differences in SARS-CoV-2 infections between the study groups (primary outcome). No outpatient visits or hospital admissions due to COVID-19 in both study groups (secondary outcome). |
| CTRI/2020/07/026667 [42] NA |
Morning and evening Pranayama yoga sessions | General fitness practices (e.g., walking, jogging, running) | Hospital, India | 280 / NA | 2020-09 to 2020-11 | Lower number of SARS-CoV-2 infections in the yoga sessions group (primary outcome). |
|
NCT04644328 [32] NA |
Facebook ads containing a short video on the importance of staying safe during Thanksgiving and Christmas by considering not traveling, social distancing, and using a mask when appropriate | No Facebook ads | Community, USA | 3,525,6399 / 1,587 | 2020-11 to 2021-01 | Lower number of SARS-CoV-2 infections in the Facebook ads group (primary outcome). |
|
NCT04668625 [43] NA |
Access to an indoor music event with systematic same-day screening of attendees with antigen-detecting rapid diagnostic tests, use of face masks, and adequate air ventilation | No access | Community, Spain | 1,047 / NA | 2020-12 to 2020-12 | Lower number of SARS-CoV-2 infections in the access to music event group (primary outcome). |
| ISRCTN18100261 [44] NA |
Daily testing of contact cases of school staff and students | Isolating contact cases of school staff and students | School, United Kingdom | 238,579 / 201 | 2021-04 to 2021-06 | No differences in SARS-CoV-2 infections between the study groups (primary outcome). |
|
NCT04630054 [45] NA |
Community-level masks promotion strategies (multiple levels of randomization, including free surgical or cloth face masks with information on the importance of masking, role modeling by community leaders and imams, and in-person reminders) | No intervention | Community, Bangladesh | 342,126 / 600 | 2020-11 to 2021-04 | Lower numbers of SARS-CoV-2 infections in the mask use promotion group (primary outcome). |
|
NCT04872075 [46] SPRING |
Access to an indoor music event with systematic antigen-screening within 3 days, medical mask-wearing and optimized ventilation | No access | Community, France | 6,678 | 2021-05 to 2021-06 | No differences in SARS-CoV-2 infections between the study groups (primary outcome). |
|
NCT04359264 [34] DISTANSE COVID |
Income support of $1000 to community-living adults suffering from COVID-19-related financial disruptions and information about reducing the spread of COVID-19 over the phone and via email | Information about reducing the spread of COVID-19 over the phone and via email alone | Community, Canada | 392 | 2020-04 to 2020-05 | No differences in COVID-19 symptoms or SARS-CoV-2 infections between the study groups (primary outcome). |
Abbreviations: N = Number; NA = Not applicable; NR = Not reported.
In sum, the foundation for potential evidence on the benefit and harms of NPIs is scarce; 57 trials, a tiny fraction of almost 5,000 trials initiated worldwide, assess NPIs to prevent COVID-19, with only a dozen having published results potentially informing evidence-based decisions [8], [10], [13], [31]. These data show that the worldwide research agenda failed to set up highly desired trials to inform political decision makers about the contribution of NPIs to prevent COVID-19.
Positive examples
However, there are some positive examples of randomized assessments of NPIs that are highlighted here to illustrate the range of creative possibilities and what could have been done.
One example is a pragmatic megatrial that was set-up in the US including more than 35 million people. Prior to Thanksgiving and Christmas holidays, Facebook users were randomly assigned to receive either ads containing a short video on the importance of “staying safe” by considering not traveling, social distancing, and using a mask when appropriate or no facebook ads. Confirmed SARS-CoV-2 infections were analysed on the zip-code level up to two weeks after the holidays. The results favor the Facebook ads indicating these measures to be an effective pubic health strategy to prevent individuals from SARS-CoV-2 infections [32].
Another example is the “GLasses Against transmission of SARS-CoV-2 in the community” (GLASSY) trial that was initiated in Norway; a pragmatic, randomized, and fully remote and virtual trial without any personal interaction between investigators and participants. It evaluates whether SARS-CoV-2 infections can be reduced when participants are asked to wear glasses (sunglasses or other glasses) when they are outside of their home and close to others (e.g., on public transport, in shopping centres) compared to participants that are asked to wear no glasses in public spaces [33].
A third example is the Canadian “Direct Income SupporT and Advice Negating Spread of Epidemic COVID-19” (DISTANSE COVID) trial; a randomized social assessment whether income support of $1000 to community-living adults suffering from COVID-19-related financial disruptions and information about reducing the spread of COVID-19 compared to information alone reduced symptoms related to COVID-19. The results indicate that COVID-19 symptoms were reduced in participants above the age of 50 [34].
Discussion
After two years of the pandemic, over 4,000 randomized trials have been registered to evaluate interventions to prevent or treat COVID-19. Unfortunately, most trials remained small and resulted in a limited number of publications. Very early in the pandemic, it became clear that there was a lack of timely coordination and structured efforts to knowledge generation – there was, with few exceptions, no integration of clinical trial research in the pandemic response. Remarkably, the development of new therapies was largely preceded by the assessment of repurposing drugs established for other diseases, such as the antimalarial hydroxychlorquine. The same question on hydroxychloroquine was investigated again and again, a rarely seen chaos-like redundancy without comprehensible justification [6]. It also became clear that the large and ambitious research effort would be accompanied by a huge waste of resources [35]. Germany played almost no role as a location for the clinical trials in the early response to the pandemic, being involved in only 2% of all over 500 clinical trials initiated in the first 100 days. Randomized assessments to find the most efficient non-pharmaceutical strategies to prevent COVID-19 was unfortunately not part of the early days response.
With steadily increasing number of trials over the first year, the number of trials with published results grew. Despite being only a small fraction of the over 2,800 trials initiated, there were still 171 trials sucessfully initiated, conducted and published within more or less one year – this is remarkable nevertheless.
In the second year, the deficits of the the COVID-19 clinical research agenda persisted with little evolution towards more coordination and efficiency. Trials remained small. Hydroxychloroquine was still included in newly registered trials. And Germany’s contribution remained limited contributing to 2.5% of registered trials nationally and internationally ranking on place 12. Germany has a long-standing tradition of excellent biomedical research in the field of basic research and preclinical studies [36]. However, with regard to practical health care decisions, a relatively small contribution of German trials was already described in 2013, with only 5% of the trials used for German Health Technology Assessments (HTA) coming from Germany [37] although such reports aim to inform benefit assessments with major relevance for health care in Germany. It seems little has improved during the COVID-19 pandemic [7].
Somewhat reassuring was the rise of internationally conducted trials which now represent 13% of the registered trials, ranging on place 2 worldwide, illustrating an improvement in collaborations and coordinations. The highest ranked position of Iran as individual country of origin with most registered trials worldwide is highly interesting and it needs a better understanding of the contributing factors.
The pandemic clinical research agenda will most certainly provide textbook examples of the missed opportunities but also the immense potential of the scientific community. On one hand, the scientific community’s inaction and continuing disinterest in conducting randomized assessments of non-pharmaceuticl interventions to prevent the spread of COVID-19 resulted in drastic preventive measures being taken without robust evidence to support these decisions. On the other hand, the incredible speed of the research agenda and the few illustrious examples, such as RECOVERY, of collaborations and coordinations, clearly illustrate the remarkable potential of the scientific community.
Our analysis here has several limitations, including that we may have missed duplicate entries across registries or of multiple national parts of an international trial, thus slightly overestimating the number of trials, or conversely, that we missed trials conducted in a country, for example because they were not registered or the registry data gave no indication of the country’s contribution. Some registry entries had missing data for sample size, interventions arms and/or country of conduct, thus, reported numbers and proportions may be imprecise. Finally, in this rapidly evolving setting, information available in a registry is not timely and sometimes never updated and we might not capture updates, adaptations and amendments to trial designs and/or interventions being assessed.
COVID-19 has highlighted the limitations of the current research system in Germany, but also challenged its status quo. It is simply not true that clinical trials need to be expensive and cumbersome [6]. Elegant and simple study designs with clinical research as standard of care can test treatments quickly and efficiently. It is obvious that priorities must be set to coordinate efforts that separate the useful from the useless and harmful.
Overall, successful clinical research does not require a revolution in methodology; we already have the tools. It is mainly the man-made problems in the research system along with the regulatory and institutional requirements that prevent us from making individual research success stories the normal state of medical progress.
Conclusions
The COVID-19 clinical research agenda was initiated at an incredible speed. The systematic assessment of the agenda highlights the challenges of the clinical research ecosystem on a world scale but also for individual countries. Most importantly, it has shed light on the ability to circumvent traditional hurdles and to make trials more useful even under extraordinary conditions. It is up to the system to adapt so that the enormous scientific potential of the clinical community can be unleashed.
The time to learn the lessons and apply them is now, and the time to demonstrate how we have improved the system is long before the next pandemic.
Funding
This work is part of the COVID-evidence project which is supported by the Swiss National Science Foundation, project ID 31CA30_196190.
Acknowledgement
The authors thank Pascal Düblin (University of Basel) for his support in managing the database and generation of Figure 3, Ulrich Dirnagl (Berlin Institute of Health at Charité BIH), and Atle Fretheim (Norwegian Institute of Public Health) for their critical feedback on a previous version of the manuscript.
Conflict of Interest
All authors declare that there is no conflict of interest.
CRediT author statement
The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
-
•
Concept and design: All authors.
-
•
Acquisition, analysis, or interpretation of data: All authors.
-
•
Drafting of the manuscript: All authors.
-
•
Critical revision of the manuscript for important intellectual content: All authors.
-
•
Supervision: All authors.
References
- 1.Ioannidis J.P.A., Bendavid E., Salholz-Hillel M., Boyack K.W., Baas J. Massive covidization of research citations and the citation elite. medRxiv. 2022 doi: 10.1101/2022.01.24.22269775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyriakidis N.C., López-Cortés A., González E.V., Grimaldos A.B., Prado E.O. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines. 2021;6:28. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ioannidis J.P.A. Why Most Clinical Research Is Not Useful. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirt J., Janiaud P., Hemkens L.G. Randomised trials on non-pharmaceutical interventions for COVID-19 as of August 2021: a scoping review. BMJ EBM. 2022 doi: 10.1136/bmjebm-2021-111825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janiaud P., Axfors C., van't Hooft J., Saccilotto R., Agarwal A., Appenzeller-Herzog C., Contopoulos-Ioannidis D.G., Danchev V., Dirnagl U., Ewald H., Gartlehner G., Goodman S.N., Haber N.A., Ioannidis A.D., Ioannidis J.P.A., Lythgoe M.P., Ma W., Macleod M., Malički M., Meerpohl J.J., Min Y., Moher D., Nagavci B., Naudet F., Pauli-Magnus C., O'Sullivan J.W., Riedel N., Roth J.A., Sauermann M., Schandelmaier S., Schmitt A.M., Speich B., Williamson P.R., Hemkens L.G. The worldwide clinical trial research response to the COVID-19 pandemic - the first 100 days [version 2; peer review: 2 approved] F1000Res. 2020;9:1193. doi: 10.12688/f1000research.26707.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janiaud P., Hemkens L.G., Ioannidis J.P.A. Challenges and lessons learned from Covid-19 trials – should we be doing clinical trials differently? Can J Cardiol. 2021;37:1353–1364. doi: 10.1016/j.cjca.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirt J., Rasadurai A., Briel M., Düblin P., Janiaud P., Hemkens L.G. Clinical trial research on COVID-19 in Germany – a systematic analysis [version 1; peer review: awaiting peer review] F1000Res. 2021;10:913. doi: 10.12688/f1000research.55541.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.J. Hirt, P. Janiaud, L.G. Hemkens, Why we urgently need a long-term research agenda on non-pharmaceutical interventions to guide policies and practices in the current and future public health emergencies: Blog entry written on: Randomized trials on non-pharmaceutical interventions for COVID-19: a scoping review, (bmjebm-2021-111825), 2022. https://blogs.bmj.com/bmjebmspotlight/2022/03/25/why-we-urgently-need-a-long-term-research-agenda-on-non-pharmaceutical-interventions-to-guide-policies-and-practices-in-the-current-and-future-public-health-emergencies/ (accessed 25 April 2022).
- 9.J. Hirt, P. Janiaud, P. Düblin, L.G. Hemkens, Randomized trials assessing non-pharmaceutical interventions to prevent COVID-19: A living systematic overview on randomized trials assessing non-pharmaceutical interventions to prevent COVID-19, 2021. https://osf.io/fq9jh/.
- 10.P. Janiaud, C. Axfors, R. Saccilotto, A.M. Schmitt, J. Hirt, L.G. Hemkens, COVID-evidence: a living database of trials on interventions for COVID-19, 2021. https://osf.io/gehfx/ (accessed 2 November 2021).
- 11.COVID-NMA, The COVID-NMA initiative: A living mapping and living systematic review of Covid-19 trials. https://covid-nma.com/ (accessed 13 July 2022).
- 12.M. Salholz-Hillel, P. Grabitz, M. Pugh-Jones, D. Strech, N.J. DeVito, Results Availability and Timeliness of Registered COVID-19 Clinical Trials: A Cross-Sectional Study: Preprint (2021). https://doi.org/10.1101/2021.04.07.21255071. [DOI] [PMC free article] [PubMed]
- 13.COVID-evidence, COVID-evidence Database: Living overview on randomized trials assessing non-pharmaceutical interventions to prevent COVID-19, 2022. https://covid-evidence.org/database?page=npi (accessed 28 February 2022).
- 14.Pierre O., Riveros C., Charpy S., Boutron I. Secondary electronic sources demonstrated very good sensitivity for identifying studies evaluating interventions for COVID-19. J Clin Epidemiol. 2022;141:46–53. doi: 10.1016/j.jclinepi.2021.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.REMAP-CAP, REMAP-CAP: A Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia, 2022. https://www.remapcap.org/ (accessed 17 March 2021).
- 16.University of Oxford, RECOVERY Trial, 2022. https://www.recoverytrial.net/ (accessed 17 March 2022).
- 17.Axfors C., Schmitt A.M., Janiaud P., van’t Hooft J., Abd-Elsalam S., Abdo E.F., Abella B.S., Akram J., Amaravadi R.K., Angus D.C., Arabi Y.M., Azhar S., Baden L.R., Baker A.W., Belkhir L., Benfield T., Berrevoets M.A.H., Chen C.-P., Chen T.-C., Cheng S.-H., Cheng C.-Y., Chung W.-S., Cohen Y.Z., Cowan L.N., Dalgard O., de Almeida F.F., e Val, de Lacerda M.V.G., de Melo G.C., Derde L., Dubee V., Elfakir A., Gordon A.C., Hernandez-Cardenas C.M., Hills T., Hoepelman A.I.M., Huang Y.-W., Igau B., Jin R., Jurado-Camacho F., Khan K.S., Kremsner P.G., Kreuels B., Kuo C.-Y., Le T., Lin Y.-C., Lin W.-P., Lin T.-H., Lyngbakken M.N., McArthur C., McVerry B.J., Meza-Meneses P., Monteiro W.M., Morpeth S.C., Mourad A., Mulligan M.J., Murthy S., Naggie S., Narayanasamy S., Nichol A., Novack L.A., O’Brien S.M., Okeke N.L., Perez L., Perez-Padilla R., Perrin L., Remigio-Luna A., Rivera-Martinez N.E., Rockhold F.W., Rodriguez-Llamazares S., Rolfe R., Rosa R., Røsjø H., Sampaio V.S., Seto T.B., Shehzad M., Soliman S., Stout J.E., Thirion-Romero I., Troxel A.B., Tseng T.-Y., Turner N.A., Ulrich R.J., Walsh S.R., Webb S.A., Weehuizen J.M., Velinova M., Wong H.-L., Wrenn R., Zampieri F.G., Zhong W., Moher D., Goodman S.N., Ioannidis J.P.A., Hemkens L.G. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021;12:2349. doi: 10.1038/s41467-021-22446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz D., Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Clin Epidemiol. 2009;62:499–505. doi: 10.1016/j.jclinepi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Mather N. How we accelerated clinical trials in the age of coronavirus. Nature. 2020;584:326. doi: 10.1038/d41586-020-02416-z. [DOI] [PubMed] [Google Scholar]
- 20.University of Oxford, The RECOVERY Trial is two years old today, 2022. https://www.recoverytrial.net/news/the-recovery-trial-is-two-years-old-today (accessed 23 March 2022).
- 21.Desai A.S., Pfeffer M.A. Beyond the P-value and the sound bite: learning from 'negative' clinical trials. Eur Heart J. 2017;38:2349–2351. doi: 10.1093/eurheartj/ehx395. [DOI] [PubMed] [Google Scholar]
- 22.Prasad V., Vandross A., Toomey C., Cheung M., Rho J., Quinn S., Chacko S.J., Borkar D., Gall V., Selvaraj S., Ho N., Cifu A. A decade of reversal: an analysis of 146 contradicted medical practices. Mayo Clinic Proc. 2013;88:790–798. doi: 10.1016/j.mayocp.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 23.M.J. Joyner, J.W. Senefeld, S.A. Klassen, J.R. Mills, P.W. Johnson, E.S. Theel, C.C. Wiggins, K.A. Bruno, A.M. Klompas, E.R. Lesser, K.L. Kunze, M.A. Sexton, J.C. Diaz Soto, S.E. Baker, J.R.A. Shepherd, N. van Helmond, C.M. van Buskirk, J.L. Winters, J.R. Stubbs, R.F. Rea, D.O. Hodge, V. Herasevich, E.R. Whelan, A.J. Clayburn, K.F. Larson, J.G. Ripoll, K.J. Andersen, M.R. Buras, M.N.P. Vogt, J.J. Dennis, R.J. Regimbal, P.R. Bauer, J.E. Blair, N.S. Paneth, D. Fairweather, R.S. Wright, R.E. Carter, A. Casadevall, Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience, 2020
- 24.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Pessoa-Amorim G., Campbell M., Fletcher L., Horby P., Landray M., Mafham M., Haynes R. Making trials part of good clinical care: lessons from the RECOVERY trial. Future Healthc J. 2021;8:e243–e250. doi: 10.7861/fhj.2021-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.[26] RECOVERY Central Coordinating Office, Randomised evaluation of COVID-19 Therapy (RECOVERY), 2022. https://www.recoverytrial.net/files/recovery-protocol-v23-1-2022-03-15.pdf (accessed 1 April 2022).
- 27.Dodd L.E., Freidlin B., Korn E.L. Platform Trials - Beware the Noncomparable Control Group. N Engl J Med. 2021;384:1572–1573. doi: 10.1056/NEJMc2102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maybaum T. Föderalismus spaltet die Gesellschaft. Deutsches Ärzteblatt. 2021;118:756. [Google Scholar]
- 29.Frankfurter Rundschau, Masken, Tests, Distanzunterricht: Diese Corona-Regeln gelten jetzt an Schulen in Deutschland, 2022. https://www.fr.de/panorama/corona-regeln-schulen-bundeslaender-masken-tests-unterricht-distanz-homescooling-mai-aenderungen-news-91511261.html (accessed 14 June 2022).
- 30.Hay M., Thomas D.W., Craighead J.L., Economides C., Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 31.Hirt J., Janiaud P., Düblin P., Hemkens L.G. A living overview of randomized trials on non-pharmaceutical interventions for COVID-19: Vortrag. Online-Pre-Conference EbM-Kongress. 2022 https://www.researchgate.net/publication/359315032_A_living_overview_of_randomized_trials_on_non-pharmaceutical_interventions_for_COVID-19 (accessed 18 March 2022) [Google Scholar]
- 32.Breza E., Stanford F.C., Alsan M., Alsan B., Banerjee A., Chandrasekhar A.G., Eichmeyer S., Glushko T., Goldsmith-Pinkham P., Holland K., Hoppe E., Karnani M., Liegl S., Loisel T., Ogbu-Nwobodo L., Olken B.A., Torres C., Vautrey P.-L., Warner E.T., Wootton S., Duflo E. Effects of a large-scale social media advertising campaign on holiday travel and COVID-19 infections: a cluster randomized controlled trial. Nat Med. 2021;27:1622–1628. doi: 10.1038/s41591-021-01487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fretheim A., Hemkens L.G., Helleve A., Elstrom P., Elgersma I.H., Kacelnik O. The GLasses Against transmission of SARS-CoV-2 in the communitY (GLASSY) trial: A pragmatic randomized trial (study protocol) medRxiv. 2022 doi: 10.1101/2022.02.04.22270120. [DOI] [Google Scholar]
- 34.Persaud N., Thorpe K.E., Bedard M., Hwang S.W., Pinto A., Jüni P., da Costa B.R. Cash transfer during the COVID-19 pandemic: a multicentre, randomised controlled trial. Fam Med Community Health. 2021;9 doi: 10.1136/fmch-2021-001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glasziou P.P., Sanders S., Hoffmann T. Waste in covid-19 research. BMJ. 2020;369 doi: 10.1136/bmj.m1847. [DOI] [PubMed] [Google Scholar]
- 36.Windeler J. Traurige Forschungskultur und fehlender politischer Wille, Observer. Gesundheit. 2021 [Google Scholar]
- 37.Herrmann K.H., Wolff R., Scheibler F., Waffenschmidt S., Hemkens L.G., Sauerland S., Antes G. All nations depend on the global knowledge pool – analysis of country of origin of studies used for health technology assessments in Germany. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0059213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ClinicalTrials.gov, Longitudinal COVID-19 Antibody Testing in Indiana University Undergraduate Students: Study Results, 2022. https://clinicaltrials.gov/ct2/show/results/NCT04620798?view=results (accessed 24 March 2022).
- 39.Gao Y., Xie J., Ye L.-S., Du J., Zhang Q.-Y., Hu B. Negative-Pressure Isolation Mask for Endoscopic Examination During the Coronavirus Disease 2019 Pandemic: A Randomized Controlled Trial. Clin Transl Gastroenterol. 2021;12 doi: 10.14309/ctg.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bundgaard H., Bundgaard J.S., Raaschou-Pedersen D.E.T., von Buchwald C., Todsen T., Norsk J.B., Pries-Heje M.M., Vissing C.R., Nielsen P.B., Winsløw U.C., Fogh K., Hasselbalch R., Kristensen J.H., Ringgaard A., Porsborg Andersen M., Goecke N.B., Trebbien R., Skovgaard K., Benfield T., Ullum H., Torp-Pedersen C., Iversen K. Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers A Randomized Controlled Trial. Ann Intern Med. 2021;174:335–343. doi: 10.7326/M20-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helsingen L.M., Løberg M., Refsum E., Gjøstein D.K., Wieszczy P., Olsvik Ø., Juul F.E., Barua I., Jodal H.C., Herfindal M., Mori Y., Jore S., Lund-Johansen F., Fretheim A., Bretthauer M., Kalager M. A Randomised Trial of Covid-19 Transmission in Training Facilities. medRxiv. 2022 doi: 10.1101/2020.06.24.20138768. [DOI] [Google Scholar]
- 42.Sarwal R., Dhamija R.K., Jain K., Basavaraddi I.V. Efficacy of Pranayama in Preventing COVID-19 in Exposed Healthcare Professionals: A Randomized Controlled Trial. OSF. 2021 doi: 10.31219/osf.io/c3qub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Revollo B., Blanco I., Soler P., Toro J., Izquierdo-Useros N., Puig J., Puig X., Navarro-Pérez V., Casañ C., Ruiz L., Perez-Zsolt D., Videla S., Clotet B., Llibre J.M. Same-day SARS-CoV-2 antigen test screening in an indoor mass-gathering live music event: a randomised controlled trial. Lancet Infect Dis. 2021;21:1365–1372. doi: 10.1016/S1473-3099(21)00268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young B.C., Eyre D.W., Kendrick S., White C., Smith S., Beveridge G., Nonnenmacher T., Ichofu F., Hillier J., Oakley S., Diamond I., Rourke E., Dawe F., Day I., Davies L., Staite P., Lacey A., McCrae J., Jones F., Kelly J., Bankiewicz U., Tunkel S., Ovens R., Chapman D., Bhalla V., Marks P., Hicks N., Fowler T., Hopkins S., Yardley L., Peto T.E.A. Daily testing for contacts of individuals with SARS-CoV-2 infection and attendance and SARS-CoV-2 transmission in English secondary schools and colleges: an open-label, cluster-randomised trial. The Lancet. 2021;398:1217–1229. doi: 10.1016/S0140-6736(21)01908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abaluck J., Kwong L.H., Styczynski A., Haque A., Kabir M.A., Bates-Jefferys E., Crawford E., Benjamin-Chung J., Raihan S., Rahman S., Benhachmi S., Bintee N.Z., Winch P.J., Hossain M., Reza H.M., Jaber A.A., Momen S.G., Rahman A., Banti F.L., Huq T.S., Luby S.P., Mobarak A.M. Impact of community masking on COVID-19: A cluster-randomized trial in Bangladesh. Science 375. 2022:eabi9069. doi: 10.1126/science.abi9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delaugerre C., Foissac F., Abdoul H., Masson G., Choupeaux L., Dufour E., Gastli N., Delarue S.M., Néré M.L., Minier M., Gabassi A., Salmona M., Seguineau M., Schmitt S., Tonglet S., Olivier A., Poyart C., Le Goff J., Lescure X., Kernéis S., Tréluyer J.-M. Prevention of SARS-CoV-2 transmission during a large, live, indoor gathering (SPRING): a non-inferiority, randomised, controlled trial. Lancet Infectious Diseases. 2022;22:341–348. doi: 10.1016/S1473-3099(21)00673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]