Summary
Background
Data on the long-term trajectories of lung function are scarce in COVID-19 survivors.
Methods
We re-analyzed the data from a prospective longitudinal cohort follow-up study of COVID-19 survivors over 2 years after infection. All participants were divided into scale 3, scale 4 and scale 5-6 groups according to seven-category ordinal scale. The changes of pulmonary function tests (PFTs), the Modified Medical Research Council (mMRC) Dyspnea Scale, 6-min walking test health-related quality of life (HRQoL) across the three serial follow-up visits were evaluated, and compared among three groups. We performed liner regression to determine potential factors that were associated with changes of PFTs and distance walked in 6 minutes (6MWD).
Findings
In this study, 288 participants generally presented an improvement of PFTs parameters from 6 months to 1 year after infection. The scale 5-6 group displayed a significantly higher increase of PFTs compared with scale 3 and scale 4 groups (all p<0.0167), and corticosteroids therapy was identified as a protective factor for the PFTs improvement with a correlation coefficient of 2.730 (0.215–5.246) for forced vital capacity (FVC), 2.909 (0.383–5.436) for total lung capacity (TLC), and 3.299 (0.211–6.387) for diffusion capacity for carbon monoxide (DLco), respectively. From 1-year to 2-year follow-up, the PFTs parameters generally decreased, which was not observed to be associated with changes of 6MWD and HRQoL. Dyspnea (mMRC≥1) generally decreased over time (23.3% [61/262] for 6-month, 27.9% [67/240] for 1-year, 13.4% [35/261] for 2-year), and 6MWD increased continuously (500.0 m vs 505.0 m vs 525.0 m).
Interpretation
Corticosteroids therapy during hospitalization was a protective factor for PFTs improvement from 6 months to 1 year. The relatively fast decline trend of PFTs from 1 year to 2 years needs to be paid attention and further validated in the future follow-up study.
Fundings
This work was supported by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2021-I2M-1-048) and the National Key Research and Development Program of China (2021YFC0864700).
Keywords: COVID-19, Lung function, Long COVID, Corticosteroids, Pulmonary function tests
Research in context.
Evidence before this study
We searched for articles in PubMed up to July 5, 2022, using the terms (COVID-19 OR SARS-CoV-2 OR Coronavirus disease 2019 OR 2019-nCoV) AND (follow up OR discharge* OR long term OR long COVID OR post-acute COVID-19 syndrome OR lung function* OR pulmonary function*) in title or abstract. After manually literature screening, only 7 longitudinal studies were identified to evaluate the temporal changes of lung function across at least two follow-up visits. However, no data on the long-term trajectories and determinants of lung-function changes has been reported, and the impact of lung-function impairment on exercise capacity and quality of life are unclear.
Added value of this study
Our study is the first to describe Post-COVID lung function trajectories over 2 years in survivors, with a variable improvement of pulmonary function tests (PFTs) across the three severity scale groups during the first year after COVID-19, then a decline trend of PFTs was observed in all scales during the second year. Participants with critical illness (ordinal scale 5–6) during hospitalization presented a higher absolute increase of PFTs than those with moderate and severe illness (ordinal scale 3 and 4) during the first year. In addition, we found that patients with corticosteroid therapy during hospitalization had a significantly greater PFTs recovery from 6 months to 1 year, but had no effect on the changes of PFTs from 1 year to 2 years. The proportion of dyspnea (mMRC ≥ 1) decreased, and exercise capacity improved over 2 years in all participants with more critical patients experiencing dyspnea, reduced exercise capacity and HRQoL at 6 months, while these differences disappeared at 2 years.
Implications of all the available evidence
The trajectories of lung function in current study trajectories that the time window of lung function recovery is from 6 months to 1 year after COVID-19 and the degrees of improvement vary according to the illness severity during hospitalization, which provides the valuable knowledge to guide the lung recovery of COVID-19 survivors. Our findings also indicate the corticosteroids therapy at acute phase as a protective factor for lung function recovery which is needed to be further validated in future trials, but ethical concerns may exist because of corticosteroids as current standard care for severe and critical COVID-19.
Alt-text: Unlabelled box
Introduction
Patients with severe and critical COVID-19 are often complicated with acute severe lung injury after SARS-CoV-2 infection,1, 2, 3, 4, 5 and whether and how the lung function rehabilitates in these survivors attract lots of attention.6,7 Previous studies of COVID-19 survivors have shown a continuously recovery in lung function though variable degrees of residual abnormalities still remain.8, 9, 10, 11, 12 The longest duration of follow-up was around 1 year and only a small number of subjects were studied.13 Moreover, no longitudinal cohort studies have provided detailed data regarding the lung-function trajectories in survivors after discharge. Here, we analyzed data from a large, longitudinal, 2-year study that included detailed assessments of participants recovered from COVID-19 to examine the Post-COVID lung-function paradigm of changes in the actual values of forced vital capacity (FVC), total lung capacity (TLC), and diffusion capacity for carbon monoxide (DLco), as well as their percentage of the predicted values. The dynamic changes of dyspnea symptom, exercise capacity assessed by distance walked in 6 minutes (6MWD) and health-related quality of life (HRQoL) among varying severity scales were also included in the comprehensive assessment of lung function. In addition, we explored the possible factors that influenced the recovery of lung function, and finally provided the trajectories of lung function change in COVID-19 survivors.
Methods
Study design and participants
This is a prospective longitudinal cohort study. The baseline data at acute phase (from onset of symptom to discharge) were extracted from electronic medical care data. The exact parameters, process and detailed cohort information have been described previously.14 The participants were classified according to the highest seven-category scale during the hospital stay (termed the severity scale)15 as follows: 1, not admitted to hospital with resumption of normal activities; 2, not admitted to hospital, but unable to resume normal activities; 3, admitted to hospital but not requiring supplemental oxygen; 4, admitted to hospital requiring supplemental oxygen; 5, admitted to hospital requiring high-flow nasal cannula (HFNC), non-invasive mechanical ventilation (NIV), or both; 6, admitted to hospital requiring extracorporeal membrane oxygenation (ECMO), invasive mechanical ventilation (IMV), or both; and 7, death. Our cohort only included participants with scale 3 to 6, but not 1, 2, 7, and participants with scale 5 or scale 6 were aggregated as one group because of the relatively small sample size during data analysis.
This longitudinal cohort study was conducted at Jin Yin-Tan hospital in Wuhan, China, involving all COVID-19 survivors discharged between Jan 7 and May 29, 2020. Of 2469 discharged alive, 1733 eligible COVID-19 survivors were enrolled at the initiation of follow-up. In order to assure the representativeness and comparability of the lung function recovery in this cohort, we contacted all severe or critical survivors (scale 5–6) and tried to invite them as many as possible to participate in the examination of pulmonary function tests (PFTs). Based on the number of available scale 5–6 participants, a stratified disproportional random sampling according to the severity scale was conducted at a ratio of 1 (scale 3): 2 (scale 4): 1 (scale 5–6). Three face-to-face follow-ups were completed at 6 months, 1 year and 2 years after symptom onset, respectively, and written informed consent of participants was obtained at each visit. The study was approved by the Research Ethics Commission of Jin Yin-tan Hospital (KY-2020-78.01, KY-2020-78.03, and KY-2020-78.05).
Procedures
PFTs were performed using Master Screen PFT (Vyaire Medical GmbH, Hoechberg, Germany) under the supervision of well-trained technicians in accordance with the standards of American Thoracic Society/European Respiratory Society guideline.16 The assessed lung function parameters included FVC, TLC, functional residual capacity (FRC), residual volume (RV), forced expiratory volume in one second (FEV1), DLco, and their percentage of the predicted values in this study. The value lower than 80% predicted would be defined as abnormal. PFTs with reduced FVC or TLC (<80% predicted) but normal or improved FEV1/FVC (>70%) was defined as restrictive ventilatory impairment, and diffusion capacity impairment was defined as DLco% pred<80% predicted.17,18 To explore the impact of lung function deficits on participants’ exercise capacity and HRQoL, we classified participants into three categories based on the change of FVC% pred and TLC% pred between two follow-up visits: stabilization or improvement (no decline), decline<5% predicted, and decline>5% predicted.19 Otherwise, decline of 10% predicted was used as the cutoff value for DLco according to a previous study.20 Taking PFTs as the main measurement, supplemented by temporal changes in dyspnea, exercise capacity and HRQoL to comprehensively evaluate lung function, and the Modified Medical Research Council (mMRC) Dyspnea Scale, 6MWD, and EuroQoL Visual Analogue Scale (EQ-VAS) were used, respectively.14,21,22 The detailed methods were described in appendix pp 1–5.
Statistical analysis
We have assessed the normality of data through Kolmogorov-Smirnova, continuous variables were presented as median (IQR), and absolute values along with percentages were used for categorical variables. Kruskal-Wallis test was employed to compare the change of PFTs, 6MWD and HRQoL between two follow-up visits among participants with three different severity scales during acute infection phase, which also used for the comparison of 6MWD and HRQoL in participants with different severity scales and various extents of lung function deficits. For the comparison of the proportion of dyspnea and 6MWD lower than LLN (the lower limit of the normal range) among severity scales, χ² test, or Fisher's exact test were used. Mann-Whitney U test, χ² test, or Fisher's exact test were used for the comparison of baseline characteristics and health outcomes at 6-month between participants enrolled and lost to follow-up, as appropriate. A liner regression analysis was employed to explore the factors associated with 6MWD at each visit and the changes of PFTs between two follow-up visits. Variables significant in univariate analysis (p<0.1) were included in the multivariate analysis. Additionally, age, sex, body-mass index (BMI), and smoking history were adjusted in the final multivariable model. Sensitivity analysis was conducted in participants who completed three follow-up visits. Propensity score matching (PSM) was used to perform a 1:1 matching analysis between patients who received and not received corticosteroids therapy with a match tolerance 0.02, and age, sex, BMI, smoking history, and the severity scale were included in PSM. The comparison of baseline characteristics and the changes of lung function between patients received corticosteroids and those not through Mann-Whitney U test, χ² test, or Fisher's exact test when appropriate. Statistical significance was defined as p<0.05, and to counteract the multiple comparisons of the PFTs results between study participants with different severity scale, we used a Bonferroni corrected α-threshold of 0.0167. We included all participants for whom the variables of interest were available in the final analysis, and missing data were not imputed. Statistical analyses were performed using SPSS statistics software (version 25.0, IBM).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Baseline characteristics and long-term outcomes of participants
Across the 6-month, 1-year and 2-year follow-ups, 349, 244, and 268 eligible participants completed the PFTs, respectively. After excluding 75 individuals who only took PFTs once data of 288 participants who completed at least two times PFTs were included in this study, none of them received intervention of pulmonary rehabilitation (Figure S1). The baseline characteristics of participants across three follow-up visits were generally similar (Table S1). The median age at discharge of participants was 55.0 years, and more than half of them were males (61.1% [176/288]), and 89.6% (258/288) were never-smokers. The common self-reported comorbidities including hypertension, diabetes and coronary heart disease, and only one participant reported COPD. 34.7% (100/288) participants had received corticosteroids during hospitalization, 7.4% (5/68) of participants with scale 3, 24.3% (34/140) of scale 4 and 76.3% (61/80) of scale 5–6. 12.5% (36/288) had been admitted into intensive care unit (ICU) with a median length of ICU stay of 17.5 (8.3 to 44.3) days (Table 1).
Table 1.
Baseline demographic characteristics of COVID-19 survivors who completed at least two follow-up visits.
| Total N=288 |
Highest seven-category scale during hospital stay |
|||
|---|---|---|---|---|
| Scale 3: not requiring supplemental oxygen N=68 |
Scale 4: requiring supplemental oxygen N=140 |
Scale 5–6: requiring HFNC, NIV, or IMV N=80 |
||
| Age at discharge, years | 55.0 (47.0 to 64.0) | 55.0 (45.0 to 63.0) | 55.5 (47.3 to 64.0) | 54.0 (46.3 to 65.0) |
| Sex, Male | 176/288 (61.1%) | 44/68 (64.7%) | 75/140 (53.6%) | 57/80 (71.3%) |
| BMI, kg/m2 | 24.6 (22.6 to 26.7) | 24.7 (22.3 to 26.6) | 24.4 (22.4 to 26.4) | 24.8 (22.8 to 27.8) |
| Education | ||||
| High school or lower | 184/282 (65.2%) | 19/67 (28.4%) | 42/135 (31.1%) | 37/80 (46.3%) |
| College or higher | 98/282 (34.8%) | 48/67 (71.6%) | 93/135 (68.9%) | 43/80 (53.7%) |
| Cigarette smoking | ||||
| Never smoker | 258/288 (89.6%) | 60/68 (88.3%) | 131/140 (93.5%) | 67/80 (83.8%) |
| Current smoker | 19/288 (6.6%) | 2/68 (2.9%) | 5/140 (3.6%) | 12/80 (15.0%) |
| Former smoker | 11/288 (3.8%) | 6/68 (8.8%) | 4/140 (2.9%) | 1/80 (1.2%) |
| Comrobidities | ||||
| Hypertension | 107/279 (38.4%) | 26/66 (39.4%) | 47/137 (34.3%) | 34/76 (44.7%) |
| Diabetes | 36/287 (12.5%) | 7/68 (10.3%) | 17/140 (12.1%) | 12/79 (15.2%) |
| Coronary heart diseases | 22/286 (7.7%) | 7/67 (10.4%) | 9/140 (6.4%) | 6/79 (7.6%) |
| Chronic kidney diseases | 14/288 (4.9%) | 4/68 (5.9%) | 8/140 (5.7%) | 2/80 (2.5%) |
| Malignancy | 6/288 (2.1%) | 1/68 (1.5%) | 3/140 (2.1%) | 2/80 (2.5%) |
| Cerebrovascular diseases | 6/287 (2.1%) | 3/68 (4.4%) | 2/139 (1.4%) | 1/80 (1.3%) |
| COPD | 1/288 (0.3%) | 0 | 1/140 (0.7%) | 0 |
| Corticosteroids during hospital stay | 100/288 (34.7%) | 5/68 (7.4%) | 34/140 (24.3%) | 61/80 (76.3%) |
| ICU admission | 36/288 (12.5%) | 0 | 4/140 (2.9%) | 32/80 (40.0%) |
| Length of ICU stay, days | 17.5 (8.3 to 44.3) | NA | 7.5 (6.3 to 11.8) | 20.0 (9.0 to 45.0) |
| Length of hospital stay, days | 14.0 (11.0 to 24.8) | 10.0 (7.0 to 14.0) | 14.0 (11.0 to 17.0) | 40.0 (24.0 to 51.8) |
| Time from symptom onset to 6-month follow-up, days | 190.0 (175.0 to 203.0) | 180.0 (170.0 to 193.3) | 189.0 (176.0 to 201.0) | 202.0 (181.0 to 215.3) |
| Time from discharge to 6-month follow-up, days | 154.0 (145.0 to 170.0) | 149.0 (133.0 to 153.0) | 167.0 (152.0 to 171.0) | 151.0 (129.3 to 167.0) |
| Time from symptom onset to 1-year follow-up, days | 357.0 (344.0 to 365.0) | 344.0 (336.8 to 357.3) | 357.0 (348.8 to 367.0) | 361.0 (355.5 to 370.5) |
| Time from discharge to 1-year follow-up, days | 324.0 (302.0 to 334.0) | 311.0 (292.0 to 325.0) | 331.0 (322.0 to 340.3) | 308.0 (294.0 to 327.5) |
| Time from symptom onset to 2-year follow-up, days | 676.0 (667.0 to 686.0) | 670.0 (659.5 to 672.0) | 682.0 (670.0 to 686.0) | 679.0 (667.0 to 689.0) |
| Time from discharge to 2-year follow-up, days | 650.0 (615.8 to 659.8) | 640.0 (613.5 to 653.5) | 657.0 (648.0 to 661.0) | 618.5 (609.3 to 648.8) |
Note. Data are median (IQR) or n/N (%). BMI=body-mass index. COPD=Chronic Obstructive Pulmonary Disease. ICU=intensive care unit. HFNC=high-flow nasal cannula. NIV=non-invasive mechanical ventilation. IMV=invasive mechanical ventilation.
The long-term health outcomes of our participants at each follow-up were presented in Table S1, the proportion of Long COVID, which is defined as having at least one sequelae symptom (appendix pp 4–5), decreased from 6 months after symptom onset (69.1% [186/269]) to 1 year (57.6% [140/243]), but increased slightly at 2-year follow-up (62.5% [163/261]). Fatigue or muscle weakness, sleep difficulties, and hair loss were the three most common sequelae symptoms reported in our previous article over 2 years. Most characteristics were similar between participants and those who lost to follow-up, except for median age, sex, smoking history, comorbidities, use of corticosteroids, sequelae symptoms and 6MWD (Table S2).
Dynamic changes of PFTs
The actual values and percentage of predicted values of Post-COVID PFTs across the three follow-up visits were shown in Table S3 stratified by severity scale. To characterize the lung-function trajectories of Post-COVID, the absolute differences of each PFT parameter from 6-month to 1-year (Value T2- Value T1) and from 1-year to 2-year (Value T3- Value T2) follow-up were presented in Table 2. In total population, the FVC increased with a median of 40.0 ml (−90.0 to 195.0) from 6 months to 1 year, and then declined with −50.0 ml (−180.0 to 70.0) during the second year. However, TLC declined continuously since 6 months to 1 year (−90.0 ml, −395.0 to 210.0) and during the second year (−220.0 ml, −440.0 to 0.0), and DLco declined with −0.1 mmol/min/kPa and −0.2 mmol/min/kPa during the two periods, respectively. Their percentage of the predicted values displayed the same pattern. In addition, the value of FEV1/FVC remained relatively stable at around 79.0% over the two years.
Table 2.
Absolute change of PFTs in COVID-19 survivors between follow-ups according to severity scale.
| Total | Highest seven-category scale during hospital stay |
|||
|---|---|---|---|---|
| Scale 3: not requiring supplemental oxygen | Scale 4: requiring supplemental oxygen | Scale 5–6: requiringHFNC, NIV, or IMV | ||
| Absolute difference (Value T2- Value T1) | N=237 | N=54 | N=117 | N=66 |
| FVC (ml) | 40.0 (−90.0 to 195.0) | 10.0 (−167.5 to 167.5) | 20.0 (−120.0 to 120.0) | 185.0 (−20.0 to 262.5) ⁎† |
| FVC (% of predicted) | 1.5 (−2.8 to 6.1) | 0.8 (−3.6 to 5.6) | 0.7 (−3.5 to 3.9) | 5.3 (−0.6 to 9.5) ⁎† |
| TLC (ml) | −90.0 (−395.0 to 210.0) | −145.0 (−400.0 to 175.0) | −210.0 (−480.0 to 100.0) | 150.0 (−90.0 to 330.0) ⁎† |
| TLC (% of predicted) | −1.4 (−6.5 to 4.0) | −2.4 (−6.4 to 3.8) | −3.4 (−8.8 to 1.7) | 2.4 (−1.2 to 6.7) ⁎† |
| DLco (mmol/min/kPa) | −0.1 (−0.6 to 0.4) | −0.3 (−0.7 to 0.4) | −0.2 (−0.8 to 0.1) | 0.3 (−0.1 to 0.8) ⁎† |
| DLco (% of predicted) | −0.6 (−7.0 to 4.9) | −2.8 (−8.3 to 4.8) | −2.3 (−8.7 to 1.8) | 3.9 (−0.7 to 9.2) ⁎† |
| Absolute difference (Value T3- Value T2) | N=216 | N=45 | N=106 | N=65 |
| FVC (ml) | −50.0 (−180.0 to 70.0) | −80.0 (−255.0 to 40.0) | −50.0 (−140.0 to 80.0) | −30.0 (−160.0 to 100.0) |
| FVC (% of predicted) | −0.9 (−4.7 to 2.9) | −1.8 (−6.9 to 1.9) | −0.5 (−4.6 to 3.0) | −0.2 (−3.7 to 3.2) |
| TLC (ml) | −220.0 (−440.0 to 0.0) | −230.0 (−510.0 to −55.0) | −220.0 (−405.0 to 25.0) | −220.0 (−485.0 to 50.0) |
| TLC (% of predicted) | −4.0 (−8.0 to 0.0) | −4.0 (−8.5 to −1.0) | −4.0 (−7.6 to 0.5) | −3.3 (−8.0 to 0.9) |
| DLco (mmol/min/kPa) | −0.2 (−0.6 to 0.0) | −0.2 (−0.7 to 0.0) | −0.3 (−0.7 to 0.1) | −0.2 (−0.5 to 0.1) |
| DLco (% of predicted) | −2.4 (−7.3 to 1.0) | −2.4 (−8.4 to 0.9) | −2.5 (−7.8 to 1.8) | −1.9 (−5.4 to 1.2) |
Note. Data are median (IQR). PFTs=pulmonary function tests. FVC=forced vital capacity. TLC=total lung capacity. DLco=diffusion capacity for carbon monoxide. T1, 6-month follow-up; T2, 1-year follow-up; T3, 2-year follow-up. HFNC=high-flow nasal cannula. NIV=non-invasive mechanical ventilation. IMV=invasive mechanical ventilation. *p<0.0167 for the comparison of scale 5–6 with scale 3. †p<0.0167 for the comparison of scale 5–6 with scale 4. ‡p<0.0167 for the comparison of scale 4 with scale 3.
For the PFTs changes among groups FVC% pred elevated numerically from 6 months to 1 year and dropped slightly at 2 years in scale 3 and scale 4 groups but the TLC% pred and DLco% pred declined continuously over the 2 years. Whereas, in participants with scale 5–6, all parameters of PFTs illustrated a same pattern, which was an increase from 6 months to 1 year, and then a decrease during 1-year and 2-year follow-up. The improvement of median absolute difference in FVC actual value was more significant in scale 5–6 group than the other two groups from 6-month to 1-year follow-up (185.0 ml [scale 5–6] vs 20.0 ml [scale 4] vs 10.0 ml [scale 3]), as well as the FVC% pred (all p<0.0167, Table 2). From 1 year to 2 years after COVID-19 (Value T3- Value T2), the changes of any parameters were only numerically different among group stratified by severity scale. The value of FEV1/FVC was stable in all three groups over the 2 years. It should be noticed that participants in scale 3 and scale 4 constantly presented with a better lung function than those in scale 5–6 at each follow-up visit.
Absolute differences of lung function parameters between 6-month and 2-year follow-up were shown according to severity scale in Table S4, all of which in scale 5–6 group were statistically different from those of scale 3 and scale 4 (all p<0.0167). Furthermore, the values of median absolute difference of FVC, FVC% pred, DLco, and DLco% pred were positive in scale 5–6 and negative in scale 3 and scale 4, indicating an improvement of lung function in critical patients during the first 2 years after COVID-19 infection, whereas the lung function of moderate-severe patients generally declined (Table S4). The pattern of the changes of lung-function in each severity scale was consistent with 210 participants who completed three times PFTs (Table S5).
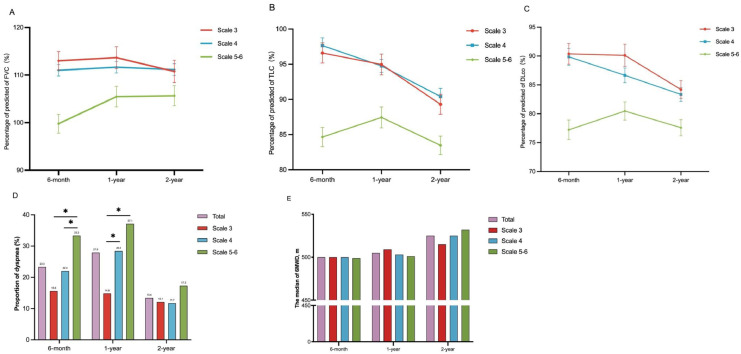
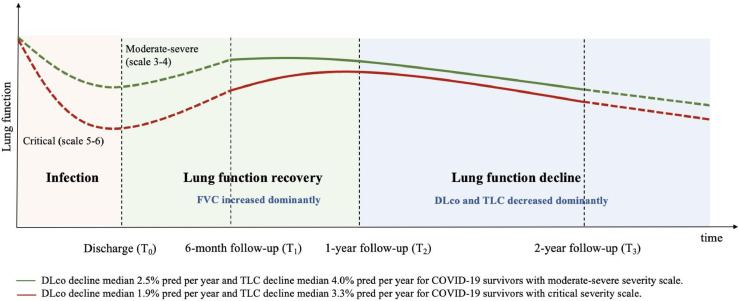
The trajectories of PFTs changes over time by severity scales according to the values of FVC% pred, TLC% pred and DLco% pred between 6 months to 2 years were showed in Figure 1A-C, and the variation trend of lung function parameters in scale 3 and scale 4 was similar. Based on the above results, we depicted the trajectories of lung function recovery in patients with different disease severities in Figure 2, and scale 3 and scale 4 were combined into moderate and severe group according to the WHO clinical management, while scale 5–6 was defined as critical group with an obviously different recovery trajectory. The change of lung function before 6 months in dotted lines refer to previous studies with similar demographic characteristics as our study, and critical COVID-19 patients still had lower lung function than those in moderate-severe COVID-19 patients over 6 months after COVID-19 infection.23, 24, 25
Figure 1.
The temporal changes in PFTs, dyspnea and 6MWD by severity scales.
Note. The temporal changes of the percentage of predicted for FVC (A), TLC (B) and DLco (C) at 6-month, 1-year and 2-year follow-up. Data are mean and error. PFTs=pulmonary function tests. FVC=forced vital capacity. TLC=total lung capacity. DLco=diffusion capacity for carbon monoxide. The temporal changes of the proportion of dyspnea (D) and the median of 6MWD (E). Dyspnea was defined as mMRC≥1. Scale 3: not requiring supplemental oxygen. Scale 4: requiring supplemental oxygen. Scale 5–6: requiring high-flow nasal cannula, non-invasive mechanical ventilation, or invasive mechanical ventilation. *p<0.05.
Figure 2.
Post-COVID lung-function trajectories over 2 years after infection.
Note. The two trajectories (red-line below: critical; green-line above: moderate-severe) present the Post-COVID lung-function patterns. Moderate-severe with smaller improvement than critical patients from T1 to T2 and reach the peak earlier. Period from T1 to T2 dominated by an increase in FVC was defined as lung function recovery, and duration between T2 and T3 dominated by a decrease in TLC and DLco was defined as lung function decline. The changes in lung function recovery period were significant differed between two trajectories, but no statistics difference been observed in lung function decline period between them. The dotted lines from T0 to T1 refer to previous studies (PMID: 32554533, 32381497 and 35018338), and the dotted lines after T3 refer to the decline rate between T2 and T3. FVC=forced vital capacity. TLC=total lung capacity. DLco=diffusion capacity for carbon monoxide.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
According to the PFTs results, the change of percentages for restrictive ventilatory impairment and diffusion capacity impairment in each group were presented in Figure S2 over the two years. The proportions of two types of lung function impairment both increased from 6 months to 2 years (restrictive ventilatory impairment from 16.0% [45/282] to 21.1% [55/261], and diffusing capacity impairment from 32.6% [92/282] to 45.2% [118/261]) (Table S1).
Factors impacting PFTs changes
To explore the potential factors impacting lung function rehabilitation, multiple liner regression analysis was used and adjusted with age, sex, BMI, and smoking history. Use of corticosteroids was identified as an independent protective factor for the improvement of lung function from 6 months to 1 year, with a correlation coefficient of 2.730 (0.2115–5.246) for FVC, 2.909 (0.383–5.436) for TLC, and 3.299 (0.211–6.387) for DLco, respectively (p<0.05) (Table 3). For the lung function change from 1-year to 2-year follow-up, severity scale, use of corticosteroids, and length of hospital stay were shown to have no influence of any parameters of PFTs (Table S6). Sensitivity analysis of 210 participants who completed three times PFTs showed that corticosteroids therapy during hospitalization was a protective factor for lung-function recovery in 1 year and no other factors contributed to the lung-function decline in all parameters of PFTs except for hypertension for TLC% pred, which is consistent with above results (Table S7, Table S8). After PSM, 58 participants received corticosteroids therapy and 58 did not, and most baseline characteristics similar between the two groups (Table S9). Participants treated with corticosteroids during hospitalization had significant improvements in the actual values and the percentage of predicted values of FVC, TLC and DLco compared with those did not, while no difference was observed in lung-function decline between two groups (Table S10).
Table 3.
Factors for the recovery of PFTs in COVID-19 survivors from 6-month to 1-year follow-up.
| Absolute difference of FVC% pred |
Absolute difference of TLC% pred |
Absolute difference of DLco% pred |
||||
|---|---|---|---|---|---|---|
| β (95%CI) | adjusted β (95%CI) | β (95%CI) | adjusted β (95%CI) | β (95%CI) | adjusted β (95%CI) | |
| Age at discharge, per 10 years | −0.129 (−1.001 to 0.742) | −0.195 (−1.047 to 0.656) | 0.131 (−0.782 to 1.044) | 0.112 (−0.751 to 0.975) | 0.111 (−1.012 to 1.234) | 0.001 (−1.052 to 1.054) |
| Sex, Female | −0.458 (−2.51 to 1.600) | 0.642 (−1.466 to 2.750) | −2.219 (−4.358 to −0.079) | −1.012 (−3.159 to 1.136) | −2.454 (−5.090 to 0.182) | −1.110 (−3.720 to 1.499) |
| BMI, kg/m2 | 0.062 (−0.181 to 0.306) | 0.018 (−0.218 to 0.254) | −0.067 (−0.323 to 0.188) | −0.136 (-0.377 to 0.105) | 0.035 (−0.279 to 0.349) | −0.085 (−0.378 to 0.208) |
| Smoking history (yes, no) | 1.432 (−1.766 to 4.630) | 0.901 (−2.328 to 4.130) | 3.622 (0.265 to 6.978) * | 1.529 (−1.776 to 4.834) | 3.405 (−0.740 to 7.550) | 0.935 (−3.085 to 4.956) |
| Comorbidities | ||||||
| Hypertension | 0.123 (−0.461 to 0.706) | 0.287 (−0.318 to 0.893) | 0.423 (−0.322 to 1.167) | |||
| Diabetes | 0.371 (−1.145 to 1.887) | −0.895 (−2.472 to 0.683) | 0.813 (−1.129 to 2.756) | |||
| Malignancy | −0.832 (−7.797 to 6.134) | 1.150 (−6.088 to 8.387) | 2.509 (−6.387 to 11.405) | |||
| Coronary heart diseases | 1.706 (0.550 to 2.862) ** | 1.447 (0.309 to 2.585) * | 0.534 (−0.689 to 1.757) | 1.982 (0.498 to 3.466) ** | 1.532 (0.133 to 2.931) | |
| Chronic kidney diseases | −1.290 (−6.046 to 3.465) | −1.752 (−6.693 to 3.188) | −2.010 (−8.087 to 4.066) | |||
| Cerebrovascular diseases | −0.712 (−2.388 to 0.964) | −0.751 (−2.501 to 1.000) | −1.015 (−3.167 to 1.138) | |||
| Severity scale§ | ||||||
| Scale 3 | Ref. | Ref. | Ref. | |||
| Scale 4 | −0.901 (−3.343 to 1.541) | −1.080 (−3.571 to 1.410) | −1.967 (−4.483 to 0.548) | −2.494 (−5.031 to 0.043) | −3.083 (−6.174 to 0.007) * | −3.568 (−6.668 to -0.469) * |
| Scale 5-6 | 4.123 (1.399 to 6.846) ** | 2.061 (−1.594 to 5.246) | 4.628 (1.837 to 7.419) ** | 0.681 (−3.018 to 4.379) | 5.106 (1.677 to 8.535) ** | −0.496 (−4.994 to 4.002) |
| Corticosteroids during hospital stay | 4.433 (2.419 to 6.447) *** | 2.730 (0.215 to 5.246) * | 5.342 (3.270 to 7.414) *** | 2.909 (0.383 to 5.436) * | 6.634 (4.088 to 9.179) *** | 3.299 (0.211 to 6.387) * |
| Length of hospital stay, days | 0.107 (0.046 to 0.167) *** | 0.009 (−0.077 to 0.094) | 0.158 (0.097 to 0.219) *** | 0.070 (−0.016 to 0.156) | 0.210 (0.135 to 0.284) *** | 0.123 (0.018 to 0.227) * |
Note. PFTs=pulmonary function tests. BMI=body-mass index. FVC=forced vital capacity. TLC=total lung capacity. DLco=diffusion capacity for carbon monoxide. Scale 3: not requiring supplemental oxygen. Scale 4: requiring supplemental oxygen. Scale 5–6: requiring high-flow nasal cannula, non-invasive mechanical ventilation, or invasive mechanical ventilation. §Highest seven-category scale and imaging feature were assessed during hospital stay. Multiple liner regression adjusted by age, sex, BMI, smoking history. *P<0.05, ** P<0.01, *** P<0.001.
The temporal changes of dyspnea, exercise capacity (6MWD) and HRQoL
The proportion of dyspnea (defined as mMRC dyspnea scale≥1 point) was increased from 23.3% (61/262) at 6-month to 27.9% (67/240) at 1-year, and then dropped to 13.4% (35/261) at 2-year. At 6-month and 1-year follow-up, significantly more critical participants (scale 5–6) reported dyspnea than moderate-severe (scale 3 and 4) participants (P<0.05), the proportion of dyspnea was reduced across groups and no differences observed among them at 2 years (Figure 1D). The median of HRQoL remained stable over 2 years, and critical participants had significantly lower HRQoL at 6-month, but the changes of HRQoL were not differed between groups over time (Table 4).
Table 4.
The temporal changes of exercise capacity and quality of life for severity scale.
| Total | Highest seven-category scale during hospital stay |
|||
|---|---|---|---|---|
| Scale 3: not requiring supplemental oxygen | Scale 4: requiring supplemental oxygen | Scale 5-6: requiringHFNC, NIV, or IMV | ||
| 6-month follow-up (T1) | ||||
| 6MWD, meters (N=280) | 500.0 (450.0 to 540.0) | 500.0 (450.0 to 542.0) | 500.0 (462.5 to 543.5) | 499.0 (438.5 to 537.5) |
| Percentage of predicted value §§, % | 87.9 (78.5 to 97.3) | 87.3 (78.3 to 99.1) | 89.3 (81.5 to 97.3) | 83.5 (75.5 to 94.7) † |
| Proportion of less than LLN || | 43/267 (16.1%) | 12/67 (17.9%) | 12/131 (9.2%) | 19/69 (27.5%) † |
| HRQoL §(N=269) | 80.0 (75.0 to 90.0) | 80.0 (75.0 to 90.0) | 80.0 (80.0 to 90.0) | 80.0 (70.0 to 85.0) *† |
| 1-year follow-up (T2) | ||||
| 6MWD, meters (N=247) | 505.0 (454.0 to 554.0) | 509.0 (448.0 to 554.3) | 503.0 (450.0 to 551.5) | 501.0 (457.0 to 557.0) |
| Percentage of predicted value §§, % | 90.8 (82.1 to 100.6) | 89.6 (80.3 to 96.7) | 91.8 (83.5 to 101.7) | 88.3 (81.0 to 98.5) |
| Proportion of less than LLN || | 31/247 (10.8%) | 9/56 (16.1%) | 11/120 (9.2%) | 11/71 (15.5%) |
| HRQoL§ (N=251) | 80.0 (75.0 to 90.0) | 82.5 (80.0 to 90.0) | 80.0 (75.8 to 90.0) | 80.0 (70.0 to 90.0) |
| 2-year follow-up (T3) | ||||
| 6MWD, meters (N=259) | 525.0 (477.0 to 585.0) | 515.0 (473.0 to 595.5) | 525.0 (454.0 to 570.0) | 532.0 (480.0 to 600.0) |
| Percentage of predicted value §§, % | 95.0 (87.4 to 104.3) | 96.0 (88.6 to 103.0) | 94.2 (87.6 to 103.1) | 95.5 (85.3 to 105.8) |
| Proportion of less than LLN || | 17/223 (77.4%) | 2/45 (4.4%) | 11/110 (10.0%) | 4/68 (5.9%) |
| HRQoL§ (N=280) | 80.0 (70.0 to 90.0) | 80.0 (70.0 to 85.0) | 80.0 (80.0 to 90.0) | 80.0 (70.0 to 90.0) |
| Absolute difference (Value T2- Value T1) | ||||
| 6MWD, m (N=243) | 3.0 (−35.0 to 56.0) | 3.0 (−46.5 to 64.8) | 0.0 (−31.0 to 55.0) | 20.0 (−27.8 to 57.5) |
| HRQoL§ (N=235) | 0.0 (−5.0 to 10.0) | 0.0 (−5.0 to 5.0) | 0.0 (−5.0 to 10.0) | 0.0 (0.0 to 10.0) |
| Absolute difference (Value T3- Value T2) | ||||
| 6MWD, m (N=220) | 11.0 (−28.5 to 57.8) | 21.0 (−13.0 to 80.5) | −1.5 (−45.0 to 39.8) ‡ | 27.0 (−14.0 to 70.0) † |
| HRQoL§ (N=243) | 0.0 (−6.0 to 5.0) | −1.0 (−10.0 to 5.0) | 0.0 (-5.0 to 5.0) | 0.0 (−10.0 to 5.0) |
| Absolute difference (Value T3- Value T1) | ||||
| 6MWD, m (N=252) | 22.5 (−26.8 to 76.5) | 32.5 (−19.5 to 79.0) | 4.0 (−33.5 to 56.5) | 55.0 (5.0 to 83.0) † |
| HRQoL§ (N=262) | 0.0 (−6.5 to 5.0) | 0.0 (−8.0 to 5.0) | 0.0 (−8.0 to 5.0) | 0.0 (−5.0 to 10.0) |
Note. Data are median (IQR) or n/N (%). HFNC=high-flow nasal cannula. NIV=non-invasive mechanical ventilation. IMV=invasive mechanical ventilation. 6MWD=distance walked in 6 minutes. HRQoL= health-related quality of life. §§The predicted value of 6MWD calculated according to the ATS reference equations. LLN = the lower limit of the normal range. ||The lower limit of the normal range was calculated by subtracting 153 m from the predicted value for men or by subtracting 139 m for women. §The EuroQol Visual Analogue Scale was used to evaluate the health-related quality of life, ranging from 0 to 100, with higher scores indicating better health status. *p<0.0167 for the comparison of scale 5–6 with scale 3. †p<0.0167 for the comparison of scale 5–6 with scale 4. ‡p<0.0167 for the comparison of scale 4 with scale 3.
Exercise capacity assessed by 6MWD continued to increase over 2 years, with a median from 500.0 m at 6-month to 525.0 m at 2-year. Although participants with scale 5–6 had numerical higher 6MWD at 2-year follow-up, but there was no statistical difference across three groups at each follow-up. The median absolute difference in 6MWD between two follow-ups indicated that critical participant had numerically higher improvement than moderate and severe participants at each visit. Statistical differences between scale 4 and scale 5–6 groups were observed between 1 year to 2 years (27.0 m vs −1.5 m)and 6 months to 2 years (55.0 m vs 4.0 m) (Table 4, Figure 1E). The percentage of predicted value and the proportion of 6MWD lower than LLN were significantly differed between participants with scale 4 and scale 5–6 ([83.5% vs 89.3%], [27.5% vs 9.2%]) at 6-month, and there were only numerical differences among varying severities at 1-year and 2-year follow-up. After adjusted by age, sex, BMI and smoking history, age and sex were identified as risk factors for 6MWD at three follow-ups (Table S11).
Impact of lung function deficits on exercise capacity (6MWD), HRQoL
During 6-month to 1-year follow-up, the median absolute change in 6MWD of participants with an obvious deficit in FVC% pred (declined>5%) (−5.0 m [−60.8 to 40.8]) was numerically lower than those with stable and improvement (5.0 m [−32.0 to 56.0]) or mild decline (declined<5%) (24.5 m [−21.5 to 72.8]). Participants with lung function deficits over the cutoff (>5% for TLC and >10% for DLco) had worsen exercise capacity rehabilitation compared to those with stable and improvement or mild decline in TLC% pred (−3.0 vs 19.0 vs 15.0 m) and DLco% pred (−3.0 vs 20.0 vs 0.0 m). No statistical difference was observed in median change of HRQoL among three groups (Table S12). For 1-year to 2-year lung function decline, exercise capacity decreased numerically in participants with obvious lung function decline, and no statistical difference was observed, compared with those with mild decline or stable and improved group. The median difference of 6MWD in participants with obvious impairment in FVC and DLco was negative (−10.0 m, −7.0 m), but positive in participants with improved (9.0 m, 13.5 m) or mild impaired (20.0 m, 13.5 m) lung function, and no statical difference was found among groups. Moreover, the median difference of HRQoL was not significant among varying degrees of lung function deficits, which indicates that the decline of lung function had minor impact on HRQoL (Table S12).
Discussion
To our best knowledge, this is the first study to describe the paradigm of Post-COVID lung function recovery, which is characterized by an improvement from 6 months to 1 year after infection, and then a decline after the observed peak of recovery. In the recovery period, the improvement of PFTs differed significantly with severity scales, as critical patients presented a higher increase than moderate and severe patients. Critical patients had significantly more dyspnea and decreased exercise capacity at 6 months than moderate-severe patients, but no difference at 2 years. Mild decline in PFTs had no significant effect on exercise capacity and HRQoL, and age and sex were risk factors for exercise capacity after adjusted by confounders. In addition, we found that corticosteroid therapy during hospitalization contributed to lung function recovery from 6 months to 1 year, whereas no variables were recognized as risk factors for the decline of lung function apart from hypertension as a risk factor for TLC% pred decline.
The trajectories of Post-COVID lung function change in current study indicated that the time window of lung rehabilitation in participants with different illness severity varied from 6 months to 1 year after illness onset. For the survivors with critical COVID-19, the lung function showed a constantly improvement during the first year after COVID-19. Previous longitudinal follow-up studies also showed that the PFTs trends in critical survivors were similar to our findings.10,13 For those with moderate-severe participants, several studies had demonstrated that lung function constantly improved in patients with mild-moderate disease from 30 days to 6 months after discharge.23, 24, 25 This study firstly caught a picture of Post-COVID lung function change that a decline trend of PFTs from 1 year to 2 years after COVID-19. The Post-COVID lung function change in current study was seemingly consistent with the trend of lung function among severe acute respiratory syndrome (SARS) survivors. Based on the results of two studies on changes of lung function after SARS infection, the PFTs improved during the first year after discharge and displayed a trend of decline in lung function from 1-year to 2-year follow-up.26,27 But, another study involved 97 SARS survivors showed that the PFTs changes remained a relatively stable from 3 months to 6 months after SARS infection, then an impairment in DLco was observed at 1-year follow-up.28 Our findings are of importance to understand the natural recovery history of Post-COVID, which provide the valuable information to guide the lung rehabilitation of COVID-19 survivors. The early lung rehabilitation after hospital discharge needs to be emphasized, even as early as during hospitalization. Our findings also suggest the time window of the measurement of PFTs for the lung rehabilitation trials.
The current study firstly demonstrated that corticosteroid therapy during hospitalization was associated with better lung function recovery in COVID-19 survivors. Corticosteroids have been recommended as a standard therapy for patients with severe or critical COVID-1929, 30, 31 based on the protective effect on mortality.32,33 A previous study including 32 of 76 COVID-19 patients received corticosteroids in acute phase observed that use of corticosteroids is helpful for improving HRQoL and relieving sequalae symptoms at 1 year after discharge,34 which is consistent with our study that corticosteroids therapy is a protected factor for the prognosis. Whereas the sample size is relatively small and lack of objective examination for lung function evaluation. Administration of corticosteroids can alleviate lung injury and improve oxygenation by reducing systemic inflammation and the plasma and bronchoalveolar lavage fluid procollagen level in acute respiratory distress syndrome.35,36 Corticosteroids therapy can also reduce mortality in hospitalized COVID-19 patients, especially in critically ill patients who received invasive mechanical ventilation or oxygen supplement.33 In addition, individuals with long COVID had obviously lower cortisol level than healthy control till to 1 year after infection, which suggests that corticosteroids replacement therapy may be beneficial for them.37,38 But the exact explanation needs to be explored in further studies, and the benefits of corticosteroids therapy for the lung-function recovery should be verified in other respiratory viral infection.
The relatively faster decline of TLC and DLco in all COVID-19 survivors is a big concern. Based on our findings, the median reduction in actual values of TLC and DLco in COVID-19 survivors were faster compared to age-relate lung function decline in the healthy adults with a similar age to our cohorts (44.0ml for TLC and 0.04mmol/min/kPa for DLco).39,40 However, the duration of this 2-year follow-up is still too short to accurately describe the changes of lung function in COVID-19 survivors. The continuous follow-up is essential for the precise trajectories of lung function changes and potential interventions for rehabilitation.
The proportion of participants with dyspnea reduced over time. A significant difference among three severity scale groups was observed during the first year after infection. Exercise capacity gradually improved over 2 years in all participants with more critical patients experiencing greater improvement in 6MWD than others, but no statistical difference among severity scales at each follow-up. Previous studies also found that the proportions of COVID-19 survivors experiencing dyspnea decreased and the 6MWD increased within 1 year after symptom onset.41 Mild decline of PFTs had no obvious impact on the changes of 6MWD. As for the analysis of factors for 6MWD at each follow-up showed advanced age and female sex were indentified with a negative relationship of 6MWD, suggesting that the recovery of exercise capacity after COVID-19 influenced by demographic characteristics.
There are several limitations in our study. Firstly, the lack of pre-COVID PFTs makes it difficult to tell whether the lung function rehabilitates to the pre-COVID status, although it is an evitable defect in nearly all emerging respiratory infectious diseases study. Secondly, due to some survivors lost to follow-up the number of participants included were relatively small for the identification of factors influence PFT changes, which might lead to a sampling bias. Fortunately, the proportion of participants with varying severity scales was roughly the same as that in stratified sampling, most demographic characteristics of enrolled COVID-19 survivors and those lost to follow-up did not differ significantly. Sensitivity analyses were performed in participants completed three follow-ups, and the results were similar to that in those attended at least twice follow-ups, which may minimize the bias. PSM analysis also showed significant lung-function improvements among participants received corticosteroids therapy, but the sample size was limited after PSM and the results should be interpreted seriously. Thirdly, this is a single center study focused on hospitalized COVID-19 patients in the early stage of the pandemic. Given the persistence of pandemic and emergence of new variants the representativeness of this cohort may limited, but a single-center study would reduce systematic error.
In conclusion, the trajectories of Post-COVID lung function were different in participants with different illness severities. Specifically, critical patients had greater increases in PFTs and 6MWD, with higher proportion of dyspnea than moderate and severe patients during the first year after COVID-19. Then a constant decline trend of PFTs was observed among all groups during the second year after acute infection, and 6MWD and HRQoL not significantly affected by the decline in PFTs. Corticosteroids therapy was identified as the independent protective factor for lung function recovery from 6 months to 1 year after adjusting confounders, and 6MWD influenced by age and sex. Finally, the relatively faster decline trend of PFTs from 1 year to 2 years needs to be paid attention and further validated in the future follow-up study.
Contributors
Conceptualization: HZ, YMW, CLH, BC; Methodology: HZ, YMW, XYG; Cohort: HZ, LH, XL; Data collection and verify: HZ, LH, XL, YiW, ML, ZBL, ZY; Analysis: HZ, XYG; Funding acquisition: BC; Writing: HZ, YMW, XYZ, BC. All authors had full access to the data in the study and confirmed the data. All authors have critically revised the content of the manuscript and had final responsibility for the decision to submit for publication.
Data sharing statement
Restrictions apply to the availability of these data and they are not publicly available. However, data are available from the corresponding author upon reasonable request and with the permission of the institution.
Declaration of interests
All authors declare no related conflict of interest in this paper.
Acknowledgments
We thank all individuals who participated in this study. The authors would like to thank all investigators who contribute to the longitudinal cohort of COVID-19 survivors. This work was supported by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2021-I2M-1-048), the National Key Research and Development Program of China (2021YFC0864700), and Changping Laboratory.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101668.
Contributor Information
Yeming Wang, Email: wwyymm_love@163.com.
Chaolin Huang, Email: 88071718@qq.com.
Bin Cao, Email: caobin_ben@163.com.
Appendix. Supplementary materials
References
- 1.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bian X-W, The COVID-19 Pathology Team, Yao X-H, et al. Autopsy of COVID-19 patients in China. Natl Sci Rev. 2020;7:1414–1418. doi: 10.1093/nsr/nwaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoniou KM, Vasarmidi E, Russell A-M, et al. European respiratory society statement on long COVID-19 follow-up. Eur Respir J. 2022;60 doi: 10.1183/13993003.02174-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George PM, Barratt SL, Condliffe R, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 8.Iversen KK, Afzal S, Ahlström MG, et al. Lung function decline in relation to COVID-19 in the general population: a matched cohort study with prepandemic assessment of lung function. J Infect Dis. 2022;225:1308–1316. doi: 10.1093/infdis/jiab636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anastasio F, Barbuto S, Scarnecchia E, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021;58 doi: 10.1183/13993003.04015-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eberst G, Claudé F, Laurent L, et al. Result of one-year, prospective follow-up of intensive care unit survivors after SARS-CoV-2 pneumonia. Ann Intensive Care. 2022;12:23. doi: 10.1186/s13613-022-00997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Writing Committee for the COMEBAC Study Group Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lombardi F, Calabrese A, Iovene B, et al. Residual respiratory impairment after COVID-19 pneumonia. BMC Pulm Med. 2021;21:241. doi: 10.1186/s12890-021-01594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 18.Taha N, D'Amato D, Hosein K, et al. Longitudinal functional changes with clinically significant radiographic progression in idiopathic pulmonary fibrosis: are we following the right parameters? Respir Res. 2020;21:119. doi: 10.1186/s12931-020-01371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8:147–157. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann-Vold A-M, Allanore Y, Alves M, et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis. 2021;80:219–227. doi: 10.1136/annrheumdis-2020-217455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10(9):863–876. doi: 10.1016/S2213-2600(22)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55 doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Liu J, Peng P, et al. Dynamic changes of pulmonary diffusion capacity in survivors of non-critical COVID-19 during the first six months. EClinicalMedicine. 2022;43 doi: 10.1016/j.eclinm.2021.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frija-Masson J, Debray M-P, Gilbert M, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56 doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngai JC, Ko FW, Ng SS, To K-W, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie L, Liu Y, Fan B, et al. Dynamic changes of serum SARS-Coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir Res. 2005;6:5. doi: 10.1186/1465-9921-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for Covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 30.Corticosteroids. COVID-19 treatment guidelines.https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/. Accessed 2 June 2022.
- 31.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis. 2022;5 doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recovery Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catalán IP, Martí CR, Sota DP de la, et al. Corticosteroids for COVID-19 symptoms and quality of life at 1 year from admission. J Med Virol. 2022;94:205–210. doi: 10.1002/jmv.27296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158:1432–1441. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 36.Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 37.Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895.e20. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein J, Wood J, Jaycox J, et al. Distinguishing features of Long COVID identified through immune profiling. medRxiv [Preprint] 2022;2022:22278592. doi: 10.1101/2022.08.09.22278592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of U.S. adults. Am J Respir Crit Care Med. 1996;153:656–664. doi: 10.1164/ajrccm.153.2.8564114. [DOI] [PubMed] [Google Scholar]
- 40.Lee JS, Kim SO, Seo JB, et al. Longitudinal lung volume changes in patients with chronic obstructive pulmonary disease. Lung. 2013;191:405–412. doi: 10.1007/s00408-013-9478-0. [DOI] [PubMed] [Google Scholar]
- 41.Lorent N, Vande Weygaerde Y, Claeys E, et al. Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res. 2022;8 doi: 10.1183/23120541.00004-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.