Abstract
Background
Patients with Parkinson’s disease (PD) show impaired performance in taste recognition tests, which suggests a possible dopaminergic influence on gustatory functioning. To experimentally test this hypothesis, we assessed whether pharmacological manipulation of dopaminergic signaling in healthy volunteers can affect performance in a standardized taste recognition test.
Methods
Physically and mentally healthy volunteers (n = 40, age 18–43 years) were randomly allocated to treatment with either pramipexole or placebo using a double-blind, parallel-group design. After 12 to 15 days of treatment (dose titrated up from 0.25 mg/d of pramipexole salt to 1.0 mg/d), taste recognition performance was assessed using a standardized and validated assay (taste strip test). Additionally, visual analogue scale ratings of subjective pleasantness and disgustingness of taste samples were obtained.
Results
Compared with the placebo group, participants receiving pramipexole showed significantly higher total recognition accuracy (medianpramipexole = 14.0, medianplacebo = 13.0, U = 264.5, P = .04). This was driven by a higher sensitivity for taste in the pramipexole group. Exploratory analysis of pleasantness and disgustingness ratings of appetitive (sweet) vs aversive (bitter) stimuli suggested that pramipexole treatment was associated with overall blunted hedonic responses, but this effect did not survive the inclusion of nausea (a side effect of treatment) as a covariate in the analysis.
Conclusions
Healthy volunteers who received subacute pramipexole treatment exhibited higher taste recognition performance compared with the placebo group. This finding is consistent with a proposed role of the dopaminergic system in gustatory functioning and could have important theoretical and clinical implications.
Keywords: Dopamine, gustation, pramipexole, taste, taste strip test
Significance Statement.
Previous research suggests that patients with Parkinson’s disease show impairments in taste function. Whether this phenomenon can be attributed to altered dopaminergic neurotransmission is not clear to date. To experimentally test the influence of dopaminergic signaling activity on gustatory processing, the present study examined the effects of subacute treatment with the dopamine D2/D3 agonist pramipexole on performance in a taste recognition test in healthy volunteers. It was found that study participants who were treated with pramipexole performed significantly better in taste recognition compared with a control group treated with placebo. This was a global effect across different taste qualities rather than a taste-specific effect. Pramipexole-treated study participants also showed blunted hedonic evaluation of pleasant and unpleasant taste stimuli, but this effect could be attributed to nausea, a common side effect of pramipexole treatment.
Introduction
Several studies have reported impaired taste recognition performance in patients with Parkinson’s disease (PD) (Lang et al., 2006; Moberg et al., 2007; Kim et al., 2011; Cecchini et al., 2014, 2015; Doty et al., 2015). These observations raise the possibility of a dopaminergic influence on gustatory functioning. However, in the aforementioned studies, patients were frequently receiving a range of medication when taste function was assessed, which means that any observed impairment could simply be a side effect of treatment. Additionally, central nervous system structures that contribute to the processing of gustatory information (e.g., nucleus tractus solitarius, operculum, insula, orbitofrontal cortex) could also be directly affected by neurodegenerative processes in PD (Shah et al., 2009; Cecchini et al., 2014; Doty et al., 2015). Furthermore, the neurochemistry of PD is complex, and a range of different neurotransmitter systems (e.g., serotonin, noradrenaline, acetylcholine)—all possibly also contributing to taste processing—is involved in the pathophysiology of the disease (Brichta et al., 2013). Thus, a direct role of dopaminergic signaling activity in gustatory functioning cannot be inferred from these observational studies.
The aim of this paper was to probe the putative causal role of dopamine in gustatory processing by assessing taste function after pharmacological manipulation of dopaminergic signaling activity. To this end, healthy volunteers were randomly allocated to subacute treatment with the dopamine agonist pramipexole or placebo and thereafter were assessed using a standardized taste assay. This approach allowed us to test the causal effects of dopaminergic activity on gustatory processing unconfounded by concurrent neuropathological processes or clinical symptoms.
Pramipexole is an orally active, non-ergoline dopamine agonist that shows selective activity at the D2 receptor subfamily, with a preferential affinity for the D3 receptor (Piercey, 1998; Tundo et al., 2019). It is an established treatment for PD and restless legs syndrome but has more recently also been suggested as a potential intervention for depressive disorders (Romeo et al., 2018; Tundo et al., 2019). A number of previous studies have used pramipexole to better understand the role of the dopaminergic system in different neurocognitive processes, including reinforcement and stimulus-response learning, impulsivity, and reward processing (Hamidovic et al., 2008; Pizzagalli et al., 2008; McCabe et al., 2013; Gallant et al., 2016; Martins et al., 2017). However, whether pramipexole can directly affect taste function has not, to our knowledge, previously been investigated.
Our working hypothesis for this paper was that, in healthy volunteers, pramipexole treatment would show opposite effects to those observed in PD, specifically, a global improvement in taste recognition. Because acute treatment with pramipexole has also been shown to blunt neural activity during the passive receipt of pleasant and unpleasant taste stimuli (McCabe et al., 2013)—including in brain areas thought to contribute to the hedonic experience of taste (Cecchini et al., 2015)—we additionally sought to explore whether pramipexole influenced subjective ratings of pleasantness and disgustingness in response to appetitive and aversive taste samples.
METHODS
Study Sample
Healthy volunteers (n = 40, 50% female) aged 18 to 43 years without a personal history of any major mental or physical disorder were recruited as part of a larger experimental medicine study that aimed to explore depression-relevant neurocognitive effects of subacute pramipexole treatment. In an initial screening visit, participants were confirmed to be mentally and physically healthy (based on a structured psychiatric interview (SCID-5 (First et al., 2015)) and a general medical interview), and basic demographic, physical, and psychological information was collected (also see Table 1).1 The trial was approved by the Oxford University research ethics committee, and all study participants gave written informed consent prior to inclusion.
Table 1.
Basic Demographic, Physical, and Psychological Characteristics of the Study Sample (Means Plus SD in Parentheses)
| Pramipexole (n = 21; 10 male) |
Placebo (n = 19; 10 male) |
Between-group comparison (t test) | |
|---|---|---|---|
| Age | 22.5 (3.7) | 24.5 (6.9) | P = .26 |
| Body mass index | 22.4 (2.6) | 24.0 (2.9) | P = .07 |
| Years in full-time education | 16.8 (2.9) | 17.5 (3.1) | P = .49 |
| IQ estimate (Spot-the-Word Test) | 108.3 (8.1) | 111.9 (7.6) | P = .16 |
| Neuroticism (Eysenck Personality Questionnaire) | 4.2 (3.7) | 4.3 (3.7) | P = .98 |
| Psychoticism (Eysenck Personality Questionnaire) | 2.5 (2.1) | 2.8 (2.1) | P = .64 |
| Extraversion (Eysenck Personality Questionnaire) | 14.7 (4.5) | 14.5 (3.7) | P = .89 |
| Lie (Eysenck Personality Questionnaire) | 9.5 (4.6) | 7.5 (3.4) | P = .12 |
| Trait anxiety (State-Trait Anxiety Inventory) | 31.2 (9.1) | 32.1 (9.1) | P = .77 |
| Depression at inclusion (Beck Depression Inventory) | 1.6 (1.7) | 2.5 (4.0) | P = .39 |
N.B.: Uneven distribution of pramipexole and placebo treatment due to random replacement of participant dropouts.
Intervention and Design
The study used a between-groups design with participants being randomly allocated to either pramipexole or placebo (lactose). Randomization was stratified by sex. Both treatments were administered in indistinguishable capsules. The daily dose of pramipexole started at 0.25 mg of pramipexole salt and was subsequently increased by 0.25 mg in a stepwise manner every 3 days until a target dose of 1.0 mg of pramipexole salt per day was reached. Participants took the target dose of 1.0 mg for at least 2 consecutive days before the taste assessment was conducted. Two participants dropped out of the study before the taste assessment because of subjectively experienced side effects and were subsequently replaced by other volunteers. Both participants who dropped out had received placebo treatment.
Assessments
Taste recognition was assessed using a validated and standardized commercial test kit (Taste Strips, Burghart Messtechnik, Holm, Germany). Briefly, this assay consists of filter paper strips impregnated with 4 basic taste qualities (sweet, sour, salty, and bitter) at 4 different intensity levels (i.e., 16 samples in total). In addition, 3 strips without any taste (no-taste samples) were included. The filter paper strips were presented to participants in a pseudo-random order, with intensity levels increasing gradually, and no-taste samples being interleaved. In each trial, participants were asked to place the paper strip on their tongue, close their mouth, move the strip on their tongue, and then identify the taste. Participants were asked to correctly classify each sample as either sweet, sour, salty, bitter, or as having no taste. A total accuracy score was calculated for each participant as the number of samples correctly identified (e.g., sour presented and sample classified as sour). In addition, we also calculated a misidentification score, defined as the total number of trials where a participant incorrectly classified a sample with a taste quality (e.g., sour presented but sample classified as sweet). Furthermore, a no-taste identification score (no-taste sample presented and classified as such) and a non-identification score (taste sample presented but classified as having no taste) were calculated as well. To complement taste recognition measures, for each taste quality, we also calculated signal detection theory measures (based on Grier, 1971; see also Stanislaw and Todorov, 1999), which are able to determine sensitivity to a taste while controlling for response bias (for formulas, Supplementary Equation 1).
Additionally, participants were asked to rate each presented taste sample on 2 visual analogue scales (based on Arrondo et al., 2015) evaluating pleasantness (ranging from “very unpleasant” to “very pleasant”) and disgustingness (ranging from “not disgusting at all” to “extremely disgusting”).
Potential side effects of treatment (including sleeping problems, abnormal dreaming, headache, dizziness, somnolence, nausea, vomiting, constipation, fatigue, impulse control problems, hallucinations, and abnormal movements) were assessed using a simple side effect questionnaire that was administered before the administration of the taste test. Potential side effects noticed since beginning of treatment were rated by participants using a 4-level Likert scale ranging from “absent” to “severe.”
Blinding to treatment was checked by means of a forced-choice guess by the study participant and a researcher.
Statistical Analysis
Data analysis was carried out in SPSS (version 25.0, IBM Corp, Armonk, NY). Taste recognition scores and hedonic ratings were compared between groups using Mann–Whitney U tests and mixed-design ANOVAs, respectively. For mixed ANOVAs, treatment group was always used as the between-participants factor and taste as the within-participant factor. To probe for a potential influence of nausea on the observed results, analyses were repeated with symptoms of nausea added as a covariate. Significant interactions in mixed-design ANOVAs were followed-up by a simple effects analysis using Bonferroni correction for multiple comparisons.
RESULTS
Taste Recognition
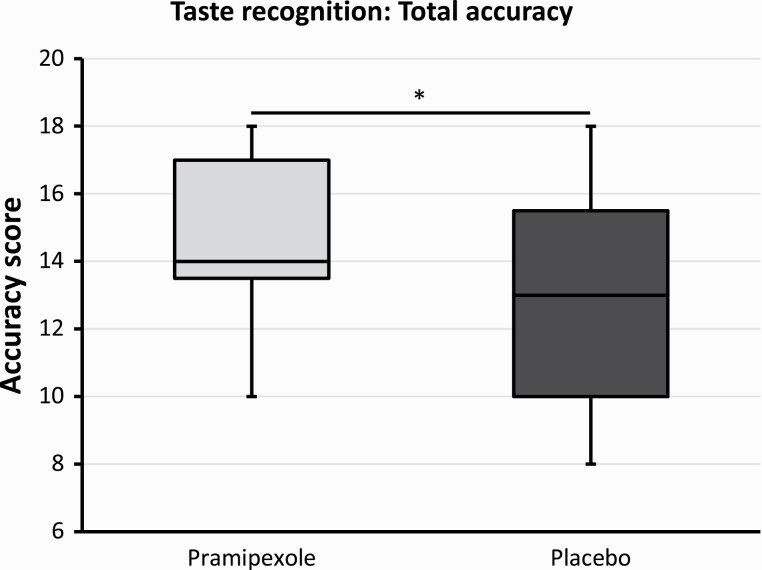
One participant (allocated to the pramipexole group) had to be excluded from the analysis because of an error in the administration of the test (not all taste samples were presented). When comparing total accuracy scores (i.e., number of all correct classifications), there was a statistically significant difference between groups (U = 264.5, P = .04), with the pramipexole group showing higher accuracy compared with the placebo group (medianpramipexole = 14.0, medianplacebo = 13.0) (also see Figure 1). This group difference was still present when the no-taste samples were excluded (U = 265.5, P = .03, medianpramipexole = 11.5, medianplacebo = 10.0). Accuracy scores for no-taste samples alone did not show a significant group difference (U = 209.0, P = .61, medianpramipexole = 3.0, medianplacebo = 3.0). Misidentification scores (i.e., number of incorrect classifications of a sample as a taste) did not differ significantly between groups (U = 188.5, P = .97, medianpramipexole = 3.0, medianplacebo = 2.0). However, non-identification scores (i.e., number of incorrect classifications of a taste sample as having no taste) differed significantly between groups (U = 111.5, P = .03). Specifically, the pramipexole group exhibited fewer non-identifications than the placebo group (medianpramipexole = 1.0, medianplacebo = 2.0).
Figure 1.
Boxplots comparing total taste recognition accuracy after subacute treatment with either pramipexole or placebo. Pramipexole group showing higher accuracy scores than the placebo group (P = .04).
To probe the potential influence of specific sample characteristics on recognition performance, we conducted group × taste mixed ANOVAs on taste accuracy, misidentifications, and non-identifications. This confirmed overall group differences (i.e., main effect of group) for accuracy (F(1,37) = 5.5, P = .02) and non-identifications (F(1,37) = 6.2, P = .02), but not misidentifications (F(1,37) = 0.1, P = .81). However, there was no significant taste-specific group difference (i.e., no group × taste interaction) for any measure (accuracy: F(3.1,113.3) = 1.4, P = .23; non-identifications: F(3,111) = 0.7, P = .53; mis-identifications: F(3,111) = 0.1, P = .95).
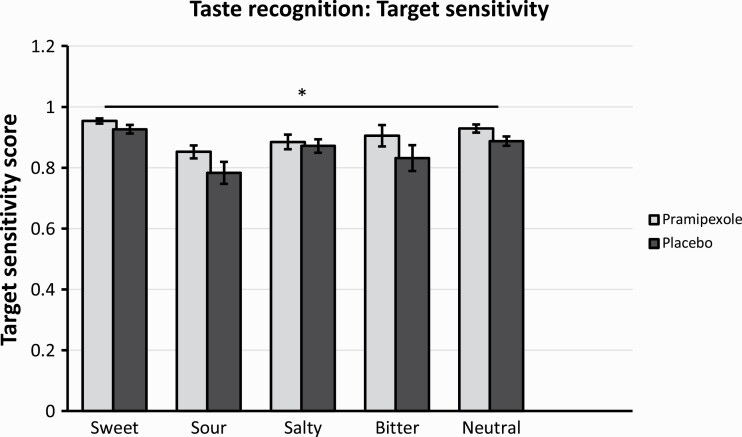
Finally, we compared groups using signal detection theory measures (i.e., target sensitivity and response bias). In line with the results reported above, there was a significant main effect of group on target sensitivity (F(1,37) = 4.2, P = .046) in the absence of a group × taste interaction (F(2.4,89.9) = 0.7, P = .55). Compared with the placebo group, the pramipexole group showed significantly higher target sensitivity scores across taste qualities (also see Figure 2). There was no main effect of group (F(1,37) = 0.2, P = .64) and no group × taste interaction (F(2.7,99.2) = 0.95, P = .41) with regards to response bias scores.
Figure 2.
Target sensitivity scores for different taste qualities after subacute treatment with either pramipexole or placebo. Bars represent means, error bars represent standard errors. Main effect of treatment group, with pramipexole group showing higher target sensitivity independent of taste quality (P = .046).
Pleasantness and Disgust Ratings of Taste Samples
To assess whether pramipexole influenced the hedonic experience of taste stimuli, we compared mean pleasantness and disgustingness ratings for sweet (i.e., appetitive) and bitter (i.e., aversive) taste samples between groups. As shown above, the placebo group classified significantly more taste samples incorrectly as having no taste, especially at the lower intensity levels (also see Supplementary Table 1), which confounds hedonic evaluations (i.e., one cannot meaningfully rate a taste that one does not perceive). Therefore, we compared only ratings for samples at the highest taste intensity level, where most participants identified taste samples correctly, and furthermore only included trials where a taste experience was reported. This analysis showed a significant group × taste interaction for both ratings, pleasantness (F(1,34) = 6.5, P = .02) and disgustingness (F(1,34) = 4.9, P = .03), but no main effect of group (pleasantness: F(1,34) = 1.2, P = .29; disgustingness: F(1,34) = 0.3, P = .62). Relative to the placebo group, the pramipexole group rated sweet samples as less pleasant and more disgusting and bitter samples as more pleasant and less disgusting. Comparing groups separately in terms of pleasantness and disgustingness ratings for sweet and bitter samples did not yield a statistically significant difference for either taste or measure (all Bonferroni-corrected P values > .05). Thus, the interaction was not driven by a group difference in a single taste quality.
Can Nausea as a Side Effect Explain the Observed Results?
Nausea is a common side effect of pramipexole treatment and was the only side effect that was observed significantly more often in participants who received pramipexole (13 out of 21 participants, i.e., 61.9%) compared with placebo (1 out of 19, i.e., 5.3%) [Fisher’s exact test: P < .01; a detailed overview of all side effects observed in this study sample, can be found in Martens et al. (2021)]. Thus, it could be the case that the experience of nausea drove the observed group differences in gustatory processing. Indeed, an exploratory analysis showed a significant correlation between nausea and disgust ratings for sweet samples in the pramipexole group (ρ = 0.5, P = .03). We therefore reran the above analyses with nausea ratings added as a covariate. This led to no qualitative change in the results reported above for taste accuracy (F(1,36) = 7.3, P = .01), non-identifications (F(1,36) = 9.0, P < .01), and mis-identifications (F(1,36) = 0.04, P = .85). However, when severity of nausea was included in the analysis for pleasantness and disgustingness ratings, the group × taste interaction reported above was no longer significant for either outcome measure (pleasantness: F(1,33) = 2.1, P = .16; disgustingness: F(1,33) = 2.2, P = .15).
Blinding
Blinding in this study sample was not fully achieved. Pramipexole-treated participants correctly guessed their treatment allocation in 61.9% of cases and placebo-treated participants in 84.2% of cases (Fisher’s exact test: P < .01). The assessor correctly guessed treatment allocation in 71.4% of cases for pramipexole-treated participants and 84.2% of cases for placebo-treated participants (Fisher’s exact test: P < .01) (also see Martens et al., 2021).
Discussion
In this paper, we used a healthy volunteer assay to study potential effects of subacute dopamine D2/D3 receptor agonism on gustatory functioning. We found that pramipexole-treated study participants exhibited enhanced taste recognition performance compared with placebo-treated volunteers. In addition, an exploratory analysis of hedonic ratings of different taste samples suggested that pramipexole-treatment was associated with blunted responses to both appetitive and aversive taste samples, but this pattern disappeared when we included symptoms of nausea as a covariate in the analysis.
As predicted, the observed effect of pramipexole was opposite to what has been described for PD patients (Lang et al., 2006; Moberg et al., 2007; Kim et al., 2011; Cecchini et al., 2014, 2015). This effect was seen across all taste qualities rather than being associated with a specific taste quality. The effect was also mainly driven by an increased sensitivity for detecting the presence of taste as evidenced by the fact that participants in the pramipexole group categorized significantly fewer samples incorrectly as having no taste. Importantly, no-taste recognition scores were comparable between groups, and, in a signal detection analysis, the pramipexole group also showed higher target sensitivity scores across all sample qualities. Therefore, enhanced taste recognition in the pramipexole group cannot be explained simply by an increased tendency to report a taste experience. The effect we observed also cannot be attributed to symptoms of nausea in the pramipexole group, because it was still observable when severity of nausea was included as a covariate in the analysis.
Taken together, the above results are in line with our initial hypothesis that gustatory processing is influenced by dopaminergic signaling activity. Therefore, decreased taste recognition performance in PD might be a direct result of impaired dopaminergic function. However, it is worth noting that a previous study that assessed taste performance in patients with early-stage PD both on and off dopamine-related medication (mostly carbidopa/levodopa) found no effect of dopaminergic drug treatment (Doty et al., 2015). Although this discrepancy could be explained by various methodological differences between that study and ours (e.g., a patient vs a healthy volunteer sample, different duration of treatment, etc.), it could also indicate that the effects we observed on taste recognition here are specific to pramipexole, for example, because of its specific affinity for the D3 receptor. Alternatively, because the neurochemistry of PD is complex and involves several other neurotransmitter systems than dopamine, it could be the case that taste recognition in PD patients is additionally influenced by non-dopaminergic pathology (Brichta et al., 2013). In line with this idea, Cecchini et al. (2019) recently demonstrated that chemosensory impairments in PD were associated with mild cognitive impairment, a symptom of PD that has also been linked to non-dopaminergic neurochemical abnormalities (Pasquini et al., 2021).
From a clinical perspective, our results suggest that pramipexole (and potentially other dopaminergic drugs) could represent a viable pharmacological treatment option for impaired gustatory function. This potential application deserves further investigation, especially given that medicine currently lacks effective therapies for taste disorders such as hypogeusia and ageusia (Kumbargere Nagraj et al., 2017). This seems particularly relevant in the context of the current COVID-19 pandemic, which will potentially leave some people suffering from long-term gustatory impairments (Vaira et al., 2020).
Interestingly, major depressive disorder is also associated with impaired chemosensory function, including reductions in both gustatory and olfactory sensitivity (Amsterdam et al., 1987; Deems et al., 1991; Berlin et al., 1998; Kohli et al., 2016). Recent work has suggested that pramipexole itself has antidepressant activity (Romeo et al., 2018; Tundo et al., 2019), raising the possibility that improved chemosensory function may be a mechanism by which dopaminergic agents, such as pramipexole, act to reduce symptoms of depression.
In addition to positive effects on taste recognition, we also observed that pramipexole-treated participants gave blunted subjective ratings of appetitive (sweet) and aversive (bitter) taste samples. Specifically, the pramipexole group showed lower pleasantness ratings for rewarding stimuli and higher pleasantness ratings for aversive stimuli, with a comparable pattern observed for ratings of disgustingness. Interestingly, this effect parallels previously reported observations at the neural level, whereby a single-dose of pramipexole reduced activity in the dorsal anterior cingulate cortex in response to both rewarding and aversive gustatory stimuli (McCabe et al., 2013). In this context, our finding is also noteworthy insofar as inhibitory effects of pramipexole on reward processing tend to be attributed to acute low-dose treatment, which is thought to primarily target presynaptic dopamine auto receptors (Pizzagalli et al., 2008; McCabe et al., 2013). Our results suggest that such an inhibitory effect can also be brought about by subacute treatment with pramipexole at a moderate dose. However, the pattern of blunted hedonic responses disappeared once nausea was included as a covariate in the analysis. Therefore, the group differences in hedonic ratings of appetitive and aversive taste stimuli could simply be due to increased nausea in the pramipexole group. Because these exploratory findings might be of clinical as well as theoretical relevance, they should be interrogated in closer detail in future trials.
Our study also has several strengths and limitations that require highlighting. To begin with, to the authors’ knowledge, this is the first assessment of gustatory function following subacute treatment with pramipexole. Because we utilized a healthy volunteer sample, as opposed to a patient sample, we were able to test potential effects of pramipexole on taste function unconfounded by neuropathology or changes in clinical symptoms. Compared with single-dose designs, commonly used for pharmacological manipulation in experimental settings, subacute drug treatment has higher clinical-ecological validity. This is especially important with pramipexole, because acute vs repeated dopaminergic manipulation might lead to differential neurochemical and neurocognitive effects (Newton et al., 2015; Martins et al., 2017). Another strength of this study is the taste test we used, which is a standardized and validated assessment system for gustatory function and is commercially available. This should make future replication efforts of our findings relatively straightforward.
There are also several limitations that need to be mentioned. First, we conducted a between-groups comparison with a moderately sized sample of participants. Although such a study design is cost-effective and practical to conduct, it might lack sensitivity to detect smaller effects (e.g., a taste-specific influence in addition to the global effect observed here). Second, as expected for a treatment with notable side effects, blinding was not fully achieved. Therefore, theoretically, treatment expectations could have confounded some of the observed results (however, one might be skeptical whether healthy volunteers are likely to hold strong expectations about pramipexole’s effect on gustatory processing). Third, by relying on a single, whole-mouth taste test and using only 1 specific target dose (i.e., 1.0 mg/d of pramipexole salt), the study was limited in terms of its methodological generalizability. Future investigations should employ alternative gustatory assessments (e.g., regional taste assessment and electrogustometry, gustatory evoked potentials, neuroimaging during taste recognition) to better understand the effects of dopaminergic manipulation on different levels of the gustatory processing hierarchy (e.g., tongue, cranial nerves, cortical areas, etc.). In addition, future studies should also try to establish a dose response curve for pramipexole’s effect on taste recognition. Fourth, the age range of our study participants was between 18 and 43 years, whereas PD typically manifests at around 50 to 60 years of age. Thus, our sample’s physiological, psychological, and social characteristics might not fully parallel those of PD patients. Finally, the investigation discussed here only utilized a healthy volunteer sample. As a next step, our findings should be independently replicated in clinical populations (e.g., in patients suffering from hypogeusia/ageusia or in patients with major depression) to explore the potential use of pramipexole (and other dopaminergic drugs) as a pharmacological treatment for impaired chemosensory function.
CONCLUSION
In conclusion, this study found that subacute treatment with the dopamine D2/D3 receptor agonist pramipexole was associated with enhanced taste recognition performance in healthy volunteers. In addition, pramipexole-treated participants also showed blunted subjective evaluation of both appetitive and aversive taste stimuli, but this pattern was linked to the experience of nausea as a side effect. Taken together, our findings indicate a direct influence of dopaminergic signaling on gustatory processing, which could have several important theoretical and clinical implications.
Supplementary Material
Acknowledgments
We thank all our study participants for dedicating their time to taking part in this study. We also thank 2 anonymous peer reviewers for their critical feedback on earlier versions of this paper.
The project was supported by the NIHR Oxford Health Biomedical Research Centre and the Medical Research Council, and we are grateful for their support. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health, or the MRC.
Footnotes
Since treatment allocation was conducted in a randomized way, no specific further screening for conditions potentially influencing taste performance was conducted.
Contributor Information
Alexander Kaltenboeck, Department of Psychiatry, University of Oxford, Oxford, United Kingdom; Clinical Division of Social Psychiatry, Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria.
Don Chamith Halahakoon, Department of Psychiatry, University of Oxford, Oxford, United Kingdom; Oxford Health NHS Foundation Trust, Warneford Hospital, Oxford, United Kingdom.
Catherine J Harmer, Department of Psychiatry, University of Oxford, Oxford, United Kingdom; Oxford Health NHS Foundation Trust, Warneford Hospital, Oxford, United Kingdom.
Philip Cowen, Department of Psychiatry, University of Oxford, Oxford, United Kingdom; Oxford Health NHS Foundation Trust, Warneford Hospital, Oxford, United Kingdom.
Michael Browning, Department of Psychiatry, University of Oxford, Oxford, United Kingdom; Oxford Health NHS Foundation Trust, Warneford Hospital, Oxford, United Kingdom.
Ethics Statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Interest Statement
Catherine Harmer reports receiving grants from Johnson & Johnson, UCB, and Sunovion and personal fees from P1vital, Sage Therapeutics, Johnson and Johnson, Pfizer, Zogenix, Compass Pathways, and Lundbeck (outside this work). Michael Browning was supported by a MRC Clinician Scientist Fellowship (MR/N008103/1), has received travel expenses from Lundbeck for attending conferences, owns shares in P1vital Products Ltd., and acted as a consultant for Johnson & Johnson and CHDR. All other authors report no conflicts of interest.
References
- Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A (1987) Taste and smell perception in depression. Biol Psychiatry 22:1481–1485. doi: 10.1016/0006-3223(87)90108-9. [DOI] [PubMed] [Google Scholar]
- Arrondo G, Murray GK, Hill E, Szalma B, Yathiraj K, Denman C, Dudas RB (2015) Hedonic and disgust taste perception in borderline personality disorder and depression. Br J Psychiatry 207:79–80. doi: 10.1192/bjp.bp.114.150433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ (1998) Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry J Assoc Eur Psychiatr 13:303–309. 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- Brichta L, Greengard P, Flajolet M (2013) Advances in the pharmacological treatment of Parkinson’s disease: targeting neurotransmitter systems. Trends Neurosci 36:543–554. doi: 10.1016/j.tins.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Cecchini MP, Osculati F, Ottaviani S, Boschi F, Fasano A, Tinazzi M (2014) Taste performance in Parkinson’s disease. J Neural Transm 121:119–122. doi: 10.1007/s00702-013-1089-7. [DOI] [PubMed] [Google Scholar]
- Cecchini MP, Fasano A, Boschi F, Osculati F, Tinazzi M (2015) Taste in Parkinson’s disease. J Neurol 262:806–813. doi: 10.1007/s00415-014-7518-1. [DOI] [PubMed] [Google Scholar]
- Cecchini MP, Federico A, Zanini A, Mantovani E, Masala C, Tinazzi M, Tamburin S (2019) Olfaction and taste in Parkinson’s disease: the association with mild cognitive impairment and the single cognitive domain dysfunction. J Neural Transm 126:585–595. doi: 10.1007/s00702-019-01996-z. [DOI] [PubMed] [Google Scholar]
- Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB Jr (1991) Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Neck Surg 117:519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- Doty RL, Nsoesie MT, Chung I, Osman A, Pawasarat I, Caulfield J, Hurtig H, Silas J, Dubroff J, Duda JE, et al. (2015) Taste function in early stage treated and untreated Parkinson’s disease. J Neurol 262:547–557. doi: 10.1007/s00415-014-7589-z. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL (2015) Structured clinical interview for DSM-5 disorders, clinician version (SCID-5-CV). Washington D.C.: American Psychiatric Association. [Google Scholar]
- Gallant H, Vo A, Seergobin KN, MacDonald PA (2016) Pramipexole impairs stimulus-response learning in healthy young adults. Front Neurosci 10:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier JB (1971) Nonparametric indexes for sensitivity and bias: computing formulas. Psychol Bull 75:424–429. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Kang UJ, de Wit H (2008) Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol 28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Jeon BS, Lee J-Y, Cho Y-J, Hong K-S, Cho J-Y (2011) Taste function in patients with Parkinson disease. J Neurol 258:1076–1079. doi: 10.1007/s00415-010-5884-x. [DOI] [PubMed] [Google Scholar]
- Kohli P, Soler ZM, Nguyen SA, Muus JS, Schlosser RJ (2016) The association between olfaction and depression: a systematic review. Chem Senses 41:479–486. doi: 10.1093/chemse/bjw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbargere Nagraj S, George RP, Shetty N, Levenson D, Ferraiolo DM, Shrestha A (2017) Interventions for managing taste disturbances. Cochrane Database Syst Rev 12:CD010470. doi: 10.1002/14651858.CD010470.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CJG, Leuschner T, Ulrich K, Stößel C, Heckmann JG, Hummel T (2006) Taste in dementing diseases and parkinsonism. J Neurol Sci 248:177–184. doi: 10.1016/j.jns.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Martens MAG, Kaltenboeck A, Halahakoon DC, Browning M, Cowen PJ, Harmer CJ (2021) An experimental medicine investigation of the effects of subacute pramipexole treatment on emotional information processing in healthy volunteers. Pharmaceuticals 14:800. doi: 10.3390/ph14080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins D, Mehta MA, Prata D (2017) The “highs and lows” of the human brain on dopaminergics: evidence from neuropharmacology. Neurosci Biobehav Rev 80:351–371. [DOI] [PubMed] [Google Scholar]
- McCabe C, Harwood J, Brouwer S, Harmer CJ, Cowen PJ (2013) Effects of pramipexole on the processing of rewarding and aversive taste stimuli. Psychopharmacology (Berl.) 228:283–290. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Balderston CC, Rick JH, Roalf DR, Weintraub D, Kleiner-Fisman G, Stern MB, Duda JE (2007) Phenylthiocarbamide (PTC) perception in Parkinson disease. Cogn Behav Neurol 20:145–148. doi: 10.1097/WNN.0b013e31812570c3. [DOI] [PubMed] [Google Scholar]
- Newton TF, Haile CN, Mahoney JJ, Shah R, Verrico CD, De La Garza R, Kosten TR (2015) Dopamine D3 receptor-preferring agonist enhances the subjective effects of cocaine in humans. Psychiatry Res 230:44–49. doi: 10.1016/j.psychres.2015.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini J, Brooks DJ, Pavese N (2021) The cholinergic brain in Parkinson’s disease. Mov Disord Clin Pract 8:1012–1026. doi: 10.1002/mdc3.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercey M (1998) Pharmacology of pramipexole, a dopamine D3-preferring agonist useful in treating Parkinson’s disease. Clin Neuropharmacol 21:141–151. [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, Culhane M (2008) Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl.) 196:221–232. 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo B, Blecha L, Locatelli K, Benyamina A, Martelli C (2018) Meta-analysis and review of dopamine agonists in acute episodes of mood disorder: efficacy and safety. J Psychopharmacol (Oxford) 32:385–396. [DOI] [PubMed] [Google Scholar]
- Shah M, Deeb J, Fernando M, Noyce A, Visentin E, Findley LJ, Hawkes CH (2009) Abnormality of taste and smell in Parkinson’s disease. Parkinsonism Relat Disord 15:232–237. doi: 10.1016/j.parkreldis.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N (1999) Calculation of signal detection theory measures. Behav Res Methods Instrum Comput 31:137–149. doi: 10.3758/BF03207704. [DOI] [PubMed] [Google Scholar]
- Tundo A, Filippis R, De Crescenzo F (2019) Pramipexole in the treatment of unipolar and bipolar depression: a systematic review and meta‐analysis. Acta Psychiatr Scand 140:116–125. [DOI] [PubMed] [Google Scholar]
- Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Chiesa-Estomba CM, Salzano G, Cucurullo M, Salzano FA, Saussez S, Boscolo-Rizzo P, et al. (2020) Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol 134:703–709. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.