Abstract
Background
Fragile X syndrome (FXS) is a genetic condition that causes a range of developmental problems, including intellectual disability, aggressive behavior, anxiety, abnormal sensory processing, and cognitive impairment. Despite intensive preclinical research in Fmr1-targeted transgenic mice, an effective treatment for FXS has yet to be developed. We previously demonstrated that ASP5736, a 5-Hydroxytryptamine (serotonin) receptor 5A receptor antagonist, ameliorated scopolamine-induced working memory deficits in mice, reference memory impairment in aged rats, and methamphetamine-induced positive symptoms and phencyclidine-induced cognitive impairment in animal models of schizophrenia. We hypothesized that ASP5736 may be effective for ameliorating similar behavior deficits in male Fmr1-targeted transgenic rats as a preclinical model of FXS.
Methods
We evaluated the effect of acute oral administration of ASP5736 on the abnormal behavior of hyperactivity (0.01, 0.1 mg/kg), prepulse inhibition (0.01, 0.03, 0.1 mg/kg), and the novel object recognition task (0.1 mg/kg) in Frmr1-knockout (KO) rats.
Results
Fmr1-KO rats showed body weight gain, hyperactivity, abnormal sensory motor gating, and cognitive impairment. ASP5736 (0.1 mg/kg) reversed the hyperactivity and ameliorated the sensory motor gating deficits (0.03–0.1 mg/kg). ASP5736 (0.01 mg/kg) also improved cognitive impairment.
Conclusions
ASP5736 is a potential drug candidate for FXS. Further studies are needed to confirm its clinical efficacy.
Keywords: 5-HT5A receptor antagonist, ASP5736, behavior, Fmr1-targeted transgenic rat, fragile X syndrome
Significance Statement.
Consistent with observations in patients with fragile X syndrome (FXS), fragile X mental retardation 1 (Fmr1) gene knockout rodent models of FXS also show disease-related abnormalities, such as seizures, abnormal visual-evoked responses, auditory hypersensitivity, and abnormal processing at multiple levels of the auditory system. In spite of intensive preclinical research using Fmr1-targeted transgenic models, an effective treatment for FXS has yet to be developed. For the first time, to our knowledge, we report that ASP5736, a 5-HT5A receptor antagonist, is effective for alleviating hyperactivity, abnormal sensory motor gating, attention deficit, and cognitive impairment in Fmr1-targeted transgenic rats.
Introduction
Fragile X syndrome (FXS) is a genetic disease that arises from an abnormal expansion of polymorphic CGG repeats (>200 CGG repeats) in the fragile X mental retardation 1 (FMR1) gene on the X chromosome (Verkerk et al., 1991; Price et al., 1996; Crawford et al., 2001), which results in hypermethylation of its promoter and a loss or reduction in the fragile X mental retardation protein (FMR). FXS affects 1 in 4000 boys and 1 in 8000 girls (Turner et al., 1996). Signs that a child might have FXS include (1) developmental delay (late sitting, walking, or talking compared with children of the same age), (2) learning disabilities (trouble learning new skills), and (3) social and behavioral problems (not making eye contact, anxiety, trouble paying attention, hand flapping, acting, and speaking without thinking, and being very active). Males who have FXS usually have some degree of intellectual disability that can range from mild to severe. In contrast, females with FXS can have normal intelligence or some degree of intellectual disability. Accordingly, patients are characterized by intellectual disability, hyperactivity, anxiety, seizures, autism-like symptoms, abnormal sensory processing (Hagerman et al., 2017; Lee et al., 2018), and body weight gain (Raspa et al., 2010). The amino acid sequence of the FMR1 protein is highly conserved across numerous evolutionary species, including humans, mice, rats, chickens, Xenopus laevis, Caenorhabditis elegans, and Drosophila melanogaster (Verkerk et al., 1991; Ashley et al., 1993; Hinds et al., 1993 ; Siomi et al., 1995; Wan et al., 2000). Fmr1-knockout (Fmr1-KO) mice have been used as a preclinical model of FXS for more than 20 years (Dahlhaus et al., 2018). Although preclinical studies have indicated the potential benefits of a multitude of interventions in Fmr1-KO mice (Erickson et al., 2017), these interventions have failed or shown only minimal effects in clinical trials.
In 2014, Fmr1-targeted transgenic rats (Fmr1-KO rats) were established by Sage Laboratories, LLC (Emmett, ID, USA) using zinc finger nuclease technology to target exon 8 of the Fmr1 gene (Hamilton et al., 2014). For drug development, rat models can be more beneficial for evaluating the safety risk of drug candidates than mouse models because toxicity studies are usually conducted in rats. However, further characterization of Fmr1-KO rats is limited, and no reports have tested potential treatments on this rat model.
The 5-Hydroxytryptamine (serotonin) receptor 5A (5-HT5A)receptor is a G-protein–coupled receptor whose human gene was cloned in 1994 (Rees et al., 1994). The 5-HT5A receptor is expressed on neurons (e.g., cortical and hippocampal pyramidal neurons) with little expression in peripheral tissues, which has provided insight into its potential roles in memory formation and emotional controls, both of which are affected in FXS patients. In addition, increased exploratory behavior in novel environments displayed by 5-HT5A receptor KO mice compared with wild-type (WT) mice (Grailhe et al., 2001), together with its widespread localization, has suggested that the 5-HT5A receptor is involved in mood and affective and cognitive function. Further, gene association studies have implicated the 5-HT5A receptor in schizophrenia and mood disturbance (Jongen-Relo et al., 2006; Rueter et al., 2006; Thomas, 2006).
We previously demonstrated that ASP5736, a 5-HT5A receptor antagonist, ameliorated scopolamine-induced working memory deficits in mice, reference memory impairment in aged rats (Yamazaki et al., 2015), and methamphetamine-induced positive symptoms (Yamazaki et al., 2014) and phencyclidine-induced cognitive impairment in animal models of schizophrenia (Yamazaki et al., 2018). Here, we characterized several behaviors of male Fmr1-KO rats as an alternative preclinical model of FXS and evaluated the effects of ASP5736 in the male Fmr1-KO rat model.
METHODS
Animals
Five-week-old Fmr1-KO and wild-type rats were purchased from Oriental Yeast Co., Ltd. (Tokyo, Japan). Fmr1-KO rats were generated using the zinc finger nuclease method (Hamilton et al., 2014), and lines were first generated on an outbred Sprague-Dawley background at Sage Laboratories, LLC. All rats were housed in groups of 3 in temperature- and humidity-controlled rooms (23°C ± 2°C and 55% ± 10%) under a 12-h-light/-dark cycle. Food and water were available ad libitum in all home cages. In the present study, we examined the efficacy of ASP5736 in Fmr1-KO rats as an animal model of FXS. We used male animals because most patients with FXS are male. Further, ethical regulations prevent the use of large numbers of female animals because of their reproductive issues.
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Astellas Pharma Inc. Tsukuba Research Center is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. A different set of animals was used for each task. We adhered to international guidelines for the use and care of laboratory animals.
Drugs
ASP5736 (N-(diaminomethylene)-1-(3,5-difluoropyridin-4-yl)-4-fluoroi-soquinoline-7-carboxamide (2E)-but-2-enedioate), was synthesized at Astellas Pharma Inc. (Tsukuba, Japan) and suspended in 0.5% (w/v) methyl cellulose. It was administered at 1 mL/kg to rats and was corrected for salt content. Oral acute administration of ASP5736 was performed 1 hour before each test.
Body Weight
Body weight was measured in 14- to 19-week-old wild-type and Fmr1-KO rats before locomotor assessment of activity, prepulse inhibition (PPI), and the novel objection recognition task (NORT). All values are expressed as mean ± standard error of the mean (SEM). An unpaired t test was used to detect significant differences between Fmr1-KO and wild-type rats. Statistical analyses were conducted using GraphPad Prism, version 5.0 (GraphPad Software, San Diego CA, USA). For all tests, P < .05 was considered significant.
Spontaneous Locomotor Activity
Locomotor activity was evaluated in 14- to 16-week-old wild-type and Fmr1-KO rats. After more than 1 hour of acclimation to a test room that was exposed to over 300 lux of light, test animals had their body weight measured before being orally administered ASP5736 (0.01 and 0.1 mg/kg). After 30 minutes, each rat was moved from their home cage to a new cage (L × W × H: 30 × 35 × 17.5 cm) where locomotor activity was measured for 60 minutes using a Supermex sensor (Muromachi Inc., Tokyo, Japan) comprising paired infrared pyroelectric detectors, data from which were analyzed using CompACT AMS, ver. 3.82 (Muromachi Inc.). All values are expressed as mean ± SEM. We used 14 (wild-type and vehicle of Fmr1-KO group) and 13 (0.01 mg/kg and 0.1 mg/kg group) rats for these experiments. Statistical analyses were conducted using GraphPad Prism, version 5.0 (GraphPad Software). Dunnett’s multiple comparisons test was used to compare multiple groups vs vehicle of Fmr1-KO group following ANOVA test. For all tests, P < .05 was considered significant.
PPI
Wild-type and Fmr1-KO rats aged 14–16 weeks old were tested in 5 startle chambers to measure their startle response (SR-LAB, San Diego Instruments, San Diego, CA, USA). The chamber was made of transparent Plexiglas tubing (diameter 9 cm, length 20 cm) mounted to a Plexiglas frame (31 × 32.5 × 45 cm) within a ventilated enclosure. Acoustic noise bursts were introduced via a speaker mounted 24 cm above the tube. A background noise level of 65 dB was maintained throughout each session. A piezoelectric accelerometer mounted below the frame detected and transduced motion within the tube. Beginning at the onset of the stimulus, 100 readings lasting 1 millisecond each were recorded. The most intense (Vmax) of the 100 readings was taken as the startle amplitude.
ASP5736 (0.01–0.1 mg/kg) was orally administered 50 minutes before the start of the test. Each rat was acclimatized to the chamber with a background noise level of 65 dB for 10 minutes. Following this period, 3 startle pulses (20 milliseconds, 120 dB) were presented. Because the most pronounced startle response typically occurs during the first 3 pulse presentations, 3 initial startle pulses served to achieve a relatively stable level of startle reactivity for the remainder of the test session. The pre-pulses were 20-millisecond broadband noise bursts of 70, 75, and 80 dB. The interval between the pre-pulse and pulse was 80 milliseconds. Each session consisted of 10 trials of pulse alone and 10 trials of pre-pulse + pulse. The different trial types were presented pseudorandomly with variable inter-trial intervals of 20–60 seconds. Each session lasted approximately 40 minutes.
Data were analyzed separately for each trial, and the percent PPI was calculated. For example, percent PPI for the pre-pulse 80 dB + pulse 120 dB (PP80-P120) trial was determined as follows: %PPI = 100-{[(startle response for pre-pulse 80 dB + pulse 120 dB trial)/(startle response for pulse 120 dB alone)]×100}. All values are expressed as mean ± SEM. We used 15 (wild type, vehicle of Fmr1-KO, 0.01 mg/kg and 0.03 mg/kg group) and 14 (0.1 mg/kg group) rats for these experiments. Statistical analyses were conducted using GraphPad Prism, version 5.0 (GraphPad Software). Dunnett’s multiple comparisons test was used to compare multiple groups vs vehicle of Fmr1-KO group following ANOVA. For all tests, P < .05 was considered significant.
NORT
The NORT is a model for assessing visual-recognition memory that takes advantage of the natural preference of rodents for novelty: mice that recognize a familiar object have been shown to instinctively spend more time exploring an unfamiliar object (Yang et al., 2017). NORT was performed in 16- to 19-week-old Fmr1-targeted transgenic rats. The NORT consists of 3 different sessions: habituation, training, and testing. Each rat was individually habituated to a box (70 × 70 cm) for 5 minutes in the absence of any objects (habituation session). After 3–4 hours, 2 different objects (*objects A and A) were placed symmetrically 17.5 cm from the 2 opposite corners of the back wall. Objects A and A were always placed in the same location. The animal was placed into the box facing a side-wall and allowed to explore the box for 5 minutes (training session). ASP5736 (0.01 mg/kg) was orally administered approximately 60 minutes before the training session. Control animals were administered vehicle instead of ASP5736. After the training session, the rat was placed back in its home cage. After a 1-hour retention interval, object A was replaced with a novel object (*object B). The animal was placed back into the same box, this time with 1 familiar object (object A) and 1 novel object (object B) and allowed to explore the box for 5 minutes (test session). The time spent exploring each object during the training and test sessions was recorded by EthoVision XT ver8 (Sophia Scientific Inc, Anjo city, Aichi prefecter, Japan). Exploration time was defined as the period for which the rat was within 3 cm of each object. Exploratory preference was calculated to assess cognitive function using the following formula:
Exploratory preference (%) = 100 × (time spent exploring novel object)/(total time exploring both objects).
We used red and yellow L-shaped Lego blocks (REGO® in Japan) (8 cm × 8 cm × 10 cm (H)) as objects A in the NOR task (see supplementary Figure 1 for a photo) and a red Lego block (8 cm × 8 cm × 10 cm (H)) as object B (see supplementary Figure 1 for a photo).
All values are expressed as mean ± SEM. We used 13 (wild-type group), 14 (vehicle of Fmr1-KO group), and 15 (0.01 mg/kg group) rats in these experiments. Statistical analyses were conducted using GraphPad Prism, version 5.0 (GraphPad Software). Figure 3A: About exploratory time (sec), ANOVA (followed by Turkey’s test) was used to detect significant differences in the time (sec) rats spent exploring the novel and familiar objects of WT rats, vehicle- and ASP5736-treated (0.01 mg/kg, po) Fmr1-KO rats. Figure 3B: About exploratory preference (%), ANOVA (followed by Turkey’s test) was used to detect significant differences in the test session among wild-type rats and vehicle- and ASP5736-treated (0.01 mg/kg, po) Fmr1-KO rats. For all tests, P < .05 was considered significant.
RESULTS
Body Weight
At the start of the locomotor activity test, Fmr1-KO and wild-type rats aged 14–16 weeks weighed 565.5 ± 13.7 g and 497.5 ± 10.0 g, respectively, with Fmr1-KO rats being significantly (P < .01) heavier than wild-type rats (Table 1). In the PPI test, Fmr1-KO and wild-type rats aged 14–16 weeks weighed 562.1 ± 8.3 g and 510.0 ± 7.2 g, respectively, similar to that observed for the locomotor activity test. Furthermore, in the NORT, Fmr1-KO and wild-type rats aged 16–19 weeks weighed 553.2 ± 10.3 g and 507.7 ± 10.9 g, respectively, with Fmr1-KO rats again being significantly (P < .01) heavier than wild-type rats (Table 1).
Table 1.
Comparisons of Fmr1-KO and wild-type rats on body weighta
| Tasks when weighing | Age | WT | Fmr1-KO |
|---|---|---|---|
| Locomotor activity | 14–16 wk | 497.5 ± 10.0 g (14) | 565.5 ± 13.7 g (14) ## |
| Prepulse inhibition | 14–16 wk | 510.0 ± 7.2 (15) | 562.1 ± 8.3 (15) ## |
| Novel object recognition | 16–19 wk | 507.7 ±10.9 (13) | 553.2 ± 10.3 (14) ## |
Abbreviations: KO, knockout; WT, wild type.
a Separate animas for each task. Values are mean ± SEM. Value in parentheses indicates the number of rats in each group. ##P < .01, statistically significant compared with WT rats (Student’s t test).
Hyperactivity in a Novel Environment
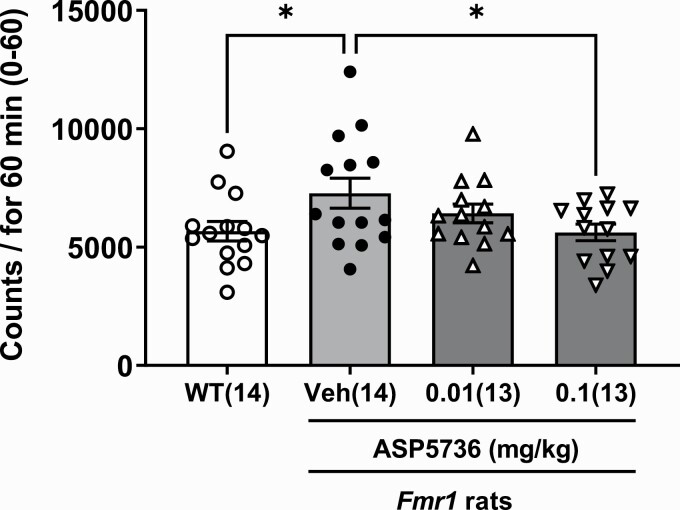
Figure 1 showed locomotor activity for 60 minutes in wild-type rats without treatments, Fmr1-KO rats without treatments, and Fmr1-KO rats with ASP5736 treatments (0.01 and 0.1 mg/kg). Those of all groups showed statistically significant differences (F(3,50) = 2.826 P < .01 by ANOVA test). Fmr1-KO rats were significantly more hyperactive than wild-type rats over an observation period of 60 minutes (Figure 1; P < .05, Dunnett’s multiple comparisons test). Treatment with ASP5736 at a dose of 0.1 mg/kg significantly reversed the hyperactivity observed in Fmr1-KO rats (Figure 1; P = .0381<0.05, Dunnett’s multiple comparisons test vs vehicle of Fmr1-KO group).
Figure 1.
Effect of ASP5736 (0.01 and 0.1 mg/kg, po) on hyperactivity across 60 minutes. Wild-type and vehicle-treated Fmr1-knockout (KO) rats were administered 0.5% methyl cellulose instead of ASP5736. Values are mean ± SEM. Values in parentheses indicate the number of rats in each group. *P < .05, compared with vehicle of Fmr1-KO rats (Dunnett’s multiple comparisons test).
Prepulse Inhibition
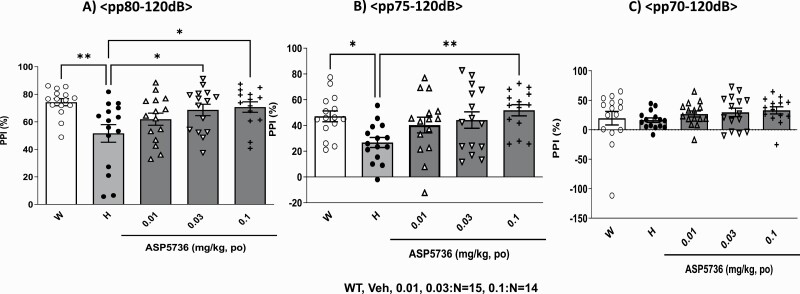
PPI is a phenomenon in which there is a reduction in the acoustic startle response to an intense acoustic stimulus (pulse) that is immediately preceded by a stimulus of lower intensity (pre-pulse) (Hoffman and Searle 1968; Graham 1975). Therefore, the amount of PPI provides an operational measure of sensorimotor gating. We confirmed the response amplitudes for every treatment (wild type, Fmr1-KO, and treatments with ASP5736 groups) did not differ (F(4,69) = 0.8195, P = .5174 by ANOVA, data not shown). Figure 2A–B show that all groups showed statistically significant differences in the PP80-P120 (F(4,69) = 4.065, P < .01, ANOVA) and PP75-P120 trials (F(4,69) = 3.443, P < .05, ANOVA), respectively. On the other hand, as shown in Figure 2C, all groups did not show statistically significant differences (F(4,69) = 0.8941, P = .4723, ANOVA). Fmr1-KO rats showed significantly reduced PPI compared with wild-type rats (Figure 2A–B). The mean percent PPI was significantly lower among Fmr1-KO rats than wild-type rats in the PP80-P120 and PP75-P120 (P < .01, ANOVA followed by Dunnett’s test). In contrast, as shown in Figure 2C, PPI was comparable between Fmr1-KO and wild-type rats in the PP70-P120 trial (P > .1). Acute administration of 0.03 mg/kg and 0.1 mg/kg of ASP5736 significantly increased Fmr1-KO rats’ lower PPI in a dose-dependent manner (P < .05, by ANOVA followed by Dunnett’s test vs vehicle) in the PP80-P120 trial (Figure 2A). As shown in Figure 2B, treatment with ASP5736 (0.1 mg/kg po, P < .01 by ANOVA followed by Dunnett’s test vs vehicle) also significantly increased the lower PPI in Fmr1-KO rats in the PP75-P120 trial. In contrast, in the PP70-P120 trial, ASP5736 showed a nonsignificant effect on increasing the lower PPI in Fmr1-KO rats with increasing doses (Figure 2C; P > .1, ANOVA followed by Dunnett’s test vs vehicle).
Figure 2.
Effect of ASP5736 (0.01–0.1 mg/kg, po) on prepulse inhibition (PPI). The prepulse was (A) 70 dB, (B) 75 dB, and (C) 80 dB. Wild-type rats and vehicle-treated Fmr1-knockout (KO) rats were administered 0.5% methyl cellulose instead of ASP5736. Values are mean ± SEM. A total of 15 (wild type, vehicle of Fmr1-KO, 0.01 mg/kg and 0.03 mg/kg group) and 14 (0.1 mg/kg group) rats were used. *P < .05, **P < .01, compared with vehicle of Fmr1-KO rats (Dunnett’s multiple comparisons test).
Novel Object Recognition Task
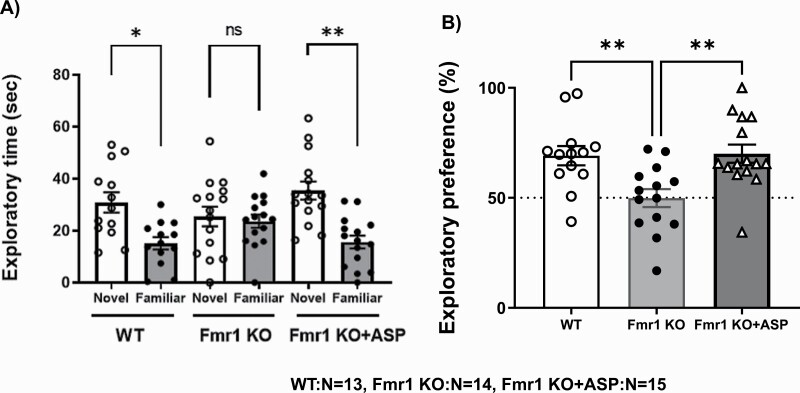
Figure 3A shows the time (seconds) rats spent exploring novel and familiar objects. All groups showed statistically significant differences (F(5,80) = 6.515 P < .01, ANOVA). Wild-type rats spent significantly more time exploring the novel object than the familiar one (P < .01, ANOVA followed by Turkey’s test). In contrast, Fmr1-KO rats spent almost the same amount of time exploring the novel object (25.46 ± 3.76) and the familiar one (23.65 ± 2.58, P > .1), suggesting impaired recognition for NORT. Acute ASP5736 administration (0.01 mg/kg, po) significantly increased the amount of time Fmr1-KO rats spent exploring the novel object (35.40 ± 3.47) compared with the familiar one (15.58 ± 2.51) (P < .01, ANOVA followed by Turkey’s test) to a level comparable with that observed in wild-type rats (Figure 3A). Whereas wild-type rats spent nearly 70% (70.7 ± 4.3) of the total time exploring the novel object in the test session, Fmr1-KO rats did so only about 50% (50.6 ± 4.2) of the time; this difference was significant (Figure 3BF(2,39) = 7.362 P < .01, ANOVA followed by Turkey’s test). This finding suggests that Fmr1-KO rats had impaired cognition. Treatment with 0.01 mg/kg (per os, oral [PO]) ASP5736 significantly improved this memory impairment in Fmr1-KO rats (P < .01, ANOVA followed by Turkey’s test; Figure 3B), with the rats appearing to remember the old object, spending nearly 70% (70.4 ± 4.3) of the total time exploring the novel object in the test session.
Figure 3.
Effect of ASP5736 (0.01 mg/kg, po) on the novel object recognition task (NORT). Wild-type rats and vehicle-treated Fmr1-knockout (KO) rats were administered 0.5% methyl cellulose instead of ASP5736. ASP indicates ASP5736. (A) Time (seconds) each group spent exploring the novel and familiar objects. Values are mean ± SEM. A total of 13 (wild-type group), 14 (vehicle of Fmr1-KO group), and 15 (0.01 mg/kg group) rats were used. *P < .05, **P < .01, statistically significant compared with the familiar object (ANOVA followed by Turkey’s test). (B) Exploratory preference (%) in each group. Values are mean ± SEM. A total of 13 (wild-type group), 14 (vehicle of Fmr1-KO group), and 15 (0.01 mg/kg group) rats were used. **P < .01, statistically significant compared with vehicle-treated Fmr1-KO rats (ANOVA followed by Turkey’s test).
Discussion
In the present study, we focused on male Fmr1-targeted transgenic rats because Fmrp is located on the X chromosome, and boys’ pathology is more severe than that of girls. We examined the neuropsychological phenotypes of Fmr1-targeted transgenic rats and demonstrated that ASP5736 ameliorated their hyperactivity, abnormal sensory motor gating, attention deficit, and cognitive impairment. Whereas the behavioral and neurophysiological characteristics of Fmr1-targeted transgenic mice have been extensively replicated, those of Fmr1-targeted transgenic rats remain limited. The present study is the first, to our knowledge, to describe the neuropsychological phenotypes of Fmr1-targeted transgenic rats.
Enhanced neuronal excitability is associated with behavioral symptoms such as increased anxiety and locomotor activity (Gibson et al., 2008; Berzhanskaya et al., 2016, 2017), representing the most consistent behavioral symptoms observed in subjects with FXS (Crawford et al., 2001).
Sensorimotor gating is a fundamental neural process that is impaired in patients with some forms of schizophrenia and other specific neuropsychiatric disorders. Previous reports have indicated that, compared with normal control subjects, schizophrenic patients exhibit lower PPI (Braff et al., 1978, 1992; Grillon et al., 1992). PPI is a cross-species phenomenon that has been studied extensively in animals (Hoffman and Ison, 1980). Evidence suggests that PPI is regulated by forebrain circuits, including those in the mesolimbic cortex, nucleus accumbens, ventral pallidum, thalamus, and pedunculopontine tegmental nucleus (Swerdlow et al., 1998).
It is known that PPI studies in FXS patients and Fmr1-KO mice do not produce the same results. For example, Frankland et al. (2004) showed that PPI and learning were enhanced rather than reduced in Fmr1-KO mice despite previous studies having revealed sensorimotor gating and learning abnormalities in these mutants. These data indicate that while mutations of the FMR1 gene have an observable impact in both humans and mice, the phenotypic consequences appear to oppose one another, suggesting that murine compensatory mechanisms following loss of FMR1 function may differ from those in humans. Potential differences in compensatory mechanisms may explain the phenotypic variations in different mutant models we described previously (Kogan et al., 2015). Based on the present finding that Fmr1-KO rats demonstrated PPI deficits resembling to those of human FXS patients, we speculate that compensatory mechanisms to Fmr1 loss/reduction in rats may be more similar to those in humans. Thus, Fmr1-KO rats may be a better model of disease conditions in human patients.
Our findings indicate that 14- to 16-week-old Fmr1-KO rats show body weight gain, hyperactivity, abnormal sensory motor gating, and cognitive impairment, as observed in FXS patients. In our previous study (Kozono et al., 2020), we also showed abnormalities in gamma power and the auditory steady-state response in these mutant rats. Therefore, Fmr1-KO rats may be one of the best models for mimicking the behavioral abnormalities and electroencephalographical/ event-related potential phenotypes of FXS patients, making them useful for drug discovery. For these reasons, we studied the effects of ASP5736, a 5-HT5A receptor antagonist, on the abnormal behavioral phenotypes of Fmr1-KO rats in this study. We did not administer ASP5736 to wild-type rats in a series of trials. Therefore, the effects of ASP5736 are limited on Fmr1-KO rats, and those on wild-type rats remain unknown; further evaluations are needed.
In our previous study, we showed that 0.001–0.03 mg/kg of ASP5736 was effective for improving cognitive performance in the water maze task in aged rats, with a maximum effect observed at 0.01 mg/kg (Yamazaki et al., 2015). In a separate study (Yamazaki et al., 2018), we showed that ASP5736 (0.001–0.01 mg/kg, po) significantly improved cognitive deficits exhibited by a subchronical phencyclidine-induced rat model in the attentional set-shifting task, with a maximum effect also observed at 0.01 mg/kg. A study by Ventura et al. (2004) demonstrated that amphetamines improve the ability of Fmr1-KO mice to discriminate between novel and familiar objects without significantly affecting locomotor activity. Consistent with their behavioral data, the amphetamine induced a greater increase in dopamine release in the medial prefrontal cortex (mPFC) of Fmr1-KO compared with wild-type mice, although only a weak striatal dopaminergic response was observed in the Fmr1-KO mice. Thus, we hypothesize that an increase in dopamine levels in the mPFC may be one mechanism by which ASP5736 restores memory impairment in Fmr1-KO rats.
We also found that 14- to 16-week-old Fmr1-KO rats showed hyperactivity similar to FXS patients and that acute administration of 0.1 mg/kg ASP5736 was effective for alleviating this symptom. In our previous study (Yamazaki et al., 2014), ASP5736 significantly reduced methamphetamin (MAP)-induced hyperactivity in mice at 0.01–0.1 mg/kg, which is consistent with the outcome of the present study.
Given that we demonstrated a similar effective dose difference in the present study using rats, we speculate that ASP5736 may use different mechanisms through different neural networks to alleviate hyperactivity and memory deficits in FXS and that these neural networks are shared across rodent species. Given the highly conserved 5-HT5A amino acid sequence and expression pattern in the central nervous system between rodents and humans, we expect that ASP5736 may exert similar amelioration effects in humans via 5-HT5A neural networks.
MK-801- and MAP-induced hyperactivity has been reported to be associated with an increase in dopamine levels in the nucleus accumbens (Mathe, 1998), however, we previously showed that ASP5736 does not directly bind to dopamine D2 receptors (Yamazaki et al., 2014). Instead, ASP5736 may block inhibitory 5-HT5A receptors on GABAergic neurons in the ventral tegmental area (VTA), thereby inhibiting dopaminergic neurons projecting from the VTA to the nucleus accumbens (Yamazaki et al., 2018). Alternatively or additionally, ASP5736 may enhance dopaminergic neuronal activity in VTA neurons projecting to the mPFC. These opposing effects may be related to a higher density of GABAA receptors within the paranigral nucleus compared with the parabrachial pigmented area (Churchill et al., 1992). From the above evidence, we speculate that ASP5736 may partially reduce the activity of dopaminergic neurons projecting from the VTA to the nucleus accumbens by using GABAergic neurons as an intermediary.
We previously showed that while acute administration of ASP5736 does not increase dopamine levels in the nucleus accumbens, it does increase dopamine levels in the prefrontal cortex (Yamazaki et al., 2018). Prefrontal pyramidal neurons in layer V are known to project to the striatum and brainstem and appear to tonically inhibit striatal dopamine synthesis/release (Carlson et al., 1991; Carr and Sesack, 2000; Bertolino et al, 2000). Because these prefrontal pyramidal neurons are positively regulated by dopamine, ASP5736 may potentially increase their activity through dopamine release in the PFC, consequently inhibiting dopamine synthesis in the striatum/nucleus accumbens (Saunders et al., 1998). This evidence suggests that 5-HT5A antagonists may improve hyperactivity.
In summary, Fmr1-targeted transgenic rats showed body weight gain, hyperactivity, PPI abnormalities, and cognitive impairment resembling those observed in FXS patients, making it a potentially useful model for studying FXS disease-related mechanisms and for drug discovery. In fact, we showed that the 5-HT5A receptor antagonist ASP5736 significantly ameliorated hyperactivity, sensory motor gating deficits, attention deficits, and cognitive impairment in Fmr1-targeted transgenic rats, potentially by affecting dopamine levels in brain areas such as the mPFC or nucleus accumbens.
Supplementary Material
Acknowledgments
This work was conducted according to a research contract with Astellas Pharma Inc.
The authors declare that, other than income received from our primary employers, this research did not receive any financial support or compensation and that they have no personal conflicts of interest.
Contributor Information
Mayako Yamazaki, Department of Neuroscience, Drug Discovery Research, Astellas Pharma Inc., Tsukuba-shi, Ibaraki, Japan.
Takatomo Arai, Department of Neuroscience, Drug Discovery Research, Astellas Pharma Inc., Tsukuba-shi, Ibaraki, Japan.
Junko Yarimizu, Department of Neuroscience, Drug Discovery Research, Astellas Pharma Inc., Tsukuba-shi, Ibaraki, Japan.
Mitsuyuki Matsumoto, Department of Neuroscience, Drug Discovery Research, Astellas Pharma Inc., Tsukuba-shi, Ibaraki, Japan; Neuroscience, La Jolla Laboratory, Astellas Research Institute of America LLC, San Diego, CA, USA.
Interest Statement
The authors declare no conflict of interest. All authors are employees of Astellas Pharma Inc. and its affiliates.
Author Disclosures
Substantial contributions to the conception or design: Yamazaki and Matsumoto. Acquisition, analysis, or interpretation of data: Yamazaki, Arai, and Yarimizu. Drafting the manuscript or revising it critically for important intellectual content: Yamazaki, Arai, Yarimizu, and Matsumoto. Final approval of the version to be published: Yamazaki, Arai, Yarimizu, and Matsumoto. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Yamazaki, Arai, Yarimizu, and Matsumoto. All authors contributed to the present study and approved the final manuscript.
References
- Ashley CT, Sutcliffe JS, Kunst CB, Leiner HA, Eichler EE, Nelson DL and Warren ST (1993) Human and murine FMR-1: alternative splicing and translational initiation downstream of the CGG repeat. Nat Genet 4:244–251. [DOI] [PubMed] [Google Scholar]
- Berzhanskaya J, Phillips MA, Shen J, Colonnese MT (2016) Sensory hypo-excitability in a rat model of fetal development in fragile X syndrome. Sci Rep 6:30769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzhanskaya J, Phillips MA, Gorin A, Lai C, Shen J, Colonnese MT (2017) Disrupted cortical state regulation in a rat model of fragile X syndrome. Cereb Cortex 27:1386–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Breier A, Callicott JH, Adler C, Mattay VS, Shapiro M, Frank JA, Pickar D and Weinberger DR (2000) The relationship between dorsolateral prefrontal neuronal N-acetylaspartate and evoked release of striatal dopamine in schizophrenia. Neuropsychopharmacology 22:125–132. [DOI] [PubMed] [Google Scholar]
- Braff DL, Stone C, Callaway E, Geyer MA, Glick ID, Bali L (1978) Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology 15:339–343. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA (1992) Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 49:206–215. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR (2000) Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neuroscience 20:3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JN, Fitzgerald LW, Keller Jr RW, Glick SD (1991) Side and region dependent changes in dopamine activation with various durations of restraint stress. Brain Res 550:313–318. [DOI] [PubMed] [Google Scholar]
- Churchill L, Dilts RP, Kalivas PW (1992) Autoradiographic localization of γ-aminobutyric acid A, receptors within the ventral tegmental area. Neurochem Res 17:101–106. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuña, JM, Sherman SL (2001) FMR1- and the fragile X syndrome: human genome epidemiology review. Genet Med 3:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhaus R (2018) Of men and mice: modeling the fragile X syndrome. Front Mol Neurosci 11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CA, Davenport MH, Schaefer T, Wink LK, Pedapati EV, Sweeney JA, et al. (2017) Fragile X targeted pharmacotherapy: lessons learned and future directions. J Neurodev Disord 9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dy EM, kens E, Ornitz M and Silva AJ (2004) Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Molecular Psychiatry 9:417–425. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM (2008) Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol 100:2615–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F (1975) The more or less startling effects of weak prestimuli. Psychophaygiology 12:238–248. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Grabtree GW, Hen R (2001) Human 5-HT5 receptors: the 5-HT5A receptor is functional but the 5-HT5B receptor was lost during mammalian evolution. Eur J Pharmacol 418:157–167. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Charney DS, Braff DL (1992) Startle gating deficits occur across prepulse intensities in schizophrenic patients. Biol Psychiatry 32:939–943. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB, Moine H, Kooy RF, et al. (2017) Fragile X syndrome. Nat Rev Dis Primers 3:17065. [DOI] [PubMed] [Google Scholar]
- Hamilton SM, Green JR, Veeraragavan S, Yuva L, McCoy A, Wu Y, et al. (2014) Fmr1- and Nlgn3 knockout rats: novel tools for investigating autism spectrum disorders. Behav Neurosci 128:103–109. [DOI] [PubMed] [Google Scholar]
- Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M (1993) Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet 3:36–43. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR (1980) Reflex modification in the dopamine of startle. I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev 87:175–189. [PubMed] [Google Scholar]
- Hoffman HS, Searle JL (1968) Acoustic and temporal factors in the evocation of startle. J Acount Soc Am 43:269–282. [DOI] [PubMed] [Google Scholar]
- Jongen-Relo AL, Bespalov AY, Rueter LE, Freeman AS, Decker MW, Gross G, Schoemaker H, Sullivan JP, vanGa- leen, MM, Wicke, KM, Zhang M, Amberg W and Garcia- Ladona FJ, (2006) Behavioral pharmacological characterization of 5-HT5A receptor antagonists in antipsychologic drug tests. In: Proceedings of the 36th Annual Meeting of Society of Neuroscience, Atlanta, USA, October 14–18, 2006. Abstract 529. 26. [Google Scholar]
- Kogan JH, Gross AK, Featherstone RE, Shin R, Chen Q, Heusner CL, Adachi M, et al. (2015) Mouse model of chromosome 15q13.3 microdeletion syndrome demonstrates features related to autism spectrum disorder. J Neurosci 35:16282–16294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozono N, Okamura A, Honda S, Matsumoto M, Mihara T (2020) Gamma power abnormalities in a Fmr1-targeted transgenic rat model of fragile X syndrome. Sci Rep 10:18799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AW, Ventola P, Budimirovic D, Berry-Kravis E, Visootsak J (2018) Clinical development of targeted fragile X syndrome treatments: an industry perspective. Brain sciences 8:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathé JM, Nomikos GG, Schilström B, Svensson TH (1998) Non-NMDA excitatory amino acid receptors in the ventral tegmental area mediate systemic dizocilpine (MK-801) induced hyperlocomotion and dopamine release in the nucleus accumbens. J Neurosci Res 51:583–592. [DOI] [PubMed] [Google Scholar]
- Price DK, Zhang F, Ashley CT, Warren ST (1996) The chicken FMR1 gene is highly conserved with a CCT 5’-untranslated repeat and encodes an RNA-binding protein. Genomics 31:3–12. [DOI] [PubMed] [Google Scholar]
- Raspa M, Bailey DB, Bishop E, Holiday D, Olmsted M (2010) Obesity, food selectivity, and physical activity in individuals with fragile X syndrome. Am J Intellect Dev Disabil 115:482–495. [DOI] [PubMed] [Google Scholar]
- Rees S, Dendaas I, Foord S, Goodson S, Bull D, Kilpatrick G (1994) Cloning and characterisation of the human 5-HT5A serotonin receptor. FEBS Lett 355:242–246. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Wicke KM, Basso AM, Jongen-Relo AL, vanGaleen MM, Gross G, Decker MW, Kling A, Schoemarker H, Sullivan JP, Amberg W, Garcia-Ladona FJ (2006) Characterization of 5-HT5A antagonists in models of depression and anxiety. In: Proceedings of the 36th Annual Meeting of Society of Neuroscience, Atlanta. USA, October 14–18, 2006. Abstract 33.3. [Google Scholar]
- Saunders RC, Kolachana BS, Bachevalier J, Weinberger DR (1998) Neonatal lesions of the medial temporal lobe disrupt prefrontal cortical regulation of striatal dopamine. Nature 393:169–171. [DOI] [PubMed] [Google Scholar]
- Siomi MC, Siomi H, Sauer WH, Srinivasan S, Nussbaum RL, Dreyfuss G (1995) FXR1, an autosomal homolog of the fragile X mental retardation gene. EMBO J 14:2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA (1998) Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull 24:285–301. [DOI] [PubMed] [Google Scholar]
- Thomas DR (2006) 5-ht5A receptors as a therapeutic target. Pharmacol Ther 111:707–714. [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H (1996) Prevalence of fragile X syndrome. Am J Med Genet 64:196–197. [DOI] [PubMed] [Google Scholar]
- Ventura R, Pascucci, T, Catania MV, Musumeci SA, Puglisi-Allegra S (2004) Object recognition impairment in Fmr1 knockout mice is reversed by amphetamine: involvement of dopamine in the medial prefrontal cortex. Behav Pharmacol 15:433–442. [DOI] [PubMed] [Google Scholar]
- Verkerk AJM, Pieretti M, Sutcliffe JS, Fu Y-H, Kuhl DPA, Pizzuti A, Reiner O, Richards S, et al. (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905–914. [DOI] [PubMed] [Google Scholar]
- Wan L, Dockendorff TC, Jongens TA, Dreyfuss G (2000) Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol 20:8536–8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Harada K, Yamamoto N, Yarimizu J, Okabe M, Shimada T, Ni K, Matsuoka N (2014) ASP5736, a novel 5-HT5A receptor antagonist, ameliorates positive symptoms and cognitive impairment in animal models of schizophrenia. Eur Neuropsychopharmacol 24:1698–1708. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Okabe M, Yamamoto N, Yarimizu J, Harada K (2015) Novel 5-HT5A receptor antagonists ameliorate scopolamine-induced working memory deficit in mice and reference memory impairment in aged rats. J Pharmacol Sci 127:362–269. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Yamamoto N, Yarimizu J, Okabe M, Moriyama A, Furutani M, Marcus MM, Svensson TH, Harada K (2018) Functional mechanism of ASP5736, a selective serotonin 5-HT5A receptor antagonist with potential utility for the treatment of schizophrenia and affective disorders. Eur Neuropsychopharmacol 28:620–629. [DOI] [PubMed] [Google Scholar]
- Yang K, Broussard JI, Levine AT, Jenson D, Arenkiel B, Dani JA (2017) Dopamine receptor activity participates in hippocampal synaptic plasticity associated with novel object recognition. Eur J Neurosci 45:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.