Abstract
Pesticides have been used in agriculture, public health programs, and pharmaceuticals for many decades. Though pesticides primarily target pests by affecting their nervous system and causing other lethal effects, these chemical entities also exert toxic effects in inadvertently exposed humans through inhalation or ingestion. Mounting pieces of evidence from cellular, animal, and clinical studies indicate that pesticide-exposed models display metabolite alterations of pathways involved in neurodegenerative diseases. Hence, identifying common key metabolites/metabolic pathways between pesticide-induced metabolic reprogramming and neurodegenerative diseases is necessary to understand the etiology of pesticides in the rise of neurodegenerative disorders. The present review provides an overview of specific metabolic pathways, including tryptophan metabolism, glutathione metabolism, dopamine metabolism, energy metabolism, mitochondrial dysfunction, fatty acids, and lipid metabolism that are specifically altered in response to pesticides. Furthermore, we discuss how these metabolite alterations are linked to the pathogenesis of neurodegenerative diseases and to identify novel biomarkers for targeted therapeutic approaches.
Keywords: Neurodegenerative disorders, Brain, Pesticides, Metabolites, Neurotoxicity, Organophosphate
Introduction
With a sharp increase in global life expectancy, the world’s population over 60 years is expected to double from 12 to 22% between 2015 and 2050 (WHO 2021). The rise in the proportion of the aging population has led to an increase in age-related neurodegenerative disorders. Various metabolic pathways like fatty acid β-oxidation pathway, oxidative stress, mitochondrial dysfunction, glycerophospholipid metabolism, tryptophan metabolism, and glutathione metabolism have been shown to play a role in neurodegenerative diseases (Widner et al. 2002; Jenner 2003; Smeyne and Smeyne 2013; Bose and Beal 2016; Yan et al. 2021). The causative factors contributing to the onset and development of complex and multigenic neurodegenerative diseases have been attributed to genetic and environmental factors (Brown et al. 2005; Cannon and Greenamyre 2013; Pihlstrøm et al. 2017).
Among the environmental factors, pesticides are one of the many factors linked to the onset of neurodegenerative disorders (Chin-Chan et al. 2015). Pesticide exposure occurs through either occupational exposure, including pesticide applicators, mixers, pesticide distributors (Maroni et al. 2006), dietary routes, or drinking water (Lewis et al. 1988). This might lead to delayed toxic manifestation at low-level and high-level exposure scenarios. Pesticides intended to eradicate agricultural pests by targeting the nervous system of pests may also directly target the nervous tissue of other organisms with similar neurochemical processes (Keifer and Fireston 2007; Bjørling-Poulsen et al. 2008). Pesticides disrupt critical cellular mechanisms that sustain the metabolic requirements and activity of the nervous system (Keifer and Fireston 2007). Studies speculate that most Alzheimer’s disease (AD) and Parkinson’s disease (PD) cases observed in the older population might have been exposed to pesticides long before diagnosis (de Pedro-Cuesta et al. 2015; Yan et al. 2021). Furthermore, Freire and Koifman (2012) reviewed several published prospective and case–control studies and found evidence of an association between pesticide exposure and PD (Freire and Koifman 2012).
Metabolomics allows monitoring the changes in the whole metabolome, reflecting the context-dependent variation in genomic, transcriptomic, and proteomic fluctuations. However, metabolomics does not reveal the cause of the disease but shows the final results of normal or altered metabolic functions. Metabolomic assessment of exposure to different classes of pesticides such as organophosphates (OPs), organochlorines (OCs), and pyrethroids (PYRs) in biofluids has shown alteration in metabolites related to oxidative stress, inflammatory reactions, and mitochondrial dysfunction (Yan et al. 2021). Furthermore, it has been noted that many of the metabolic pathways altered by pesticides are common to neurodegeneration (Kori et al. 2016; Yan et al. 2021). So, metabolomics can detect metabolite level deviation and identify the affected metabolic pathways, which helps determine the etiology of a neurodegenerative disorder (Jové et al. 2014). Furthermore, diagnostic markers are unavailable for early detection of neurological syndromes such as AD, PD, and amyotrophic lateral sclerosis (ALS); therefore metabolomics is now slowly gaining importance in biomarker identification. The present review provides an overview of specific metabolic pathways, including tryptophan metabolism, glutathione metabolism, dopamine metabolism, energy metabolism, mitochondrial dysfunction, fatty acids, and lipid metabolism altered in response to pesticides and their link to neurodegenerative disorders.
Methodology
For the present review, we scoured the online scientific databases like Pubmed, Scopus, and Web of Science with a set of keywords pertaining to the topic of the review. The keywords like “pesticides, neurodegeneration, metabolomics,” “pesticides, neurotoxicity, metabolomics,” “pesticides, hippocampus, neurodegeneration,” and “pesticides, mitochondria, metabolism, brain” were used in different combinations in order to obtain studies showing alterations in different metabolic pathways. Studies conducted between 1970 and 2022, including both in vivo and in vitro studies, were considered. Only studies reporting changes in the metabolomic profile of the brain or metabolites affecting the brain were included. The web-based reviewing tool Rayyan (Ouzzani et al. 2016) was used to import and screen all the search results as per our inclusion criteria. A total of 42 studies that looked into the metabolomic changes induced by pesticides in different experimental models were found and included in the study (Fig. 1). Furthermore, we looked for studies where these metabolic changes are associated with neurodegeneration.
Fig. 1.
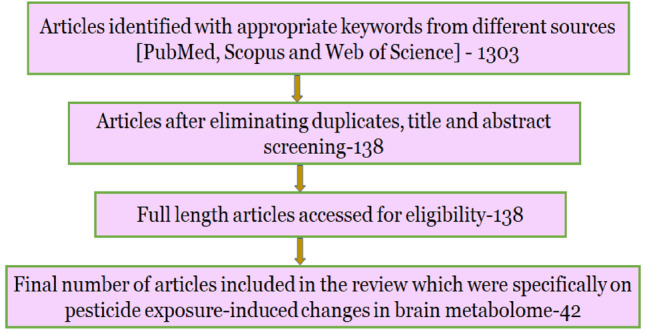
Flowchart depicting the literature search strategy for the review
Specific Metabolomic Changes
Amino Acid Metabolism: Tryptophan Metabolism
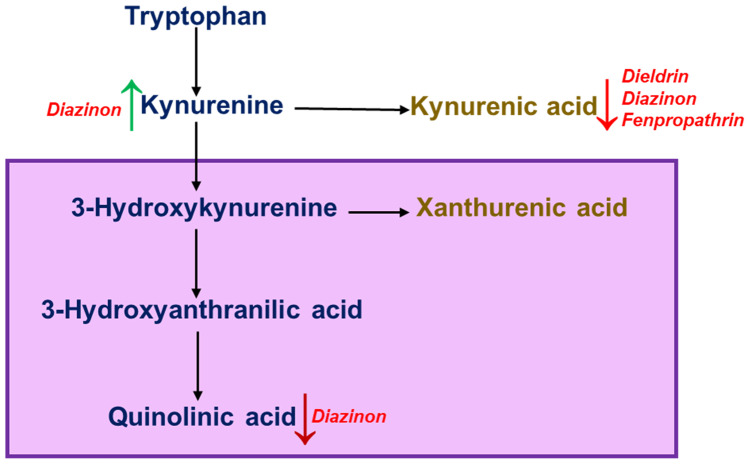
In Tryptophan (TRT) metabolism, enzymes tryptophan 2,3-dioxygenase and the indoleamine 2,3-dioxygenase, metabolizes L-tryptophan into its metabolite L-kynurenine (KYN) (Vamos et al. 2009). KYN is further metabolized into either kynurenic acid (KYNA) or quinolinic acid (QUINA) (Sas et al. 2007; Zádori et al. 2009). KYNA binds to excitatory amino acid receptors such as N-methyl-D-aspartate (NMDA) receptors, kainite receptors, quisqualate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Prescott et al. 2006; Rózsa et al. 2008). NMDA receptors are induced by QUINA, causing glutamate excitotoxicity and inhibiting glutamate reuptake (Tavares et al. 2002). Lipid peroxidation and free radical production are promoted by QUINA, promoting neurotoxicity (Rios and Santamaria 1991; Behan et al. 1999). A study conducted on 22 PD patients and 11 age-matched controls displayed a higher kynurenine/tryptophan ratio (kyn/trp ratio), indicating that altered kynurenine pathway affecting TRT metabolism plays a role in PD (Widner et al. 2002).
Pyrethroids are a class of pesticides that cause modulation of sodium channel activity. When adult male Wistar rats were exposed to deltamethrin and fenpropathrin, the metabolite KYNA production was decreased by 31% (Zielińska et al. 2005). On dosage of mice with diazinon, plasma metabolites indicated that the levels of L-tryptophan metabolites were altered (Seifert and Pewnim 1992). As more than 40% of the brain kynurenine originates from the circulatory route (Gál and Sherman 1978), the changes in the levels affect the biosynthesis of the metabolites KYNA and QUINA (Seifert and Pewnim 1992).
Comparing cerebrospinal fluid (CSF) levels of tryptophan in PD patients vs. healthy controls, it was determined that tryptophan levels were severely reduced in PD patients (Trupp et al. 2014). PD patients’ CSF and brain tissue showed reduced KYNA synthesis in the kynurenine pathway (Ogawa et al. 1992). Pyrethroids inhibit KYNA synthesis and tryptophan metabolism, leading to de novo generation of nicotinamide adenine dinucleotide coenzyme (NAD+), leading to 3-hydroxykynurenine synthesis. Studies on PD indicate that increased 3-hydroxykynurenine levels caused excitotoxicity and increased oxidative stress (Zinger et al. 2011; LeWitt et al. 2013), which are usually countered by the metabolite KYNA (Szabó et al. 2011). A metabolomics study using plasma and CSF from 20 PD patients detected reduced levels of tryptophan 3-hydroxyisovaleric acid compared to the control group leading to increased 3-hydroxykynurenine synthesis (Trupp et al. 2014). Altered tryptophan metabolism is thus a connecting link between pesticide-induced changes in metabolism and neurodegeneration. The effect of various pesticides on quinolinic acid and kynurenic acid levels is depicted in Fig. 2.
Fig. 2.
Alterations in quinolinic acid and kynurenic acid levels due to pesticide exposure
Metabolism of Other Amino Acids
Apart from tryptophan metabolism, pesticides also drastically affect metabolism of other amino acids. Amino acids are building blocks for several other metabolic pathways, including neurotransmitter synthesis as well as purine and pyrimidine synthesis, and thus alterations in their levels can impair other necessary pathways within the brain (Rose 2019). Exposure to p,p’-dichloro-diphenyl-trichloroethane (DDE) led to an increase in the glutamine levels and metabolism of glutamate, aspartate, and alanine in the brain (Rodríguez-Moro et al. 2019). This pathway is the major regulator of glutamate levels and the maintenance of the homeostasis of glutamate levels through the subsequent conversion into γ-aminobutyrate (GABA), one of the main inhibitory neurotransmitters (Pardo et al. 2011). The increased levels of glutamine could be to deal with the excitotoxicity effects of the pesticide. Increased glutamine also finds its use in the purine and pyrimidine biosynthesis, Krebs, and urea cycle (Cruzat et al. 2018). Furthermore, proline, which has a prominent role in scavenging reactive oxygen species (Liang et al. 2013), was found to increase, which may indicate oxidative stress in the cell (Rodríguez-Moro et al. 2019). A group of pregnant rats exposed to a mixture of pesticides showed decreased levels of amino acids like glutamine, serine, lysine, and ethanolamine in the brain, indicating altered the amino acid metabolites (Bonvallot et al. 2018). Chlorfenapyr-treated zebrafish brain metabolome revealed a significant decrease in the metabolites like lysine, alanine, tyrosine, leucine, phenylalanine, and valine, while 7-methylxanthine and taurine were shown to be upregulated (Chen et al. 2021). Dichlorvos-poisoned broilers showed variations in amino acids like gamma-glutamylcysteine, glutathione disulfide, and dipeptide compound (Huang et al. 2022). In Drosophila melanogaster too, paraquat-induced reduced GABA levels with several metabolites altered in amino acid and pentose phosphate metabolism (Shukla et al. 2016).
The changes in the amino acid levels correlate with neurodegenerative conditions wherein patients of Alzheimer’s exhibited reduced levels of methionine and tryptophan along with the reduced ratio of plasma taurine with methionine and serine plasma product levels (TSM ratio); and reduced plasma tyrosine and large neutral amino acids ratio (LNAA) (Fekkes et al. 1998). Significant levels of reduction were also noted in the CSF/plasma ratio of amino acids like glutamine, alanine, phenylalanine, and valine, giving a clear indication of amino acid metabolism disruption in neurodegenerative conditions (Basun et al. 1990). Serum profiles of PD patients also displayed changes in the levels of alanine, arginine, phenylalanine, and threonine in different severity stages (Figura et al. 2018), indicating the importance of amino acid in neurodegenerative diseases.
Glutathione Metabolism Induces Oxidative Stress
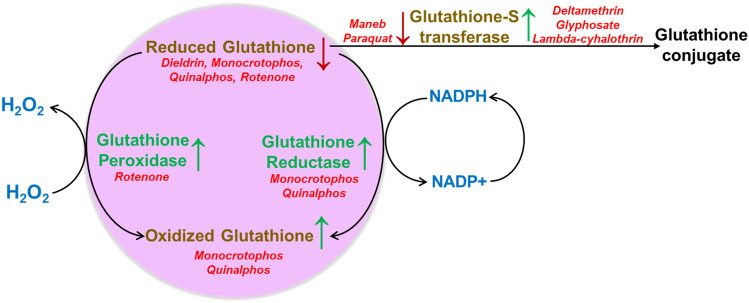
Glutathione S-transferase (GST) and reduced glutathione (GSH) systems work together to maintain redox homeostasis. In the event of increased free radicals, GST dimerizes to bind and interact with GSH and reduces the level of free radicals (Coles et al. 1988). Glutathione oxidizes to glutathione disulfide while reducing H2O2, thus reducing oxidative stress (Mishra and Srivastava 2013). An increased rate of glutathione biosynthesis is inversely correlated with decreased oxidative stress (Smeyne and Smeyne 2013), which also signals the need to remove reactive oxidative species (ROS). In neurons, approximately 85 to 90% of cellular oxygen is consumed by the mitochondria to produce energy as adenosine triphosphate molecules (ATP), resulting in the formation of ROS. Oxidative stress has been associated with PD as indicated by elevated oxidation end-products (Smeyne and Smeyne 2013).
Paraquat is known to cause Parkinson-like symptoms via oxidative and nitrosative stress (Peng et al. 2004; Shukla et al. 2014). The three pesticides, deltamethrin, glyphosate, and lambda-cyhalothrin, showed decreased GST activity (Diken et al. 2017). When male Swiss albino mice were treated with maneb and paraquat at a dose of 30 mg/kg and 10 mg/kg, their brain samples showed increased GST levels (Singhal et al. 2011). GST worked to counteract the oxidative stress caused by maneb and paraquat treatment in the nigrostriatal tissues by increasing GST levels (Patel et al. 2006). GST binding to GSH is affected due to maneb, as it mimics the properties of GSH, but due to the structural and chemical differences between maneb and GSH the normal fuctioning is inhibited (Anderson et al. 2021). Diminished GST activity in CSF (Mazzetti et al. 2015) and brain specimens (Lovell et al. 1998) has been observed in AD patients.
A group of pregnant rats exposed to a mixture of pesticides simulating the exposure scenario in Brittany in 2004 (cropland and vegetable and fruit contamination) showed decreased levels of several metabolites, including higher levels of oxidized glutathione (Bonvallot et al. 2018). Rotenone administration in Sprague–Dawley male rats showed decreased GSH levels in the cortex and midbrain (Khurana and Gajbhiye 2013). Dieldrin administration showed reduced levels of GSH in specimen striatal tissue and an increase in oxidative damage following exposure in C57BL/6 J mice (Hatcher et al. 2007). Decreasing levels of GSH in the erythrocytes were observed in ALS patients (Babu et al. 2008). Interestingly, proton magnetic resonance spectroscopy in the motor cortex of ALS patients compared with healthy controls showed decreased levels of GSH (Weiduschat et al. 2014). GSH levels were reduced in studies conducted in the hippocampus and cerebral cortex of Kunming mice, which showed neurodegeneration and cognitive decline (Li et al. 2014). A study conducted in C57BL/6 mice also showed similar results in the hippocampus cerebral cortex in neurodegenerative conditions (Jhoo et al. 2004). Male Wistar albino rats treated with monocrotophos and quinalphos caused a reduction in the levels of GSH (Mishra and Srivastava 2013). In contrast, an increase in the oxidized glutathione levels (GSSG) was observed in the brain specimens (Mishra and Srivastava 2013). Decreased GSH levels are observed in neurodegeneration in patients’ CSF and substantia nigra (Sian et al. 1994; LeWitt et al. 2013). In the early stages of PD, glutathione levels are elevated, suggesting an attempt to protect the brain against the mounting oxidative stress (Rae 2014). Subsequently, ROS accumulation induced by pesticide exposure can lead to a decrease in glutathione synthesis, causing neurodegeneration.
The γ-glutamyl cycle involves 5-oxoproline and is associated with glutathione metabolism and oxidative stress (Cassol et al. 2014). Metabolic profiling of human saliva and urine samples from fifty-two pesticide sprayers predominantly exposed to various pesticides, including profenofos, cypermethrin, endosulfan, kilthion, and pendimethalin, showed upregulation in the metabolite 5-oxoproline (Ch et al. 2019). Increased levels of the metabolites 5-oxoproline were detected in plasma samples of 20 PD patients compared to the control (Trupp et al. 2014). A study investigating the CSF samples of 31 patients with PD revealed an increase in 5-oxoproline levels during the early disease progress (Willkommen et al. 2018).
Male CFT-Swiss mice, when treated with rotenone, showed an increase in the levels of glutathione peroxidase (GPx) in both the hippocampus and striatum tissue samples (Gokul and Muralidhara 2014). Glutathione reductase (GR), an important antioxidant, showed reduced levels and enzyme activity in the brain specimens of mice treated with a combination of both monocrotophos and quinalphos (Mishra and Srivastava 2013). GPx and GR levels in the blood samples of 50 AD subjects showed statistically lower levels of the metabolites involved in glutathione metabolism (Casado et al. 2008), thus showing that a decrease in the antioxidant enzymes increases oxidative stress leading to neurodegeneration. Hence, oxidative stress can be the common pathological feature between pesticide-induced metabolic rewiring and neurodegeneration. The effect of various pesticides on glutathione metabolism is depicted in Fig. 3.
Fig. 3.
Alterations in the glutathione and glutathione S-transferase levels due to pesticide exposure
Dopamine Metabolism
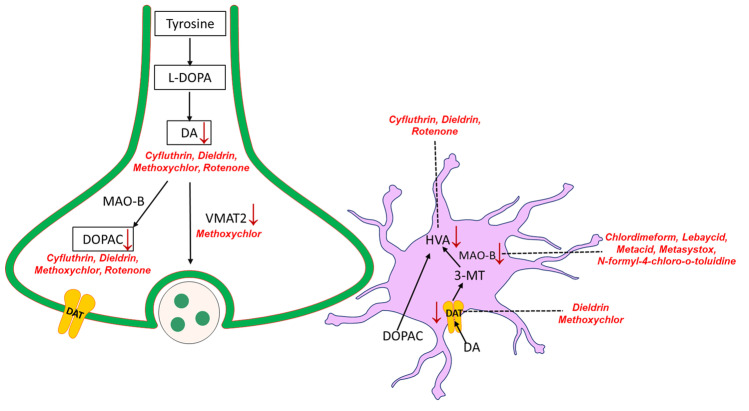
Tyrosine is an amino acid that produces levodopa (L-DOPA). Formamidine pesticides such as chlordimeform (CDM) and their metabolites have been implicated in the inhibition of monoamine oxidase (MAO) (Beeman and Matsumura 1973). MAO catalyzes the oxidative deamination of neuroactive monoamines like dopamine (DA), serotonin, melatonin, and norepinephrine in the brain (Cho et al. 2021). Inhibition of MAO by CDM is reversible, but its metabolite, N-formyl-4-chloro-o-toluidine (CT), is more potent against MOA (Hollingworth et al. 1979). A study by Hirata and Nagatsu showed that 1 µM rotenone dosage decreased the levels of dopamine (DA) and dopamine metabolites, dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and noradrenaline (NA) (Hirata and Nagatsu 2005). A study found that the reduced levels of HVA may be related to neurodegeneration and thus a factor in PD (Scatton et al. 1983). Interestingly, organophosphate pesticides such as lebaycid, metacid, and metasystox inhibited MOA and increased catecholamine concentration (Nag and Nandi 1987). Due to affected dopamine metabolism, striatal and cortical HVA levels were reduced. DA is involved in the neuroprotection of dopaminergic neurons, and lower DA levels are involved in neurodegeneration (Segura-Aguilar et al. 2014). The overall DA metabolite level reduction supports the conclusion of DA deficiency in dopaminergic innervation receiving regions (Scatton et al. 1983). The substantia nigra pars compacta displayed reduced dopamine levels in PD patients confirming metabolomic changes in dopamine metabolism leading to neurodegeneration (Gröger et al. 2014).
Methoxychlor is a synthetically produced organochloride insecticide, and its role in dopamine metabolism alteration was assessed in striatal samples of female CD1 mice where levels of DA, DOPAC, dopamine transporter (DAT), and vesicular monoamine transporter 2 (VMAT2) were reduced (Schuh et al. 2009). Dieldrin treatment in C57BL/6J mice showed decreased dopamine metabolites DA, DAT, DOPAC, and HVA in the mouse cortical tissue specimens (Hatcher et al. 2007). In male Wistar rats, administration of the pyrethroid insecticide cyfluthrin showed a decrease in DA, DOPAC, and HVA metabolites (Rodríguez et al. 2016). Paraquat induced PD Drosophila model showed reduced DA and increased DOPAC level (Chaudhuri et al. 2007; Shukla et al. 2014). Furthermore, the reduction in DOPAC levels was also observed in PD patients, indicating their role in the neurodegenerative disorder (Eldrup et al. 1995). Hence, altered metabolites in dopamine metabolism may be a common node between pesticide altered metabolism and neurodegeneration. The effect of various pesticides on dopamine metabolism is depicted in Fig. 4.
Fig. 4.
Changes in different metabolite levels involved in dopamine metabolism due to pesticide exposure
Mitochondrial Dysfunction
Mitochondria are essential in providing cellular energy generated through oxidative phosphorylation. Apart from ROS, metabolic dysregulation has been implicated in causing mitochondrial dysfunction (Moon and Paek 2015). Over the years, studies have shown significant involvement of mitochondrial dysfunction during the pathogenesis of PD (Anandhan et al. 2017b). The intact mitochondria produces ATP and aids in ROS clearance (Moon and Paek 2015). Zebrafish, on exposure to organochlorine pesticides like endosulfan and tetrachlorodibenzo-p-dioxin (TCDD) showed alteration in the tricarboxylic acid pathway metabolites by affecting the oxygen consumption rate (Lee et al. 2020). On exposure to malathion, activity of the enzymes glycogen phosphorylase (GP), phosphoglucomutase (PGM), and hexokinase (HK) was increased, which caused a significant increase in the lactate levels with no changes in the pyruvate levels, thus indicating that it affected cellular respiration in the brain leading to neurodegeneration (Matin et al. 1990). Furthermore, another study supported this observation by showing an increase in HK activity in the brain mitochondria exposed to malathion (Azadbar et al. 2009). Mitochondrial dysfunction, along with changes in other pathways, including creatine phosphate biosynthesis and pyruvate fermentation to lactate, was affected in brain homogenates of mice model mimicking the effects of Gulf War agents (pyridostigmine bromide and permethrin) (Abdullah et al. 2016).
A mass difference enrichment analysis compared 243 masses of control and PD samples and found that metabolites like pyruvate, 2-ketosuccinate, and α-ketoglutarate involved in the TCA cycle were over-represented, causing metabolic dysregulation in PD patients (Willkommen et al. 2018). A study by González-Domínguez et al. showed an increase in the α-ketoglutarate levels causing the alteration of metabolic pathway leading to AD (González-Domínguez et al. 2015). Furthermore, elevated malate levels in plasma samples of PD patients were reported (Trupp et al. 2014). Wu et al. found increased citrate concentrations in CSF samples of 22 PD patients (Wu et al. 2016). However, conflicting results regarding decreased levels of plasma TCA cycle metabolites (citrate, isocitrate, malate, succinate), which were correlated to alteration of pyruvate dehydrogenase activity, were highlighted in a study (Ahmed et al. 2009). Thus, mitochondrial dysfunction due to pesticides is also found in neurodegenerative diseases.
Energy Metabolism
PD model created by exposure to paraquat was analyzed for any changes in the energy metabolism in dopaminergic cells. The study indicated an increase in glucose, myoinositol, and sedoheptulose concentrations (Lei et al. 2014). A community-based case–control analysis by high-resolution metabolomic assessment was performed for three classes of pesticides indicating that higher organophosphate and organochlorine exposure altered glycolysis and gluconeogenesis metabolites (Yan et al. 2021). Paraquat-treated black mice showed metabolomic alterations in the midbrain and the striatum region. Paraquat decreased metabolites like alanine and lactate within the glycolysis cycle and glutamate within the TCA cycle led to increased citrate levels, increased pAMPK and acetyl-CoA carboxylase (pACC) substrate levels, all of which led to subsequent dopaminergic cell death (Anandhan et al. 2017a). Paraquat also induced changes in energy and pyruvate metabolism in Drosophila melanogaster (Shukla et al. 2016).
Metabolomic analysis in mice model mimicking the effects of Gulf War agents showed that several metabolites involved in Krebs cycle like citric acid, malic acid, fumaric acid, succinic acid, and isocitric acid were significantly lowered with respect to control. Furthermore, β-hydroxybutyrate, lactate, glycerol-3-phosphate, and glyceric acid 3-phosphate levels were also lowered (Abdullah et al. 2016). Brain homogenates in dichlorvos-poisoned broilers showed alterations in energy metabolites like acetylcarnitine, dihydroxyacetone phosphate, and glucose-6-phosphate (Huang et al. 2022). Pregnant rats treated with a mixture of pesticides showed decreased levels of adenosine di-phosphate/adenosine monophosphate (ADP/AMP), ATP, lactate, succinate, and aspartate affecting the TCA cycle and energy production (Bonvallot et al. 2018).
On the other hand, metabolites of energy metabolism in CSF samples of 31 PD patients indicated a decrease in the sedoheptulose and an increase in D-glucose-6-sulfate and α-mannosylglycerate (Willkommen et al. 2018). Fructose and mannose are present in increased levels in the CSF of PD patients, and both fructose and mannose metabolisms are involved in the synthesis of α-mannosylglycerate (Trezzi et al. 2017). Glycolysis in PD patients is affected due to the altered level of these metabolites linked to glycolysis (Izumi and Zorumski 2009). Such alterations are influenced by oxidative stress to suppress oxidative phosphorylation in mitochondria (Mazzio and Soliman 2003). A study by Ahmed et al. found that sorbitol concentrations were increased by altering metabolites of the fructose and mannose pathway (Ahmed et al. 2009). Michell et al. observed increased levels of different monosaccharides by analyzing serum metabolites (Michell et al. 2008). Taken together, the loss of energy metabolism leads to persistent dysregulation of neuronal pathways and may lead to neurodegeneration.
Fatty Acids and Lipid Metabolism
Lipid metabolism is necessary to activate receptors, signal transduction and, modulation for maintaining several biological functions, including cognition (Yadav and Tiwari 2014). Studies on lipid metabolism in neurodegenerative diseases have shown decreased brain cholesterol, galactosylceramide, and sulfatide levels (Colombelli et al. 2015). Rotenone treatment in mice showed alterations in fatty acids linoleic acid, arachidonic acid, and docosahexaenoic acid levels (Tyurina et al. 2015). Furthermore, rotenone also acted with cardiolipins (CLs), increasing CL oxidation metabolites (Tyurina et al. 2015). Polyunsaturated fatty acids CLs were also depleted in rats on exposure to rotenone (Tyurina et al. 2015). Chlorophenotane (DDT) administration in rhesus monkeys also showed alteration in the lipid metabolism metabolites (Sanyal et al. 1986). Another pesticide, paraquat, also altered fatty acid ener metabolism in Drosophila melanogaster (Shukla et al. 2016). Furthermore, in brain homogenates of diisopropylfluorophosphate-treated Sprague–Dawley rats, a significant increase in the pro-inflammatory lipid mediators and a small class of anti-inflammatory lipid mediators were seen, indicating the neuroinflammatory response to pesticides (Yang et al. 2019). Metabolites like phosphocholine and glycerophosphocholine of the lipid metabolism were altered in pregnant rats treated with a combination of pesticides (Bonvallot et al. 2018). Metabolomic profiles of the brain of bifenthrin-fed juvenile steelhead trout also showed alteration in lipid metabolism with reduced levels of related metabolites docosahexaenoic acid (DHA) and acetyl-L-carnitine (ALC) (Magnuson et al. 2020).
Increased concentrations of arachidonic acid (ARA), decanoic acid, dihomo-γ-linolenic acid (DGLA), quinic acid, valerenic acid, and 10-hydroxydecanoic acid were noted in PD samples (Willkommen et al. 2018). The increase in ARA and DGLA was correlated to neuroinflammation and oxidative stress, and due to the inflammatory process, the release of ARA from membranes was higher. DGLA is a PUFA that can form pro-inflammatory ARA and is released before the anti-inflammatory compounds (Bazinet and Laye 2014). Increased ARA metabolism due to oxidative stress and neuroinflammation caused neurodegeneration (Bosetti 2007). PUFA depletion has also been indicated to cause mitochondrial dysfunction contributing to PD pathogenesis (Tyurina et al. 2015). Furthermore, the role of the fatty acid metabolism in CNS disorders and the diagnostic and therapeutic potential of metabolomics data were reviewed by Bogie et al. (Bogie et al. 2020). Many pesticides affect more than one metabolic pathway; for example, imidacloprid altered six metabolic pathways in mice hippocampus, including lipid metabolism, amino acid metabolism, nucleotide metabolism, carbohydrate metabolism, energy metabolism, and metabolism of cofactors and vitamins (Zheng et al. 2020).
The summary of various pesticides and the selected metabolic pathways affected by their exposure is listed in Table 1.
Table 1.
Studies on the pesticide-induced changes in the metabolites in the nervous tissue
| Pesticides | Model/cells—sample type | Pathway affected | Changes induced | References |
|---|---|---|---|---|
| In vitro studies | ||||
| Paraquat | SK-N-SH cell line | Pentose phosphate pathway | Glucose↑, myoinositol↑, sedoheptulose↑ | Lei et al. (2014) |
| In vivo studies | ||||
| Paraquat | Oregon R + (wild-type) Drosophila flies | Fatty acid and amino acid metabolism |
Glycerolipid, inositol phosphate, glycerophospholipid, fatty acid ↑ Proline, arginine, valine, aspartate, alanine, glutamate, leucine, isoleucine ↓ |
Shukla et al. (2016) |
| Endosulfan and tetrachlorodibenzo-p-dioxin | Zebrafish | Tricarboxylic acid pathway | OCR↓ | Lee et al. (2020) |
| Chlorfenapyr | Zebrafish | Amino acid metabolism |
Alanine, tyrosine, lysine, leucine, phenylalanine, and valine ↑ 7-Methylxanthine and taurine ↓ |
Chen et al. (2021) |
| Dieldrin | C57BL/6 J mice striatal tissue | Glutathione pathway | GSH↓ | Hatcher et al. (2007) |
| Dieldrin | C57BL/6 J mice striatal tissue | Dopamine pathway | DA↓, DOPAC↓, DAT↓, HVA↓ | Hatcher et al. (2007) |
| Maneb | Male Swiss albino mice brain samples | Glutathione pathway | GST↑ | Singhal et al. (2011) |
| Methoxychlor | female CD1 mice striatal samples | Dopamine pathway | DA↓, DOPAC↓, DAT↓, VMAT2↓ | Schuh et al. (2009) |
| Rotenone | CFT-Swiss mice hippocampus and striatum samples | Glutathione pathway | GPx↑ | Gokul and Muralidhara (2014) |
| N-Formyl-4-chloro-o-toluidine | Male Swiss white mice | Dopamine pathway | MAO↓ | Hollingworth et al. (1979) |
| Paraquat | Male Swiss albino mice brain samples | Glutathione pathway | GST↑ | Singhal et al. (2011) |
| Paraquat | C57BL/6 J mice | Glycolysis and TCA cycle |
Citrate, pAMPK, and acetyl-CoA carboxylase ↑ Alanine, lactate and glutamate ↓ |
Anandhan et al. (2017a) |
| Permethrin | C57BL6 mice | TCA cycle, Glycolysis and energy cycle |
Citric acid, malic acid, fumaric acid, succinic acid, and isocitric acid ↓ β-hydroxybutyrate, lactate, glycerol-3-phosphate, and glyceric acid 3-phosphate ↓ |
Abdullah et al. (2016) |
| Cyfluthrin | Male Wistar rats | Dopamine pathway | DA↓, DOPAC↓, HVA↓ | Rodríguez et al. (2016) |
| Deltamethrin | Adult male Wistar rat cortical tissue | L-Kynurenine pathway | KYNA↓ | Zielińska et al. (2005) |
| Fenpropathrin | Adult male Wistar rat cortical tissue | L-Kynurenine pathway | KYNA↓ | Zielińska et al. (2005) |
| Mixture of acetochlor, bromoxynil, carbofuran, chlormequat, ethephon, fenpropimorph, glyphosate, imidacloprid | Pregnant Wistar rats | TCA cycle, lipid and amino acid metabolism |
Lysine, n-acetylaspartate, inosine, ethanolamine, and oxidized glutathione ↑ Lipids, aspartate, lactate, glutamine, succinate, serine, phosphocholine, glycerophosphocholine, urine, ADP/AMP, and ATP ↓ |
Bonvallot et al. (2018) |
| Monocrotophos | Wistar albino male adult rats brain samples | Glutathione pathway | GSH↓, GSSG↑ | Mishra and Srivastava (2013) |
| Monocrotophos and quinalphos | Wistar albino male adult rats brain samples | Glutathione pathway | GR↑ | Mishra and Srivastava (2013) |
| Quinalphos | Wistar albino male adult rats brain samples | Glutathione pathway | GSH↓, GSSG↑ | Mishra and Srivastava (2013) |
| Rotenone | Male Wistar rat striatal tissue | Dopamine pathway | DA↓, DOPAC↓, HVA↓, NA↓ | Hirata and Nagatsu (2005) |
| Lebaycid, metacid, and metasystox | Male albino rat brain mitochondria | Dopamine pathway | MAO↓ | Nag and Nandi (1987) |
| Malathion | Adult female albino rat brain | Mitochondrial dysfunction | Lactate↑ | Matin et al. (1990) |
| Diazinon | Swiss-Webster male mice plasma | L-Kynurenine pathway | QA↓, KYNA↓, KYN↑ | Seifert and Pewnim (1992) |
| Rotenone | Male Lewis rats | Fatty acid metabolism | Linoleic acid↓, arachidonic acid↓, docosahexaenoic acid↓ | Tyurina et al. (2015) |
| Rotenone | Male Lewis rats | Mitochondrial signaling pathway | PUFA CLs ↓ | Tyurina et al. (2015) |
| Rotenone | Sprague–Dawley male rat cortex and midbrain | Glutathione pathway | GSH↓ | Khurana and Gajbhiye (2013) |
| Dichlorvos | Broilers | Energy and amino acid and nucleic acid metabolism |
Dihydroxyacetone phosphate, glucose 6-phosphate ↑, acetylcarnitine ↓ Gamma-glutamylcysteine, glutathione disulfide, dipeptide compound ↑ Uridine ↓ Inosine 5′-monophosphate, hypoxanthine, uridine 5′-monophosphate ↑ |
Huang et al. (2022) |
| Human studies | ||||
| Deltamethrin | Human blood | Glutathione pathway | GST↓ | Diken et al. (2017) |
| Glyphosate | Human blood | Glutathione pathway | GST↓ | Diken et al. (2017) |
| Lambda-cyhalothrin | Human blood | Glutathione pathway | GST↓ | Diken et al. (2017) |
| Cypermethrin, endosulfan, kildor, kilthion, pendimethalin, and profenofos | Pesticide sprayers saliva and urine samples | Glutathione pathway | 5-Oxoproline↑ | Ch et al. (2019) |
DA dopamine, DAT dopamine transporter, DOPAC dihydroxyphenylacetic acid, GSH reduced glutathione, GR glutathione reductase, GSSG oxidized glutathione, GST glutathione S-transferase, HVA homovanillic acid, KYN kynurenine, KYNA kynurenic acid, MAO monoamine oxidase, NA noradrenaline, OCR oxygen consumption rate, PUFA CLs polyunsaturated fatty acids cardiolipins, QA quinolinic acid, SK-N-SH human dopaminergic neuroblastoma cell line, VMAT2 vesicular monoamine transporter 2
Discussion and Conclusion
The review summarizes the epidemiological and experimental studies looking for the correlation between metabolites altered by pesticide exposure and their link with neurodegenerative disorders. Reports indicate that less than 10% of PD cases are purely genetics (Simon et al. 2020).However, environmental factors play a significant role, which acts independently or combined with genetic factors (Caudle et al. 2011). Furthermore, aging is detrimental to neurodegenerative disorders due to increased and accumulated toxicant exposure which can aggravate symptoms of neurodegeneration. The epidemiological studies identify pesticide exposure as an essential cause of neurodegenerative diseases (Freire and Koifman 2012; Moretto and Colosio 2013). However, the mechanisms and the metabolites involved in pesticide-induced neurodegeneration are poorly explored.
Neurodegenerative disorders have previously been associated with metabolic abnormalities. Hence, the metabolomic approach is a promising tool that helps identify abnormal metabolite levels and pathways associated with major neurodegenerative diseases (Cai et al. 2012). Therefore, metabolomic studies will be helpful in identifying the abnormal levels of metabolites that are indicative of specific underlying disease processes and aid in the management of neurodegenerative diseases. In addition, the differentially altered metabolites can then act as biomarkers for the early detection of neurodegenerative disorders and could help counter damage caused (Patti et al. 2012).
Inflammation-related pathways like tryptophan catabolism, arachidonic acid metabolism, and histidine metabolism, when altered by different pesticides, lead to the accumulation of neurotoxic products driving towards neurodegeneration. Pesticide exposure-induced oxidative stress can cause mitochondrial dysfunction wherein the use of organochlorines and organophosphates has shown alteration of mitochondrial energy metabolism pathways. Mitochondrial dysfunction is often accompanied by oxidative stress and impaired cellular respiration and also leads to apoptosis/autophagy, which is often some of the symptoms of neurodegenerative disorders. Samples from the brain tissue, CSF, serum, blood, saliva, and urine samples all have shown changes in the various metabolite levels. The role of altered dopamine metabolism in Parkinson’s has been well established. Organochlorine pesticides have been shown to affect dopamine metabolism, which could hamper neurotransmission and dopaminergic neuronal survival as a consequence of pesticide resultant oxidative stress. Furthermore, pesticides alter glutathione synthesis and metabolism, further exacerbating the production of reactive oxygen species, thus displaying their role in inducing neurodegenerative disorders. The brain and the activities of the resident neurons are energy driven, meaning they depend on mitochondria for a continuous supply of energy for their abundant activities. The majority of the metabolomic studies have shown that along with mitochondrial dysfunction, the TCA cycle, which is the source of ATP for the cellular activities of these neurons, is impaired due to variations in the metabolite levels within the cycle. Studies show that glycolysis is similarly altered in PD patients, which exhibits another parallel between the pesticide effects and neurodegenerative symptoms. Studies also reveal the alteration of several pro- and anti-inflammatory mediators upon pesticide exposure that are recruited as part of the immediate immune response of the cell. Most importantly, amino acid metabolism, which serves a key role as precursors or intermediates in several overlapping metabolic pathways, like purine and pyrimidine synthesis, neurotransmitter metabolism, citric acid cycle, and urea biosynthesis, is also affected. Altered levels of various amino acids serve as essential early biomarkers for various diseases like Alzheimer’s and PD, although when it comes to human studies, there are several cofactors like the diet that may affect the levels of the metabolites within an individual. However, these pathway changes often culminate in neuronal death, a primary factor for the onset of neurodegenerative diseases.
Therefore, this review summarizes the molecular pathways that are vulnerable to chronic pesticide toxicity to understand their physiology, leading to better prediction of pesticide-related health outcomes. Metabolomics data focusing on the nervous system are still scarce though the available evidence supports the existence of specific mechanistic associations between neuronal damage and pesticide exposure leading to neurodegeneration. However, it is also important to validate how the changes in the individual metabolite associated with a particular neurodegenerative phenotype have any measurable influence on the phenotype. A further combined approach of different omics will help better understand the physiological and pathologic processes involved in the human brain.
Acknowledgements
The authors would like to thank Manipal School of Life Sciences, Manipal Academy of Higher Education (MAHE), Manipal, India, for support, and TIFAC-CORE, DST, Govt. of India, and BUILDER, DBT, Govt. of India, for infrastructure facilities. Rekha would like to thank MAHE, Manipal, for the Dr. T.M.A Pai fellowship and KSTePS, Govt. of India, for the scholarship. Kamalesh DM is grateful to Manipal Academy of Higher Education for the intramural grant.
Author Contribution
KDM had the idea for the article; JAR performed the literature search and data analysis, and drafted the manuscript; HSD, KDM, MBJ, and RKN edited and critically revised the work.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. This work was supported by the Science and Engineering Research Board (grant no: ECR/2017/001239/LS), Government of India. RKN thanks Dr. T.M.A Pai PhD Scholarship and Karnataka Science and Technology Promotion Society (KSTePS) for the DST-PhD fellowship.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdullah L, Evans JE, Joshi U, et al. Translational potential of long-term decreases in mitochondrial lipids in a mouse model of Gulf War Illness. Toxicology. 2016;372:22–33. doi: 10.1016/j.tox.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Ahmed SS, et al. Metabolic profiling of Parkinson’s disease: evidence of biomarker from gene expression analysis and rapid neural network detection. J Biomed Sci. 2009;16:63. doi: 10.1186/1423-0127-16-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandhan A, Lei S, Levytskyy R, et al. Glucose metabolism and AMPK signaling regulate dopaminergic cell death induced by gene (α-synuclein)-environment (paraquat) interactions. Mol Neurobiol. 2017;54:3825–3842. doi: 10.1007/s12035-016-9906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandhan A, et al. Metabolic dysfunction in Parkinson’s disease: bioenergetics, redox homeostasis and central carbon metabolism. Brain Res Bull. 2017;133:12–30. doi: 10.1016/j.brainresbull.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CC, et al. Maneb alters central carbon metabolism and thiol redox status in a toxicant model of Parkinson’s disease. Free Radic Biol Med. 2021;162:65–76. doi: 10.1016/j.freeradbiomed.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadbar M, et al. Interaction of phosphodiesterase 5 inhibitor with malathion on rat brain mitochondrial-bound hexokinase activity. Pesticide Biochem Physiol. 2009;95:121–125. doi: 10.1016/j.pestbp.2009.08.001. [DOI] [Google Scholar]
- Babu GN, et al. Oxidant-antioxidant imbalance in the erythrocytes of sporadic amyotrophic lateral sclerosis patients correlates with the progression of disease. Neurochem Int. 2008;52:1284–1289. doi: 10.1016/j.neuint.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Basun H, Forssell LG, Almkvist O, et al (1990) Amino acid concentrations in cerebrospinal fluid and plasma in Alzheimer’s disease and healthy control subjects. Journal of Neural Transmission - Parkinson’s Disease and Dementia Section 1990 2:4 2:295–304. 10.1007/BF02252924 [DOI] [PubMed]
- Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- Beeman RW, Matsumura F. Chlordimeform: a pesticide acting upon amine regulatory mechanisms. Nature. 1973;242:273–274. doi: 10.1038/242273a0. [DOI] [PubMed] [Google Scholar]
- Behan WM, et al. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenyl. Br J Pharmacol. 1999;128:1754–1760. doi: 10.1038/sj.bjp.0702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørling-Poulsen M, et al. Potential developmental neurotoxicity of pesticides used in Europe. Environ Health. 2008;7:50. doi: 10.1186/1476-069X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogie J, et al. Fatty acid metabolism in the progression and resolution of CNS disorders. Adv Drug Deliv Rev. 2020;159:198–213. doi: 10.1016/j.addr.2020.01.004. [DOI] [PubMed] [Google Scholar]
- Bonvallot N, Canlet C, Blas-Y-Estrada F, et al. Metabolome disruption of pregnant rats and their offspring resulting from repeated exposure to a pesticide mixture representative of environmental contamination in Brittany. PLoS ONE. 2018;13:1–21. doi: 10.1371/journal.pone.0198448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem. 2016;139:216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- Bosetti F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J Neurochem. 2007;102:577–586. doi: 10.1111/j.1471-4159.2007.04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, et al. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect. 2005;113:1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai HL, et al. Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naive schizophrenia patients after treatment with risperidone. J Proteome Res. 2012;11:4338–4350. doi: 10.1021/pr300459d. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. Gene-environment interactions in Parkinson’s disease: specific evidence in humans and mammalian models. Neurobiol Dis. 2013;57:38–46. doi: 10.1016/j.nbd.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado A, Encarnación López-Fernández M, Concepción Casado M, de La Torre R (2008) Lipid peroxidation and antioxidant enzyme activities in vascular and Alzheimer dementias. Neurochem Res 33(3):450–8. 10.1007/s11064-007-9453-3 [DOI] [PubMed]
- Cassol E, et al. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014;28:1579–1591. doi: 10.1097/QAD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle WM. Using ‘omics’ to define pathogenesis and biomarkers of Parkinson’s disease. Expert Rev Neurother. 2011;10:925–942. doi: 10.1586/ern.10.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch R, et al. Saliva and urine metabolic profiling reveals altered amino acid and energy metabolism in male farmers exposed to pesticides in Madhya Pradesh State. India Chemosphere. 2019;226:636–644. doi: 10.1016/j.chemosphere.2019.03.157. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O'Donnell JM (2007) Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci : The Official Journal of the Society for Neuroscience 27(10):2457–2467. 10.1523/JNEUROSCI.4239-06.2007 [DOI] [PMC free article] [PubMed]
- Chen X, Zheng J, Teng M, et al. Bioaccumulation, Metabolism and the toxic effects of chlorfenapyr in zebrafish (Danio rerio) J Agric Food Chem. 2021;69:8110–8119. doi: 10.1021/acs.jafc.1c02301. [DOI] [PubMed] [Google Scholar]
- Chin-Chan M, et al. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci. 2015;9:124. doi: 10.3389/fncel.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HU, Kim S, Sim J et al (2021) Redefining differential roles of MAO-A in dopamine degradation and MAO-B in tonic GABA synthesis. Experi Molecul Med 2021 53:7 53:1148–1158. 10.1038/s12276-021-00646-3 [DOI] [PMC free article] [PubMed]
- Coles B, et al. The spontaneous and enzymatic reaction of N-acetyl-p-benzoquinonimine with glutathione: a stopped-flow kinetic study. Arch Biochem Biophys. 1988;264:253–260. doi: 10.1016/0003-9861(88)90592-9. [DOI] [PubMed] [Google Scholar]
- Colombelli C, et al. Defective lipid metabolism in neurodegeneration with brain iron accumulation (NBIA) syndromes: not only a matter of iron. J Inherit Metab Dis. 2015;38:123–136. doi: 10.1007/s10545-014-9770-z. [DOI] [PubMed] [Google Scholar]
- Cruzat V, Rogero MM, Keane KN et al (2018) Glutamine: metabolism and immune function, supplementation and clinical translation Nutrients 10. 10.3390/NU10111564 [DOI] [PMC free article] [PubMed]
- de Pedro-Cuesta J, et al. Comparative incidence of conformational, neurodegenerative disorders. PLoS ONE. 2015;10:e0137342. doi: 10.1371/journal.pone.0137342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diken ME, et al. In vitro effects of some pesticides on glutathione-s transferase activity. Fresenius Environ Bull. 2017;26:408–414. [Google Scholar]
- Eldrup E, et al. CSF and plasma concentrations of free norepinephrine, dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), 3,4-dihydroxyphenylalanine (DOPA), and epinephrine in Parkinson’s disease. Acta Neurol Scand. 1995;92:116–121. doi: 10.1111/j.1600-0404.1995.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Fekkes D, van der Cammen TJM, van Loon CPM, et al. Abnormal amino acid metabolism in patients with early stage Alzheimer dementia. J Neural Transm. 1998;105:287–294. doi: 10.1007/s007020050058. [DOI] [PubMed] [Google Scholar]
- Figura M, Kuśmierska K, Bucior E et al (2018) Serum amino acid profile in patients with Parkinson’s disease PLoS ONE 13. 10.1371/JOURNAL.PONE.0191670 [DOI] [PMC free article] [PubMed]
- Freire C, Koifman S. Pesticide exposure and Parkinson’s disease: epidemiological evidence of association. Neurotoxicology. 2012;33:947–971. doi: 10.1016/j.neuro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Gál EM, Sherman AD. Synthesis and metabolism of L-kynurenine in rat brain. J Neurochem. 1978;30:07–613. doi: 10.1111/j.1471-4159.1978.tb07815.x. [DOI] [PubMed] [Google Scholar]
- Gokul K, Muralidhara, Oral supplements of aqueous extract of tomato seeds alleviate motor abnormality, oxidative impairments and neurotoxicity induced by rotenone in mice: relevance to Parkinson’s disease. Neurochem Res. 2014;39:1382–1394. doi: 10.1007/s11064-014-1323-1. [DOI] [PubMed] [Google Scholar]
- González-Domínguez R, et al. Metabolite profiling for the identification of altered metabolic pathways in Alzheimer’s disease. J Pharm Biomed Anal. 2015;107:75–81. doi: 10.1016/j.jpba.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Gröger A, et al. Dopamine reduction in the substantia nigra of Parkinson’s disease patients confirmed by in vivo magnetic resonance spectroscopic imaging. PLoS ONE. 2014;9:e84081. doi: 10.1371/journal.pone.0084081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JM, et al. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007;204:619–630. doi: 10.1016/j.expneurol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Nagatsu T. Rotenone and CCCP inhibit tyrosine hydroxylation in rat striatal tissue slices. Toxicol. 2005;216:9–14. doi: 10.1016/j.tox.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Hollingworth RM, et al. Mode of action of formamidine pesticides: an evaluation of monoamine oxidase as the target. Chem Biol Interact. 1979;24:35–49. doi: 10.1016/0009-2797(79)90101-7. [DOI] [PubMed] [Google Scholar]
- Huang L, Guo X, Liu P, et al. Correlation between acute brain injury and brain metabonomics in dichlorvos-poisoned broilers. J Hazard Mater. 2022;422:126849. doi: 10.1016/j.jhazmat.2021.126849. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Glial-neuronal interactions underlying fructose utilization in rat hippocampal slices. Neuroscience. 2009;161:847–854. doi: 10.1016/j.neuroscience.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53:26–38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Jhoo JH, et al. Beta-amyloid (1–42)-induced learning and memory deficits in mice: involvement of oxidative burdens in the hippocampus and cerebral cortex. Behav Brain Res. 2004;155:185–196. doi: 10.1016/j.bbr.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Jové M, et al. Metabolomics of human brain aging and age-related neurodegenerative diseases. J Neuropathol Exp Neurol. 2014;73:640–657. doi: 10.1097/NEN.0000000000000091. [DOI] [PubMed] [Google Scholar]
- Keifer MC, Firestone J. Neurotoxicity of Pesticides J Agromedicine. 2007;12:17–25. doi: 10.1300/J096v12n01_03. [DOI] [PubMed] [Google Scholar]
- Khurana N, Gajbhiye A. Ameliorative effect of Sida cordifolia in rotenone induced oxidative stress model of Parkinson’s disease. Neurotoxicology. 2013;39:57–64. doi: 10.1016/j.neuro.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Kori M, et al. Metabolic biomarkers and neurodegeneration: a pathway enrichment analysis of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. OMICS. 2016;20:645–661. doi: 10.1089/omi.2016.0106. [DOI] [PubMed] [Google Scholar]
- Lee H, et al. Differential mitochondrial dysregulation by exposure to individual organochlorine pesticides (OCPs) and their mixture in zebrafish embryos. Environ Pollut. 2020;277:115904. doi: 10.1016/j.envpol.2020.115904. [DOI] [PubMed] [Google Scholar]
- Lei S, et al. Alterations in energy/redox metabolism induced by mitochondrial and environmental toxins: a specific role for glucose-6-phosphate-dehydrogenase and the pentose phosphate pathway in paraquat toxicity. ACS Chem Biol. 2014;9:2032–2048. doi: 10.1021/cb400894a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RG, Bond AE, Johnson DE, Hsu JP (1988) Measurement of atmospheric concentrations of common household pesticides: A pilot study. Environ Monit Assess 10(1):59–73. 10.1007/BF00394257 [DOI] [PubMed]
- LeWitt PA, et al. 3-hydroxykynurenine and other Parkinson’s disease biomarkers discovered by metabolomic analysis. Mov Disord. 2013;28:1653–1660. doi: 10.1002/mds.25555. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Schisantherin A recovers Aβ-induced neurodegeneration with cognitive decline in mice. Physiol Behav. 2014;132:10–16. doi: 10.1016/j.physbeh.2014.04.046. [DOI] [PubMed] [Google Scholar]
- Liang X, Zhang L, Natarajan SK, Becker DF. Proline mechanisms of stress survival. Antioxid Redox Signal. 2013;19:998. doi: 10.1089/ARS.2012.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, et al. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer’s disease. Neurology. 1998;51:1562–1566. doi: 10.1212/wnl.51.6.1562. [DOI] [PubMed] [Google Scholar]
- Magnuson JT, Cryder Z, Andrzejczyk NE, et al. Metabolomic profiles in the brains of juvenile steelhead (Oncorhynchus mykiss) following bifenthrin treatment. Environ Sci Technol. 2020;54:12245–12253. doi: 10.1021/acs.est.0c04847. [DOI] [PubMed] [Google Scholar]
- Maroni M, et al. Risk assessment and management of occupational exposure to pesticides in agriculture. Med Lav. 2006;97:430–437. [PubMed] [Google Scholar]
- Matin MA, et al. Modification of malathion induced neurochemical changes by adrenalectomy in rats. Mol Chem Neuropathol. 1990;13:119–128. doi: 10.1007/BF03159913. [DOI] [PubMed] [Google Scholar]
- Mazzetti AP, et al. Glutathione transferases and neurodegenerative diseases. Neurochem Int. 2015;82:10–18. doi: 10.1016/j.neuint.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Mazzio E, Soliman KF. The role of glycolysis and gluconeogenesis in the cytoprotection of neuroblastoma cells against 1-methyl 4-phenylpyridinium ion toxicity. Neurotoxicology. 2003;24:137–147. doi: 10.1016/s0161-813x(02)00110-9. [DOI] [PubMed] [Google Scholar]
- Michell AW, et al. Metabolomic analysis of urine and serum in Parkinson’s disease. Metabolomics. 2008;4:191–201. doi: 10.1007/s11306-008-0111-9. [DOI] [Google Scholar]
- Mishra V, Srivastava N. Organophosphate pesticides-induced changes in the redox status of rat tissues and protective effects of antioxidant vitamins. Environ Toxicol. 2013;30:472–482. doi: 10.1002/tox.21924. [DOI] [PubMed] [Google Scholar]
- Moon HE, Paek SH. Mitochondrial dysfunction in Parkinson’s disease. Exp Neurobiol. 2015;24:103–116. doi: 10.5607/en.2015.24.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto A, Colosio C. The role of pesticide exposure in the genesis of Parkinson’s disease: epidemiological studies and experimental data. Toxicol. 2013;307:24–34. doi: 10.1016/j.tox.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Nag M, Nandi N. In vitro and in vivo effect of organophosphate pesticides on monoamine oxidase activity in rat brain. Biosci Rep. 1987;7:801–803. doi: 10.1007/BF01116753. [DOI] [PubMed] [Google Scholar]
- Ogawa T, et al. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology. 1992;42:1702–1706. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:1–10. doi: 10.1186/S13643-016-0384-4/FIGURES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo B, Rodrigues TB, Contreras L, et al. Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. J Cereb Blood Flow Metab. 2011;31:90. doi: 10.1038/JCBFM.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, et al. Status of antioxidant defense system and expression of toxicant responsive genes in striatum of maneb- and paraquat-induced Parkinson’s disease phenotype in mouse: mechanism of neurodegeneration. Brain Res. 2006;1081:9–18. doi: 10.1016/j.brainres.2006.01.060. [DOI] [PubMed] [Google Scholar]
- Patti GJ. Innovation: metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012;13:263–269. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, et al. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J Biol Chem. 2004;279:32626–32632. doi: 10.1074/jbc.M404596200. [DOI] [PubMed] [Google Scholar]
- Pihlstrøm L, et al. Genetics of neurodegenerative diseases: an overview. Handb Clin Neurol. 2017;145:309–323. doi: 10.1016/B978-0-12-802395-2.00022-5. [DOI] [PubMed] [Google Scholar]
- Prescott C, et al. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci Lett. 2006;402:108–112. doi: 10.1016/j.neulet.2006.03.051. [DOI] [PubMed] [Google Scholar]
- Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014;39:1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- Rios C, Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- Rodríguez JL, et al. Effects of exposure to pyrethroid cyfluthrin on serotonin and dopamine levels in brain regions of male rats. Environ Res. 2016;146:388–394. doi: 10.1016/j.envres.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moro G, Abril N, Jara-Biedma R, et al. Metabolic impairments caused by a "chemical cocktail" of DDE and selenium in mice using direct infusion triple quadrupole time-of-flight and gas chromatography-mass spectrometry. Chem Res Toxicol. 2019;32:1940–1954. doi: 10.1021/acs.chemrestox.9b00102. [DOI] [PubMed] [Google Scholar]
- Rose AJ (2019) Amino acid nutrition and metabolism in health and disease Nutrients 11. 10.3390/NU11112623 [DOI] [PMC free article] [PubMed]
- Rozsa E, Robotka H, Nagy D, Farkas T, Sas K, Vecsei L, Toldi J (2008) The pentylenetetrazole-induced activity in the hippocampus can be inhibited by the conversion of L-kynurenine to kynurenic acid: an invitro study. Brain Res Bull 76(5):474–479. 10.1016/j.brainresbull.2007.12.001 [DOI] [PubMed]
- Sanyal S, et al. Effect of acute sublethal and chronic administration of ddt (chlorophenotane) on brain lipid metabolism of rhesus monkeys. Toxicol Lett. 1986;34:47–54. doi: 10.1016/0378-4274(86)90144-x. [DOI] [PubMed] [Google Scholar]
- Sas K, et al. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007;257:221–239. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Scatton B, et al. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Schuh RA, et al. Effects of the organochlorine pesticide methoxychlor on dopamine metabolites and transporters in the mouse brain. Neurotoxicology. 2009;30:274–280. doi: 10.1016/j.neuro.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Aguilar J, et al. Protective and toxic roles of dopamine in Parkinson’s disease. J Neurochem. 2014;129:898–915. doi: 10.1111/jnc.12686. [DOI] [PubMed] [Google Scholar]
- Seifert J, Pewnim T. Alteration of mice L-tryptophan metabolism by the organophosphorous acid triester diazinon. Biochem Pharmacol. 1992;44:2243–2250. doi: 10.1016/0006-2952(92)90353-k. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Ratnasekhar C, Pragya P, et al. Metabolomic analysis provides insights on paraquat-induced Parkinson-like symptoms in Drosophila melanogaster. Mol Neurobiol. 2016;53:254–269. doi: 10.1007/s12035-014-9003-3. [DOI] [PubMed] [Google Scholar]
- Shukla AK, et al. A mutation in Drosophila methuselah resists paraquat induced Parkinson-like phenotypes. Neurobiol Aging. 2014;35:2419.e1–2419.e16. doi: 10.1016/j.neurobiolaging.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Sian J. Glutathione-related enzymes in brain in Parkinson’s disease. Ann Neurol. 1994;36:356–361. doi: 10.1002/ana.410360306. [DOI] [PubMed] [Google Scholar]
- Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36:1–12. doi: 10.1016/j.cger.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal NK, et al. Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson’s disease phenotype in the mouse. J Pineal Res. 2011;50:97–109. doi: 10.1111/j.1600-079X.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- Smeyne M, Smeyne RJ. Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó N, et al. Altered tryptophan metabolism in Parkinson’s disease: a possible novel therapeutic approach. J Neurol Sci. 2011;310:256–260. doi: 10.1016/j.jns.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Tavares RG, et al. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int. 2002;40:621–627. doi: 10.1016/s0197-0186(01)00133-4. [DOI] [PubMed] [Google Scholar]
- Trezzi JP, et al. Distinct metabolomic signature in cerebrospinal fluid in early Parkinson’s disease. Mov Disord. 2017;32:1401–1408. doi: 10.1002/mds.27132. [DOI] [PubMed] [Google Scholar]
- Trupp M, et al. Metabolite and peptide levels in plasma and CSF differentiating healthy controls from patients with newly diagnosed Parkinson’s disease. J Parkinsons Dis. 2014;4:549–560. doi: 10.3233/JPD-140389. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, et al. LC/MS analysis of cardiolipins in substantia nigra and plasma of rotenone-treated rats: implication for mitochondrial dysfunction in Parkinson’s disease. Free Radic Res. 2015;49:681–691. doi: 10.3109/10715762.2015.1005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamos E, et al. The role of kynurenines in disorders of the central nervous system: possibilities for neuroprotection. J Neurol Sci. 2009;283:21–27. doi: 10.1016/j.jns.2009.02.326. [DOI] [PubMed] [Google Scholar]
- Weiduschat N, et al. Motor cortex glutathione deficit in ALS measured in vivo with the J-editing technique. Neurosci Lett. 2014;570:102–107. doi: 10.1016/j.neulet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- WHO (2021) Ageing and health. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. (Accessed 14 Feb 2022)
- Widner B, et al. Increased neopterin production and tryptophan degradation in advanced Parkinson’s disease. J Neural Transm. 2002;109:181–189. doi: 10.1007/s007020200014. [DOI] [PubMed] [Google Scholar]
- Willkommen D, et al. Metabolomic investigations in cerebrospinal fluid of Parkinson’s disease. PLoS ONE. 2018;13:e0208752. doi: 10.1371/journal.pone.0208752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, et al. NMR analysis of the CSF and plasma metabolome of rigorously matched amyotrophic lateral sclerosis, Parkinson’s disease and control subjects. Metabolomics. 2016;12:1–13. doi: 10.1007/s11306-016-1041-6. [DOI] [PubMed] [Google Scholar]
- Yadav RS, Tiwari NK. Lipid integration in neurodegeneration: an overview of Alzheimer’s disease. Mol Neurobiol. 2014;50:168–176. doi: 10.1007/s12035-014-8661-5. [DOI] [PubMed] [Google Scholar]
- Yan Q, et al. High-resolution metabolomic assessment of pesticide exposure in Central Valley. California Chem Res Toxicol. 2021;34:1337–1347. doi: 10.1021/acs.chemrestox.0c00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bruun DA, Wang C, et al. Lipidomes of brain from rats acutely intoxicated with diisopropylfluorophosphate identifies potential therapeutic targets. Toxicol Appl Pharmacol. 2019;382:114749. doi: 10.1016/j.taap.2019.114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zádori D, et al. Kynurenines in chronic neurodegenerative disorders: future therapeutic strategies. J Neural Transm. 2009;116:1403–1409. doi: 10.1007/s00702-009-0263-4. [DOI] [PubMed] [Google Scholar]
- Zheng M, et al. Metabolic disturbance in hippocampus and liver of mice: a primary response to imidacloprid exposure. Sci Rep. 2020;10:5713. doi: 10.1038/s41598-020-62739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska E, et al. Effect of pesticides on kynurenic acid production in rat brain slices. Ann Agric Environ Med. 2005;12:177–179. [PubMed] [Google Scholar]
- Zinger A et al (2011) The involvement of neuroinflammation and the kynurenine pathway in Parkinson’s disease. Parkinsons Dis 716859. 10.4061/2011/716859 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.