Abstract
Background:
The association between socioeconomic factors and PAD has not been as well characterized as other cardiovascular conditions. We sought to define how annual income and education level are associated with PAD in a well-characterized diverse set of adults with CKD.
Methods:
The Chronic Renal Insufficiency Cohort Study (CRIC) is a multicenter, prospective cohort study designed to examine risk factors for progression of CKD and cardiovascular disease. Demographic, income and education-level data, as well as clinical data including ankle-brachial index (ABI) were collected at baseline. Annual income was categorized as: < $25,000, $25,000-50,000, $50,000-100,000, or above $100,000; educational level was categorized as: some high school, high school graduate, some college, or college graduate. Participants with missing income data or incompressible ABI (>1.4) were excluded from initial analysis. Logistic regression was used to estimate the association of income and/or education level with PAD, defined as an enrollment ABI of < 0.90, history of PAD, or history of PAD intervention.
Results:
A total of 4,122 were included, mean age of participants was 59.5 yrs, 56% were male, and 44% were Black. There were 763 CRIC participants with PAD at study enrollment (18.5%). In the final multivariable logistic regression model, Black race (OR = 1.3, 95% CI 1.1-1.6, p = 0.004) and level of annual household income remained independently associated with PAD at the time of enrollment (income <$25,000 OR = 1.9, 95% CI 1.3-2.8, p < 0.001; income $25,000-50,000 OR = 1.6, 95% CI 1.1-2.3, p = 0.011; income $50,000-100,000 OR = 1.2, 95% CI 0.9-1.8, p = 0.246), relative to a baseline annual income of >$100,000 (overall p-value<0.001). Decreasing level of educational attainment was not independently associated with increased PAD at enrollment, but lower level of educational attainment was associated with increased PAD when income data was not adjusted for (p = 0.001). Interestingly, Black race (OR = 0.7, 95% CI 0.6-0.8, p < 0.001), female gender (OR = 0.8, 95% CI 0.7-0.9, p = 0.007), and income <$25,000 (OR = 0.7, 95% CI 0.5-0.9, p = 0.008) were significantly associated with decreased statin use even after controlling for cardiovascular conditions.
Conclusions:
In this prospectively followed CKD cohort, lower annual household income and Black race were significantly associated with increased PAD at study enrollment. In contrast, educational level was not associated with PAD when adjusted for patient income data. Black race, female gender, and low income were independently associated with decreased statin use, populations which could be targets of future public health programs.
1.0. Introduction
Despite continued improvements in cardiovascular care in the US, substantial disparities remain, as advances in cardiovascular care are not experienced equally by all socioeconomic groups.1 Patient income, education level, race, and gender have been consistently demonstrated to be important contributors to overall health and CVD outcomes.2 Peripheral arterial disease (PAD) is a common disease in the US population, with an estimated prevalence of approximately 8 million in the United States, and is a major source of morbidity and mortality, resulting in functional impairment, limb loss, as well as death.3 While low socioeconomic status (SES) has been linked with an increased prevalence of coronary artery disease (CAD) and increased CAD mortality,4 there are few studies that have investigated the relationship between SES and PAD.5,6,7 In addition, the study of associations between socioeconomic deprivation and PAD is hindered since PAD screening or active surveillance is not conducted in any state or nation.5
Chronic kidney disease (CKD) patients are at particularly high risk for PAD. Data from the National Health and Nutrition Examination Survey (NHANES) showed that 24% of patients with CKD stage 3 or higher had PAD as defined by an ankle brachial index (ABI) < 0.9, compared to 4% in those with normal renal function.8 The Chronic Renal Insufficiency Cohort (CRIC) is a multi-center, prospective cohort study of CKD participants which is well-balanced with regard to education level, race, gender, age, and has detailed data regarding lower extremity arterial circulation obtained at standardized time intervals. These data allow for the diagnosis as well as enumeration of the severity of PAD. As such, it is an ideal population to study SES-based differences in PAD prevalence and severity. The objective of this study is assess the association of SES factors on PAD prevalence in those with CKD.
2.0. Methods:
The CRIC Study is an observational study that examines risk factors for progression of cardiovascular disease (CVD) among patients with CKD. Participants include a racially and ethnically diverse group of men and women who were aged 21 to 74 years old at the time of inclusion and have CKD based on age-based estimated GFR (eGFR) thresholds.10 Age-based GFR was defined as the following: for age 21 to 44, GFR 20 to 70 mL/min per 1.73 m2, age 45 to 64, GFR 20 to 60 mL/min per 1.73 m2; and age 65 to 74, GFR 20 to 50 mL/min per 1.73 m2. A total of 5,499 participants were recruited between 2003-2013 from seven clinical centers in the US.11 Participants were identified through searches of laboratory databases, medical records, and referrals from health care providers. Patients with cirrhosis, HIV infection, polycystic kidney disease, renal cell carcinoma, those on dialysis or recipients of a kidney transplant, and those taking immunosuppressant drugs were excluded. All study data were collected by trained study staff during the CRIC clinical visits. Annual household income was derived as a four-level categorical variable: below $25,000, $25,000-50,000, $50,000-100,000, or above $100,000. Highest level of educational attainment was also treated as a four-level categorical variable: some high school, high school graduate, some college, or college graduate. Racial designation was done by self-identification during study enrollment. All data collection procedures and equipment were standardized across study sites. Baseline characteristics of participants were summarized as means (standard deviation, SD) for continuous variables and percentages for categorical variables by PAD status. Statistical significance was tested using either t-test or ANOVA for continuous variables and the χ2 test for categorical variables. Statistical analyses were performed using STATA version 15 (College Station, TX). All p-values were two-sided and statistical significance was defined as p<0.05.
2.1. PAD Assessment
The ankle brachial index (ABI) is the standard test utilized to diagnose PAD. After lying supine for 5 minutes, systolic blood pressure was measured in both arms and in the dorsalis pedis artery and posterior tibial artery using a Doppler probe. The ABI was calculated by dividing the systolic blood pressure for each sided pedal artery by the higher systolic blood pressure of the brachial artery. In participants with functioning arteriovenous fistula or grafts, the available contralateral brachial artery blood pressure was used. An ABI below 0.9 has been well established to be diagnostic for PAD with a sensitivity of 68% and a specificity of 99%.11,12
Patients were classified as having PAD if they had a history of a PAD intervention prior to enrollment (history of PAD-related lower extremity amputation, angioplasty, or bypass) or if patients had an enrollment ABI of less than 0.9 in either lower extremity. Those with an enrollment ABI >1.4 were excluded due to their uncertainty regarding PAD status in those with noncompressible vasculature. Logistic regression was performed to explore the association of PAD at CRIC enrollment with socioeconomic factors. Traditional CVD (CAD, diabetes, hyperlipidemia, CKD level) and socioeconomic factors were also initially modeled using univariate logistic regression analysis. A multivariable model was then created using backwards selection that contained all variables that had a univariate p-value below 0.20 with variables eliminated in stepwise fashion if found to be non-significant.
3.0. Results:
There were 5,499 participants enrolled with a mean age of 59.5 years, 56.4% were males, and 44.3% were Black. There were 1,377 patients who were excluded from initial analysis, 833 with missing income information and 544 with non-compressible ABIs (> 1.4), resulting in a study population of 4,122. There were 763 participants (18.5%) in the CRIC cohort that had PAD at study enrollment, patient demographic and clinical history by annual income are shown in Table 1 and patient enrollment ABI by annual income is displayed in Figure 1. There were 63 patients (1.1%) with an ABI below 0.5, 245 (4.5%) with an ABI between 0.5 and 0.7, 509 (9.3%) with an ABI between 0.7 and 0.9, 4, 408 (80.2%) with an ABI between 0.9 and 1.4, and 274 (6.2%) with an ABI > 1.4. Increased PAD severity (lower ABI), increased prevalence of cerebrovascular disease, diabetes mellitus, hyperlipidemia, hypertension, and smoking were noted in those with lower income.
Table 1:
Summary statistics of demographic variables and clinical characteristics for patients entering the CRIC, stratified by annual household income.
| Below $25,000 (n = 1652) | $25,000-$50,000 (n=1360) | $50,000-$100,000 (n=1033) | Above $100,000 (n=621) | p-value | ||
|---|---|---|---|---|---|---|
| Age (years) | 59.2 (10.3) | 60.5 (11.1) | 58.7 (10.9) | 59.6 (10.2) | <0.001 | |
|
| ||||||
| Education Level | Some High School | 655 (39.6%) | 177 (13.0%) | 33 (3.2%) | 3 (0.5%) | <0.001 |
| High School Graduate | 384 (23.2%) | 328 (24.1%) | 106 (10.3%) | 27 (4.3%) | ||
| Some College | 437 (26.5%) | 499 (36.7%) | 345 (33.4%) | 109 (17.6%) | ||
| College Graduate | 176 (10.7%) | 356 (26.2%) | 549 (53.1%) | 482 (77.6%) | ||
|
| ||||||
| Enrollment ABI (lowest in either extremity) | Below 0.7 | 141 (8.5%) | 88 (6.5%) | 31 (3.0%) | 9 (1.4%) | <0.001 |
| 0.7 to 0.9 | 198 (12.0%) | 122 (9.0%) | 73 (7.1%) | 29 (4.7%) | ||
| 0.9 to 1.4 | 1202 (72.8%) | 1075 (79.0%) | 899 (87.0%) | 563 (90.7%) | ||
| Above 1.4 | 111 (6.7%) | 75 (5.5%) | 30 (2.9%) | 20 (3.2%) | ||
|
| ||||||
| Male Gender | 787 (47.6%) | 776 (57.1%) | 658 (63.7%) | 447 (72.0%) | <0.001 | |
|
| ||||||
| Black Race | 932 (56.4%) | 647 (47.6%) | 341 (33.0%) | 99 (15.9%) | <0.001 | |
|
| ||||||
| eGFR (mL/min per 1.73 m2) | Below 30 | 383 (23.2%) | 194 (14.3%) | 109 (10.6%) | 33 (5.3%) | <0.001 |
| 30-60 | 1048 (63.4%) | 898 (66.0%) | 628 (60.8%) | 338 (54.4%) | ||
| Above 60 | 221 (13.4%) | 268 (19.7%) | 296 (28.7%) | 250 (40.3%) | ||
|
| ||||||
| History of CVD | 681 (41.2%) | 459 (33.8%) | 285 (27.6%) | 142 (22.9%) | <0.001 | |
|
| ||||||
| History of DM | 1011 (61.2%) | 692 (50.9%) | 446 (43.2%) | 243 (39.1%) | <0.001 | |
|
| ||||||
| History of Hypertension | 1513 (91.6%) | 1206 (88.7%) | 852 (82.5%) | 464 (74.7%) | <0.001 | |
|
| ||||||
| History of High Cholesterol | 1337 (80.9%) | 1106 (81.3%) | 801 (77.5%) | 469 (75.5%) | 0.004 | |
|
| ||||||
| Tobacco Use | 320 (19.4%) | 149 (11.0%) | 77 (7.5%) | 39 (6.3%) | <0.001 | |
|
| ||||||
| Medication Use | Statin | 940 (57.3%) | 819 (60.4%) | 580 (56.6%) | 358 (58.0%) | 0.22 |
| Antiplatelet | 826 (50.0%) | 685 (50.4%) | 454 (43.9%) | 294 (47.3%) | 0.007 | |
| Anticoagulant | 124 (7.5%) | 95 (7.0%) | 75 (7.3%) | 35 (5.6%) | 0.48 | |
|
| ||||||
| PAD at Study Enrollment | 422 (25.5%) | 252 (18.5%) | 133 (12.9%) | 53 (8.5%) | <0.001 | |
|
| ||||||
| Cause of Renal Disease | Diabetes | 551 (33.4%) | 344 (25.3%) | 219 (21.2%) | 100 (16.1%) | <0.001 |
| Hypertension | 242 (14.6%) | 209 (15.4%) | 148 (14.3%) | 78 (12.6%) | ||
| Other | 142 (8.6%) | 177 (13.0%) | 166 (16.1%) | 142 (22.9%) | ||
| Unknown | 717 (43.4%) | 630 (46.3%) | 500 (48.4%) | 301 (48.5%) | ||
|
| ||||||
| CRIC Site | University of Pennsylvania | 154 (9.3%) | 186 (13.7%) | 138 (13.4%) | 103 (16.6%) | <0.001 |
| The Johns Hopkins University | 190 (11.5%) | 216 (15.9%) | 143 (13.8%) | 74 (11.9%) | ||
| Case Western Reserve University | 222 (13.4%) | 183 (13.5%) | 172 (16.7%) | 76 (12.2%) | ||
| University of Michigan | 150 (9.1%) | 185 (13.6%) | 182 (17.6%) | 110 (17.7%) | ||
| University of Illinois at Chicago | 617 (37.3%) | 210 (15.4%) | 72 (7.0%) | 26 (4.2%) | ||
| Tulane University Health Science Center | 243 (14.7%) | 179 (13.2%) | 99 (9.6%) | 44 (7.1%) | ||
| Kaiser Permanente of Norther California | 76 (4.6%) | 201 (14.8%) | 227 (22.0%) | 188 (30.3%) | ||
Figure 1:
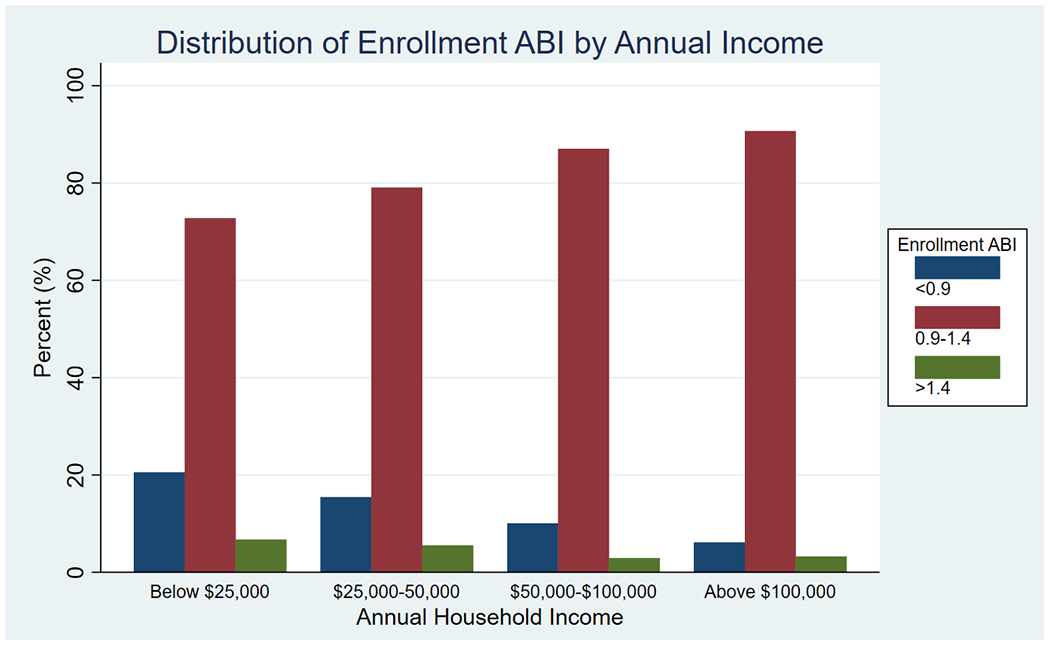
Distribution of enrollment ABIs by annual household income.
On univariate logistic regression analysis, lower level of annual household income (p < 0.001), lower level of educational attainment (p <0.001), and Black race (OR 1.6, 95% CI 1.3-1.8, p <0.001) were significantly associated with PAD. Annual household income below $25,000 had the strongest association with PAD at study enrollment (OR 3.6, 95% CI 2.6-5.0, p <0.001), followed by income $25,000-50,000 (OR 2.4, 95% CI 1.8-3.4, p <0.001), and income $50,000-100,000 (OR 1.5, 95% CI 1.1-2.1, p =0.021), relative to income >$100,000 as a baseline. Education level of less than high school graduate had the highest association with PAD (OR 2.9, 95% CI 2.3-3.6, p <0.001), followed by high school graduate (OR 2.1, 95% CI 1.7-2.8, p <0.001), and those with some level of college education (OR 1.7, 95% CI 1.3-2.1, p <0.001), relative to college graduate as a baseline.
In the final multivariable logistic regression model, which included demographic and clinical factors relevant from the univariate analysis, Black race (OR 1.3, 95% CI 1.1-1.6, p = 0.004) and lower level of annual household income (p < 0.001) remained independently associated with PAD at the time of study enrollment (Table 2). When compared to a baseline annual income of >$100,000, lower levels of annual household income were associated with PAD (income < $25,000 OR 1.9, 95% CI 1.3-2.8, p = 0.001; income $25,000-50,000 OR 1.6, 95% CI 1.1-2.3, p = 0.011; income $50,000-100,000 OR 1.2, 95% CI 0.9-1.8, p = 0.246). In contrast, level of educational attainment was not associated with PAD in the adjusted analysis (p = 0.31) with inclusion of patient income data. The full multivariable model is shown in supplement Table 1, with history of MI, diabetes mellitus, hyperlipidemia, and smoking all determined to be independently associated with increased PAD at CRIC enrollment. No significant interaction was found between age, race, or gender with either annual household income or level of educational attained. While decreased income and Black race were associated with increased tobacco use, no significant interactions were found in their association with PAD. Further, Black race (OR = 0.7, 95% CI 0.6-0.8, p < 0.001), female gender (OR = 0.8, 95% CI 0.7-0.9, p = 0.007), and income <$25,000 (OR = 0.7, 95% CI 0.5-0.9, p = 0.008) were significantly associated with decreased statin use even after controlling for cardiovascular conditions (including PAD and coronary artery disease).
Table 2:
Univariate and multivariable logistic regression model results for association between PAD at CRIC study enrollment and selected demographic and socioeconomic factors
| Univariate Logistic OR | Univariate p-value | Multivariable Logistic OR | Multivariable p-value | ||
|---|---|---|---|---|---|
| Annual Household Income | Below $25,000 | 3.6 (2.6-5.0) | <0.001 | 1.9 (1.3-2.8) | <0.001 |
| $25,000-$50,000 | 2.4 (1.8-3.4) | 1.6 (1.1-2.3) | |||
| $50,000-$100,000 | 1.5 (1.1-2.1) | 1.2 (0.9-1.8) | |||
| Above $100,000 | Ref | Ref | |||
|
| |||||
| Education Level | Some High School Education | 2.9 (2.3-3.6) | <0.001 | 1.3 (1.0-1.7) | 0.31 |
| High School Graduate | 2.1 (1.7-2.8) | 1.2 (0.9-1.6) | |||
| Some College Education | 1.7 (1.3-2.1) | 1.1 (0.9-1.4) | |||
| College Graduate | Ref | Ref | |||
|
| |||||
| Black Race | 1.6 (1.3-1.8) | <0.001 | 1.3 (1.1-1.6) | 0.004 | |
|
| |||||
| Female Gender | 1.0 (0.8-1.2) | 0.847 | 1.1 (0.9-1.3) | 0.188 | |
To determine if there were differences amongst the individuals diagnosed with PAD, we performed subset analysis of those patients that were diagnosed based on ABI<0.9 (newly diagnosed PAD) versus those that came with a preexisting diagnosis of PAD or a history of PAD intervention (previously diagnosed PAD). There were 763 participants (18.5%) in the CRIC cohort with PAD at study enrollment, with 237 participants (31%) having previously diagnosed PAD and 492 participants (69%) with newly diagnosed PAD (i.e. found at CRIC enrollment to have ABI below 0.9). Those with newly diagnosed PAD were significantly more likely to be of Black race (OR 1.7, 95% CI 1.2-2.4, p = 0.004) and female gender (OR 1.5, 95% CI 1.1-2.1, p = 0.026), even after adjustment for demographic factors and other cardiovascular conditions in multivariable analyses. Further, those with previously diagnosed PAD had a higher rate of statin use compared to those with newly diagnosed PAD (77% vs 69%, p=0.014). Black race was independently associated with decreased statin use in both the previous and newly diagnosed PAD cohorts: (Previously diagnosed PAD OR 0.4, 95% CI 0.2-0.8, p = 0.007; Newly diagnosed PAD OR 0.5, 95% CI 0.4-0.8, p = 0.006) even when accounting for comorbid cardiovascular conditions and demographic factors. In contrast, female gender was associated with decreased statin use in newly diagnosed PAD (OR 0.6, 95% CI 0.4-0.9, p = 0.010), but not in those with previous diagnosed PAD. For those with either previous or newly diagnosed PAD, statin use did not vary by income level or educational level.
3.1. Sensitivity Analysis
3.1.1. PAD Prevalence by education level
Income was missing in 15% of the study cohort limiting the study population size. Further, increased education level was found to have a positive correlation with increased income level (r = 0.54, p = <0.001). Given this, multivariable logistic analysis was performed to assess the association between PAD prevalence and education level, without inclusion of patient income data in the model (sample size of 4,857 patients). In this multivariable logistic regression model, lower levels education attainment was significantly associated with PAD (p = 0.001). Education level of less than high school graduate had the strongest association with PAD (OR 1.6, 95% CI 1.2-2.1, p <0.001), followed by high school graduate (OR 1.4, 95% CI 1.1-1.8, p = 0.003), and those with some level of college education (OR 1.2, 95% CI 1.1-1.5, p = 0.078), relative to college graduate as a baseline. Black race (OR 1.4, 95% CI 1.1-1.6, p < 0.001) remained independently associated with PAD in this adjusted model. Multivariable model performance did not significantly vary significantly when income was included in the multivariable model compared to models in which income level was excluded and education level was used (ROC of 0.758 vs 0.755).
3.1.2. PAD Prevalence with imputation of missing income information
Income was missing in 15% of the study cohort. Imputation of missing income data was performed using multiple imputation with chained equation methods.14 Using 10 chained equations, imputation of 581 missing income levels (70%) was successful for a total sample size of 4,634 patients. In the multivariable logistic regression model with imputation, lower levels of annual household income were associated with PAD (income < $25,000 OR 1.9, 95% CI 1.3-2.7, p = 0.001; income $25,000-50,000 OR 1.6, 95% CI 1.1-2.3, p = 0.008; income $50,000-100,000 OR 1.2, 95% CI 0.8-1.8, p = 0.267) compared to those with an annual income above $100,000 (overall p-value of 0.001). There was no association between education level and risk of incident PAD (p = 0.17) in the adjusted imputed model with imputation. Black race (OR 1.3, 95% CI 1.1-1.6, p = 0.001) remained independently associated with PAD in the adjusted model with imputation.
3.1.3. PAD Prevalence with inclusion of patients with ABI>1.4
There were 544 participant enrolled in the CRIC cohort who had enrollment ABI above 1.4 in either lower extremity and were excluded from our index analysis. Of these, 97 (17.3%) had a prior diagnosis of PAD. Multivariable logistic regression analysis was repeated with inclusion of these patients for a sample size of 4,636 patients. Similar to the above findings, an annual income above $100,000 was less associated with PAD compared to those with an income < $25,000 (OR = 2.1, 95% CI 1.4-2.9, p < 0.001), $25,000-50,000 (OR = 1.6, 95% CI 1.2-2.3, p = 0.004), and $50,000-100,000 PAD (OR = 1.3, 95% CI 0.9-1.9, p = 0.110) with an overall p-value of 0.005. There was no association between education level and PAD (group p = 0.330), while Black race (OR 1.4, 95% CI 1.1-1.6, p = 0.001) remained independently associated with PAD.
4.0. Discussion
In this study, we demonstrate the associations between socioeconomic factors, including income, education level, race, and PAD. Even with multivariable adjustment, annual household income remains a significant and impactful SES factor, with lower income strongly associated with PAD at CRIC enrollment. As demonstrated in previous literature, the association between educational level and PAD appears to be largely mediated through demographic and cardiovascular risk factors, as there was an association between education level and risk of PAD on univariate but no association in the multivariable models.14 The relationship between annual household income and risk of PAD showed an exposure-response relationship on the multivariable model, with decreased levels of annual income more strongly associated with PAD, with the greatest burden of disease in those with an annual income below $25,000. This association has been described in other cardiovascular diseases, even with adjustment of comorbid conditions.4,15 Further, we found no interaction between annual income, education, or race with regard to PAD. The etiology of the association between low annual income and cardiovascular disease is likely multifactorial. Possible contributing factors include: the documented association between low annual income and chronic low-grade inflammation,15 the association between lower SES with fewer social supports and decreased likelihood of engaging in healthy lifestyle choices,15–17 those with lower income level experiencing increased difficulty in gaining access to community resources and/or exercise accessories due to employment and financial constraints,5,16,18 and increased difficulty in attaining access to preventive care or screening initiaives.5,18 Our results support the need for innovative solutions to address the increased prevalence of PAD observed among these disadvantaged patients. This may entail expansion of existing cardiovascular health outreach services as well as enactment of new health and social policies to mitigate disparities associated with lower income.
On both univariate and multivariable analysis, Black race was associated with PAD. Further, Black race was associated with new PAD diagnosis at study enrollment, suggesting that there may be a component of delayed recognition of this disease in this cohort. Additionally, we noted that Black patients with PAD were significantly less likely to be receiving statin therapy compared to their non-Black counterparts even with strict control of comorbid conditions, a pattern which has been suggested elsewhere in literature.28 The continued association between Black race and cardiovascular disease, even when baseline comorbid conditions and other socioeconomic factors are accounted for, has been seen in other areas of cardiovascular health.15,19 The persistence of this disadvantage has often been attributed to worse control of comorbid conditions in the Black population (including increased levels of baseline blood pressure in those with hypertension and increased frequency of central obesity in those with elevated BMI) and the increased baseline levels of psychological distress experienced by this population.15,19 Additionally, the Black population has been noted to have decreased access to medical care, even with adjustment of other socioeconomic factors, which may contribute to these findings.20,21
Overall, Female gender was not found to be associated with PAD at study enrollment. Prior reports have shown conflicting results in regard to the association between gender and PAD. There have been previous studies demonstrating an increased association between female gender and PAD, but this was attributed to increased risk of comorbid cardiovascular conditions and decreased cardiovascular medication use in women.22,23 Our results seem to agree with prior population based studies demonstrating no difference in prevalence of PAD by gender, which is likely secondary to the stringent tracking of cardiovascular conditions that is done in the CRIC cohort, as well as thorough history and serial ABI measurements in a balanced gender cohort.24,25
The disparities highlighted in this study demonstrate the need for improved approaches to raise PAD awareness, as well as focused research endeavors and treatment strategies that target low SES individuals, as they are the most at risk for PAD as well as disease progression. It has previously been shown that those afflicted with PAD with low SES are more likely to receive amputations compared to those with higher SES, who are more likely to undergo revascularization or debridement.26,27 Continued efforts to identify and implement programs such as increased and earlier statin use in these subpopulations would reduce the morbidity from delayed diagnosis. Given the high prevalence of PAD in those with CKD, as well as the at times asymptomatic nature of PAD, earlier ABI screening in this subpopulation may be warranted. In this study, over 2/3rds of those found to have PAD at CRIC enrollment did not have a previous PAD diagnosis, highlighting the sometimes indolent or asymptomatic nature of the early stages of PAD which may contribute to delayed diagnosis. In this population with CKD, we found the overall PAD prevalence to be 18.6%, which is similar to that in the previous literature and increased from the general US population.8
There are important limitations to our study. Annual income and education level are self-reported and only collected upon participant enrollment into the CRIC, thus, misclassification is possible. However, prior studies have shown minimal bias and error between self-reported income and true wage or salary. In addition, annual household income may not always approximate total wealth and varies by family size, especially in those who may be retired. Patients with CKD and/or diabetes can have calcified, noncompressible vessels, which can lead to an overestimation of the ABI and misclassification. However, in sensitivity analyses with inclusion of noncompressible vessels, the same associations were found between SES factors and PAD. The CRIC is a longitudinal study designed for those with CKD, and thus may limit generalizability to the general US populace. Additionally, patients able to comply with the yearly visits for CRIC participation may not be generalizable to those in the US who are at most risk for PAD development. While we were able to control for presence or absence of traditional cardiovascular risk factors, we were unable to control for severity of these comorbidities or level of medical compliance. Thus, there may have been residual confounding by disease severity that could explain the associations seen in this study. However, this is a rich dataset which lends important insight into a highly relevant population which is commonly afflicted by PAD.
5.0. Conclusions
In this prospectively followed CKD cohort, we found that lower annual household income and Black race were significantly associated with PAD. In contrast, level of education and gender were not associated with PAD after multivariable adjustment. Additionally, we found that Black race and female gender were associated with previously undiagnosed PAD and decreased statin use. Improved public awareness efforts that are targeted toward these populations will lower the adverse impact of PAD that these vulnerable patients experience. Additionally, given the high prevalence of PAD in this cohort, the role of periodic ABI screening for PAD in high risk populations should be considered.
Supplementary Material
Sources of Funding:
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902 and U24DK060990). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM R01DK119199
Appendix:
The CRIC Study Investigators include Lawrence J. Appel, MD, MPH: Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Medical Institutions, Baltimore, MD; Alan S. Go, MD: Departments of Epidemiology, Biostatistics, and Medicine, University of California, San Francisco, CA; Jiang He, MD, PhD: Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, LA; John W. Kusek, PhD: National Institutes of Health, Bethesda, MD; James P. Lash, MD: Department of Medicine, University of Illinois College of Medicine, Chicago, IL; Mahboob Rahman, MD: Department of Medicine, Case Western Reserve University, Cleveland, OH.
Footnotes
Disclosures:
None
References:
- [1].Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of Disparities in Cardiovascular Health in the United States. Circulation. 2005;111:1233–1241. [DOI] [PubMed] [Google Scholar]
- [2].Alter DA, Franklin B, Ko DT, Austin PC, Lee DS, Oh PI, Stukel TA, Tu JV. Socioeconomic Status, Functional Recovery, and Long-Term Mortality among Patients Surviving Acute Myocardial Infarction. PLoS One. 2013;8:e65130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001. Sep 19;286(11):1317–24. [DOI] [PubMed] [Google Scholar]
- [4].Kaplan GA, Keil JE. Socioeconomic Factors and Cardiovascular Disease: A Review of the Literature. Circulation. 1993;88:1973–1998. [DOI] [PubMed] [Google Scholar]
- [5].Pande RL, Creager MA. Socioeconomic inequality and peripheral artery disease prevalence in US adults. Circ Cardiovasc Qual Outcomes. 2014;7:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kröger K, Dragano N, Stang A, Moebus S, Möhlenkamp S, Mann K, Siegrist J, Jöckel KH, Erbel R; Heinz Nixdorf Recall Study Investigator Group. An unequal social distribution of peripheral arterial disease and the possible explanations: results from a population-based study. Vasc Med. 2009;14:289–296. [DOI] [PubMed] [Google Scholar]
- [7].Kaplan GA, Keil JE. Socioeconomic Factors and Cardiovascular Disease: A Review of the Literature. Circulation. 1993;88:1973–1998. [DOI] [PubMed] [Google Scholar]
- [8].O’Hare Ann M, Glidden David V, Fox Caroline S, Hsu Chi-yuan. High Prevalence of Peripheral Arterial Disease in Persons With Renal Insufficiency. Circulation. 2004;109(3):320–323. doi: 10.1161/01.CIR.0000114519.75433.DD [DOI] [PubMed] [Google Scholar]
- [9].Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. JASN. 2003;14(suppl 2):S148–S153. doi: 10.1097/01.ASN.0000070149.78399.CE [DOI] [PubMed] [Google Scholar]
- [11].Khan TH, Farooqui FA, Niazi K. Critical Review of the Ankle Brachial Index. Curr Cardiol Rev. 2008;4(2):101–106. doi: 10.2174/157340308784245810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schröder F, Diehm N, Kareem S, et al. A modified calculation of ankle-brachial pressure index is far more sensitive in the detection of peripheral arterial disease. J Vasc Surg. 2006;44(3):531–536. doi: 10.1016/j.jvs.2006.05.016 [DOI] [PubMed] [Google Scholar]
- [13].Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–213. doi: 10.1007/s11121-007-0070-9 [DOI] [PubMed] [Google Scholar]
- [14].McLafferty RB, Moneta GL, Taylor LM, Porter JM. Ability of ankle-brachial index to detect lower-extremity atherosclerotic disease progression. Arch Surg. 1997;132(8):836–840; discussion 840-841. doi: 10.1001/archsurg.1997.01430320038005 [DOI] [PubMed] [Google Scholar]
- [15].Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and Ethnic Disparities in Cardiovascular Risk In the United States, 2001–2006. Annals of Epidemiology. 2010;20(8):617–628. doi: 10.1016/j.annepidem.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cohen B, Vittinghoff E, Whooley M. Association of Socioeconomic Status and Exercise Capacity in Adults With Coronary Heart Disease (from the Heart and Soul Study). The American Journal of Cardiology. 2008;101(4):462–466. doi: 10.1016/j.amjcard.2007.09.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shishehbor MH, Gordon-Larsen P, Kiefe CI, Litaker D. Association of neighborhood socioeconomic status with physical fitness in healthy young adults: The Coronary Artery Risk Development in Young Adults (CARDIA) study. American Heart Journal. 2008;155(4):699–705. doi: 10.1016/j.ahj.2007.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Winkleby MA, Cubbin C, Ahn DK, Kraemer HC. Pathways by Which SES and Ethnicity Influence Cardiovascular Disease Risk Factors. Annals of the New York Academy of Sciences. 1999;896(1):191–209. doi: 10.1111/j.1749-6632.1999.tb08116.x [DOI] [PubMed] [Google Scholar]
- [19].Rooks RN, Simonsick EM, Miles T, et al. The Association of Race and Socioeconomic Status With Cardiovascular Disease Indicators Among Older Adults in the Health, Aging, and Body Composition Study. J Gerontol B Psychol Sci Soc Sci. 2002;57(4):S247–S256. doi: 10.1093/geronb/57.4.S247 [DOI] [PubMed] [Google Scholar]
- [20].Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN). American Heart Journal. 2005;149(6):1066–1073. doi: 10.1016/j.ahj.2004.08.027 [DOI] [PubMed] [Google Scholar]
- [21].Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and Ethnic Disparities in Cardiovascular Risk In the United States, 2001–2006. Annals of Epidemiology. 2010;20(8):617–628. doi: 10.1016/j.annepidem.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jelani Q, Petrov M, Martinez SC, Holmvang L, Al-Shaibi K, Alasnag M. Peripheral Arterial Disease in Women: an Overview of Risk Factor Profile, Clinical Features, and Outcomes. Curr Atheroscler Rep. 2018;20(8). doi: 10.1007/s11883-018-0742-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sadrzadeh Rafie AH, Stefanick ML, Sims ST, et al. Sex differences in the prevalence of peripheral artery disease in patients undergoing coronary catheterization. Vasc Med. 2010;15(6):443–450. doi: 10.1177/1358863X10388345 [DOI] [PubMed] [Google Scholar]
- [24].Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172(1):95–105. doi: 10.1016/S0021-9150(03)00204-1 [DOI] [PubMed] [Google Scholar]
- [25].Sigvant B, Wiberg-Hedman K, Bergqvist D, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. Journal of Vascular Surgery. 2007;45(6):1185–1191. doi: 10.1016/j.jvs.2007.02.004 [DOI] [PubMed] [Google Scholar]
- [26].Ferguson HJM, Nightingale P, Pathak R, Jayatunga AP. The Influence of Socio-economic Deprivation on Rates of Major Lower Limb Amputation Secondary to Peripheral Arterial Disease. European Journal of Vascular and Endovascular Surgery. 2010;40(1):76–80. doi: 10.1016/j.ejvs.2010.03.008 [DOI] [PubMed] [Google Scholar]
- [27].Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. Journal of Vascular Surgery. 2011;54(2):420–426.e1. doi: 10.1016/j.jvs.2011.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gober L, Bui A, Ruddy JM. Racial and Gender Disparity in Achieving Optimal Medical Therapy for Inpatients with Peripheral Artery Disease. Ann Vasc Med Res [Internet]. 2020. [cited 2021 May 13];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7877491/ [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


