Abstract
Context.—
The incidence of human epidermal growth factor receptor 2 (HER2) positivity in gastric cancers differs widely across various populations and is unknown in many low-resource settings.
Objective.—
To evaluate the rates of HER2 positivity in gastric and gastroesophageal adenocarcinoma at a national referral hospital in East Africa. We also assessed the association between HER2 overexpression and patient clinicopathologic characteristics.
Design.—
A retrospective review of cases diagnosed as either gastric or gastroesophageal adenocarcinoma between 2013 and 2017 was performed at Muhimbili National Hospital in Dar es Salaam, Tanzania. Of 1205 specimens meeting inclusion criteria, stratified random sampling was conducted to select 150 cases for HER2 immunohistochemistry and clinicopathologic analysis.
Results.—
The median age of patients was 56.5 years, with 65.3% (98 of 150) of the cohort composed of male patients, and 34.7% (52 of 150) of female patients. HER2 overexpression was identified in 6.0% (9 of 150) of cases. Approximately half of the tumors (51.3%; 77 of 150) were intestinal-type gastric adenocarcinoma, and 36.0% (54 of 150) were moderately differentiated. Intestinal-type (P = .01) and well-differentiated tumors (P = .001) were associated with HER2 overexpression.
Conclusions.—
HER2 overexpression was primarily seen in intestinal-type and well-differentiated tumors. Therefore, prioritizing HER2 testing for patients with intestinal-type, well-differentiated, or moderately differentiated gastric and gastroesophageal adenocarcinomas may be appropriate in Tanzania in efforts to allocate testing for patients who are most likely to benefit from trastuzumab therapy.
Gastric cancer is the sixth most common cancer worldwide and the second leading cause of cancer-related death globally.1 Although the estimated age-standardized incidence rate of gastric cancer is the same in both Tanzania and the United States (4.2 per 100 000 persons per year), the estimated age-standardized mortality rate in Tanzania is more than twice the rate in the United States (3.8 per 100 000 persons per year versus 1.7 per 100 000 persons per year).1 Moreover, the number of new gastric cancers in Tanzania is projected to increase from 1091 in 2020 to 2529 cases in 2040, an increase of 132%.1 In Tanzania, the vast majority of patients with gastric cancer (92.1%) present with advanced, unresectable disease and are treated with palliative intent.2
For patients with human epidermal growth factor receptor 2 (HER2)–positive advanced gastric cancer, the Trastuzumab for Gastric Cancer (ToGA) trial demonstrated that trastuzumab, a humanized monoclonal antibody against HER2, in combination with chemotherapy, resulted in improved median overall survival and progression-free survival, compared with chemotherapy alone.3 Historically, most patients in low-resource settings have not had access to HER2-targeted therapies, primarily because of cost. In 2015, trastuzumab was added to the World Health Organization’s (WHO) Essential Medicines List, with the caveat that inclusion was based on the possibility of access to lower-cost biosimilar product(s).4 Now, with the advent of trastuzumab biosimilars and expectations for improved access to targeted therapies in low-resource settings, there is an urgent need to generate context-specific data to evaluate the role for targeted therapeutics in the management of gastric cancer in sub-Saharan Africa.
Reported rates of HER2 positivity by immunohistochemistry (IHC) in gastric cancers range widely from 4.4% to 53.4%, with most of the studies conducted in East Asian or Western populations.5 Of note, studies conducted in Africa have also reported a wide range of HER2 positivity, from 11% to 42.4%.6–10 Small sample sizes and technical challenges, including the inability to perform fluorescence in situ hybridization (FISH) testing for cases equivocal by IHC (2+), stand as some of the major limitations in these studies.
Recognizing the limited data on HER2 expression in gastric cancer patients from East Africa, we aimed to evaluate the rate of HER2 positivity among patients with gastric and gastroesophageal adenocarcinoma in Tanzania and to determine its association with clinicopathologic factors, such as age, sex, tumor grade, and histologic subtype. Although HER2 IHC is available for breast cancer specimens in Tanzania, it is not routinely performed on gastric cancer samples; as a result, trastuzumab is not used to treat gastric cancer patients. Elucidating the rate of HER2 positivity in gastric cancers in Tanzania will influence the allocation of resources and determine the utility of routinely performing HER2 IHC.
MATERIALS AND METHODS
Study Setting and Design
This was a retrospective study involving archived specimens previously reported as gastric adenocarcinoma or gastroesophageal adenocarcinoma at Muhimbili National Hospital (MNH) in Dar es Salaam, Tanzania. MNH, besides being a national referral hospital, is the public teaching hospital for Muhimbili University of Health and Allied Sciences (MUHAS) in Dar es Salaam, Tanzania. All suspected cancer cases referred to MNH must be evaluated by the MNH Central Pathology Laboratory for pathologic confirmation prior to referral for treatment at Ocean Road Cancer Institute in Dar es Salaam, Tanzania. Central Pathology Laboratory receives approximately 250 to 350 gastric carcinoma biopsies and resections per year, which are obtained either by endoscopic biopsy or surgical resection performed at MNH. Because resources for IHC are limited, HER2 is currently only performed on breast cancer, and it is not routinely done on gastric carcinoma specimens.
This study received approvals from the Institutional Review Boards at MUHAS (MUHAS-REC-4-2020-204) and the University of California, San Francisco (19-29285).
Study Population
Gastric or gastroesophageal biopsies and resection specimens reported as adenocarcinoma from patients ages ≥18 years from 2013 to 2017 were identified from a pathology database. Cases with unavailable clinical data, processing artifacts that precluded evaluation of the tumor, insufficient tumor in the sample, and unavailable formalin-fixed, paraffin-embedded tissue blocks were excluded (Figure 1). Pathology reports and hematoxylin-eosin (H&E) slides were reviewed for determination of eligibility. Tumors that met initial quality criteria were subsequently categorized into histologic subtypes according to the Lauren classification system,11 and histologic tumor grades were assigned using the WHO 2010 criteria.12 Stratified random sampling was conducted to select a total of 150 cases, with each histologic subtype proportionally represented. Clinical and pathologic data were collected, including age, sex, histologic subtype, and histologic grade.
Figure 1.
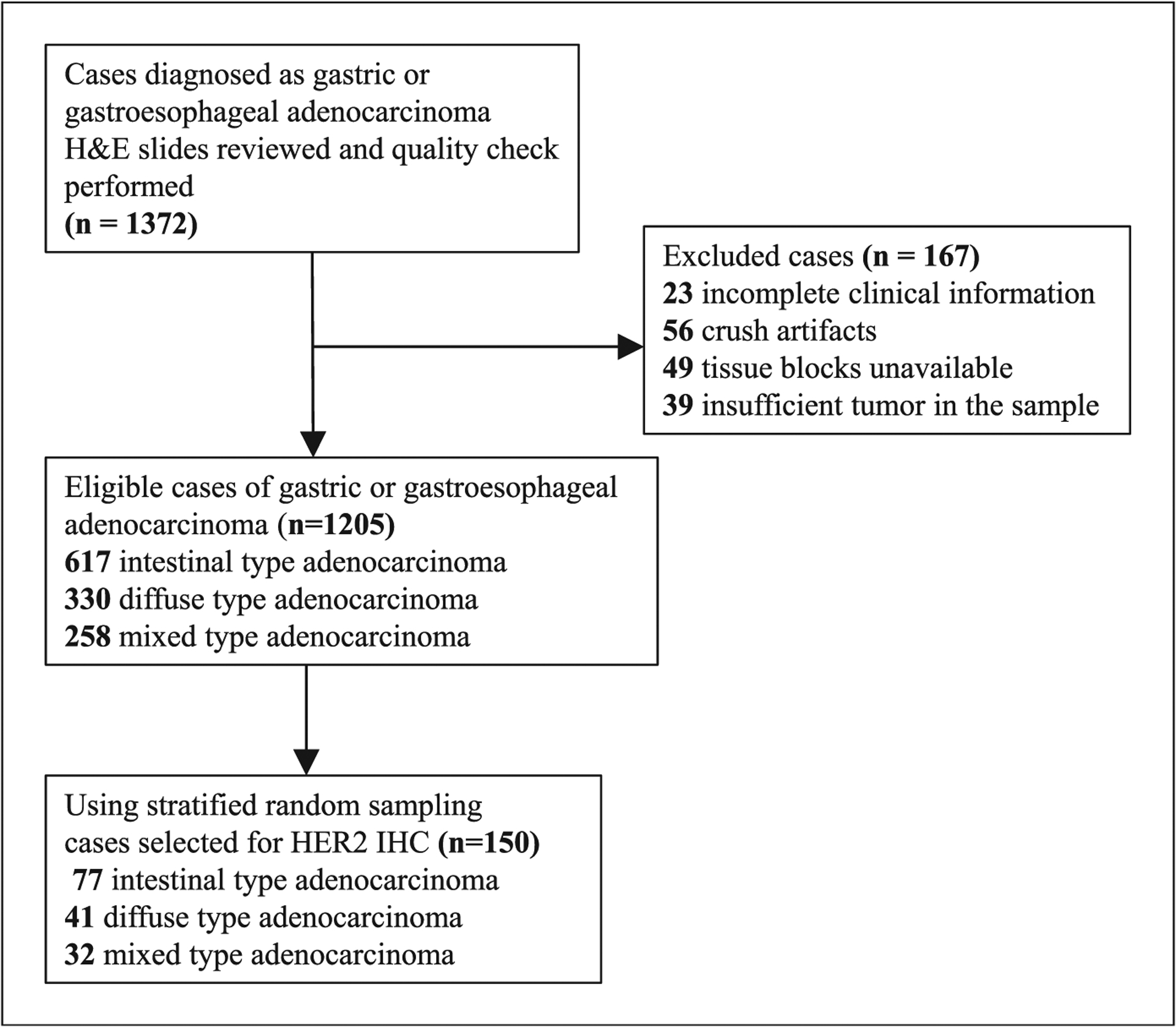
Sample collection and human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) testing. Abbreviation: H&E, hematoxylin-eosin.
Histopathologic and Immunohistochemical Studies
H&E slides were retrieved, or, if unavailable, the formalin-fixed, paraffin-embedded tissue block was sectioned at 4 μm and the section was stained with H&E. The H&E slides were reviewed independently by 2 pathologists who were blinded to clinical information and the previous histologic diagnosis.
IHC was performed in the Central Pathology Laboratory at MNH in Tanzania by manual morphometry as previously described.13 Formalin-fixed, paraffin-embedded tissue blocks were sectioned at 4 μm, deparaffinized in xylene, and rehydrated through descending grades of alcohol. The slides were then rinsed in running tap water for 1 hour. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 minutes and then rinsed in tap water for 5 minutes twice. Heat-induced epitope retrieval was performed using a microwave and Pharmingen Retrievagen A (BD Biosciences, San Jose, California) and heated to 95°C. The slides were incubated at 95°C for 10 minutes, and then allowed to slowly cool to room temperature for 20 minutes. The slides were rinsed in phosphate buffer solution for 5 minutes, 3 times. Then, the primary antibody (Polyclonal Rabbit Anti-Human c-erbB-2 Oncoprotein, ready-to-use, Cat A0485, Dako) was added onto the tissue slides and was incubated for 1 hour. The slides were rinsed in phosphate buffer solution for 5 minutes, and then 2 drops of horse rabbit peroxidase were added to each section for 30 minutes. The slides were again washed with phosphate buffer solution for 5 minutes. Phosphate buffer solution was drained from the sections. This was followed by adding 2,3-diaminobenzidine to the sections and incubating them for 5 minutes. The sections were washed in distilled water, counterstained in hematoxylin for 10 seconds, and differentiated by 2 dips into 1% acid alcohol. The sections were blued in warm water for 2 minutes, dehydrated in ethanol, and then cleared in xylene for 10 minutes. Positive and negative controls were performed simultaneously. Previously tested HER2+ breast cancer tissue was used as a positive control and normal lymph node tissue was used as a negative control.
HER2 status was scored independently by 2 pathologists using College of American Pathologists/American Society of Clinical Oncology scoring guidelines.14 A score of 0 was defined as no reactivity in any tumor cells in biopsy specimens, or no reactivity or membranous reactivity in <10% of tumor cells in surgical specimens. A score of 1+ was defined as tumor cell cluster with faint or barely membranous reactivity in biopsy specimens, or faint/barely perceptible membranous reactivity in ≥10% of tumor cells in surgical specimens. A score of 2+ was defined as tumor cell cluster with weak to moderate, complete basolateral or lateral membranous activities irrespective of percentage of tumor cells stained, or weak to moderate, complete basolateral or lateral membranous reactivity in ≥10% of tumor cells in surgical specimens. A score of 3+ was defined as tumor cell cluster with strong, complete basolateral or lateral membranous activity irrespective of percentage of tumor cells stained, or strong, complete basolateral or lateral membranous reactivity in ≥10% of tumor cells in surgical specimens. In cases where there was disagreement, a pathologist subspecializing in molecular and gastrointestinal pathology served as the adjudicator. Final determination or HER2 expression was reported according to existing standards for gastric and gastroesophageal cancers: negative (0, 1+), equivocal (2+), or positive. (3+).
Statistical Analysis
De-identified data were electronically transcribed from the data collection sheets into SPSS for analysis (version 20, SPSS Inc). Relationships between clinical and pathologic variables and HER2 IHC results were assessed using χ2 and Fisher exact tests, where appropriate. Statistical significance was declared for all P values <.05.
RESULTS
A total of 1372 gastric or gastroesophageal specimens with a diagnosis of adenocarcinoma were identified in the pathology database at MNH. A total of 167 specimens were excluded because of unavailable clinical data (23 of 167; 13.8%), crush or processing artifacts precluding morphologic evaluation (56 of 167; 33.5%), missing formalin-fixed, paraffin-embedded tissue blocks (49 of 167; 29.3%), or insufficient tumor in the biopsy (39 of 167; 23.4%; Figure 1). The H&E slides and clinical data of the remaining 1205 samples were reviewed and categorized into histologic subtypes based on the Lauren classification (intestinal, diffuse, and mixed). Through stratified random sampling, 150 cases were selected, proportionally representing each histologic subtype (Figure 1).
The demographic and histopathologic characteristics of patients are summarized in Table 1. The median age of the patients was 56.5 years (range, 19–97 years). Most patients were male, 65.3% (98 of 150). Most of the samples were resection specimens (109 of 150; 72.7%), and 41 of 150 samples (27.3%) were endoscopic biopsies. There were 123 of 150 cases (82.0%) that were designated as gastric, and 27 of 150 (18.0%) as gastroesophageal junction. Intestinal type adenocarcinoma was the most common subtype, 51.3% (77 of 150), followed by diffuse type, which comprised 27.3% of cases (41 of 150). Furthermore, we observed that the distribution of tumor grade was fairly even in which 36.0% (54 of 150) of gastric or gastroesophageal adenocarcinomas were moderately differentiated (grade 2), 33.3% (50 of 150) were poorly differentiated (grade 3), and 30.6% (46 of 150) were well differentiated (grade 1).
Table 1.
Demographic and Histopathologic Characteristics of the Patients and Human Epidermal Growth Factor Receptor 2 (HER2) Overexpression (N = 150)
| Variable | Frequency (n) | Percentage (%) |
|---|---|---|
| Age | ||
| 19–40 y | 23 | 15.3 |
| 41–60 y | 72 | 48.0 |
| ≥61 y | 55 | 36.7 |
| Sex | ||
| Male | 98 | 65.3 |
| Female | 52 | 34.7 |
| Histologic subtype | ||
| Intestinal type | 77 | 51.3 |
| Diffuse type | 41 | 27.3 |
| Mixed type | 32 | 21.4 |
| Histologic grade | ||
| Well differentiated | 46 | 30.7 |
| Moderately differentiated | 54 | 36.0 |
| Poorly differentiated | 50 | 33.3 |
| Type of specimen | ||
| Biopsy | 41 | 27.3 |
| Resection | 109 | 72.7 |
| Tumor location | ||
| Gastric | 123 | 82.0 |
| Gastroesophageal junction | 27 | 18.0 |
| HER2 status | ||
| Negative (0 and 1+) | 139 | 92.7 |
| Equivocal (2+) | 2 | 1.3 |
| Positive (3+) | 9 | 6.0 |
The overall rate of HER2 3+ positivity was 6.0% (9 of 150; Figure 2, A and B). In addition, 2 cases (1.3%) were noted to demonstrate equivocal (2+) expression of HER2 (Figure 2, C and D), and the remaining 92.7% (139 of 150) were negative (0 and 1+; Figure 2, E and F; Table 1). Most of the HER2+ cases were from gastric specimens (8 of 123; 6.5%), whereas 1 of 27 cases (3.7%) was from the gastroesophageal junction (P > .99). HER2 expression was positive in 7 of 41 biopsy cases (17.1%), and only 2 of 109 of the resection cases (1.8%; P = .002).
Figure 2.
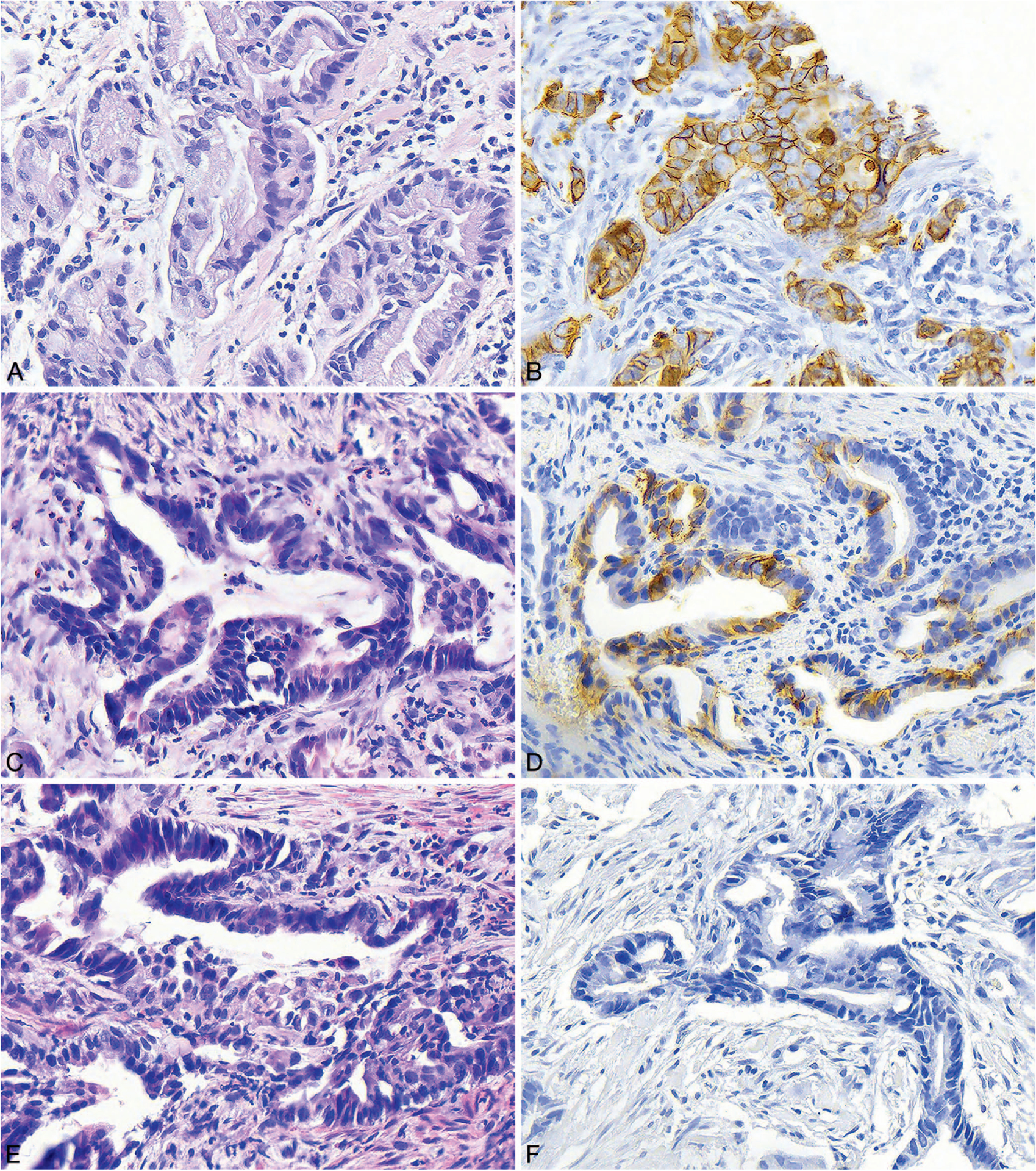
A and B, Histologic section and human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) from a biopsy specimen showing well-differentiated, intestinal-type gastric adenocarcinoma. The IHC shows malignant glands with strong 3+ staining. C and D, Histologic section and HER2 IHC from a biopsy specimen showing moderately differentiated, intestinal-type gastric adenocarcinoma. The IHC shows malignant glands with equivocal 2+ staining. E and F, Histologic section and HER2 IHC performed on a biopsy specimen showing moderately differentiated, intestinal-type gastric adenocarcinoma. The IHC shows malignant glands with no staining (hematoxylin-eosin, original magnification ×400 [A, C, and E]; HER2 IHC, original magnification ×400 [B, D, and F]).
We then evaluated the association of demographic and histopathologic characteristics with HER2 positivity (Table 2). HER2 overexpression was not associated with age (P = .79). Likewise, there was no association between sex and HER2 positivity (P = .42). Although 77.8% (7 of 9) of HER2+ gastric/gastroesophageal cancers were observed in men, most of the patients in this cohort were also male. We also found that there was association between HER2 positivity and intestinal type (P = .01) as well as well-differentiated adenocarcinoma (P < .001). Among the 9 cases that were determined to be positive for HER2, all were of intestinal type, and 8 cases were well differentiated (Figure 2, A and B).
Table 2.
Association of Demographic and Histopathologic Characteristics With Human Epidermal Growth Factor Receptor 2 (HER2) Overexpression (3+ Only) for Patients With Gastric Carcinoma (N = 150)
| Variable | HER2 Status | P Value | |
|---|---|---|---|
| Positive, No. (%) | Negative, No. (%) | ||
| Age | .80 | ||
| 19–40 y | 2 (8.7) | 21 (91.3) | |
| 41–60 y | 3 (4.2) | 69 (95.8) | |
| ≥61 y | 4 (7.3) | 51 (92.7) | |
| Sex | .42 | ||
| Male | 7 (7.1) | 91 (92.9) | |
| Female | 2 (3.8) | 50 (96.2) | |
| Histologic subtype | .01 | ||
| Intestinal type | 9 (11.7) | 68 (88.3) | |
| Diffuse type | 0 (0.0) | 41 (100.0) | |
| Mixed type | 0 (0.0) | 32 (100.0) | |
| Histologic grade | |||
| Well differentiated | 8 (17.4) | 38 (82.6) | |
| Moderately differentiated | 1 (1.9) | 53 (98.1) | |
| Poorly differentiated | 0 (0.0) | 50 (100.0) | |
| Type of specimen | .002 | ||
| Biopsies | 7 (17.1) | 34 (82.9) | |
| Resection | 2 (1.8) | 107 (98.2) | |
| Tumor location | >.99 | ||
| Gastric | 8 (6.5) | 115 (93.5) | |
| Gastroesophageal junction | 1 (3.7) | 26 (96.3) | |
DISCUSSION
Although most patients in Tanzania present with advanced gastric cancer and HER2 testing is recommended by the National Cancer Comprehensive Network Harmonized Guidelines for sub-Saharan Africa, Tanzania’s current national cancer guidelines do not include recommendations for routine HER2 testing and do not make specific recommendations regarding the use of targeted therapies, including trastuzumab or trastuzumab biosimilars.15,16 Trastuzumab has been shown to increase overall survival in patients with advanced HER2+ gastric cancer. As a result, HER2 testing is now recommended as part of the standard diagnostic evaluation for advanced gastric cancers in developed countries.14 The omission of HER2 testing in gastric cancer from the recently published Tanzanian National Cancer Treatment Guidelines is likely attributable to the lack of routine access to trastuzumab in this setting. However, following the addition of trastuzumab and trastuzumab biosimilars to the WHO Essential Medicines list, it is important to assess the rate of HER2 positivity in gastric cancers in Tanzania to determine the utility of routine HER2 testing.
In this study, more than half of gastric cancers were intestinal type, with diffuse-type carcinomas as the second most common. Other studies conducted globally across a diverse array of populations have also reported a similar distribution of histologic subtypes.5,6,8,9,17–25 We also noted the distribution among categories of differentiation was fairly even, which is similar to a study conducted in India, but not in East Asian or Western populations, which have higher proportions of poorly differentiated carcinomas (55%–65%).17,18,25–27 Our results demonstrated that HER2 positivity was primarily found in intestinal-type gastric cancers, which also correlated with prior studies.19,24,28–30 Our group observed a higher rate of HER2 positivity in intestinal than in diffuse-type carcinomas, which is also similar to the reported literature, including the ToGA trial, which found that 34% of intestinal type gastric cancers were HER2+, 6% of diffuse type, and 20% of mixed type.24
The HER2 positivity rate in gastric cancers differs widely across various populations. In our cohort, the HER2 positivity rate of 6% is lower than what has been previously reported in most regions, including in prior studies conducted in sub-Saharan Africa. In studies performed in Ghana and Nigeria it was found that the prevalence of HER2 expression was 41.4% (41 of 99) and 11% (4 of 36), respectively.9,10 Furthermore, in the ToGA study, which included patients from Europe, Africa, Australia, and South America, the overall HER2 positivity rate was 22.1%.24 Although the differences in HER2 positivity are unknown, there may be a number of contributing factors, which include variations in tumor biology due to differences in genetic ancestry as well as tumor heterogeneity and sampling, but also technical variables, such as specimen handling, fixation, IHC technique, and interpretation.
Of note, studies from Kenya, by Ali Hussein et al,6 and Zambia, by Kasochi et al,8 which are both neighboring countries of Tanzania, reported rates of HER2 positivity at 42.4% (28 of 66) and 22.8% (13 of 57), respectively. Moreover, the study in Zambia observed that moderately differentiated, rather than well-differentiated, adenocarcinoma had the highest rates of HER2 overexpression. Although the etiology of this difference is unclear, several preanalytic and analytic variables may be considered. In the study conducted by Kasochi et al,8 49.1% (28 of 57) of the patients were female, as opposed to 34.7% (52 of 150) in our cohort, and their cohort (47.3%; 27 of 57) had more individuals older than 60 years than in the current study (36.7%; 55 of 150). However, there was no statistically significant association between age, sex, or anatomic site and HER2 overexpression in the current study as well as the studies conducted in Zambia and Kenya. Sample type may have been another preanalytic variable that contributed to the differences observed. In particular, most the of cases in the studies from Zambia and Kenya were endoscopic biopsies as opposed to surgical resections. Formalin permeates biopsy specimens more readily, and they are less subject to variations in handling and fixation than surgical specimens. Analytic variables that may have also impacted IHC results include differences in scoring criteria and IHC technique. Although the studies in Zambia and Tanzania used the Ruschoff/Hofmann method for scoring,31 it is unclear what scoring criteria were used in the study by Ali Hussein et al.6 In addition, all 3 laboratories used different HER2 immunohistochemical antibodies, and the laboratory in Zambia adhered to the manufacturer’s instructions rather than conducting a site-specific immunohistochemical stain validation.
Limitations in the study include its retrospective design. Because of the limitations in resources, a larger cohort of gastric and gastroesophageal adenocarcinomas could not be tested by HER2 IHC. Because most patients present with clinically advanced stage gastric cancer, it was difficult to definitively determine whether the tumor arose from the stomach or gastroesophageal junction in many cases. In addition, studies have shown that both delayed fixation and prolonged fixation can significantly reduce HER2 IHC staining.14,32,33 The formalin fixation process itself can also affect HER2 IHC results by masking antigen epitopes, which can sometimes be difficult to reverse during the antigen retrieval step.33 These variables may have contributed to a low HER2 positivity rate. Although it is unclear why a higher number of HER2+ cases was observed among biopsy specimens than in surgical specimens, this difference may be attributable to better formalin penetration and shorter ischemic times in biopsy samples than in resection specimens. Intratumoral heterogeneity may also impact interpretation of HER2 expression in small tissue samples, such as endoscopic biopsies. Moreover, fluorescence in situ hybridization was not available to adjudicate equivocal IHC (2+) results; thus, a small number of cases may have been reported as false negatives.
CONCLUSIONS
In summary, this study provides valuable pilot data on HER2 positivity in gastric and gastroesophageal cancers in Tanzania, which will need to be validated in larger prospective studies. We found that HER2 overexpression was primarily seen in intestinal-type and well-differentiated tumors. Therefore, prioritizing HER2 testing to patients with intestinal-type, well-differentiated, or moderately differentiated gastric and gastroesophageal adenocarcinomas may be appropriate in Tanzania in efforts to allocate testing for patients who are most likely to benefit from trastuzumab therapy. However, a larger prospective cohort study will be critical in establishing recommendations for HER2 diagnostic testing in gastric and gastroesophageal cancers in sub-Saharan Africa and other resource-limited settings.
Acknowledgments
Funded by a Pilot Award from the Global Cancer Program at the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center. Vuhahula and Ng receive research support from Cepheid Inc. The funding is not related to this manuscript.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Mabula JB, Mchembe MD, Koy M, et al. Gastric cancer at a university teaching hospital in northwestern Tanzania: a retrospective review of 232 cases. World J Surg Oncol. 2012;10:1–10. doi: 10.1186/1477-7819-10-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 4.Bazargani YT, de Boer A, Schellens JHM, Leufkens HGM, Mantel-Teeuwisse AK. Essential medicines for breast cancer in low and middle income countries. BMC Cancer. 2015;15(1):1–8. doi: 10.1186/s12885-015-1583-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22(19):4619–4625. doi: 10.3748/wjg.v22.i19.4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali Hussein A, Emily R, Tm O, Ndaguatha P. HER2/Neu protein overexpression in patients with gastric and gastro-esophageal junction carcinoma seen at Kenyatta National Hospital, Kenya. J Carcinog Mutagen. 2014;5:1–10. [Google Scholar]
- 7.Kitinya JN, Lauren PA, Jones ME, Paljarvi L. Epidemiology of intestinal and diffuse types of gastric carcinoma in the Mount Kilimanjaro area, Tanzania. Afr J Med Sci. 1988;17(2):89–95. [PubMed] [Google Scholar]
- 8.Kasochi C, Julius P, Mweemba I, Kayamba V. Human epidermal growth factor receptor 2 overexpression in gastric and gastroesophageal junction adenocarcinoma in patients seen at the University Teaching Hospital, Lusaka, Zambia. Afr Health Sci. 2020;20(4):1857–1864. doi: 10.4314/ahs.v20i4.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpong DL, Asmah RH, Krampah C, et al. HER-2 Protein overexpression in patients with gastric and oesophageal adenocarcinoma at a tertiary care facility in Ghana. Sci World J. 2018;2018:1564150. 10.1155/2018/1564150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogun GO, Afuwape OO, Ayandipo OO, Oluwasola OA. HER2 expression status in gastric carcinomas in Ibadan, Nigeria: a preliminary study using immunohistochemistry. Niger Postgrad Med J. 2014;21(3):231–234. [PubMed] [Google Scholar]
- 11.Lauren P The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. Doi: 10.1111/apm.1965.64.1.31 [DOI] [PubMed] [Google Scholar]
- 12.Bosman F, Carneiro F, Hruban R, Theise N. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: World Health Organization; 2010. [Google Scholar]
- 13.Mwakigonja AR, Lushina NE, Mwanga A. Characterization of hormonal receptors and human epidermal growth factor receptor-2 in tissues of women with breast cancer at Muhimbili National Hospital, Dar es Salaam, Tanzania. Infect Agents Cancer. 2017;12:60. doi: 10.1186/s13027-017-0170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartley AN, Washington MK, Ventura CB, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Arch Pathol Lab Med. 2016;140(12): 1345–1363. doi: 10.5858/arpa.2016-0331-CP [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Comprehensive Network Guidelines Harmonized Guidelines for Sub-Saharan Africa: breast cancer: version 1.2019. https://www.nccn.org/global/what-we-do/harmonized-guidelines. Accessed June 23, 2021.
- 16.Ministry of Health, Community Development, Gender, Elderly and Children, the United Republic of Tanzania. National Cancer Treatment Guidelines. January 2020. https://www.orci.or.tz/wp-content/uploads/2020/02/National-Cancer-Treatment-Guidelines.pdf. Accessed October 25, 2021.
- 17.Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW, Heiss MM. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol. 2000;18(11):2201–2209. doi: 10.1200/JCO.2000.18.11.2201 [DOI] [PubMed] [Google Scholar]
- 18.Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value–conclusions from 924 cases of two independent series. Cell Oncol. 2010;32(1–2):57–65. doi: 10.3233/CLO-2009-0497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jørgensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer–a systematic analysis of data from the literature. J Cancer. 2012;3:137–144. doi: 10.7150/jca.4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KC, Koh YW, Chang HM, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18(10): 2833–2840. doi: 10.1245/s10434-011-1695-2 [DOI] [PubMed] [Google Scholar]
- 21.Kurokawa Y, Matsuura N, Kimura Y, et al. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 2015;18(4):691–697. doi: 10.1007/s10120-014-0430-7 [DOI] [PubMed] [Google Scholar]
- 22.Baykara M, Benekli M, Ekinci O, et al. clinical significance of HER2 overexpression in gastric and gastroesophageal junction cancers. J Gastrointest Surg. 2015;19(9):1565–1571. doi: 10.1007/s11605-015-2888-y [DOI] [PubMed] [Google Scholar]
- 23.Janjigian YY, Werner D, Pauligk C, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA international collaborative analysis. Ann Oncol. 2012;23(10):2656–2662. doi: 10.1093/annonc/mds104 [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Bang YJ, Feng-yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–484. doi: 10.1007/s10120-014-0402-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy PS, Nyodu T, Hazarika M, et al. Prevalence of HER2 expression and its correlation with clinicopathological parameters in gastric or gastroesophageal junction adenocarcinoma in north-east Indian population. Asian Pac J Cancer Prev. 2019;20(4):1139–1145. doi: 10.31557/APJCP.2019.20.4.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng F, Liu J, Wang F, et al. Prognostic value of differentiation status in gastric cancer. BMC Cancer. 2018;18(1):865. doi: 10.1186/s12885-018-4780-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajadurai P, Fatt HK, Ching FY. Prevalence of HER2 positivity and its clinicopathological correlation in locally advanced/metastatic gastric cancer patients in Malaysia. J Gastrointest Cancer. 2018;49(2):150–157. doi: 10.1007/s12029-017-9921-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J, Zhang J, Zhang T, Zheng Z. Clinicopathological and prognostic significance of HER2 overexpression in gastric cancer: a meta-analysis of the literature. Tumor Biol. 2014;35(5):4849–4858. doi: 10.1007/s13277-014-1636-3 [DOI] [PubMed] [Google Scholar]
- 29.Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes–a systematic review. Int J Cancer. 2012;130(12):2845–2856. doi: 10.1002/ijc.26292 [DOI] [PubMed] [Google Scholar]
- 30.Gu J, Zheng L, Wang Y, Zhu M, Wang Q, Li X. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumor Biol. 2014;35(6):5315–5321. doi: 10.1007/s13277-014-1693-7 [DOI] [PubMed] [Google Scholar]
- 31.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805. doi: 10.1111/j.1365-2559.2008.03028.x [DOI] [PubMed] [Google Scholar]
- 32.Yamashita-Kashima Y, Shu S, Yorozu K, et al. Importance of formalin fixing conditions for HER2 testing in gastric cancer: immunohistochemical staining and fluorescence in situ hybridization. Gastric Cancer. 2014;17(4):638–647. doi: 10.1007/s10120-013-0329-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moatamed NA, Nanjangud G, Pucci R, et al. Effect of ischemic time, fixation time, and fixative type on HER2/neu immunohistochemical and fluorescence in situ hybridization results in breast cancer. Am J Clin Pathol. 2011;136(5):754–761. doi: 10.1309/AJCP99WZGBPKCXOQ [DOI] [PubMed] [Google Scholar]


