Abstract
Does use of Facial nerve monitors during parotidectomy decrease incidence of facial paralysis/paresis without use of facial paresis? This study was done to compare the incidence, grade and risk factors of facial palsy in patients undergoing parotidectomy for benign parotid lesions with and without use of facial nerve monitor. This is a retrospective study. Eighty parotid patients operated for benign parotid lesions from 2013 to 2020 were retrospectively analysed. Demography details, history of the patients, history of addictions, clinical examination findings, investigation findings like the biopsy report, FNAC report, imaging i.e., CT / MRI / USG, use of intraoperative facial nerve monitor, time taken to identify the facial nerve, postoperative facial nerve palsy, facial nerve stimulation test and recovery time were analysed. Fifty patients were operated without use of facial nerve monitor, and 30 patients were operated using facial nerve monitor. Postoperative facial nerve complications were seen in 28 out of 80 patients (35%). Postoperative facial nerve complications were observed in 5 out of 30 patients (20%) in whom facial nerve monitoring was used. Marginal mandibular nerve palsy was observed in 4 patients and 1 patient had weakness of both marginal mandibular and orbital branches. While in postoperative facial nerve complications were observed in 25 out of 50 patients (50%), marginal mandibular nerve palsy was observed in 15 patients (40%), grade 3 facial palsy was observed in 3 out of 50 patients (6%), and grade 4 facial palsy were observed in 2 out of 50 patients (4%). The use of intraoperative FNM significantly lowered the incidence of paralysis.
Keywords: Parotidectomy, Benign lesions, Postoperative facial palsy, Intraoperative facial nerve monitoring, Incidence, Factors
Introduction
Facial nerve palsy is the most feared complication of parotid surgery which may significantly impact the quality of life of the patient. Temporary facial nerve palsy occurs in 20–40% of patients undergoing parotid surgery, and permanent facial nerve palsy is seen in 0–4% of patients [1]. Marginal mandibular nerve is the most commonly affected branch of facial nerve injured during parotidectomy [2].
Facial nerve injury during parotid surgery is usually due to nerve division, stretching of nerve, compression of nerve, ligature entrapment, thermal and electrical burns and ischemia of the nerve [3]. This occurs due to failure to identify the facial nerve trunk or its branches or inadequate haemostasis or careless technique [3]. The operating surgeon has control over most of these causes of facial nerve injury, and safe surgical technique can prevent facial nerve injury during parotidectomy.
Facial nerve monitoring is an adjunctive method to prevent both temporary and permanent facial nerve palsy during parotidectomy [4]. It can help the operating surgeon in early identification of facial nerve, warning to the surgeon of unpredicted facial nerve stimulation, delineate the course of the facial nerve, depletion of mechanical trauma to the facial nerve and assessment and prognosis of facial nerve function at the end of the procedure. However, facial nerve monitor cannot replace sound anatomical knowledge [5].
In this retrospective analysis we aim to see usefulness of facial nerve monitor during parotidectomy.
Material and Methods
This is a retrospective review of charts of patients presenting to the with parotid tumours from January 2013 to January 2020.
All consecutive patients undergoing parotidectomy (superficial and total) for parotid lesions presenting with intact facial nerve and who underwent superficial/total parotidectomy under general anaesthesia performed by single surgeon during the period of January 2013 to January 2020 were included in this study.
A total of 100 patients operated during this period. Patients with parotid tumours who had preoperative facial nerve weakness or those requiring facial nerve sacrifice for disease clearance were excluded.
Charts of all the patients were reviewed and data with respect to demographic details, history of the patients’ complaints, history of addictions, clinical examination findings, documentation of various investigation findings like the biopsy report, FNAC report, imaging i.e., CT / MRI / USG, operative records, House-Brackmann Grading [6] for facial paralysis/weakness in immediate post op period and House-Brackmann Grading at 3 months were used for analysis.
House-Brackmann grading:
Grade I: Normal
Grade II slight dysfunction: Forehead motion, mod to good function; eye, complete closure with minimal effort; mouth, slight asymmetry
Grade III (moderate dysfunction): Forehead motion, slight to moderate movement; eye, complete closure with effort; mouth, slightly weak with maximum effort
Grade IV (moderate to severe dysfunction): forehead motion, none; eye, incomplete closure; mouth, asymmetric with maximum effort
Grade V (severe dysfunction): forehead motion, none; eye, incomplete closure; mouth, slight movement
Grade VI (total paralysis): No movement
Only 80 patients had a post op follow up data for at least 3 months. In 30 patients from 2017–2020 (Group I) nerve monitoring was used to assist the surgeon for identification of facial nerve and in 50 patients from 2013–2018 (Group II) no monitor was used for identification of facial nerve.
Procedure for Nerve Monitoring
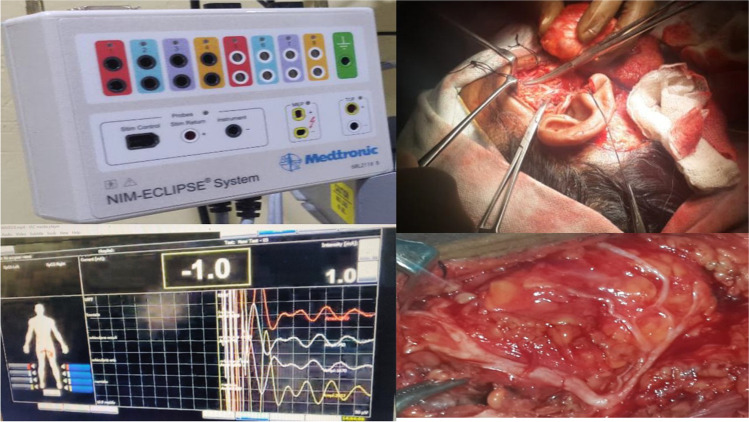
Intraoperative facial nerve monitoring was performed using NIM ECLIPSE device from MEDTRONIC XOMED, INC. No muscle relaxant was used during the procedure. Needle electrodes were placed to record activity from the facial muscles typically in the 4 areas innervated by the facial nerve: Frontal, zygomatic, buccal, and marginal mandibular and facial muscles monitored were frontalis, orbicularis oculi, orbicularis oris and mentalis. Dual needle electrodes were inserted in these muscles to record specific muscle activity, and other end of the electrode was connected to recording box of the NIM system. Ground electrode was placed over chest wall near sternum. Stimulator anode electrode was placed in the nearby muscles in operating field; the one end of the electrode was connected to the circuit box. A stimulation bipolar probe was used on the sterile operative field for precise stimulation of a discrete area. The current was kept at 1 mA and the probe was used to touch the surgical field in the vicinity of the nerve. If facial nerve is nearby, current will stimulate the nerve, causing muscle contraction. This contraction is recorded by the needle electrode placed in the muscle, shown on the monitor as a signal depicting action potential signifying the integrity of facial nerve. Typical parameters used were as follows: stimulus intensity, 0.5 to 2 mA; duration, 100 microseconds; rate 4bursts/s; and event threshold, 100 microvolts (Fig. 1).
Fig. 1.
showing intraoperative facial nerve monitoring during parotidectomy
Results
A total of 100 patients operated during period of January 2013 to January 2020 were operated for parotid tumours. Twenty patients had no available follow-up data and so were excluded, and so charts of 80 patients were included for final analysis.
We had 30 patients in group I (facial monitor group) and 50 patients in group II (no facial monitor group). The mean age of the patients in Gr I was 42.3 years (18–60 years), and the mean age of patients in group II was 49.5 (20–65 years).
Of the total of 80 patients, there were 46 females (57.5%) and 34 males (42.5%). In group I, there was 17 females (56.6%) and 13 males (43.4%), while in group II, there was 29 females (58%) and 21 males (42%).
Of the 80 parotid tumours, 48 patients (60%) had left sided lesion, and 32 (40%) had right sided lesion. In group I, 18 out of 30 patients (60%) had left-sided parotid lesion, and 12 out of 30 patients (40%) had right-sided parotid lesion, while in group II, 30 out of 50 patients (60%) had left-sided parotid tumour, and 20 out of 50 patients (40%) had right-sided parotid tumour. Superficial lobe of the parotid gland was involved in 80/80 (100%), and superficial parotidectomy was done in all patients.
Preoperative fine needle cytology was done in all patients. Pleomorphic adenoma was the most common diagnosis seen in 70/80 (87%) patients, 4 patients had Warthin’s tumour, 3 had facial nerve schwannoma, 2 had Basal cell adenoma, and 1 patient had Lipoma of parotid region.
Facial nerve function was assessed by House-Brackmann grading scale during immediate postoperative period and 3 months after surgery for permanent weakness.
On day 1, in group I (FNM), 5 out of 30 patients (16.6%) had immediate facial nerve weakness, marginal mandibular nerve weakness seen in 4/30 patients (16.6%), and 1 out of 30 patients (3.3%) had Gr IV palsy. While in group II (WIFNM), 20 out of 50(40%) had immediate facial nerve weakness, out of which marginal mandibular weakness was seen in 15 out of 50 patients (30%), 3 out of 50 patients (8%) had gr III, and 2 out of 50 had GR IV palsy (p value < .05).
At 3 months in the FNM group only, 1 out of 30 had persistent facial nerve palsy which was GR IV, while in WFNM group, 4 out of 50 had persistent facial nerve palsy of which 3 had GR III and 1 had Gr IV facial palsy (Table 1).
Table 1.
Faial nerve complications in both groups assessed by House-Brackmann grading
| Day | FNM | WFNM | ||
|---|---|---|---|---|
| Day 1 | Facial palsy | No facial palsy | Facial palsy | No facial palsy |
|
5/30 (16.6%) Marginal mandibular palsy 4/30 (13.3%) Gr I-3 Gr II-1 Gr III-0 Gr IV-1 |
25/30 (83%) |
20/50 (40%) Marginal mandibular palsy 15/50 (30%) Gr I-10 Gr I-5 Gr III-3 Gr IV-2 |
35/50 (70%) | |
| Day 90 | 1/30 | 29/30 (96%) |
4/50 (8%) Gr III-3 Gr IV-1 |
46/50 (92%) |
Preoperative and postoperative facial nerve stimulation test after 3 months was available for all 30 patients in group I and 35 out of 50 patients of group II. The preoperative FNST and the postoperative FNST values for all branches were compared between both study and control group. In group I (FNM), the significance in paired samples test was observed only in the mandibular nerve. (p value 0.012). In group II (WFNM,) the significance in paired samples test was observed in upper buccal nerve (p value 0.010), lower buccal nerve (p value 0.002), and mandibular nerve (p value 0.000) (Table 2).
Table 2.
Pre and postoperative FNST difference among both groups
| Facial nerve stimulation test Mean difference Pre op-post op |
FNM | WFNM |
|---|---|---|
|
Frontal nerve Zygomatic nerve |
-.025 (-.064 to -.014) p value .204 -.030 (-.082 to -.022) p value .249 |
-.135 (-.281 to -.011) p value .069 -.155 (-.341 to -.031) p value .098 |
|
Upper buccal nerve Lower buccal nerve |
-.055 (.002 to -1.99) p value .061 -.080 (-.158 to -.001) p value -.056 |
-.455 (-.789 to -.120) p value .010 -.565 (-.892 to -.237) p value -.002 |
| Mental nerve |
-.2650 (-.465 to -.064) p value .012 |
-1.06 (-1.38 to -.74) p value .000 |
Discussion
Parotidectomy with preservation of facial nerve function is the standard treatment of all benign lesions involving the parotid gland. In spite of best efforts of the surgeon, the incidence of temporary facial nerve paralysis is 20–40%, and permanent facial weakness is 0–4% [1]. Facial nerve paresis and paralysis have cosmetic and functional consequence and significantly impact the quality of the life of patient.
Intraoperative facial nerve monitoring during parotidectomy monitors the electrophysiological activity of the facial muscles and warns the surgical team by visual and audio alerts [7, 8].
The facial nerve can is injured during parotidectomy secondary to dissection, transection, laceration, clamp compression, retraction, electrocautery injury, ligature entrapment, suction trauma and ischemia. It can help the surgeon for early identification of the nerve, avoiding excessive facial nerve stimulation while dissecting over the nerve, mapping the course of facial nerve and its branches, reduces mechanical trauma over the nerve and helps to evaluate and prognosticate the function of nerve at the end of parotidectomy [8]. It is now being increasing used during parotidectomy for medicolegal reasons and also gives increased sense of safety to surgeon and has now become the standard of care [9]. But the opponents of FNM have suggested that it gives false sense of security that may result in less meticulous surgical nerve dissection [10].
In this retrospective analysis, we tried to look into the incidence of immediate and the permanent facial nerve palsy in group I (FNM) and group II (WFNM). We found significantly more incidence of immediate facial nerve weakness in group II (WFNM) compared to group I (FNM) (30% vs 16%). The incidence of marginal mandibular paresis was also found to be significantly higher in group II (WFNM) as compared to group I (FNM) (33% vs 50%) (p value < .05). The incidence of permanent facial weakness was also found to be significantly higher in group II (WFNM) when compared to group I (FNM) (3.3% vs 8%) (p value < .05).
This is consistent with results of meta-analysis published by Sood et al. [11] and Savaaset al. [12] In contrast, there are multiple studies in literature that conclude that use of a facial nerve stimulator had no effect on preventing or promoting postoperative facial nerve paralysis [13–15].
We also performed the maximum facial nerve stimulation test in both the groups preoperative and 3 months after surgery to look for evidence of electrical weakness in the branches of facial nerve. In group I (FNM), statistically significant difference was observed only in the marginal mandibular nerve, while in group II (WFNM), the statistically significant difference was seen in the upper buccal (p value .02), lower buccal (p value .07) and marginal mandibular nerve (p value .01) branches of facial nerve. This suggest that inherent weaknesses of facial nerve branches were found more in WFNM group when compared to FNM group though these electrical weakness in facial nerve branches were not apparent clinically.
We suggest that monitoring may increase the surgeon’s caution during the identification of nerve’s trunk and its major branches, resulting in lesser risk of facial nerve weakness as compared to parotidectomy done without facial nerve monitoring.
Conclusion
Facial paralysis is one of the most serious complications that can occur in parotid gland surgery. Electrophysiological facial nerve monitoring can reduce the occurrence of immediate or late facial nerve palsy for benign parotid lesions. Hence, we strongly recommend intraoperative use of FNM in primary as well as revision parotid surgery.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guntinas-Lichius O, Klussmann JP, Wittekindt C, Stennert E. Parotidectomy for benign disease at a university teaching hospital: outcome of 963 operations. Laryngoscope. 2006;116:534–540. doi: 10.1097/01.mlg.0000200741.37460.ea. [DOI] [PubMed] [Google Scholar]
- 2.Rahman MA, Alam MM, Joarder AH. Study of the nerve injury in parotid gland surgery. NepaleseJ ENT Head NeckSurg. 2011;2(1):17–19. doi: 10.3126/njenthns.v2i1.6779. [DOI] [Google Scholar]
- 3.Guntinas-Lichius O, Kick C, Klussmann JP, Jungehuelsing M, Stennert E. Pleomorphic adenoma of the parotid gland: a 13- year experience of consequent management by lateral or total parotidectomy. Eur Arch Otorhinolaryngol. 2004;261:143–146. doi: 10.1007/s00405-003-0632-9. [DOI] [PubMed] [Google Scholar]
- 4.Olsen KD, Daube JR. Intraoperative monitoring of the facial nerve: an aid in the management of parotid gland recurrent pleomorphic adenomas. Laryngoscope. 1994;104:229–232. doi: 10.1288/00005537-199402000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Wolf SR, Schneider W, Suchy B, Eichhorn B. Intraoperative facial nerve monitoring in parotid surgery. HNO. 1995;43:294–298. [PubMed] [Google Scholar]
- 6.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 7.Meier JD, Wenig BL, Manders EC, Nenonene EK. Continuous intraoperative facial nerve monitoring in predicting postoperative injury during parotidectomy. Laryngoscope. 2006;116:1569–1572. doi: 10.1097/01.mlg.0000231266.84401.55. [DOI] [PubMed] [Google Scholar]
- 8.Macdonald DB, Skinner S, Shils J, Yingling C. Intraoperative motor evoked potential monitoring—a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol. 2013;124:2291–2316. doi: 10.1016/j.clinph.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Lowry TR, Gal TJ, Brennan JA. Patterns of use of facial nerve monitoring during parotid gland surgery. Otolaryngol Head Neck Surg. 2005;133:313–318. doi: 10.1016/j.otohns.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Preuss SF, Guntinas-Lichius O. On the diagnosis and treatment of parotid gland tumors: results of a nationwide survey of ENT hospitals in Germany. HNO. 2006;54:868–874. doi: 10.1007/s00106-006-1394-7. [DOI] [PubMed] [Google Scholar]
- 11.Sood AJ, Houlton JJ, Nguyen SA, et al. Facial nerve monitoring during parotidectomy: a systematic review and metaanalysis. Otolaryngol Head Neck Surg. 2015;152:631–637. doi: 10.1177/0194599814568779. [DOI] [PubMed] [Google Scholar]
- 12.Savvas E, Hillmann S, Weiss D, Koopmann M, Rudack C, Alberty J. Association between facial nerve monitoring with postoperative facial paralysis in parotidectomy. JAMA Otolaryngol Head Neck Surg. 2016;142(9):828–833. doi: 10.1001/jamaoto.2016.1192. [DOI] [PubMed] [Google Scholar]
- 13.Grosheva M, Klussmann JP, Grimminger C. Electromyographic facial nerve monitoring during parotidectomy for benign lesions does not improve the outcome of postoperative facial nerve function: a prospective two-center trial. Laryngoscope. 2009;119:2299–2305. doi: 10.1002/lary.20637. [DOI] [PubMed] [Google Scholar]
- 14.Deneuve S, Quesnel S, Depondt J, et al. Management of parotid gland surgery in a university teaching hospital. Eur Arch Otorhinolaryngol. 2010;267(4):601–605. doi: 10.1007/s00405-009-1088-3. [DOI] [PubMed] [Google Scholar]
- 15.Meier JD, Wenig BL, Manders EC, Nenonene EK. Continuous intraoperative facial nerve monitoring in predicting postoperative injury during parotidectomy. Laryngoscope. 2006;116(9):1569–1572. doi: 10.1097/01.mlg.0000231266.84401.55. [DOI] [PubMed] [Google Scholar]