Abstract
Breast reconstruction with an autologous lower dermal sling (ALDS) is an established one-stage procedure in patients with moderate to large ptotic breasts. However, this technique is difficult to perform in small and non/minimally ptotic breasts. We describe our experiences from a single institution about a novel Advanced Autologous Lower Dermal Sling (A-ALDS) technique for reconstruction in small breasts. We performed one-stage nipple/skin sparing mastectomies in 61 patients with immediate reconstruction either by Conventional Immediate Breast Reconstruction Surgery or A-ALDS technique. Mean age of study patients was 46.9 years. We observed significantly better cosmetic score and lower immediate complication rate vis-a-vis skin necrosis, implant loss with the A-ALDS technique (i.e., nil versus 3 in Conventional Immediate Breast Reconstruction Surgery (IBRS)). Forty patients completed 12-month follow-up. The PROMs — Patient Reported Outcome Measures (Breast-Q) revealed good to excellent scores for satisfaction with breast, cosmetic outcome, and psychosocial well-being in patients operated with both these techniques. However, sexual well-being was significantly better in the A-ALDS group. The A-ALDS is a novel, cost-effective, and safe technique for immediate one-stage implant-based reconstruction for small breasts. It provides a dermal barrier flap and hence, ensures less complications, excellent cosmetic results, and patient satisfaction.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13193-022-01524-8.
Keywords: Breast cancer, Small breast, Advanced-Autologous Lower Dermal Sling, Immediate Breast Reconstruction Surgery, Patient Reported Outcome Measures
Introduction
The well-established autologous lower dermal sling (ALDS) is an ideal technique for implant-based Immediate Breast Reconstruction Surgery (IBRS) in breast cancer (BC) patients with moderate-to-large-sized breasts with significant ptosis (grade 2 +) in which the distance from the nipple to the infra-mammary fold (IMF) may be significantly greater than 5–7 cm. The ALDS provides robust stability to the implant ensuring excellent contouring, projection, and fullness of the lower pole of the reconstructed breast with correction of ptosis. This approach reduces the risk of a high-riding implant, provides excellent cosmetic results, and ensures good symmetry. Therefore, ALDS is now considered as a safe and cost-effective single-stage reconstructive technique especially in moderate-to-large ptotic breasts [1–3].
However, implant-based breast reconstruction for small and non-ptotic breasts is a surgical challenge due to non-availability of excess lower pole length that aids in the creation of an appropriately sized dermal sling. Conventionally, breast reconstruction in such scenarios is performed by placing the implant either in a complete or partial sub-muscular pocket. These techniques have been shown to result in sub-optimal cosmetic outcomes that were attributed to poor expansion and tightness of the inferior pole [4]. As a result, it is now a common practice employed by several oncoplastic surgeons to use acellular dermal matrices (ADMs) that function as a sling to cover the implant inferiorly and provide robust support to the inferior pole [4].
In developing countries such as in India, ADMs are not available and their high cost can prohibit its use in breast reconstruction. Hence, in such low-resource settings, we have initially approached reconstruction in minimally ptotic or non-ptotic small breasts by placing the implant in a sub-muscular pocket that is formed by pectoralis major muscle above, the fascia or superficial fibers of the serratus anterior muscle laterally and the fascia over rectus abdominis muscle inferiorly. Later, we developed a technique of Advanced-Autologous Lower Dermal Sling (A-ALDS) to provide a double layer of vascularized tissue cover over the implant inferiorly. It has been previously reported in the ALDS procedure that dermal sling provides an advantage over ADMs as it acts as a vascularized flap eliminating the risk of implant exposure even if superficial skin necrosis does occur [1, 2]. Therefore, the ALDS technique has been utilized routinely to improve breast reconstruction outcomes. Similarly, our modified A-ALDS technique was able to maintain the natural breast shape, projection, and symmetrization of the reconstruction with respect to the opposite breast thereby, obviating the need for contralateral surgery.
In this study, we report the application of this novel A-ALDS technique for reconstruction in patients with small breasts. Furthermore, we present the post-surgical outcomes in our study patients who have undergone either A-ALDS technique or Conventional IBRS.
We present the following article/case in accordance with the STROBE reporting checklist.
Material and Methods
Study Design
This is a longitudinal cohort study involving a retrospective analysis of prospective data from a single institution. This study was approved by an independent ethics committee associated with the institution. Patients were considered eligible for analysis if they underwent unilateral and bilateral mastectomies with implant-based IBRS (Immediate Breast Reconstruction Surgery) and fulfilled the criteria laid down for a small breast. Small breast for the purpose of this study was defined as the one with a cup size of B or smaller and/or mastectomy weight of less than 350 g with either no or normal ptosis.
Written informed consent was obtained from all patients for collection of study-relevant medical data associated with disease management and routine follow-up visits. The study recruitment period was defined as the day informed consent was obtained from the patient until 1 year of follow-up. Study sample size represented all eligible cases during the study period from year 2016 to 2018. Data collection included demography, medical history, clinicopathological characteristics, surgical notes, post-surgery evaluations, and follow-up details for Patient Reported Outcome Measures (PROMs) and aesthetic scores. To minimize bias in data collection, Standard Operating Procedures (SOPs) were implemented by well-trained researchers.
During the study period, a total of 61 BC patients with small breasts underwent implant-based IBRS at our breast unit. Three patients underwent bilateral mastectomies. Only those patients with small breasts who were recommended for mastectomy and opted for IBRS were included in the study. The selection criteria for A-ALDS (Advanced-Autologous Lower Dermal Sling) or Conventional IBRS as an appropriate surgical technique were as follows: (a) Conventional IBRS was performed when the tumor was located in the upper outer quadrant and lower quadrant close to the skin, warranting skin removal with the tumor. (b) The A-ALDS procedure for IBRS was applied to all other patients including those that had the presence of tumor in the lower pole but at an optimal distance from skin. In such situations, the skin could be preserved to create the desired dermal sling. The surgical margins — especially the anterior margins — were evaluated by frozen sections in selected cases and re-ascertained on the paraffin sections in all cases.
Out of these 61 patients, 40 completed 1-year post-surgery follow-up (22 with Conventional IBRS and 18 with A-ALDS) and were analyzed for surgical outcomes and PROMs. These patients underwent chemotherapy and/or radiation therapy treatment according to NCCN guidelines under the supervision of a multidisciplinary clinical team.
Conventional IBRS Technique
Conventional IBRS (i.e., one-stage sub-muscular implant reconstruction) involves placement of the breast implant in a sub-muscular pocket. The mastectomy is performed preserving the skin along with nipple areola complex in which the lower extent of mastectomy is the infra-mammary fold (IMF). The Conventional IBRS technique involves splitting pectoralis major muscle in the middle along its fibers. This is in contrast to the usual practice of most plastic surgeons that involves lifting the pectoralis major starting from its lateral edge and then dissecting beneath it to create a sub-muscular pocket. Laterally, the dissection is carried under the fascia/superficial fibers of serratus anterior, hence providing a continuous pocket laterally. The dissection then continues under the fascia of lower thoracic and upper abdominal wall approximately 2 cm below IMF. This procedure ensures the provision of an appropriate cover to the implant to prevent high riding and imparts fullness to the lower quadrant of the reconstructed breast. In this way, the implant is partially covered by muscle and partially by fascia. We have adopted a single-stage procedure by inserting a dual-lumen expendable implant so as to create lower pole fullness.
Advanced-Autologous Lower Dermal Sling (A-ALDS) Technique
The A-ALDS procedure is described for implant-based reconstruction in patients with small non-ptotic or minimally ptotic breasts where the distance of the lower segment of the breast is inadequate to perform the regular ALDS technique.
The A-ALDS technique begins by first marking out the usual pre-operative landmarks on the breast with appropriate measurements. The IMF is marked and the IMF-to-nipple distance is ascertained (typically small; 5 to 7 cm). The desired dermal sling (breadth of 3 to 4 cm) is marked out in a semi-circular fashion above the IMF on the lower pole of the breast. This area is de-epithelialized to constitute the lower dermal sling. The mastectomy proceeds from above the lower dermal sling and the flaps are raised in the appropriate sub-cutaneous plane maintaining the sub-dermal blood supply of the flaps. At the areola, the plane becomes more superficial and then dips into the nipple to core out the ducts as recommended [5]. The lower dermal sling is dissected off the lower breast tissue in the correct plane up to the IMF. Then, mastectomy is performed and the nipple is cored out. During this procedure, the lower dermal sling is then advanced by mobilizing the skin and sub-cutaneous tissue above the fascia of the lower thoracic and upper abdomen by 3 to 4 cm, thereby advancing the dermal sling to cover the implant. We recommend that during this mobilization the perforators of medial and lateral thoracic region should be maintained scrupulously as they contribute significantly to the vascularity of A-ALDS flap. The advanced skin mobilized (from lower thoracic and upper abdomen) equals the breadth of the dermal sling (i.e., 3 to 4 cm), maintains the required ideal length of the lower segment, and helps to symmetrize the reconstructed breast to the contralateral side. Finally, the IMF is recreated by anchoring the mobilized skin (from the lower thoracic and upper abdominal wall) with 3 sub-cutaneous sutures to the chest wall at the level of the original IMF.
After this step, a breast pocket is created by lifting up the pectoralis major muscle from the chest wall and cutting its attachments inferiorly. The pocket is continued under the fascia and superficial fibers of the serratus anterior muscles without detaching the pectoralis major muscle at its lateral attachment providing a continuous uninterrupted pocket laterally. Medially, the dissection is carried under the pectoralis major muscle up-to the medial perforators without damaging them. An appropriate size and type of dual-lumen anatomical implant is placed in the resultant sub-muscular dermal pocket. To avoid excess tension inside the sub-muscular pocket, the volume of the pocket is kept proportionate to the skin envelope and to implant dimension.
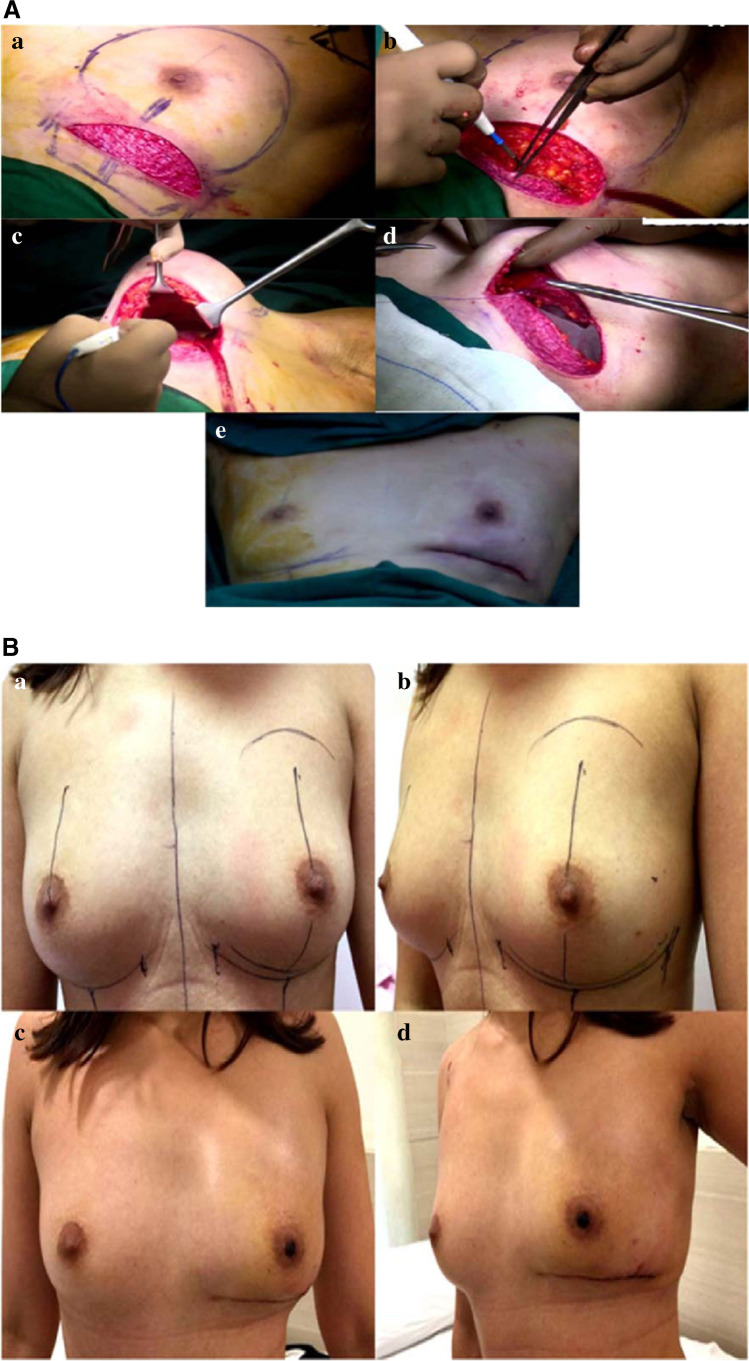
In the final surgical step, the inferior border of pectoralis major muscle and superficial fibers of serratus anterior or its fascia muscle is sutured to the de-epithelialized A-ALDS generated earlier. The suction drains are placed under skin flaps of the patient and the skin flap is sutured down at the infra-mammary crease (Fig. 1A (a)–(e)) (Online Resource 1–3).
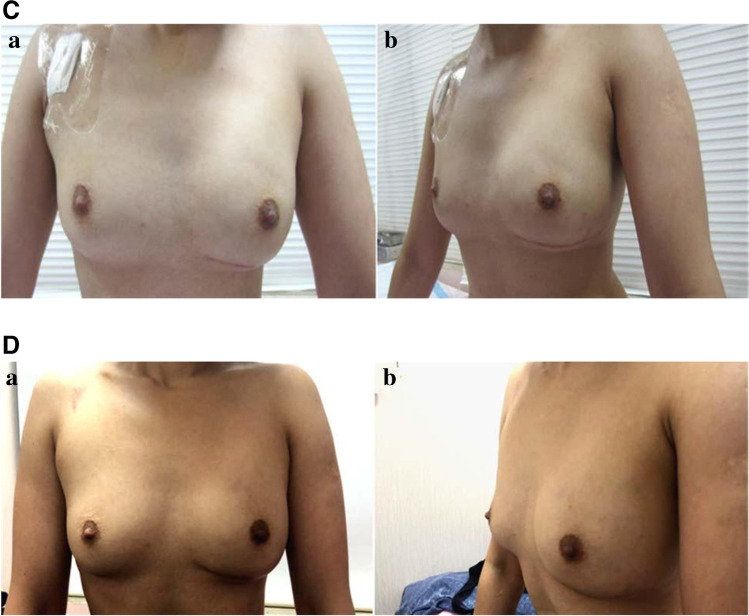
Fig. 1.
Representative case study for A-ALDS-based IBRS. A Intraoperative images. (a) De-epithelialization of lower dermal flap from IMF to 3 cm onto the breast. (b) Advancement of lower dermal flap to create of new-IMF by mobilizing skin from lower thoracic and upper abdominal wall. (c) Formation of sub-pectoral pocket. (d) Insertion of implant under pectoralis major muscle. (e) Completion of IBRS with A-ALDS procedure after skin suturing. B Pre-operative and post-operative images. (a and b) Pre-operative images. (c and d) Post-operative images after 1 month of the surgery. Lower pole flattening seen in early phase. C Post-operative image of the same patient after 3-month follow-up. (a and b) Post-operative images after 3 months of the surgery and lower pole fullness achieved. D Post-operative image of the same patient after 1-year follow-up. (a and b) Post-operative images after 1 year of the surgery
Study Assessments
Surgical outcomes were assessed by a team of onco-surgeons for post-surgery outcomes. Early complications such as hematoma, seroma, wound dehiscence, and wound infection were recorded. Complications were classified as “major” when they required surgical intervention and “minor” when they were managed conservatively. Major immediate complications include implant loss and skin dehiscence that required re-suturing. Minor complications include minor skin/wound dehiscence, minor flap necrosis healing with secondary intention, and epidermolysis.
The late complications such as capsular contracture and late infections were also noted. The capsular contracture was assessed using the modified Baker classification system [6]. While Baker 3 and 4 observations were considered as major complications, Baker 2 observations were considered as minor. We also noted the time between completion of the surgery and start of the adjuvant therapy to ascertain any delays in the adjuvant therapy.
The Patient Reported Outcome Measures (PROMs) were used to evaluate patient satisfaction and quality of life, 1 year after IBRS. We are grateful to the MAPI Research Trust for permission to use BREAST-Q (http://www.mapitrust.org). It is a standardized tool. To assess PROMs, a standardized Breast-Q questionnaire was utilized. The Breast-Q reconstruction module was divided into multiple independent scales. Higher scores indicate greater patient satisfaction and functionality [7, 8]. PROM patient interview was conducted by a well-trained study coordinator after obtaining informed consent. BREAST-Q captured meaningful and reliable information from the patients’ who could read and comprehend the English language. For the patients who had difficulty in following the questions in English the coordinator translated the PROMS questionnaire in local language.
Aesthetic outcomes were measured with 5 different variables that included breast reconstruction volume, contour, implant placement, scarring, and appearance of infra-mammary fold [9]. Post-operative cosmetic assessment was performed by the clinical team during visual inspection of the patient in the sitting position. Photographic data were scored by 3 independent clinical observers using the 3-point scale (Online Resource 4). Two of them were not directly involved with direct patient care. Aesthetic scores of 5 different variables were pooled and analyzed for statistical significance. Cosmetic score was assessed individually using the pre-determined criteria. The seven criteria included were shape with brassierie, shape without brassierie, symmetry to the opposite breast, mobility, condition of infra-mammary fold, consistency, and overall appearance [10].
Statistical Analysis
Statistical analysis was performed using Student’s t-test and a p-value of < 0.05 was considered significant.
Results
Representative Case Study
A 39-year-old patient with B-cup breasts and no ptosis presented with a lump in the left breast. On radiological investigations, it showed multicentric tumors in the upper outer quadrant and diffuse microcalcifications. She underwent nipple-areola-sparing mastectomy followed by IBRS with A-ALDS technique. Post-surgery histo-pathological examination revealed that the sentinel lymph nodes were free of tumor (0/3) and with clear tumor margins. The intraoperative, pre- and post-surgery images for this patient are depicted in Fig. 1A, B, C, and D, respectively.
Study Cohort Demography
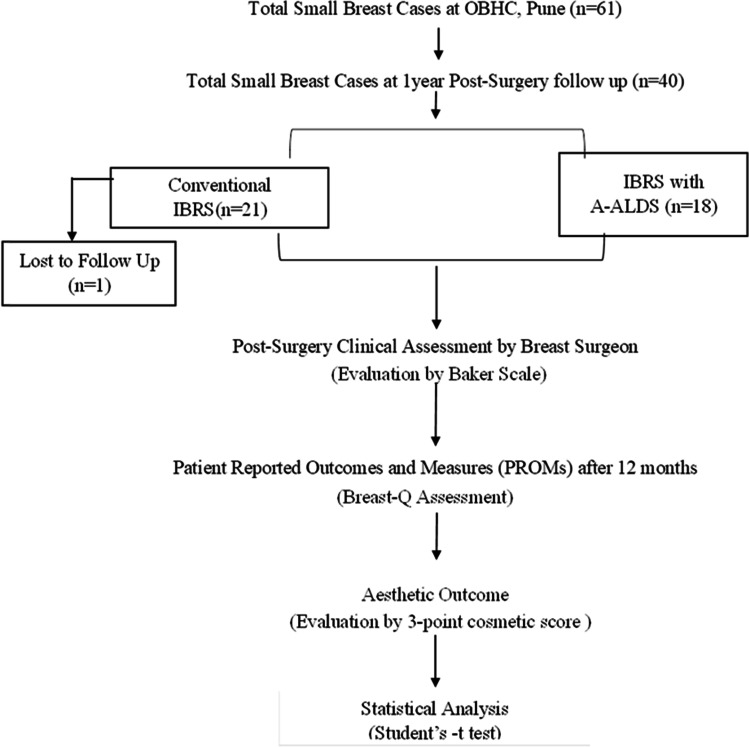
Forty patients with small breasts who completed 12-month post-surgery follow-up were included in the study. Of these, 22 patients underwent implant-based Conventional IBRS and 18 patients underwent surgeries with the A-ALDS technique. Out of 40, data of 39 patients was available. Demographic distribution of patients and clinico-pathological characteristics are summarized in Fig. 2 and Table 1. None of the patients in our study cohort experienced any delays in their adjuvant therapies.
Fig. 2.
Study flow chart: distribution of study participants
Table 1.
Demography and clinico-pathological profile of study participants
| Variables | Data sources | Class intervals | Conventional IBRS (n = 22*) Number (%) | IBRS with A-ALDS (n = 18) Number (%) |
|---|---|---|---|---|
| Age (years) | Scheduled interview (post-informed consent) | ≤ 35 | 4 (18.1) | 3 (16.7) |
| 36–50 | 7 (31.8) | 9 (44.4) | ||
| ≥ 51 | 10 (45.4) | 6 (33.3) | ||
| 45.6 | 48.2 | |||
| Tumor pathology | Clinico-pathological report (histo-pathology) | IDC | 16 (72.7) | 9 (50.0) |
| DCIS | 2 (9.1) | 2 (11.1) | ||
| IDC + DCIS | 2 (9.1) | 7 (38.8) | ||
| Others | 1 (4.5) | - | ||
| ER | Clinico-pathological report (IHC) | Positive | 18 (81.8) | 15 (83.3) |
| PR | Clinico-pathological report (histo-pathology) | Positive | 6 (27.3) | 13 (72.2) |
| Her2 | Clinico-pathological report (histo-pathology) | Positive | 3 (13.6) | 2 (11.1) |
| TNBC | Clinico-pathological report (histo-pathology) | - | 2 (9.1) | 2 (11.1) |
| Grade | Clinico-pathological report (histo-pathology) | I | 2 (9.1) | - |
| Clinico-pathological report (histo-pathology) | II | 15 (68.2) | 15 (83.3) | |
| Clinico-pathological report (histo-pathology) | III | 4 (18.2) | 3 (16.7) | |
| Stage | Pathology lab report (according to AJCC staging guidelines 7th ed) | I | 9 (40.9) | 4 (22.2) |
| Pathology lab report and according to AJCC staging guidelines 7th ed | II | 4 (18.2) | 10 (55.5) | |
| Pathology lab report and according to AJCC staging guidelines 7th ed | III | 8 (36.4) | 4 (22.2) | |
| Neo-adjuvant therapy | Medical oncologist consultation and records | 4 (18.2) | 4 (22.2) | |
| Adjuvant therapy | Medical oncologist consultation and records | 21 (95.5) | 15 (83.3) | |
| Radiation therapy | Radiation oncologist consultation and records | 12 (54.5) | 8 (44.4) |
*Data available for 21 patients only
PROMs and Aesthetic Score
PROM data was collected from the study participants at 12 months post-surgery with the Breast-Q questionnaire. Out of 40 study participants, 36 (90%) responded to the questionnaire. PROM data indicated that all study participants, irrespective of the type of reconstruction, reported good-to-excellent satisfaction for the breast cosmetic outcomes and psychosocial well-being.
However, we found that the Breast-Q parameters scored higher for A-ALDS patients as compared to Conventional IBRS.
The sexual well-being scores were significantly higher for the A-ALDS patients (62.8 ± 21.9 versus 52.2 ± 28.4) in comparison to Conventional IBRS procedure (p = 0.0139). In addition,
A-ALDS group scored significantly higher for aesthetic score over Conventional IBRS group (i.e., 6.7 ± 1.3 versus 4.6 ± 2.3, p = 0.0009) (Table 2).
Table 2.
Inter-group comparison of PROMs (Conventional-IBRS versus A -ALDS) and aesthetic score
| Serial no | Variables | Conventional IBRS | IBRS with A-ALDS | p-value | |
|---|---|---|---|---|---|
| 1 | Breast-Q | Satisfaction with breast | 69.2 ± 22.6 | 69.4 ± 14.3 | 0.7271 |
| 2 | Satisfaction with outcome | 80.0 ± 24.2 | 86.9 ± 13.1 | 0.3017 | |
| 3 | Psychosocial well-being | 71.2 ± 26.2 | 80.4 ± 21.6 | 0.2769 | |
| 4 | Sexual well-being | 52.2 ± 28.4 | 62.8 ± 21.9 | 0.0139* | |
| 5 | Aesthetic score | 4.6 ± 2.3 | 6.7 ± 1.3 | 0.0009* | |
Data: mean + S.D.; *statistically significant
Assessment of Post-IBRS Complications
No major complications (immediate or delayed) were observed in the patients that underwent A-ALDS (n = 18) procedure. However, out of the 22 patients that underwent Conventional IBRS, immediate major complications were observed in 3 patients (13.6%) with implant loss and delayed major complications in 1 patient (4.5%) with grade III capsular contracture (Table 3). No delay in time between completion of the surgery and start of the adjuvant therapy were observed in patients from both groups.
Table 3.
Summary of post-operative complications
| Variables | Conventional IBRS (n = 22) Number (%) | A-ALDS (n = 18) Number (%) | ||
|---|---|---|---|---|
| Major complications | Immediate | Implant loss | 3 (13.6) | 0 (0) |
| Skin dehiscence requiring re-suture or skin graft or minor local flap | 0 (0) | 0 (0) | ||
| Delayed | Capsular contracture: severe (grade III) | 1 (4.5) | 0 (0) | |
| Total | 4 (18.1) | 0 (0) | ||
| Minor complications | Capsular contracture: mild (grade I/II) | 2 (9.09) | 1 (5.5) | |
| Minor skin/wound dehiscence (treated conservatively) | 3 (13.6) | 1 (5.5) | ||
| Late infections (treated with antibiotics) | 2 (9.09) | 0 (0) | ||
| Total | 7 (31.78) | 2 (11.0) | ||
As described above, 18 patients with small breasts from our study cohort have successfully undergone the reconstruction with the novel A-ALDS technique without any major complications. These observations indicate that the use of A-ALDS procedure during IBRS is a safe and feasible technique.
Discussion
Breast reconstruction in patients with small breasts is challenging because of insufficient tissue access in the lower pole to create a dermal sling. In this study, we have described in detail a novel A-ALDS technique, which represents an innovative modification to the routine lower dermal sling procedure for application in the reconstruction of small, non-ptotic breasts. In our study cohort, we have observed an early trend towards lower rates of capsular contracture (grades II and III) and implant loss in A-ALDS patients in comparison to Conventional IBRS patients.
A-ALDS provides a stable, double-layered vascularized tissue cover for the implant. This likely allows effective in situ placement of the implant in the breast pocket with robust mechanical stability and helps in symmetrization with the contralateral breast.
The A-ALDS was performed by advancing the de-epithelialized dermal sling over the implant by mobilizing the skin and sub-cutaneous tissue from the lower thoracic and upper abdominal wall. This modification results in the recreation of a well-defined IMF to facilitate symmetry vis-a-vis shape and size with contralateral breast. In our technique, the A-ALDS flap was sutured with pectoralis major/serratus anterior muscle to provide desired expansion to the lower pole for implant placement that provides mechanical stability to the implant. The A-ALDS flap allows the expansion of the inferior pole of the pocket and prevents high riding of the implant. Hence, this modification provides a good contour and natural shape and symmetry to the breast. It is well reported that thoraco-epigastric flaps maintain vascularity from perforators of the intercostal, lumbar, epigastric arcade, and inferior epigastric arteries. As a result, these flaps have been previously used in correcting mastectomy defects by small-volume replacements [11].
We now present evidence of successful use of A-ALDS flap in breast reconstruction without detaching and advancing it by preserving the vascularity. In our cohort, A-ALDS patients demonstrated significantly higher aesthetic scores (6.7 ± 1.3 versus 4.6 ± 2.3, p = 0.009) compared with Conventional IBRS after a 12-month follow-up.
In addition to optimal post-surgery outcomes, patient acceptance of the A-ALDS technique is equally important. The PROM (Breast-Q) data from our study indicates significantly higher satisfaction with sexual well-being in patients who have undergone reconstruction with the A-ALDS technique. Other parameters such as satisfaction with breast, satisfaction with outcome, and psycho-social well-being showed a positive trend in favor of A-ALDS-based reconstruction.
Based on these observations, we hypothesize that use of A-ALDS flap with an implant may provide a viable, vascularized tissue cover to the implant. This flap lowers the risk of implant exposure, thereby lowering the rates of implant loss and capsular contracture. This well-vascularized autologous flap is expected to render the tissue environment more favorable for implant placement and improve wound healing, thereby reducing the risks of fibrosis, capsular contracture, wound breakdown, and infection and may reduce the implant-related complication after RT [12]. By extension, the higher complication rate in our study patients who have undergone Conventional IBRS may be attributed to the thin fascia covering the implant, which may predispose the implant to exposure in case of infection or necrosis.
In low-resource settings such as India, ADMs are not yet available. This prompted us to innovate the breast reconstruction technique for women with small breasts using the A-ALDS technique. Indeed, our collective results indicating superior cosmetic scores, lower rates of immediate complications, and trend towards better patient acceptance after A-ALDS-based reconstruction are encouraging. It is conceivable that our novel A-ALDS technique may provide a cost-effective alternative to ADM-based IBRS. The economic advantage of this ALDS technique is apparent as it can be performed in a single setting without compromising the patient outcomes and obviating the need for contralateral procedure.
Our main aim was to report the surgical details of the innovative A-ALDS-based IBRS procedure in small or minimally ptotic breasts. We propose that the A-ALDS-based IBRS procedure may be routinely employed in the patients with small breasts that will ensure a good cosmetic outcome with minimal early complications, no implant loss, and lesser capsular contracture rate with an overall positive impact on quality of life.
Despite the important findings, our study has few limitations. This study represents the post-IBRS follow-up data only for a period of 12 months. To substantiate the promising clinical and PROM observations, long-term follow-up, i.e., 3 to 5 years post-surgery in the same cohort, is needed. Secondly, this study represents a single-institutional cohort in which all A-ALDS procedures were performed by the same surgical team which may represent investigator bias. To validate our observations and minimize bias, this study needs to be replicated in multicentric settings.
The main objective of this pilot observational study was to generate proof-of-concept data to report any significant positive trends in post-surgical outcomes after application of novel A-ALDS procedure in comparison to the Conventional IBRS technique.
In addition to the Conventional IBRS technique, the novel A-ALDS technique was implemented by the same surgical team. Hence, the optimal data collection was only possible using a case series approach with available cases over a stipulated study duration.
More importantly, the study design strengths include data collections of PROMs via the BREAST-Q in BC patients who have undergone a minimum duration of 12 months post-surgery. Hence, the inclusion criteria had to be stringent, thereby limiting the number of study participants who had undergone A-ALDS procedure but who have not completed a 12-month follow-up. Given these logistical challenges and the study objectives, it was not possible to apply statistical sample size calculations for comparisons between the 2 groups, namely, Conventional IBRS and the A-ALDS groups.
Conclusion
In conclusion, our study reports application of a novel A-ALDS technique for implant-based breast reconstruction in small, non/minimally ptotic small breasts. Preliminary observations from post-surgery evaluations and PROMs demonstrate that A-ALDS technique may potentially reduce post-surgery complications, improve aesthetic outcomes, and improve overall patient satisfaction. This technique may serve as a cost-effective alternative to ADM-based reconstruction in low-resource settings. Long-term follow-up and study replication in other breast surgery units will be necessary for further substantiating our early observations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 16470 KB) Koppiker etal -A-ALDS Video 1.mp4: Pre-Operative Marking and Creation of A-ALDS Flap. Pre-Operative markings and skin de-epithelialization to form A-ALDS flap.
Supplementary file2 (MP4 122492 KB) Koppiker etal -A-ALDS Video 2.mp4: Mastectomy. Mastectomy is carried out with the removal of breast tissue.
Supplementary file3 (MP4 74034 KB) Koppiker etal -A-ALDS Video 3.mp4: Correction of Completely Vascularized Pocket with A-ALDS Technique. Implant is placed in sub-pectoral pocket and advancement of A-ALDS flap. Pectoralis major and serratus anterior muscle are sutured to de-epithelialized A-ALDS to create a complete vascularized cover.
Acknowledgements
The study authors would like to thank all participants who consented to participate in this study. We express our appreciation to Dr. Madhura Kulkarni, Dr. Dhara Patel, and Dr. Sneha Joshi for their help in editing the manuscript. We are grateful to the MAPI Research Trust for permission to use BREAST-Q (http://www.mapitrust.org). We would also like to thank the management and staff of Ruby Hall Clinic, Pune where all surgeries were performed.
Abbreviations
- ALDS
Autologous lower dermal sling
- IBRS
Immediate Breast Reconstruction Surgery
- BC
Breast cancer
- IMF
Infra-mammary fold
- ADMs
Acellular dermal matrices
- A-ALDS
Advanced-Autologous Lower Dermal Sling
- PROMs
Patient Reported Outcome Measures
Funding
Bajaj auto Ltd. provided support to research activities at PCCM (Grant #PCCM529).
Data Availability
All data will be available and can be shared on request. Supplementary tables and figures are provided in support of data presented in the manuscript.
Declarations
Ethics Approval
Study approval was granted by the Independent Ethics Committee of Prashanti Cancer Care Mission (DCGI/CDSCO Registration Number: ECR/298/Indt/MH/2018 (dated May 14, 2018)).
Consent to Participate
All subjects gave their consent for the use of their personal and medical information including images in the publication of this study.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A part of this work was presented as a poster at the Breast Cancer Coordinated Care conference in Washington, DC on March 1–3, 2018.
Contributor Information
Chaitanyanand B. Koppiker, Email: obs.koppiker@gmail.com
Aijaz Ul Noor, Email: aeijazul@gmail.com.
Santosh Dixit, Email: sgdixit@gmail.com.
Laleh Busheri, Email: lalehbusheri@gmail.com.
Gautam Sharan, Email: dr.gautamsharan@gmail.com.
Upendra Dhar, Email: upendradhar@hotmail.com.
Hari Kiran Allampati, Email: dr.harikiran89@gmail.com.
Smeeta Nare, Email: smeetanare@hotmail.com.
Nutan Gangurde, Email: dr.nutang@gmail.com.
References
- 1.Dietz J, Lundgren P, Veeramani A, O’Rourke C, Bernard S, Djohan R, et al (2012) Autologous inferior dermal sling (autoderm) with concomitant skin-envelope reduction mastectomy: an excellent surgical choice for women with macromastia and clinically significant ptosis. Ann Surg Oncol 19(10):3282-8 [DOI] [PubMed]
- 2.King IC, Harvey JR, Bhaskar P (2014) One-stage breast reconstruction using the inferior dermal flap, implant, and free nipple graft. Aesthetic Plast Surg 38(2):358-64 [DOI] [PubMed]
- 3.Roy PG. Modified lower pole autologous dermal sling for implant reconstruction in women undergoing immediate breast reconstruction after mastectomy. Int J Breast Cancer. 2016;2016:9301061. doi: 10.1155/2016/9301061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spear SL, Sher SR, Al-Attar A. Focus on technique: supporting the soft-tissue envelope in breast reconstruction. Plast Reconstr Surg. 2012;130(5 Suppl 2):89S–94S. doi: 10.1097/PRS.0b013e3182625852. [DOI] [PubMed] [Google Scholar]
- 5.Rusby JE, Smith BL, Gui GP. Nipple-sparing mastectomy. Br J Surg. 2010;97(3):305–16. doi: 10.1002/bjs.6970. [DOI] [PubMed] [Google Scholar]
- 6.Spear SL, Baker JL., Jr Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg. 1995;96(5):1119–1124. doi: 10.1097/00006534-199510000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Shekhawat L, Busheri L, Dixit S, Patel C, Dhar U, Koppiker C. Patient-reported outcomes following breast reconstruction surgery and therapeutic mammoplasty: prospective evaluation 1 year post-surgery with BREAST-Q questionnaire. Indian J Surg Oncol. 2015;28(2):121–127. doi: 10.1007/s13193-015-0432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen KT, Mioton LM, Smetona JT, Seth AK, Kim JY. Esthetic outcomes of ADM-assisted expander-implant breast reconstruction. Eplasty. 2012;12:e58. [PMC free article] [PubMed] [Google Scholar]
- 10.Adimulam G, Challa VR, Dhar A, Chumber S, Seenu V. A Srivastava Assessment of cosmetic outcome of oncoplastic breast conservation surgery in women with early breast cancer: a prospective cohort study. Indian J Cancer. 2014;51(1):58–62. doi: 10.4103/0019-509X.134629. [DOI] [PubMed] [Google Scholar]
- 11.Matros E, Disa JJ. Uncommon flaps for chest wall reconstruction. Semin Plast Surg. 2011;25(1):55–59. doi: 10.1055/s-0031-1275171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang DW, Barnea Y, Robb GL. Effects of an autologous flap combined with an implant for breast reconstruction: an evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg. 2008;122(2):356–362. doi: 10.1097/PRS.0b013e31817d6303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (MP4 16470 KB) Koppiker etal -A-ALDS Video 1.mp4: Pre-Operative Marking and Creation of A-ALDS Flap. Pre-Operative markings and skin de-epithelialization to form A-ALDS flap.
Supplementary file2 (MP4 122492 KB) Koppiker etal -A-ALDS Video 2.mp4: Mastectomy. Mastectomy is carried out with the removal of breast tissue.
Supplementary file3 (MP4 74034 KB) Koppiker etal -A-ALDS Video 3.mp4: Correction of Completely Vascularized Pocket with A-ALDS Technique. Implant is placed in sub-pectoral pocket and advancement of A-ALDS flap. Pectoralis major and serratus anterior muscle are sutured to de-epithelialized A-ALDS to create a complete vascularized cover.
Data Availability Statement
All data will be available and can be shared on request. Supplementary tables and figures are provided in support of data presented in the manuscript.