Abstract
Surgical resection is a generally accepted treatment for residual masses after chemotherapy for metastatic testicular germ cell tumour (GCT). About half the patients have necrosis in post-chemotherapy residual masses, whereas rest have viable tumour and teratoma. The likelihood of leaving behind teratoma with its subsequent complications such as growing teratoma syndrome necessitates resection outweighing its surgical complications. Ours is a retrospective observational study and aims at assessing post-chemotherapy residual masses in testicular GCTs and to predict importance of teratomatous and non-seminomatous components. A total of 62 cases of testicular GCTs resected after chemotherapy between January 2012 and June 2019 were included. Demographic, clinical, biochemical and imageological findings were noted and categorised according to WHO classification (2016). They were divided into two groups — those who underwent retroperitoneal lymph node dissection (RPLND) post-high inguinal orchidectomy (HIO) and chemotherapy (CT) as group 1 (n = 40) and those who underwent HIO and/or RPLND post-chemotherapy as group 2 (n = 22). The gross and microscopic examination was carried out to assess response to chemotherapy in terms of residual viable tumour, necrosis and teratoma. Viable tumour, necrosis and teratoma were 10%, 62.5% and 35% respectively in group 1 and in group 2, the same were 15%, 70% and 25% respectively in HIO specimen and 7%, 50% and 21% respectively in RPLND specimen. All the cases with viable tumour were proven to be yolk sac tumours (YST) based on morphology and immunohistochemistry (IHC).Twenty cases had teratoma in the post-CT residual masses out of which 11 cases had teratoma despite reduction in size. At a median follow-up of 47.85 months, 5 cases in group 1 and 2 cases in group 2 showed relapse and it was observed that group 1 had a prolonged relapse-free survival over group 2. Our study re-emphasises the importance of performing resection of residual mass post-CT irrespective of the size, imageological or biochemical evidence of tumour regression. There does not appear to be reliable predictors of post-chemotherapy histology of residual masses indicating the continued need for surgical resection in specialised centres.
Keywords: Testicular germ cell tumours, Residual, Post-chemotherapy
Introduction
A solid intratesticular mass in a post-pubertal male should be considered a GCT until proven otherwise. With rare exceptions, inguinal orchidectomy with high ligation of the spermatic cord should be performed in men suspected of having GCT.
Seminoma is associated with increased sensitivity to radiation therapy and platinum-based chemotherapy compared with NSGCT and may be adopted in higher stage before performing surgery. If elevated before orchidectomy, serum tumour marker levels should be measured after orchidectomy to determine if levels are declining, stable or rising. Rising post-orchidectomy serum tumour marker levels indicate the presence of metastatic GCT and these patients should receive chemotherapy [1]. CT imaging is the optimal staging for retroperitoneum, abdomen and chest [2, 3].
Unresected teratoma has the potential to exhibit rapid growth, undergo malignant transformation and/or cause late relapse, all of which are lethal. Also in men with embryonal carcinoma predominance, it is possible to reduce the recurrence rate from 30 to 60% down to about 2 to 3% by performing RPLND [4].
Subjects and Methods
All testicular GCTs diagnosed and treated through surgical (high inguinal orchidectomy [HIO] ± retroperitoneal lymph node dissection [RPLND]) and medical (primary and/or salvage chemotherapy) modalities between January 2012 and June 2019 were included in the study. The demographic, clinical, biochemical and imageological findings were noted. The testicular GCTs were categorised according to the latest WHO classification (2016) [5]. The gross and microscopic examination of the post-CT residual masses was carried out to assess the response to chemotherapy in terms of presence of residual viable disease, necrosis and teratoma. All the cases were divided into two groups — those who underwent RPLND post-HIO and CT as group 1 and those who underwent HIO and/or RPLND post-chemotherapy as group 2.
Results
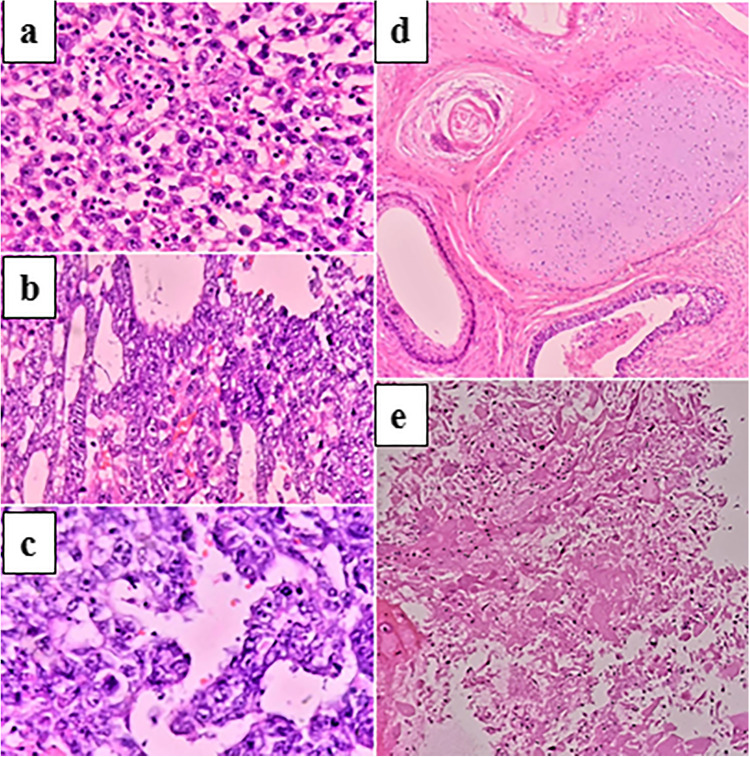
A total of 73 patients were available during the study period, out of which 11 were excluded due to non-availability of desired parameters from the records. The remaining 62 patients were divided into 2 groups based on the treatment provided as per the clinician’s decision taking into account the various clinical factors. The study included 40 patients in group 1 and 22 patients in group 2 with mean age at diagnosis being 27.25 years and 26.85 years respectively. Viable tumour, necrosis and teratoma were 10%, 62.5% and 35% respectively in group 1 and in group 2, the same was 15%, 70% and 25% respectively in HIO specimen and 7%, 50% and 21% respectively in RPLND specimen. All the cases with residual viable tumour were proven to be YST based on morphology and IHC. A mixed or a single predominant morphology in post-chemotherapy patients has been seen (Fig. 1).
Fig. 1.
Post chemotherapy morphology in residual mass. a Seminoma b Yolk sac tumour c Embryonal carcinoma d Teratoma e Necrosis (H and E, 40x magnification)
Cases showing necrosis in post-chemotherapy residual masses showed reduction in size with a significant p value of 0.03.
Presence of teratoma, viable tumour and necrosis was recorded in both the groups (Table 1).
Table 1.
Distribution of viable tumour, necrosis and teratoma across group 1 and group 2
| Variables | Group 1 (n = 42) | Group 2 (n = 20) |
|---|---|---|
| Viable tumour | 4 (1%) | 3 (15%) |
| Viable tumour present despite size reduction of residual mass | 1 | 2 |
| Necrosis | 25 (60%) | 15 (75%) |
| Teratoma | 14 (33%) | 6 (30%) |
| Teratoma present despite size reduction of residual mass | 7 | 4 |
The post-chemotherapy residual status was correlated with the pre-chemotherapy and post-surgery serum marker values and was found to have a significant correlation (p value — 0.039). However, the pattern of marker normalization in group 1 was that β-hcg and AFP normalise after chemotherapy in all the patients post-HIO and CT, whereas LDH remained elevated in 9 patients. In group 2, β-hcg and AFP normalise after chemotherapy in all the patients post-CT, whereas LDH remained elevated in 4 patients.
Follow-up data was available for 54 patients and the disease-free survival and overall survival in both the groups were recorded (Table 2). It was found that patients treated with CT before RPLND (group 1) had a prolonged relapse-free interval as compared with group 2. A Kaplan–Meier analysis of the same was also analysed which show similar findings although the p value was not significant (p value of DFS — 0.486, p value of OS — 0.453).
Table 2.
Follow-up data of testicular GCTs with final outcome
| Group | Stage | Number of patients | Lost to follow-up | DFS (months) | OS (months) | %relapse | Deaths |
|---|---|---|---|---|---|---|---|
| 1 (n = 42) | I | 1 | 64 | 65 | 0 | ||
| II | 26 | 6 | 48.5 | 59.5 | 12 | ||
| III | 15 | 3 | 38.5 | 45 | 13 | 1 | |
| 2 (n = 20) | II | 11 | 3 | 40 | 45 | 9 | |
| III | 9 | 1 | 38.5 | 42.5 | 0 |
The morphological types were assessed in relation to follow-up outcomes and were found that most patients had EC and/or YST as components. Teratoma was found in one case each in both the groups. Cases with seminoma as a component had the longest relapse-free intervals (Tables 3 and 4).
Table 3.
Morphological types in relation to follow-up status in group 1
| Years of follow-up | 1 | 3 | 5 | |
|---|---|---|---|---|
| Asymptomatic | Seminoma | 1 | 2 | 1 |
| YST | 1 | - | 2 | |
| EC | - | - | 2 | |
| Mixed | ||||
| EC + YST | 1 | 9 | 4 | |
| EC + Sem | 2 | - | 1 | |
| EC + Chorio | - | 1 | - | |
| YST + Chorio | - | - | 1 | |
| YST + Sem | - | - | 1 | |
| Teratoma | 1 | 2 | 5 | |
| Relapse | Seminoma | - | - | - |
| YST | - | - | - | |
| EC | 1 | - | - | |
| Mixed (EC + YST) | 2 | - | - | |
| Teratoma | 1 | - | - | |
| Death | EC + YST | 1 | - | - |
Table 4.
Morphological types in relation to follow-up status in group 2
| Years of follow-up | 1 | 3 | 5 | |
|---|---|---|---|---|
| Asymptomatic | Seminoma | 1 | 6 | 4 |
| NSGCT | - | 2 | - | |
| Teratoma | - | - | 1 | |
| Relapse | Seminoma | - | - | - |
| NSGCT | 3 | - | - | |
| Teratoma | 1 | - | - |
Out of 62 patients, five patients relapsed, received salvage chemotherapy with two patients underwent surgical resection and chemotherapy both. Rest of the 57 patient received primary chemotherapy (BEP) were without any relapse.
Discussion
In men with a testicular mass, hydrocele or unexplained scrotal symptoms or signs, scrotal ultrasonography should be considered an extension of the physical examination. Heterogeneous echotexture within a lesion is more commonly associated with NSGCT, because seminomas usually have a homogenous echotexture. The presence of increased flow within the lesion on colour Doppler sonography is suggestive of malignancy, although its absence does not exclude GCT [6]. In cases in which the sonographic findings are equivocal or suboptimal, magnetic resonance imaging (MRI) is a helpful tool [7].
Testicular cancer is one of the few malignancies associated with serum tumour markers (lactate dehydrogenase [LDH], AFP and HCG) that are essential in its diagnosis and management. Serum tumour marker levels should be obtained at diagnosis, after orchidectomy to monitor response to chemotherapy and for relapse in patients on surveillance [1].
The probability of cure in young males, even in the presence of metastatic disease, has also led to an aggressive approach with regard to the administration of chemotherapy and the performance of post-chemotherapy surgery to resect all residual masses [8, 9] even if this involves multiple anatomic sites.
The potential for seminoma to transform into NSGCT elements is an important consideration in the management of patients who fail to respond to chemotherapy or who relapse after radiation therapy. Of patients with metastatic seminoma who relapse after treatment, approximately 10 to 15% have NSGCT elements at the site(s) of relapse. An autopsy study has shown that 30% of patients who die from seminoma have NSGCT elements at metastatic sites [10].
Teratoma is not sensitive to chemotherapy and the outcome of patients with metastatic teratoma is related to the completeness of surgical resection [1].
Our study represents the outcomes of post-chemotherapy in two groups of patients treated differently and our results are comparable to similar other studies (Table 5).
Table 5.
Post-chemotherapy outcome in comparison with other studies
| Variables | Stenning et al. [11] | Steyerberg et al. [12] | Luz et al. [13] | Alquasem et al. [14] | Miranda et al. [15] | Present study |
|---|---|---|---|---|---|---|
| Number of patients | 153 | 556 | 73 | 56 | 32 | 42 |
| Mean age (years) | - | - | 30.4 | 30 | 29.7 | 27 |
| Mean size of the residual mass (cm) | - | - | 4 | 6 | 4.94 | 3.8 |
| Findings in residual mass (%) | ||||||
| - Viable tumour | 17.6 | 13 | 21.9 | 34 | 6.4 | 10 |
| - Necrosis | 76.5 | 45 | 37 | 30 | 47 | 62.5 |
| - Teratoma | 50 | 42 | 41.1 | 36 | 47 | 35 |
In non-seminoma cases, the rate of viable tumour after chemotherapy is considerably higher than in seminoma (40% teratoma, 20% viable cancer). Surgery after chemotherapy has a higher morbidity compared to initial retroperitoneal lymph node dissection, as well as a higher rate of additional procedures (e.g. nephrectomy) and a higher percentage of loss of ejaculation due to resection of sympathetic nerve fibres during RPLND [13].
Predictors for necrosis were a teratoma-negative primary tumour, normal pre-chemotherapy. If mature teratoma or cancer is present in the residual mass, the patient is expected to benefit from resection. The prognosis then is generally favourable, with 5-year relapse-free survival greater than 85% after resection of mature teratoma, and 50 to 80% after resection of cancer. Resection of viable cancer cells is usually followed by two additional cycles of chemotherapy. If resection is not performed, masses that contain mature teratoma may start to grow during a follow-up of months or even years (growing teratoma syndrome). Resection may then be more complicated than it would have been shortly after the end of chemotherapy [12].
The absence of teratoma in the orchidectomy specimen does not reliably predict the absence of teratoma in the surgical specimen at post-chemotherapy retroperitoneal lymph node dissection. Post-chemotherapy surgery is indicated if retroperitoneal tumour remains after chemotherapy irrespective of the presence or absence of teratoma in the orchidectomy specimen. Approximately 50% of these patients had teratomatous elements in the retroperitoneum regardless of tumour volume in the retroperitoneum. Therefore, neither absence of teratoma in the orchiectomy specimen nor volume of retroperitoneal tumour serves as a reliable selection factor to exclude patients from the likely benefit of post-chemotherapy retroperitoneal lymph node dissection. For low stage testis cancer managed by primary retroperitoneal lymph node dissection, the absence of teratoma in the orchiectomy specimen was predictive of the absence of teratoma in the retroperitoneum [16].
Follow-up was available for 31 patients in group 1 and showed that 5 (16%) of them relapsed at a mean of 11 months. Two of them were treated with surgical excision along with salvage CT, while two were treated only with salvage CT. One of them died at 4 months while on salvage CT. In group 2, 4 out of 17 patients (23.5%) relapsed. Three of them were treated with salvage CT, while one who relapsed with teratoma was treated with RPLND. Our observation correlates with similar other studies (Tables 6 and 7).
Table 6.
Follow-up data of group 1 in comparison with other studies
Table 7.
Follow-up data of group 2 in comparison with other studies
| Miller et al. [19] | Present study | |
|---|---|---|
| No. of patients | 38 | 17 |
| Median follow-up (months) | 49 | 43 |
| %asymptomatic | 57% | 76% |
| %relapse | 36% | 23.5% |
A higher LDH level has been found to indicate a lower probability of complete response and a worse survival in patients with metastatic disease, analyzing from the start of primary chemotherapy. It may therefore be postulated that patients with residual masses who respond to chemotherapy (as indicated by normalization of AFP/HCG levels) form a favourable subgroup of patients with a pre-chemotherapy high LDH level [12].
Newer markers such as hMLH1 and mdr-1 have been found to be useful as prediction markers of viable tumour in post-CT residual masses [20]. Further studies on molecular markers of the primary may assist as prognostic and predictive markers in future.
Conclusion
Our study re-emphasises the importance of performing resection of residual mass and RPLND post-chemotherapy irrespective of the size, imageological or biochemical evidence of tumour regression. There does not appear to be reliable predictors of post-chemotherapy histology of residual masses indicating the continued need for surgical resection in specialised centres. The fact that growing teratoma syndrome poses a much increased morbidity on long-term follow-up also necessitates resection of any post-chemotherapy residual mass.
Author Contribution
Conceptualization: B. Vishal Rao; data curation: Ranjitha Vodigenahalli Nagaraj, Jayakarthik Yoganarsimha; formal analysis: Ranjitha Vodigenahalli Nagaraj, B. Vishal Rao, Jayakarthik Yoganarsimha; investigation: B.Vishal Rao, Ashwin Giridhar, Rakesh Sharma; methodology: B. Vishal Rao, Ranjitha Vodigenahalli Nagaraj; project administration: Suseela Kodandapani; supervision: Thammineedi Subramanyeshwar Rao; validation: B. Vishal Rao; visualization: Challa Sundaram, Senthil Rajappa; writing — original draft: Ranjitha Vodigenahalli Nagaraj; writing — review and editing: B. Vishal Rao, Challa Sundaram, Daphne Fonseca.
Declarations
Ethics Declaration
The submitted work is an original work done at our institute after approval from the Institute Ethical Committee, and has not been published elsewhere in any form or language. All authors whose names appear on the submission made substantial contributions to the conception, design, acquisition of data, analysis, interpretation of data, drafted the work, revised it and critically reviewed it for important intellectual content.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Key Messages
In the wake of finding viable tumour and teratoma in specimens post-chemotherapy irrespective of the size and biomarker status, a thorough grossing with adequate sampling in HIO specimens (pre-CT), testicular residual masses and RPLND specimens (post-CT) is a must. Adjunct investigations such as IHC may be used to identify the GCT components to provide additional information regarding prognosis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Partin AW, Wein AJ, Kavoussi LR (2020) Campbell-Walsh Urology: ISBN: 9780323672269; Publisher : Elsevier US/UK. Ed 12th
- 2.Hermans BP, Sweeney CJ, Foster RS, et al. Risk of systemic metastases in clinical stage I nonseminoma germ cell testis tumor managed by retroperitoneal lymph node dissection. J Urol. 2000;163:1721–1724. doi: 10.1016/S0022-5347(05)67528-3. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney CJ, Hermans BP, Heilman DK, et al. Results and outcome of retroperitoneal lymph node dissection for clinical stage I embryonal carcinoma–predominant testis cancer. J Clin Oncol. 2000;18:358–362. doi: 10.1200/JCO.2000.18.2.358. [DOI] [PubMed] [Google Scholar]
- 4.Honecker F, Aparicio J, Berney D et al (2018) ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol 29:1658–1686 [DOI] [PubMed]
- 5.Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO classification of tumours of the urinary system and male genital organs. 4. Lyon: International Agency for Research on Cancer; 2016. [Google Scholar]
- 6.DeCastro BJ, Peterson AC, Costabile RA. A 5-year followup study of asymptomatic men with testicular microlithiasis. J Urol. 2008;179:1420–1423. doi: 10.1016/j.juro.2007.11.080. [DOI] [PubMed] [Google Scholar]
- 7.Park SB, Lee WC, Kim JK, et al. Imaging features of benign solid testicular and paratesticular lesions. Eur Radiol. 2011;21:2226–2234. doi: 10.1007/s00330-011-2155-x. [DOI] [PubMed] [Google Scholar]
- 8.de Wit R, Roberts JT, Wilkinson PM, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol. 2001;19:1629–1640. doi: 10.1200/JCO.2001.19.6.1629. [DOI] [PubMed] [Google Scholar]
- 9.Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:1287–1293. doi: 10.1200/JCO.1998.16.4.1287. [DOI] [PubMed] [Google Scholar]
- 10.Bredael JJ, Vugrin D, Whitmore WF., Jr Autopsy findings in 154 patients with germ cell tumors of the testis. Cancer. 1982;50:548–551. doi: 10.1002/1097-0142(19820801)50:3<548::AID-CNCR2820500327>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Stenning SP, Parkinson MC, Fisher C, Mead GM, Cook PA, Fossa SD, Horwich A, Jones WG, Newlands ES, Oliver RT, Stenwig AE. Postchemotherapy residual masses in germ cell tumor patients: content, clinical features, and prognosis. Cancer Interdiscip Int J Am Cancer Soc. 1998;83(7):1409–19. [PubMed] [Google Scholar]
- 12.Steyerberg E, Keizer HJ, Fossa S, Sleijfer D, Toner GC, Schraffordt Koops H, Mulders PF, Ney K, Donohue JP. Prediction of residual retroperitoneal mass histology after chemotherapy for metastatic nonseminomatous germ cell tumor: multivariate analysis of individual patient data from six study groups. J Clin Oncol. 1995;13(5):1177–1187. doi: 10.1200/JCO.1995.13.5.1177. [DOI] [PubMed] [Google Scholar]
- 13.Luz MA, Kotb AF, Aldousari S, Brimo F, Tanguay S, Kassouf W, Aprikian AG. Retroperitoneal lymph node dissection for residual masses after chemotherapyin nonseminomatous germ cell testicular tumor. World J Surg Oncol. 2010;8(1):97. doi: 10.1186/1477-7819-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alqasem K, Abukhiran I, Jasser J, Bisharat T, Ellati RT, Khzouz J, Al-Saidi I, Al-Daghamin A. Clinico-pathological outcomes of post-primary and salvage chemotherapy retroperitoneal lymph node dissection for mixed germ cell tumors, King Hussein Cancer Center experience. Turk J Urol. 2016;42(4):256. doi: 10.5152/tud.2016.64188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Paula Miranda E, Abe DK, Nesrallah AJ, dos Reis ST, Crippa A, Srougi M, Dall’Oglio MF. Predicting necrosis in residual mass analysis after retroperitoneal lymph node dissection: a retrospective study. World J Surg Oncol. 2012;10(1):1–5. doi: 10.1186/1477-7819-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck SD, Foster RS, Bihrle R, Ulbright T, Koch MO, Wahle GR, Einhorn LH, Donohue JP. Teratoma in the orchiectomy specimen and volume of metastasis are predictors of retroperitoneal teratoma in post-chemotherapy nonseminomatous testis cancer. J Urol. 2002;168(4):1402–1404. doi: 10.1016/S0022-5347(05)64458-8. [DOI] [PubMed] [Google Scholar]
- 17.Rick O, Bokemeyer C, Weinknecht S, Schirren J, Pottek T, Hartmann JT, Braun T, Rachud B, Weissbach L, Hartmann M, Siegert W. Residual tumor resection after high-dose chemotherapy in patients with relapsed or refractory germ cell cancer. J Clin Oncol. 2004;22(18):3713–3719. doi: 10.1200/JCO.2004.07.124. [DOI] [PubMed] [Google Scholar]
- 18.El Sayed S, Grando JP, De Almeida SH, Mortati Neto N, Moreira HA. Post-cheotherapy residual mass in non-siinomatous testicular cancer: the role of retroperitoneal lymph node dissection. Int Braz J Urol. 2004;30(5):384–388. doi: 10.1590/S1677-55382004000500005. [DOI] [PubMed] [Google Scholar]
- 19.Miller RE, Dudderidge T, Huddart R, Seckl MJ, Rustin GJ, Christmas TJ. Pathological findings after primary chemotherapy in patients undergoing simultaneous orchidectomy and retroperitoneal lymph node dissection for advanced germ cell tumours. BJU Int. 2013;111(4b):E152–E157. doi: 10.1111/j.1464-410X.2012.11537.x. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich AN, Pfister D, Thüer D, Engelmann UH, Ohlmann CH. Prediction of residual retroperitoneal mass histology following postchemotherapy retroperitoneal surgery for metastatic nonseminomatous germ cell tumors: role of MDR-1 and mismatch repair genes. J Clin Oncol. 2007;25(18_suppl):5088. doi: 10.1200/jco.2007.25.18_suppl.5088. [DOI] [Google Scholar]