Abstract
Clinicopathologic classification of endometrial cancer imperfectly reflects the tumor biology. Pathologic categorization — especially in high-grade tumors — results in an imprecise estimation of the risk of disease, recurrence, and death. Molecular subtyping is emerging as the standard of care in diagnosis and treatment of endometrial cancers. Molecular markers are important prognostic factors in tumor dissemination and early recurrence of endometrial cancers. TP53 mutation is an important prognostic factor for both serous and endometrioid cancers. The study aims to compare the clinical profile and overall survival of endometrial cancers with and without p53 mutation. Sixty-three patients who underwent surgical staging for carcinoma endometrium were included in the study.TP53 mutation status was determined based on p53 expression by immunohistochemistry (IHC) as a p53 wild or p53 mutant type. Data were analyzed for the clinical profile, p53 mutation status on IHC, histological pattern, tumor grade, stage of the disease, lymph node spread, recurrence pattern, treatment received, 2-year disease-free survival, and overall survival. Recurrence was noted in 12.7% patients after 2-year follow-up, of which 75% patients had p53 mutation. Significant association was seen between p53 expression and high-grade tumors, stage, cervical involvement, and adnexal involvement. The 2-year overall survival of the p53 wild type was 97.2% and the p53 mutant type was 91.7%. The 2-year disease-free survival for the p53 wild type was 94.3% and the disease-free survival of the p53 mutant variety was 83.5%. The 2-year disease-free survival for endometrioid carcinoma with p53 wild type was 100% and p53 mutant variety was 86.2% (p value 0.033). About 15.9% (10) patients were reassigned to the high-risk group needing chemotherapy and radiation according to the ESGO ESTRO 2021 consensus classification, due to their p53 mutation status. IHC to assess somatic p53 mutation may be done in endometrial biopsies irrespective of their histology. This may help to identify that the aggressive tumors thereby help in tailoring surgery, planning adjuvant treatment, and follow-up.
Keywords: Endometrial cancer, Survival, p53 mutation
Introduction
Endometrial carcinoma is the most common malignancy of the female genital tract in developed countries [1]. The estimated cases per year in India according to Globocan 2020 are 16,413 and the estimated deaths are 6385 [1]. The majority of cases are present in the 6th and 7th decades of life. An increasing trend has been noted for endometrial cancers even in developing countries over the past few years. The reasons proposed for this are an increase in exogenous estrogen use in peri and postmenopausal women, changes in the reproductive pattern like nulliparity, lower parity, increase in the prevalence of diabetes mellitus, and obesity, particularly in low and middle-income countries [2].
The 5-year overall survival rate for women with early-stage endometrial cancer exceeds 80%. Recurrence is seen in 10–15% of cases [3]. The stage, histological type, tumor grade, myometrial invasion, lymphovascular invasion, and lymph node metastasis at the time of treatment are independent prognostic factors in patients with endometrial carcinoma [4]. Endometrial cancers are heterogeneous groups of tumors not only in terms of histology, biology, and clinical behavior but also concerning their genetic makeup. The Cancer Genome Atlas (TCGA) has reported a comprehensive genomic and transcriptomic analysis of endometrial cancers by the whole-exome massively parallel sequencing analysis, copy number alterations, and microsatellite instability. Endometrial cancers were categorized into four genomic subtypes: POLE (ultra-mutated) tumors, microsatellite-instable (hypermutated) tumors, copy-number low tumors, and copy-number high tumors [5]. Copy number high tumors show recurrent mutations affecting the TP53 gene. Tumor suppressor TP53 plays an important role in the preservation of genomic stability from various damages through the regulation of cell-cycle checkpoints, DNA repair, senescence, and apoptosis [6]. Mutant TP53 loses its antitumor transcriptional activity and often acquires oncogenic functions to promote tumor proliferation, invasion, and drug resistance [7, 8]. TP53 mutation is detected in about 25% of all endometrial cancer patients [9]. The frequency of TP53 mutation in type I endometrial cancer is about 10–40%, whereas that in type II endometrial cancer is about 90% [10]. Somatic mutation in TP53 can be the best predictive biomarker for poor prognosis in endometrial cancer patients [8]. Identification of TP53 mutation would be very useful for the selection of the most appropriate therapy and for the development of novel treatment modalities [11]. TP53 missense mutations results in the nuclear accumulation of p53 protein that can be detected as overexpression by IHC. Overexpression and complete absence are interpreted as mutation-type, with p53 expression levels in between these extremes are taken as wild type [12]. IHC for p53 protein is available in most pathology labs. It can be easily done on initial endometrial biopsy samples. This will help us to decide the extent of surgery as complete pelvic and paraaortic node dissection may be considered in endometrial cancers with myometrial invasion having p53 mutation [13]. It will also help to determine the adjuvant treatment, the chance of recurrence, and survival [14].
In this context, we aimed to analyze the clinic pathological factors and survival outcome of p53 wild and mutant types among the patients who underwent surgical staging in the gynecological oncology department in a tertiary-care center.
Methodology
This retrospective study was done after obtaining Institutional Review Board approval. We reviewed the medical records of 63 patients who underwent surgical staging in our department for endometrial cancer for a period of 2 years from October 2016 to December 2018. All patients had undergone hysterectomy with bilateral salphingo-oophorectomy and pelvic node dissection. Paraaortic node dissection was done in only 48 patients. Paraaortic node dissection was omitted in low-risk patients with grade 1,2 tumors and less than 50% on MRI and patients with morbid obesity. TP53 mutation status was determined based on p53 expression by IHC as a p53 wild or p53 mutant type. Data were analyzed for the clinical profile, p53 mutation status on IHC, histological pattern, tumor grade, stage of the disease, lymph node spread, treatment received, recurrence pattern, 2-year disease-free survival, and overall survival. Risk stratification was done using the ESGO ESMO ESTRO consensus of 2016. The patients received adjuvant treatment according to 2016 consensus recommendations [15]. The patients were reclassified according to ESGO ESTRO ESP 2021 consensus which included molecular classification [13].
Statistical Methods
Descriptive statistics were generated. The statistically significant association between p53 expression and the various factors was assessed using Pearson’s chi-square and for small samples Fisher’s exact test. The overall and 2-year disease-free survival for p53 mutated and p53 wild types were calculated using the Kaplan–Meier method.
Results
A total of 63 patients who underwent surgical staging for carcinoma endometrium were included in the study. The mean age of the study group was 59.4 years. Of the patients, 57.1% (36/63) were p53 wild type and 42.8% (27/63) of the patients were p53 mutant. Of the women, 95.2% (60) were postmenopausal. The most common presenting complaint was postmenopausal bleeding seen in 88.9% (56) patients.
All patients had undergone pelvic node dissection. Only 76% (48) patients had undergone paraaortic nodal evaluation. Pelvic nodal metastasis was seen in 23.8% (15) patients and paraaortic nodal metastasis was seen in 14.58% (7/48) patients.
Endometrioid adenocarcinoma was the most common histological variant seen in 76.1% (48) patients. In a total of 48 endometrioid adenocarcinomas, 37.5% (18) patients were p53 mutant. A high-grade tumor (including grade 3 endometrioid, serous, and clear cell types) was seen in 65.1% (41). p53 mutation was seen in 55.5% of the high-grade endometrioid tumors.
Risk stratification was done for endometrial cancers by ESMO ESGO ESTRO consensus 2016 as low, intermediate, high intermediate, and high risk [15]. The patients received no adjuvant in the low-risk group, vaginal brachytherapy in the intermediate-risk group, EBRT in the high-risk group, vaginal brachytherapy + EBRT in stage II, and chemotherapy ± radiation in stage III and above according to the risk category according to ESMO ESGO ESTRO consensus 2016 recommendations [15]. Of the patients, 17.4% (11) received no adjuvant treatment and were kept on follow-up. Of the patients, 38.1% (24) received radiation and 38.1% (24) patients received chemoradiation.
On 2-year follow-up, 81% (51) patients were alive disease-free, 7.9% (5) were alive with disease, and 7.9% (5) patients had died, among which 3 were due to disease, and 2 were due to other causes. Of the patients, 3.2% (2) were lost to follow-up (Table 1).
Table 1.
Clinical characteristics of patients evaluated
| Clinical characteristics | Subgroups | No. (n = 63) | Percentage (%) |
|---|---|---|---|
| Age |
< 60 > 60 |
35 28 |
|
| p53 expression |
p53 wild p53 mutant |
36 27 |
57.1 42.8 |
| Histology |
Endometrioid grade1 Endometrioid grade 2 Endometrioid grade 3 Serous Clear cell carcinoma |
2 20 26 8 7 |
3.1 31.7 41.3 12.7 11.1 |
| Stage |
I II III IV |
31 11 19 2 |
49.2 17.5 30.2 3.1 |
| Tumor size |
< 2 cm ≥ 2 cm |
6 57 |
9.5 90.5 |
| Myometrial Infiltration |
Nil Less than half More than or equal to half |
3 25 35 |
4.8 39.7 55.6 |
| Lymphovascular invasion | Present | 26 | 41.3 |
| Cervical involvement | Present | 13 | 20.6 |
| Adnexal involvement | Present | 11 | 17.5 |
| Vaginal or parametrial involvement | Present | 5 | 7.9 |
| Nodal metastasis |
Pelvic Paraaortic |
15/63 7/48 |
23.8 14.58 |
| Adjuvant treatment |
Nil Radiation only Chemotherapy only Chemoradiation |
11 24 4 24 |
17.4 38.1 6.3 38.1 |
| Recurrence |
p53 wild p53 mutant |
2 6 |
3 9.5 |
| Follow-up |
Alive disease-free Alive with disease Died due to disease Died due to other cause Lost to follow-up |
51 5 3 2 2 |
81 7.9 4.7 3.2 3.2 |
A significant association was demonstrated between histology, stage, cervical, and adnexal involvement (Table 2).
Table 2.
Comparison of p53 wild and p53 mutant type
| Clinical characteristics | Subgroups | p53 wild (n = 36) | p53 mutant (n = 27) | P-value* |
|---|---|---|---|---|
| Age |
< 60 > 60 |
17 19 |
11 16 |
0.608 |
| Histology |
Endometrioid grade 1 Endometrioid grade 2 Endometrioid grade 3 Serous Clear cell carcinoma |
2 18 14 0 2 |
0 2 12 8 5 |
0.001* |
| Stage |
I II III IV |
23 6 6 1 |
8 5 12 2 |
0.025* |
| Tumor size |
< 2 cm ≥ 2 cm |
4 32 |
2 25 |
0.693 |
| Myometrial infiltration |
Nil Less than half More than or equal to half |
1 18 17 |
2 7 18 |
0.70 |
| Lymphovascular invasion | Present | 12 | 14 | 0.140 |
| Cervical involvement | Present | 4 | 9 | 0.031* |
| Adnexal involvement | Present | 2 | 9 | 0.006* |
| Vaginal or parametrial involvement | Present | 3 | 2 | 1.000 |
| Nodal metastasis |
Pelvic Paraaortic |
5 2 |
6 5 |
0.507 0.215 |
| Adjuvant treatment |
Nil Radiation Chemotherapy Chemoradiation |
10 15 0 11 |
1 9 4 13 |
0.069 |
| Recurrence | 2 | 6 | 0.065 | |
| Follow-up |
Alive disease-free Alive with disease Died due to disease Died due to other cause Lost to follow-up |
30 0 2 2 2 |
21 5 1 0 0 |
Although only p53 mutation had been checked, we also reclassified the patients according to risk stratification for endometrial cancers by ESGO ESTRO ESP consensus 2021 which included the molecular classification which had taken POLE mutation MMRd and p53 mutation into consideration [13]. Of the patients, 15.9% (10) were reassigned to the high-risk group needing chemotherapy and radiation according to the latest classification, due to their p53 mutation status (Table 3).
Table 3.
Risk stratification of patients
| ESGO-ESMO ESTRO consensus 2016 | ESGO-ESTRO ESP 2021 (molecular unknown) | ESGO-ESTRO ESP 2021 (molecular known—including p53) | |
|---|---|---|---|
| Low | 11 | 11 | 10 |
| Intermediate | 1 | 8 | 7 |
| High intermediate | 9 | 19 | 11 |
| High | 42 | 25 | 35 |
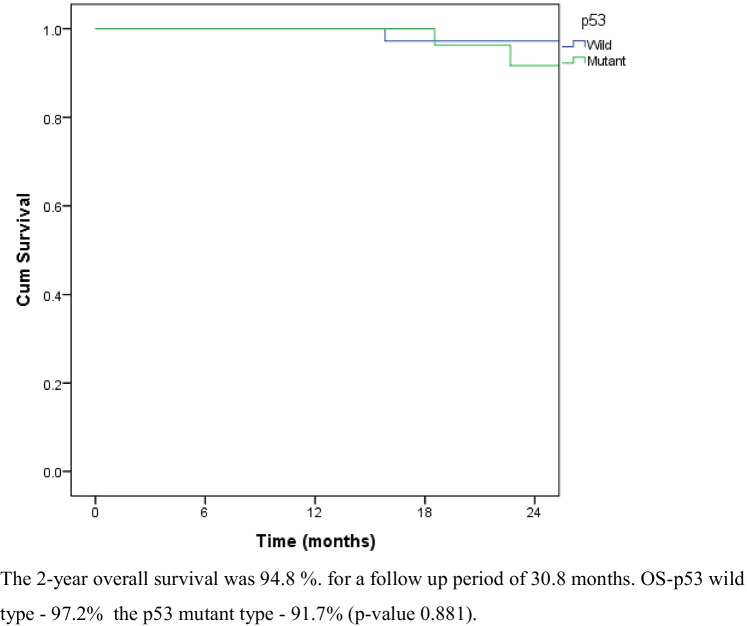
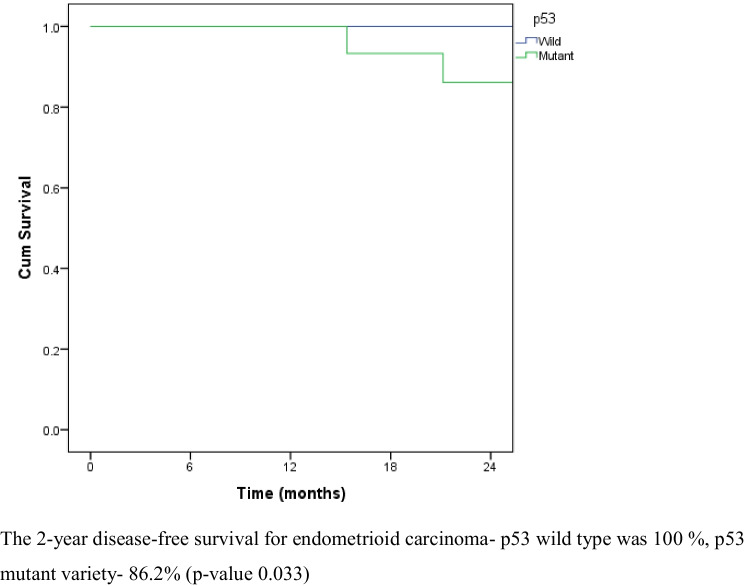
Recurrence was noted in 12.7% (8) patients after 2 years. Of the patients, 6/8 (75%) had p53 mutation. Recurrence was noted at the paraaortic area in 2 patients. Peritoneal recurrence was seen in 2 patients and lung metastasis in 4 patients (Table 4). The 2-year overall survival was 94.8%. The 2-year overall survival of the p53 wild type and p53 mutant type was 97.2% and 91.7%, respectively (p-value 0.881) (Fig. 1). The disease-free survival was 89.4% for a median follow-up of 30.8 months. The 2-year disease-free survival for p53 wild type and p53 mutant varieties were 94.3% and 83.5%, respectively (p-value 0.105) (Fig. 2). The 2-year disease-free survival for endometrioid carcinoma with p53 wild type was 100% and the disease-free survival of the p53 mutant variety was 86.2%. The difference was statistically significant (p-value 0.033) (Fig. 3).
Table 4.
Recurrence pattern
| Initial stage | Adjuvant taken | Recurrence area | |
|---|---|---|---|
| p53 wild | IIIC1 | Chemoradiation | Peritoneal deposits |
| p53 wild | IIIC2 | Chemoradiation | Peritoneal deposits |
| p53 mutant | IV | Chemotherapy | Paraaortic |
| p53 mutant | II | EBRT + vaginal brachytherapy | Paraaortic |
| p53 mutant | IIIA | Chemoradiation | Lung |
| p53 mutant | IIIA | Chemoradiation | Lung |
| p53 mutant | IIIA | Chemoradiation | Lung |
| p53 mutant | IIIC2 | Chemoradiation | Lung |
Fig. 1.
Overall survival for p53 wild and p53 mutant. The 2-year overall survival was 94.8% for a follow-up period of 30.8 months. OS-p53 wild type — 97.2%, the p53 mutant type — 91.7% (p-value 0.881)
Fig. 2.
Disease-free survival of p53 wild and p53 mutant. The disease-free survival was 89.4% for a median follow-up of 30.8 months. DFS of p53 wild type — 94.3%, p53 mutant variety — 83.5% (p-value 0.105)
Fig. 3.
Disease-free survival of endometrioid histology with p53 wild and p53 mutant. The 2-year disease-free survival for endometrioid carcinoma — p53 wild type was 100%, p53 mutant variety was 86.2% (p-value 0.033)
For p53 mutated tumors, overall survival at 2 years for endometrioid histology was 100% followed by serous with a survival of 83.3% and clear cell with a survival of 75%. The disease-free survival of p53 mutated tumors with endometrioid histology was 86.2%, serous with a survival of 83.3%, and clear cell with a survival of 75%. The 2-year survival probability for p53 mutated patients with cervical involvement and nodal involvement was was74.1% (p-value 0.029) and 62.5% (p-value 0.004), respectively.
Discussion
In our study, we could demonstrate a significant association between p53 mutation, high-grade tumors, stage, cervical, and adnexal involvement. In similar studies, there was a significant association between p53 mutation and advanced stage, non-endometrioid histology, and high grade [16, 17]. p53 may contribute to better tumor characterization and thus precisely determine clinical behavior.
In our study, p53 mutations were found in 29.1% of the endometrioid cancers, which was comparable to a study by Schulthei et al. [18]. Endometrioid tumors with p53 mutations behaved more aggressively than those with wild-type p53. There was also a significant difference in the 2-year disease-free survival by around 14% between the two groups. The 2-year overall survival of the p53 wild type was 97.2% and the p53 mutant type was 91.7%. The 2-year disease-free survival for p53 wild type was 94.3% and the p53 mutant variety was 83.5%. We could demonstrate a difference in overall and disease-free survival between the two groups, but we could not demonstrate statistical significance probably because of the shorter follow-up period and smaller sample size. Similar results were obtained in studies by Garg et al. and Akiyama [19, 20]. p53 immunohistochemistry assays in morphologically ambiguous endometrial carcinomas can be helpful for prognostic assessment and therapeutic decision making [19].
The standard algorithm for molecular testing of endometrial cancers which includes sequential testing of POLE mutation by Sanger sequencing, MMRd, and p53 by immunohistochemistry is resource-intensive and not routinely done in all centers. According to the latest molecular classification [13], p53 mutation itself in the endometrial cancers will make it intermediate risk if there is no myometrial infiltration. If p53 mutation is present and if there is myometrial infiltration, it will make the patient at high risk of requiring chemotherapy. If we at least do p53 IHC alone in all patients we will be able to pick up these patients who may otherwise be included in intermediate or high intermediate risk and may require chemotherapy [13, 14].
A longer follow-up of a larger cohort of patients is needed to determine the exact overall and disease-free survival as even a 2-year follow-up could demonstrate a difference in overall and disease-free survival in our population. Abnormal p53 accumulation may influence patient survival via unfavorable biological tumor properties, including rapid progression and radioresistance [20].
The limitations of this study are that instead of following the standard protocol only p53 mutation was done for molecular assessment. IHC overexpression of p53 was used as a surrogate for TP53 mutation. Splice site mutation can result in p53 wild-type staining and hence may be missed [12]. The sample size and the follow-up period were relatively small. Further studies preferably prospective studies are required to strengthen the current findings.
Conclusion
p53 mutation is significantly associated with high-grade histology, myometrial invasion, adnexal involvement, stage of the disease, and cervical involvement. p53 mutated tumors have lower overall survival, lower disease free survival and increased recurrence rate. Although the standard protocol for molecular assessment is recommended, it is resource-intensive. Identification of at least p53 mutation will help to categorize high-risk groups. It can be easily done as IHC on all endometrial biopsies. It may help to tailor surgery, adjuvant treatment, and follow-up. Newer studies and precision medicine to find targeted therapies against the p53 mutated tumors will help in bringing about a better disease response to adjuvant treatment [14].
Data Availability
The data and material are available for review.
Code Availability
The data was coded and analyzed with SPSS software.
Declarations
Ethics Approval
The study was conducted after institutional scientific and research board approval.
Consent to Participate
The study has been conducted after attaining consent to participate.
Consent for Publication
All authors consent for publication once it is accepted.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 71(3):209–49. [DOI] [PubMed]
- 2.Mathew A, George PS, Kalavathy MC, Padmakumari G, Jagathnath Krishna KM, Sebastian P. Cancer incidence and mortality: district cancer registry, Trivandrum, South India. Asian Pacific Journal of Cancer Prevention: APJCP. 2017;18(6):1485. doi: 10.22034/APJCP.2017.18.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, Reznek RH. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol. 2007;62(1):28–34. doi: 10.1016/j.crad.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Werner HM, Salvesen HB. Current status of molecular biomarkers in endometrial cancer. Curr Oncol Rep. 2014;16(9):403. doi: 10.1007/s11912-014-0403-3. [DOI] [PubMed] [Google Scholar]
- 6.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao D, Tahaney WM, Mazumdar A, Savage MI, Brown PH. Molecularly targeted therapies for p53-mutant cancers. Cell Mol Life Sci. 2017;74(22):4171–4187. doi: 10.1007/s00018-017-2575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15(1):2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 9.Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology. 2013;62(1):111–123. doi: 10.1111/his.12053. [DOI] [PubMed] [Google Scholar]
- 10.Levine DA, Cancer Genome Atlas Research Network (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497(7447):67–73 [DOI] [PMC free article] [PubMed]
- 11.Remmerie M, Janssens V. Targeted therapies in type II endometrial cancers: too little, but not too late. Int J Mol Sci. 2018;19(8):2380. doi: 10.3390/ijms19082380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Köbel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of p53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol. 2019;38(1 Suppl 1):S123. doi: 10.1097/PGP.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razumova Z, Bizzarri N, Kacperczyk-Bartnik J, Pletnev A, Martin AG, Persson J. Report from the European Society of Gynaecological Oncology (ESGO) 2020 State-of-the-Art Virtual Meeting. International Journal of Gynecologic Cancer. 2021;31(5). [DOI] [PubMed]
- 14.van den Heerik AS, Horeweg N, Nout RA, Lutgens LC, van der Steen-Banasik EM, Westerveld GH, van den Berg HA, Slot A, Koppe FL, Kommoss S, Mens JW (2020) PORTEC-4a: international randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. International Journal of Gynecologic Cancer:ijgc-2020. [DOI] [PMC free article] [PubMed]
- 15.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C (2016) ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. International Journal of Gynecologic Cancer. 26(1) [DOI] [PMC free article] [PubMed]
- 16.Ray S, Jha A, Islam AA, Sengupta M. Study of prognostic and diagnostic significance of P53 and PTEN mutation in proliferative lesions of endometrium. Journal of Current Medical Research and Opinion. 2020;3(08):563–9. doi: 10.15520/jcmro.v3i08.321. [DOI] [Google Scholar]
- 17.Watanabe T, Nanamiya H, Kojima M, Nomura S, Furukawa S, Soeda S, Tanaka D, Isogai T, Imai JI, Watanabe S, Fujimori K. Clinical relevance of oncogenic driver mutations identified in endometrial carcinoma [DOI] [PMC free article] [PubMed]
- 18.Schultheis AM, Martelotto LG, De Filippo MR, Piscuoglio S, Ng CK, Hussein YR, Reis-Filho JS, Soslow RA, Weigelt B. TP53 mutational spectrum in endometrioid and serous endometrial cancers. International journal of gynecological pathology: official journal of the International Society of Gynecological Pathologists. 2016;35(4):289. doi: 10.1097/PGP.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg K, Leitao MM, Wynveen CA, Sica GL, Shia J, Shi W, Soslow RA. p53 overexpression in morphologically ambiguous endometrial carcinomas correlates with adverse clinical outcomes. Mod Pathol. 2010;23(1):80–92. doi: 10.1038/modpathol.2009.153. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama A, Minaguchi T, Fujieda K, Hosokawa Y, Nishida K, Shikama A, Tasaka N, Sakurai M, Ochi H, Satoh T. Abnormal accumulation of p53 predicts radioresistance leading to poor survival in patients with endometrial carcinoma. Oncol Lett. 2019;18(6):5952–5958. doi: 10.3892/ol.2019.10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and material are available for review.
The data was coded and analyzed with SPSS software.