Graphical abstract
Keywords: Coronavirus disease 2019 (COVID-19), Vaccine, Chronic kidney disease, Kidney transplant, Anti-spike RBD IgG
Abstract
Background
Vaccination of patients with chronic kidney disease (CKD) and kidney transplants (KTs) may achieve a less robust immune response. Understanding such immune responses is crucial for guiding current and future vaccine dosing strategies.
Methods
This prospective, observational study estimated the immunogenicity of humoral and cellular responses of two SARS-CoV-2 vaccines in different patient groups with CKD compared with controls. Secondary outcomes included adverse events after vaccination and the incidence of COVID-19 breakthrough infection, including illness severity.
Results
In total, 212 patients received ChAdOx1 nCoV-19 (89.62 %) or inactivated vaccines (10.38 %).The antibody response against the S protein was analyzed at T0 (before the first injection), T1 (before the second injection), and T2 (12 weeks after the second injection). Seroconversion occurred in 92.31 % of controls at T2 and in 100 % of patients with CKD, 42.86 % undergoing KT, 80.18 % of hemodialysis (HD), and 0 % of patients undergoing continuous ambulatory peritoneal dialysis (CAPD) at T2 of the ChAdOx1 nCoV-19 vaccine. Neutralizing antibody levels by surrogate virus neutralization test were above the protective level at T2 in each group. The KT group exhibited the lowest neutralizing antibody and T cell response. Blood groups O and vaccine type were associated with good immunological responses. After the first dose, 14 individuals (6.6 out of the total population experienced COVID-19 breakthrough infection.
Conclusion
Immunity among patients with CKD and HD after vaccination was strong and comparable with that of healthy controls. Our study suggested that a single dose of the vaccine is not efficacious and delays may result in breakthrough infection. Some blood groups and types of vaccine can affect the immune response.
1. Introduction
Patients with chronic kidney disease (CKD), including kidney transplant (KT) recipients, and those on dialysis represent a special subgroup of patients requiring protection during the severe coronavirus disease 2019 (COVID-19) pandemic [1], [2]. Patients with CKD usually have a compromised immune response [3], [4], require higher dosages of vaccine and more frequent dosing because the vaccine response is short lived and achieves a lower response, especially among patients undergoing dialysis [5], [6].
Related reports of vaccination among patients with CKD mainly considered mRNA vaccines [8], [7]. Recent reports describing seroconversion rates among patients undergoing dialysis receiving two doses of the BNT 162b2 vaccine (Pfizer BioNtech) were lower than those of controls [9], [10]. One study reported a weak antibody response of patients with HD to the viral vector COVID-19 vaccine [11]. In Thailand, the main vaccines available are Coronavac (Sinovac Life Science, Beijing, China), BBIBP-Cor V vaccine (Sinopharm) and ChadOx1 nCoV-19 (Oxford-Astra Zeneca). Zhang et al. conducted a pilot, prospective study to survey the safety and humoral response to inactivated SARS-CoV-2 vaccine among 45 patients with CKD receiving a 2-dose immunization of inactivated (Sinovac and Sinopharm). They showed that the majority (84 %) of patients with CKD acquired detectable neutralizing antibody lower than those of controls [12]. Bruminhent et al. studied immune responses among 31 patients with KT, 28 with PD, 31 with HD and 16 controls with two-dose inactivated SARS-CoV-2 vaccine (V2) and a third dose of ChAdOx1 nCoV-19 vaccine (V3) at 1–2 months after V2. The anti-receptor binding domain antibody levels significantly increased from V2 across all groups (p < 0.05). Seroconversion and neutralization positivity rates were impaired among patients with KT in contrary to the other groups [13]. This study aimed to measure the antibody and cellular responses among patients with CKD, including those undergoing dialysis therapy and kidney transplantation, and to monitor the adverse events (AEs) after the first and second doses of vaccination. The incidence rate of SARS-CoV-2 postvaccination was also observed.
2. Materials and methods
This prospective cohort study included four different patient groups: patients with CKD, those on hemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD), recipients of KT, and a control group without kidney failure from the Faculty of Medicine, Vajira Hospital, Navamindradhiraj University. Participants were enrolled between July and December 2021. The inclusion criteria were CKD stages 3–5 (eGFR ≤ 60 mL/min/1.73 m3), patients with stage 5 CKD undergoing HD, CAPD and KT > 3 months. The healthy control group consisted of volunteer healthcare workers that had eGFR ≥ 60 mL/min/1.73 m3. Participants in every group were 18–90 years old. Every participant received the same vaccine type in both first and second doses. The exclusion criteria included allergy to the components of the vaccines, inability to receive the vaccine according to their schedule, fever or concomitant serious illnesses and side effects from the first dose of vaccination. Patients or individuals contracting COVID-19 (diagnosed via patient history and serological tests to detect the nucleocapsid [NCP] antibody) were excluded from the study. The study protocol was approved by the Vajira Ethics Committee, approval number 94/2564, and the participants were enrolled after obtaining written informed consent. This study was conducted following the principles of the Declaration of Helsinki and Good Clinical Practice. The vaccine used was authorized by the Thai Food and Drug Administration and Department of Medical Sciences. Each type of vaccine was allocated by patient’s preference. Vaccine response was defined as anti-SARS-CoV-2 S1 (IgG) antibody titer ≥35.2 BAU/ml. Seroconversion means that levels of antibody titers to be significantly higher in serum of recipients after vaccination. The reference levels as detailed in Appendix.
2.1. Trial procedure
The enrolled patients received the COVID-19 vaccine according to the vaccination protocol approved in Thailand, that is, two doses of ChAdox-1 nCOV-19 vaccine, 12-week interval, Coronavac, 3-week interval or BBIBP-Cor V, 4-week interval. All participants provided a blood sample for antibody and cellular immunity measurement at the following time periods: T0 (before the first injection), T1(before the second injection) and T2 (12 weeks after the second injection). Immunogenicity analysis was performed at one and three months post-infection.
2.2. Assessment of antibody responses
We classified responses as negative anti-SARS-CoV2-NCP IgG (NCP, Euroimmun, Hausen Bernsten CO; Ltd, Germany); (index value < 0.8), borderline (index value 0.8–1.1) and positive (index value > 1.1). We chose anti-SARS-CoV2 Spike S1/receptor binding domain (RBD) IgG < 25.6 binding antibody unit/mL BAU/mL as the negative cut-off point, values between 25.6 and 35.2 were considered borderline, and levels ≥35.2 BAU/mL were considered positive. A percent inhibition (%IH) > 35 for SARS CoV2 neutralizing antibodies (NA) was considered positive, index values ranging 20–25 % IH were defined as borderline, and values <20 % were considered negative.
2.3. Determination of antibodies against SARS-CoV-2
All SARS-CoV-2 antibody assays were performed and analyzed using the EUROIMMUN Analyzer I-2P® (Euroimmun Medizinische Labordiagnostika, Lubeck, Germany) at the Central Laboratory and Blood Bank, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University. Controls and calibrators were used in the test kit for each run. The ratios of diluted serum, optical density and cut-off values in this study were used according to the manufacturer’s instructions.
2.4. Detecting anti-SARS-CoV-2 nucleocapsid protein IgG
The IgG antibody against the nucleocapsid protein of SARS-CoV-2 in plasma samples was detected using an enzyme-linked immunosorbent assay (ELISA) (Euroimmun, Lübeck, Germany) (see Appendix).
2.5. Quantitative determination of anti-SARS-CoV-2 S1 (IgG)
The anti-SARS-CoV-2 S1/ (RBD) IgG QuantiVac ELISA IgG (Euroimmun, Lübeck, Germany) Kit was used for quantitative determination of human antibodies against immunoglobulin class IgG against the S1 domain of the SARS-CoV-2 spike protein of in serum samples (see Appendix).
2.6. Determining neutralizing antibodies against SARS-CoV-2
To detect the presence of NA against the S1 receptor-binding domain (RBD) of SARS-CoV-2 to ACE2 receptors in the plasma samples, the ELISA-based surrogate virus neutralization test was used (SARS-CoV-2 NeutraLISA (Euroimmun, Lübeck, Germany) (see Appendix).
2.7. Assessment of the T cell response by quantitative determination of interferon-γ release by SARS-CoV-2-specific T cells
Cellular immunogenicity was measured by calculating the secretion of interferon gamma (IFN-γ) using peripheral blood mononuclear cells upon SARS-Co-V2 glycoprotein stimulation and subsequent determination of released IFN-γ by ELISA (Euroimmun, Lübeck, Germany) (see Appendix).
2.8. Participants
Demographic information, including age, sex and body mass index, was obtained at the first enrolment. The vaccine type, date of vaccination, use of immunosuppressed agents, number and types of comorbidities and history of transplantation were recorded. Primary outcomes included humoral and cellular responses after COVID-19 vaccination at T0, T1 and T2, as measured by SARS-CoV2 spike S1-specific IgG antibody levels and the viral neutralization test by surrogate virus neutralization test. The percentages of responders in different cohorts (CKD, HD, CAPD and KT) were compared with the controls, within and between cohorts to define the seropositivity rate (individuals who developed detectable anti-SARS-Co-V antibodies). The secondary outcomes were rates of AEs after vaccination and the incidence of COVID-19 breakthrough infection after vaccination, including illness severity.
2.9. Statistical analysis
The sample size calculation is provided in detail in the Appendix. Values are presented as median (interquartile range) for continuous variables. Antibody levels were compared between timepoints and analyzed using the paired sample t-test or Wilcoxon matched-pairs signed-ranks test. Categorical variables were reported as frequencies and percentages. Proportions were compared using Fisher’s exact test (or the Kruskal-Wallis test as appropriate). Correlation between two continuous parameters was calculated using Spearman’s correlation. Logistic regression models were used in both univariate and multivariate analyses, and statistical significance was set at p < 0.05. Statistical analysis was performed using STATA (Version 13.0; StataCorp College Station, TX, USA).
2.10. Monitoring of adverse events
AE assessments, including vaccine and drug side effects after the first and second vaccine doses, were monitored.
3. Results
3.1. Baseline characteristics
Between June 2021 and December 2021, 212 patients with CKD at various stages and controls were vaccinated with COVID-19 vaccines, CoronaVac, BBIBP-Cor V, or ChAdOx1 nCoV-19 vaccine (AZD 1222). Totally, 31 patients (15.20 %) had underlying heart problems and none of the patients had either lung or liver diseases. Fourteen patients were lost to follow-up. Eleven patients died during the study period (COVID-19, eight;underlying diseases, two;sepsis,one:underlying disease). Finally, 212 patients (104 men, 49.06 %) with a mean age 54.8 ± 16.07 years were enrolled in the study (Fig. 1 ). The vaccination distribution was as follows: 190 patients (89.62 %) received ChAdOx1 nCoV-19, 20 (9.43 %) CoronaVac and two (0.94 %) BBIBP-Cor V. One hundred and thirty-four (63.20 %) patients were undergoing HD, four (1.88 %) were undergoing CAPD and seven (3.30 %) were KT recipients, twelve (5.66 %) were nondialysis patients with CKD, and 55 (25.94 %) were the controls. The median duration of HD was 3.04 years (IQR 1.42–5.29 years). Almost all patients and the control group received the ChAdOx1 nCoV-19 vaccine being the main vaccine scheme adopted in our country at the time of the study; the baseline characteristics of the population are detailed in Table 1 .
Fig. 1.
Study flowchart.
Table 1.
Baseline characteristics of patients.
| Overall (n = 212) |
Control (n = 55) |
CKD (n = 12) |
KT (n = 7) |
HD (n = 134) |
CAPD (n = 4) |
P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n (%) | n | n (%) | | n | n (%) | n | n (%) | n | n (%) | n | n (%) | ||
| Sex | 0.159a | ||||||||||||
| M | 104 (49.06) | 22 (40.00) | 3 (25.00) | 4 (57.14) | 73 (54.48) | 2 (50.00) | |||||||
| F | 108 (50.94) | 33 (60.00) | 9 (75.00) | 3 (42.86) | 61 (45.52) | 2 (50.00) | |||||||
| Age (years) (mean ± SD) | 208 | 54.8 ± 16.07 | 52 | 46.69 ± 17.65 | 12 | 63.08 ± 13.66 | 7 | 50.86 ± 11.11 | 133 | 57.34 ± 14.84 | 4 | 58 ± 11.66 | <0.001b |
| Weight (kg) (mean ± SD) | 205 | 62.76 ± 14.49 | 51 | 64.3 ± 13.89 | 12 | 57.4 ± 15.43 | 7 | 71.39 ± 14.8 | 131 | 61.97 ± 14.44 | 4 | 69.75 ± 16.9 | 0.198b |
| Height (cm) (mean ± SD) | 205 | 162.08 ± 9.31 | 51 | 163 ± 9.04 | 12 | 158.58 ± 9.86 | 7 | 165.29 ± 11.43 | 131 | 161.85 ± 9.34 | 4 | 162.5 ± 7.14 | 0.544b |
| BMI (kg/m2) (mean ± SD) | 205 | 23.75 ± 4.49 | 51 | 24.12 ± 4.35 | 12 | 22.52 ± 3.84 | 7 | 26.13 ± 5.58 | 131 | 23.52 ± 4.53 | 4 | 26.14 ± 4.38 | 0.322b |
| Dialysis vintage (year) [median (IQR)] | -0 | -0 | 0 | -0 | -0 | 7 | 9.83 (5.08–20.5) | 124 | 3.04 (1.42–5.29) | 3 | 1.58 (0.58–2.92) | ||
| Kidney disease | <0.001a | ||||||||||||
| No | 49 (23.9) | 46 (95.83) | 0 | 1 (14.29) | 2 (1.49) | 0 | |||||||
| Yes | 156 (76.1) | 2 (4.17) | 12 (100.00) | 6 (85.71) | 132 (98.51) | 4 (100.00) | |||||||
| Diabetes mellitus | <0.001a | ||||||||||||
| No | 144 (70.59) | 45 (95.74) | 7 (58.33) | 7 (1 0 0) | 82 (61.19) | 3 (75) | |||||||
| Yes | 60 (29.41) | 2 (4.26) | 5 (41.67) | 0 (0) | 52 (38.81) | 1 (25) | |||||||
| Heart disease | 0.013a | ||||||||||||
| No | 173 (84.80) | 46 (97.87) | 11 (91.67) | 6 (85.71) | 106 (79.1) | 4 (100.00) | |||||||
| Yes | 31 (15.20) | 1 (2.13) | 1 (8.33) | 1 (14.29) | 28 (20.9) | – | |||||||
| Hypertension | <0.001a | ||||||||||||
| No | 98 (48.04) | 43 (91.49) | 5 (41.67) | 3 (42.86) | 46 (34.33) | 1 (25.00) | |||||||
| Yes | 106 (51.96) | 4 (8.51) | 7 (58.33) | 4 (57.14) | 88 (65.67) | 3 (75.00) | |||||||
| Lung disease | NA | ||||||||||||
| No | 204 (100.00) | 47 (100.00) | 12 (100.00) | 7 (100.00) | 134 (100.00) | 4 (100.00) | |||||||
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Liver disease | NA | ||||||||||||
| No | 204 (100.00) | 47 (100.00) | 12 (100.00) | 7 (100.00) | 134 (100.00) | 4 (100.00) | |||||||
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Cancer | 0.554a | ||||||||||||
| No | 200 (98.04) | 45 (95.74) | 12 (100.00) | 7 (100.00) | 132 (98.51) | 4 (100.00) | |||||||
| Yes | 4 (1.96) | 2 (4.26) | 0 | 0 | 2 (1.49) | 0 | |||||||
| Blood group | 0.842a | ||||||||||||
| A | 40 (19.70) | 11 (23.4) | 0 | 2 (28.57) | 26 (19.40) | 1 (25.00) | |||||||
| B | 66 (32.51) | 14 (29.79) | 5 (45.45) | 1 (14.29) | 44 (32.84) | 2 (50.00) | |||||||
| AB | 21 (10.34) | 5 (10.64) | 2 (18.18) | 1 (14.29) | 13 (9.70) | – | |||||||
| O | 76 (37.44) | 17 (36.17) | 4 (36.36) | 3 (42.86) | 51 (38.06) | 1 (25.00) | |||||||
| BUN [median (IQR)] | 45 (27–59) | 14 (11–16.5) | 27 (23.5–36.5) | 21 (17–33) | 51 (37–65) | 36 (30–55) | <0.001c | ||||||
| Creatinine [median (IQR)] | 7.83 (3.37–10.3) | 0.75 (0.64–0.9) | 1.63 (1.23–2.02) | 1.28 (1.04–1.86) | 8.91 (7–11.4) | 10.1 (6.84–11.4) | <0.001c | ||||||
| eGFR [median (IQR)] | 6 (4.54–11) | 97.9 (87.3–100.8) | 32 (25–47) | 57 (39–65) | 5 (4–7) | 5 (4–9) | <0.001c | ||||||
| CKD Staging | |||||||||||||
| G1 (≥90) | 17 (10.43) | 16 (72.73) | 0 | 0 | 0 | 0 | |||||||
| G2 (60–89) | 8 (4.91) | 6 (27.27) | 1 (9.09) | 2 (40.00) | 0 | 0 | |||||||
| G3a (45–59) | 4 (2.45) | 0 | 3 (27.27) | 1 (20.00) | 0 | 0 | |||||||
| G3b (30–44) | 4 (2.45) | 0 | 2 (18.18) | 1 (20.00) | 0 | 0 | |||||||
| G4 (15–29) | 4 (2.45) | 0 | 3 (27.27) | 1 (20.00) | 0 | 0 | |||||||
| G5 (<15) | 126 (77.30) | 0 | 2 (18.18) | 0 | 122 (1 0 0) | 3 (100.00) | |||||||
| Hb [median (IQR)] | 10.7 (9.3–12.5) | 13.25 (12.6–14.05) | 11.9 (11.4–12.05) | 11.5 (11.5–12.5) | 9.95 (8.7–11.2) | 9.4 (7.9–11.3) | <0.001c | ||||||
| Hct [median (IQR)] | 33.1 (29–38.5) | 40.35 (38.35–41.85) | 36.45 (34.35–37.95) | 38 (35.3–39.5) | 31.1 (27.9–34.4) | 28 (25.1–34.5) | <0.001c | ||||||
| WBC [median (IQR)] | 6,275 (4,700–7,760) | 6,065 (,5055–7,215) | 6,250 (5,080–9,715) | 7,130 (5,630–7,760) | 6,295 (4,495–7,990) | 6980 (6490–8570) | 0.636c | ||||||
| Platelet [median (IQR)] | 227,500 (180,500–286,500) |
246,500 (28,900–328,500) |
260,000 (185,000–2770,00) |
187,000 (179,000–256,000) |
220,000 (184,000–282,500) |
287,000 (251000–335000) |
0.544c | ||||||
| SGOT [median (IQR)] | 21 (17–26) | 23 (21–32) | 23 (20–26) | 0 | 19 (14–21) | 0 | 0.008c | ||||||
| SGPT [median (IQR)] | 10 (7–24) | 22.5 (10–30) | 14.5 (5–24) | 0 | 10 (6–19) | 0 | 0.033c | ||||||
| Bilirubin [median (IQR)] | 0.48 (0.3–0.57) | 0.57 (0.29–0.68) | 0 | 0 | 0.4 (0.3–0.55) | 0 | 0.216c | ||||||
| Albumin [median (IQR)] | 4.1 (3.8–4.3) | 4.3 (4.2–4.7) | 4.3 (4.1–4.5) | 4.2 (4.1–4.3) | 4.1 (3.8–4.3) | 2.6 (2.5–4.10) | 0.010c | ||||||
| Globulin [median (IQR)] | 3.3 (2.9–3.7) | 3.1 (2.9–3.35) | 3.4 (3.2–4.1) | 3 (2.6–3.3) | 3.3 (2.9–3.8) | 3.4 (3.3–3.6) | 0.261c | ||||||
| ALP [median (IQR)] | 71 (57–88.5) | 65 (49–72) | 104.5 (49–160) | 0 | 74.5 (58–92.5) | 0 | 0.369c | ||||||
| FBS [median (IQR)] | 108 (93–137) | 99.5 (89.5–126) | 109.5 (95–130) | 97 (91–101) | 112 (94–139) | 109 (83–362) | 0.585c | ||||||
| Cholesterol [median (IQR)] | 158 (135–185.5) | 192 (161–206) | 162 (130–174.5) | 181 (175–206) | 154.5 (132–176) | 177 (131–282) | 0.015c | ||||||
| Triglyceride [median (IQR)] | 98 (69–138) | 91 (64–147.5) | 129.5 (104–183) | 96 (81–130) | 98 (69–134) | 47 (45–245) | 0.297c | ||||||
| Uric acid [median (IQR)] | 5.85 (4.85–7.4) | 5.7 (3.9–7.2) | 6.6 (5.7–9) | 9.3 (6.4–9.7) | 5.8 (4.8–7.4) | 5.5 (5.1–6) | 0.288c | ||||||
| Vaccine type | |||||||||||||
| AstraZeneca | 190 (89.62) | 41 (74.55) | 11 (91.67) | 7 (100.00) | 127 (94.78) | 4 (100.00) | 0.003a | ||||||
| Sinovac | 20 (9.43) | 14 (25.45) | 1 (8.33) | 0 | 5 (3.73) | 0 | |||||||
| Sinopharm | 2 (0.94) | 0- | 0 | 0 | 2 (1.49) | 0 | |||||||
Chi-square test.
ALP, Alkaline phosphatase; BMI, Body mass index;CKD,Chronic kidney disease; Hb, FBS,Fasting blood sugar;Haemoglobin; Hct; HaematocriT;SGOT,Aspartate aminotransferase.
SGPT,Alanine transaminase.
Fisher exact test.
One-way ANOVA.
Kruskal-Wallis H test.
The KT recipient group revealed an average age of 50.86 ± 11.11 years; 42.86 % were women; and median time since transplantation was 9.83 years (IQR 5.08–20.5) The maintenance immunosuppressant regimens included calcineurin inhibitors (87 %), corticosteroids (45.4 %), antimetabolites (82.4 %) and mTor inhibitors (10.4 %). The antimetabolite treatments used included mycophenolate mofetil (85.2 %), mycophenolic acid (11.5 %) and azathioprine (3.3 %). The mean age in the HD group was 57.34 ± 14.84 years, 45.52 % were women.Subjects in the control group were aged 46.69 ± 17.65 years and 60 % were women. Only four patients were treated with peritoneal dialysis, with a mean age of 58.00 ± 11.66 years. None of the patients had a prior or current diagnosis of COVID-19 and all tested negative for the anti-SARS-CoV-2 NCP IgG.
Diabetes is the most common cause of end-stage renal disease (ESRD). The median eGFR in the control group was 97.90 [IQR 87.30–100.80] mL/min/1.73 m2 compared with 32.0 [IQR 25–47] mL/min/1.73 m2 in the CKD group (p = 0.001). The median eGFR in the KT group was 57.0 [IQR 39–65] mL/min/1.73 m2. As expected, the eGFR in the HD and CAPD groups was the lowest, with a median eGFR of 5.00 [IQR 4–9] mL/min/1.73 m2 (Table 1, Fig. 2 ).
Fig. 2.
Estimated glomerular filtration rate (eGFR) according to different patient groups.
3.2. Anti-SARS-CoV-2 antibody response
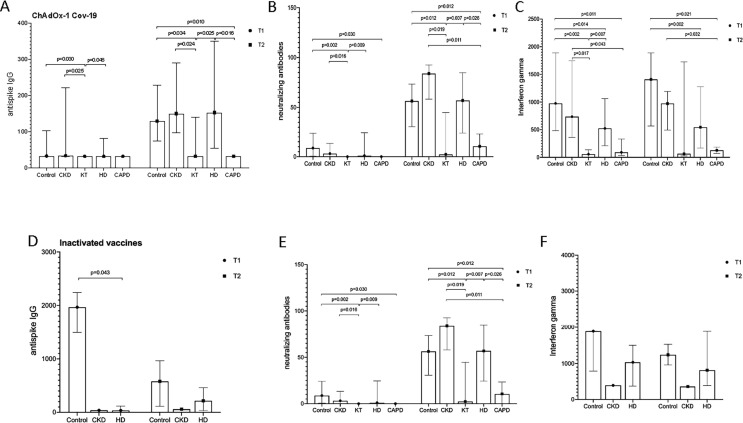
Patients on HD and nondialysis patients with CKD exhibited nonsignificant different antibody responses compared with those in the control group. In the CKD group, the median antibody titer was 3.20 BAU/mL [IQR 3.2–3.2] at T0, 34.10 BAU/mL [IQR 32.00–221.70] at T1 and 150.13 BAU/mL [IQR 97.12–290.65] at T2 (Fig. 3 ).The antibody levels in the CAPD group at T2 were significantly lower than those in the control and HD groups (p = 0.01; CAPD vs control, p = 0.016 CAPD vs HD). A positive antibody level was detected in only one KT recipient at T2.
Fig. 3.
(A–B) Humoral immune responses against SARS-CoV-2 was assessed by Euroimmune ELISA for (A) antispike IgG antibodies and (B) neutralizing activities level in different groups of CKD patients compared to controls receiving ChAdOx1 nCoV-19 vaccine at T1 and T2 (C) Cellular immune response was assessed by interferon-gamma levels in different groups of patients compared to controls at T1 and T2. (D–F) Humoral and cellular responses to inactivated vaccines in different groups of CKD patients compared to controls at T1 and T2.
Vaccine response was evaluated for 151 patients after the second dose in vaccine types. The response rate was 70.59 % in the control group of the ChAdOx1 nCoV-19 vaccine. The CKD and dialysis group had similar response rates of 60 and 59.62 % respectively. The KT group revealed a weak response of 33.33 % (Fig. 4 ). The CAPD group also showed a poor immunological response, with none being seropositive at T2. The NA and IFNγ seropositive rates followed a similar pattern to anti-SARS-CoV-2 antibodies with the lowest response rate in the KT and CAPD groups, and the level of immunity and response rate in the inactivated vaccine groups were satisfactory in CKD, HD, and KT groups compared with controls (Table 2 ).
Fig. 4.
(A–C) Seropositivity rates in vaccine serum samples elicited by ChAdOx1 nCoV-19. (D–F) Seropositivity rates in vaccine serum samples elicited by inactivated vaccines in different groups of patients at T1 and T2.
Table 2.
Proportion of rat seroconversion of anti-spike IgG antibody response, neutralizing capacity, and gamma-interferon among patients receiving dialysis, CKD, kidney transplantation, and healthy controls after vaccination with ChAdOx1 nCoV-19 and inactivated vaccines.
| Control |
CKD |
KT |
HD |
CAPD |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | ||
| ChAdOx1 nCoV-19 vaccine | |||||||||||
| Antispike IgG | 0.153 | ||||||||||
| Increase <35 | 5 | 29.41 | 4 | 40.00 | 4 | 66.67 | 42 | 40.38 | 3 | 100.00 | |
| Increase 35+ | 12 | 70.59 | 6 | 60.00 | 2 | 33.33 | 62 | 59.62 | – | ||
| NeutraLISA | 0.270 | ||||||||||
| Increase <35.2 | 9 | 52.94 | 4 | 40.00 | 5 | 71.43 | 50 | 45.45 | 3 | 100.00 | |
| Increase 35.2+ | 8 | 47.06 | 6 | 60.00 | 2 | 28.57 | 60 | 54.55 | – | ||
| Interferon | 0.529 | ||||||||||
| Increase ≤ 200 | 11 | 68.75 | 7 | 70.00 | 2 | 50.00 | 73 | 77.66 | 2 | 100.00 | |
| Increase > 200 | 5 | 31.25 | 3 | 30.00 | 2 | 50.00 | 21 | 22.34 | 0 | ||
| Inactivated Vaccines | |||||||||||
| Antispike IgG | 0.545 | ||||||||||
| Increase <35 | 5 | 100.00 | 1 | 100.00 | 0 | 3 | 60.00 | 0 | |||
| Increase 35+ | 0 | 0 | 0 | 2 | 40.00 | 0 | |||||
| Neutralizing Abs | 0.545 | ||||||||||
| Increase <35.2 | 5 | 100.00 | 1 | 100.00 | 0 | 3 | 60.00 | 0 | |||
| Increase 35.2+ | 0 | 0 | 0 | 2 | 40.00 | 0 | |||||
| Interferon | 0.583 | ||||||||||
| Increase ≤ 200 | 3 | 100.00 | 1 | 100.00 | 0 | 3 | 60.00 | 0 | |||
| Increase ≤ 200 | 0 | 0 | 0 | 2 | 40.00 | ||||||
Fisher's exact test.
Significant if p <0.05.
CAPD, continuous ambulatory peritoneal dialysis; CKD, chronic kidney disease; HD, haemodialysis; KT, kidney transplantation.
For quantitative determination of anti-SARS-CoV-2 S1 (IgG), samples with a ratio of<25.6 BAU/mL were interpreted as negative. Ratios of 25.6–35.2 BAU/mL was considered borderline, and a ratio ≥35.2 BAU/mL was considered positive.
For the SARS-CoV-2 neutralizing antibody, the interpreting results were as follows: % inhibition (IH) <20: Negative, % IH ≥20 to <35: Borderline % IH ≥35: positive.
For the SARS-CoV-2 interferon-gamma ELISA, the interpreting results were as follows: negative:<100 mlU/mL, Borderline: 100–200 mlU/mL, Positive: >200 mlU/mL.
3.3. Neutralizing antibodies test (NA)
NA showed a good correlation with levels of anti-spike IgG antibodies at T1 and T2 (r = 0.876 at T1, r = 0.819 at T2, p <0.001) (Fig. 5 ).The median NA in the control group was 8.7 % [IQR 0.51–24.24] at T1 and 56.34 % [IQR 30.61–73.47] at T2. NA levels in the CKD and dialysis groups overlapped with those of controls (Table 3 ). The NA level in the KT was lowest with a median level of 2.31 % [IQR 0–44.46].
Fig. 5.
(A) Correlation of anti-spike IgG levels with neutralizing antibodies. (B) Correlation of anti-spike IgG levels with interferon-gamma. (C) Correlation of neutralizing antibodies with interferon-gamma.
Table 3.
Comparison of neutralizing antibodies between different groups of patients and the control group.
| n | Median (IQR) | n | Median (IQR) | P-value | |
|---|---|---|---|---|---|
| Neutralizing antibody (%Inhibition) | |||||
| Control vs CKD | |||||
| T0 | 30 | 1.1 (0–3.53) | 10 | 0 (0–3.86) | 0.336 |
| T1 | 22 | 8.7 (0.51–24.24) | 10 | 3.1 (0–13.36) | 0.388 |
| T2 | 26 | 56.34 (30.61–73.47) | 10 | 83.93 (58.22–92.73) | 0.148 |
| ΔT0–T1 | 20 | 5.5 (0–16.96) | 10 | 1.17 (0–13.36) | 0.627 |
| ΔT1–T2 | 17 | 34.33 (21.19–70.37) | 10 | 59.07 (32.22–82.21) | 0.228 |
| Control vs KT | |||||
| T0 | 30 | 1.1 (0–3.53) | 7 | 0 (0–7.78) | 0.692 |
| T1 | 22 | 8.7 (0.51–24.24) | 7 | 0 (0–0) | 0.002 |
| T2 | 26 | 56.34 (30.61–73.47) | 7 | 2.31 (0–44.76) | 0.012 |
| ΔT0–T1 | 20 | 5.5 (0–16.96) | 7 | 0 (−7.78 to 0) | 0.003 |
| ΔT1–T2 | 17 | 34.33 (21.19–70.37) | 7 | 2.31 (0–44.76) | 0.053 |
| Control vs HD | |||||
| T0 | 30 | 1.1 (0–3.53) | 124 | 0 (0–0.89) | <0.001 |
| T1 | 22 | 8.7 (0.51–24.24) | 115 | 1.01 (0–24.59) | 0.121 |
| T2 | 26 | 56.34 (30.61–73.47) | 111 | 56.93 (24.26–84.89) | 0.906 |
| ΔT0–T1 | 20 | 5.5 (0–16.96) | 115 | 0.63 (0–22.54) | 0.429 |
| ΔT1–T2 | 17 | 34.33 (21.19–70.37) | 110 | 39.33 (10.65–62.69) | 0.453 |
| Control vs CAPD | |||||
| T0 | 30 | 1.1 (0–3.53) | 4 | 0 (0–0) | 0.021 |
| T1 | 22 | 8.7 (0.51–24.24) | 3 | 0 (0–0) | 0.030 |
| T2 | 26 | 56.34 (30.61–73.47) | 3 | 10.5 (0–23.3) | 0.012 |
| ΔT0–T1 | 20 | 5.5 (0–16.96) | 3 | 0 (0–0) | 0.096 |
| ΔT1–T2 | 17 | 34.33 (21.19–70.37) | 3 | 10.5 (0–23.3) | 0.064 |
| CKD vs KT | |||||
| T0 | 30 | 1.1 (0–3.53) | 7 | 0 (0–7.78) | 0.743 |
| T1 | 22 | 8.7 (0.51–24.24) | 7 | 0 (0–0) | 0.016 |
| T2 | 26 | 56.34 (30.61–73.47) | 7 | 2.31 (0–44.76) | 0.019 |
| ΔT0–T1 | 20 | 5.5 (0–16.96) | 7 | 0 (−7.78 to 0) | 0.036 |
| ΔT1–T2 | 17 | 34.33 (21.19–70.37) | 7 | 2.31 (0–44.76) | 0.040 |
| CKD vs HD | |||||
| T0 | 30 | 1.1 (0–3.53) | 124 | 0 (0–0.89) | 0.394 |
| T1 | 22 | 8.7 (0.51–24.24) | 115 | 1.01 (0–24.59) | 0.879 |
| T2 | 26 | 56.34 (30.61–73.47) | 111 | 56.93 (24.26–84.89) | 0.120 |
| ΔT0–T1 | 20 | 5.5 (0–16.96) | 115 | 0.63 (0–22.54) | 0.963 |
| ΔT1–T2 | 17 | 34.33 (21.19–70.37) | 110 | 39.33 (10.65–62.69) | 0.074 |
| CKD vs CAPD | |||||
| T0 | 30 | 1.1 (0–3.53) | 4 | 0 (0–0) | 0.156 |
| T1 | 22 | 8.7 (0.51–24.24) | 3 | 0 (0–0) | 0.098 |
| T2 | 26 | 56.34 (30.61–73.47) | 3 | 10.5 (0–23.3) | 0.011 |
| ΔT0–T1 | 20 | 5.5 (0–16.96) | 3 | 0 (0–0) | 0.297 |
| ΔT1–T2 | 17 | 34.33 (21.19–70.37) | 3 | 10.5 (0–23.3) | 0.011 |
| KT vs HD | |||||
| T0 | 7 | 0 (0–7.78) | 124 | 0 (0–0.89) | 0.291 |
| T1 | 7 | 0 (0–0) | 115 | 1.01 (0–24.59) | 0.009 |
| T2 | 7 | 2.31 (0–44.76) | 111 | 56.93 (24.26–84.89) | 0.007 |
| ΔT0–T1 | 7 | 0 (−7.78 to 0) | 115 | 0.63 (0–22.54) | 0.005 |
| ΔT1–T2 | 7 | 2.31 (0–44.76) | 110 | 39.33 (10.65–62.69) | 0.066 |
| KT vs CAPD | |||||
| T0 | 7 | 0 (0–7.78) | 4 | 0 (0–0) | 0.149 |
| T1 | 7 | 0 (0–0) | 3 | 0 (0–0) | 1.000 |
| T2 | 7 | 2.31 (0–44.76) | 3 | 10.5 (0–23.3) | 0.814 |
| ΔT0–T1 | 7 | 0 (−7.78 to 0) | 3 | 0 (0–0) | 0.207 |
| ΔT1–T2 | 7 | 2.31 (0–44.76) | 3 | 10.5 (0–23.3) | 0.814 |
| HD vs CAPD | |||||
| T0 | 124 | 0 (0–0.89) | 4 | 0 (0–0) | 0.214 |
| T1 | 115 | 1.01 (0–24.59) | 3 | 0 (0–0) | 0.084 |
| T2 | 111 | 56.93 (24.26–84.89) | 3 | 10.5 (0–23.3) | 0.026 |
| ΔT0–T1 | 115 | 0.63 (0–22.54) | 3 | 0 (0–0) | 0.240 |
| ΔT1–T2 | 110 | 39.33 (10.65–62.69) | 3 | 10.5 (0–23.3) | 0.116 |
Mann-Whitney U test.
CAPD, continuous ambulatory peritoneal dialysis; CKD, chronic kidney disease; HD, haemodialysis; KT, kidney transplantation.
T0: Before the first vaccination.
T1: Before the second vaccination.
T2: After T1 for 3 months.
For the SARS-CoV-2 neutralizing antibody, the interpreting results were as follows: % inhibition (IH) <20: negative, % IH ≥20 to <35: borderline, % IH ≥35: positive.
3.4. T cell responses
The median INFγ level in the CKD group was lower than that in the control group at T2 (973 mIU/mL, IQR 494.48–1191.11), while the level decreased by one half among patients on HD (median value 544.36 mIU/mL IQR 168.96–1273.58). Patients on CAPD and KT recipients indicated the lowest median INFγ value at 127.99 mIU/mL [IQR 68.46–187.52]. <50 % seropositive individuals were detected in our cohort (Table 2, Table 4 ).
Table 4.
Comparison of interferon gamma levels between different groups of patients and the control group.
| Interferon-gamma (mIU/mL) | n | Median (IQR) | n | Median (IQR) | P-value |
|---|---|---|---|---|---|
| Control vs CKD | |||||
| T1 | 19 | 975.82 (480.06–1890) | 10 | 736.02 (358.53–1749.45) | 0.345 |
| T2 | 24 | 1409.76 (565.84–1890) | 10 | 973 (494.48–1191.11) | 0.209 |
| ΔT1–T2 | 16 | 61.2 (0–239.38) | 10 | 77.78 (−452.89 to 251.48) | 0.711 |
| Control vs KT | |||||
| T1 | 19 | 975.82 (480.06–1890) | 6 | 57.1 (0–137.58) | 0.002 |
| T2 | 24 | 1409.76 (565.84–1890) | 5 | 64.94 (25.15–1728.22) | 0.088 |
| ΔT1–T2 | 16 | 61.2 (0–239.38) | 4 | 481.44 (−23.75 to 1391.01) | 0.297 |
| Control vs HD | |||||
| T1 | 19 | 975.82 (480.06–1890) | 109 | 523.8 (208.25–1062.24) | 0.014 |
| T2 | 24 | 1409.76 (565.84–1890) | 99 | 544.36 (168.96–1273.58) | 0.002 |
| ΔT1–T2 | 16 | 61.2 (0–239.38) | 94 | 0 (8130.98 to 166.56) | 0.248 |
| Control vs CAPD | |||||
| T1 | 19 | 975.82 (480.06–1890) | 3 | 91.5 (36.62–329.75) | 0.011 |
| T2 | 24 | 1409.76 (565.84–1890) | 2 | 127.99 (68.46–187.52) | 0.021 |
| ΔT1–T2 | 16 | 61.2 (0–239.38) | 2 | 63.93 (31.84–96.02) | 1.000 |
| CKD vs KT | |||||
| T1 | 10 | 736.02 (358.53–1749.45) | 6 | 57.1 (0–137.58) | 0.017 |
| T2 | 10 | 973 (494.48–1191.11) | 5 | 64.94 (25.15–1728.22) | 0.426 |
| ΔT1–T2 | 10 | 77.78 (−452.89 to 251.48) | 4 | 481.44 (−23.75 to 1391.01) | 0.396 |
| CKD vs HD | |||||
| T1 | 10 | 736.02 (358.53–1749.45) | 109 | 523.8 (208.25–1062.24) | 0.472 |
| T2 | 10 | 973 (494.48–1191.11) | 99 | 544.36 (168.96–1273.58) | 0.252 |
| ΔT1–T2 | 10 | 77.78 (−452.89 to 251.48) | 94 | 0 (−130.98 to 166.56) | 0.834 |
| CKD vs CAPD | |||||
| T1 | 10 | 736.02 (358.53–1749.45) | 3 | 91.5 (36.62–329.75) | 0.043 |
| T2 | 10 | 973 (494.48–1191.11) | 2 | 127.99 (68.46–187.52) | 0.032 |
| ΔT1–T2 | 10 | 77.78 (−452.89 to 251.48) | 2 | 63.93 (31.84–96.02) | 0.830 |
| KT vs HD | |||||
| T1 | 6 | 57.1 (0–137.58) | 109 | 523.8 (208.25–1062.24) | 0.007 |
| T2 | 5 | 64.94 (25.15–1728.22) | 99 | 544.36 (168.96–1273.58) | 0.341 |
| ΔT1–T2 | 4 | 481.44 (−23.75 to 1391.01) | 94 | 0 (−130.98 to 166.56) | 0.166 |
| KT vs CAPD | |||||
| T1 | 6 | 57.1 (0–137.58) | 3 | 91.5 (36.62–329.75) | 0.604 |
| T2 | 5 | 64.94 (25.15–1728.22) | 2 | 127.99 (68.46–187.52) | 0.699 |
| ΔT1–T2 | 4 | 481.44 (−23.75 to 1391.01) | 2 | 63.93 (31.84–96.02) | 1.000 |
| HD vs CAPD | |||||
| T1 | 109 | 523.8 (208.25–1062.24) | 3 | 91.5 (36.62–329.75) | 0.072 |
| T2 | 99 | 544.36 (168.96–1273.58) | 2 | 127.99 (68.46–187.52) | 0.130 |
| ΔT1–T2 | 94 | 0 (−130.98 to 166.56) | 2 | 63.93 (31.84–96.02) | 0.572 |
Mann-Whitney U test.
CAPD, continuous ambulatory peritoneal dialysis; CKD, chronic kidney disease; HD, haemodialysis; KT, kidney transplantation.
T0: Before the first vaccination.
T1: Before the second vaccination.
T2: After T1 for 3 months.
For the SARS-CoV-2 interferon-gamma ELISA, the interpreting results were as follows: negative: <100 mlU/mL, borderline: 100–200 mlU/mL, positive: >200 mlU/mL.
3.5. Factors affecting the immune response
Variables significantly associated with vaccine response from multivariate regression analysis (anti-spike IgG and NA) included blood group O (OR.8.21;95 % CI,2.12–31.78; p = 0.012) (Table 5 ) and vaccine type (OR, 9.02; 95 % CI, 2.00–50.10; p = 0.002). The ChAdOx1 nCoV-19 vaccine was associated with higher levels of antispike IgG while the NA and IFNγ did not differ.
Table 5.
Factors affecting anti-spike IgG levels (level difference between T2 and T1) by uni-multivariable analysis.
| Crude OR (95 %CI) | P-value | Adjusted OR (95 %CI) | P-value | |
|---|---|---|---|---|
| Type of patient | ||||
| Control | 2.20 (0.33–14.73) | 0.416 | 2.17 (0.10–45.01) | 0.617 |
| CKD | 2.80 (0.36–21.73) | 0.325 | 7.86 (0.72–86.49) | 0.092 |
| HD | 2.84 (0.50–16.2) | 0.239 | 4.74 (0.67–33.66) | 0.120 |
| CAPD | NA | NA | NA | NA |
| Age | 1.01 (0.99–1.03) | 0.552 | 1.01 (0.98–1.03) | 0.635 |
| Sex: M | 1.36 (0.71–2.58) | 0.354 | 1.65 (0.76–3.59) | 0.203 |
| BMI | 1.04 (0.96–1.12) | 0.338 | 1.08 (0.98–1.19) | 0.104 |
| Kidney Diseases | 1.07 (0.45–2.58) | 0.875 | 0.60 (0.04–8.93) | 0.709 |
| Heart Diseases | 1.87 (0.75–4.65) | 0.177 | 1.92 (0.65–5.62) | 0.235 |
| Hypertension | 0.77 (0.40–1.47) | 0.427 | 0.44 (0.19–1.02) | 0.056 |
| Cancer | 0.39 (0.04–4.41) | 0.448 | 0.67 (0.04–10.37) | 0.773 |
| Blood group | ||||
| A | 2.80 (0.75–10.48) | 0.126 | 4.10 (0.95–17.78) | 0.059 |
| B | 2.29 (0.70–7.53) | 0.173 | 2.87 (0.78–10.57) | 0.112 |
| O | 4.40 (1.34–14.49) | 0.015 | 8.21 (2.12–31.78) | 0.002 |
| Vaccine: | 6.36 (1.33–30.54) | 0.021 | 9.02 (1.62–50.10) | 0.012 |
Multiple logistics regression.
Significant if p < 0.05.
BMI, body mass index; CAPD, continuous ambulatory peritoneal dialysis; CKD, chronic kidney disease; HD, haemodialysis; KT, kidney transplantation.
3.6. Sars-CoV2 infection
After the first dose, 14 patients (6.6 %) experienced breakthrough COVID −19 infection and 57.14 % of these patients died. Of these, 13 (92.85 %) received only one dose of a vaccine, with a median interval of 52 [IQR 44–61] days after the first vaccination. One patient developed COVID-19 after completing the second dose on day 64. Infection in two controls was resolved uneventfully. Overall, 85.17 % of cases were in the HD group. Factors associated with SARS-CoV2 infection were male sex and blood group (p = 0.005 and p = 0.039, respectively) (Supplementary Table 1). Only one patient received an inactivated vaccine. Among the patients diagnosed with COVID-19 during follow-up, the median anti-spike IgG, NA and IFNγ levels significantly increased at 1one and three months after diagnosis, and natural immunity was robust and significantly higher than vaccine-induced immunity for as long as three months (Table 6 ).
Table 6.
Comparison of immunogenicity between non-COVID-19 and COVID-19 patients.
| Non-COVID-19 (n = 198) |
COVID-19 (n = 14) |
P-value | |||
|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | ||
| ChAdOx-1 nCov-19 vaccine | |||||
| S1/RBD IgG (BAU/mL) | |||||
| T0 | 155 | 3.2 (3.2–3.2) | 13 | 3.2 (3.2–3.73) | 0.525 |
| T1 | 145 | 32 (32–73.6) | 6 | 2673.55 (865–3840) | <0.001 |
| T2 | 152 | 130.93 (48.06–274.02) | 5 | 2952.9 (588.28–3619.7) | 0.001 |
| ΔT0–T1 | 142 | 28.8 (28.8–62.22) | 6 | 2667.11 (861.8–3836.8) | 0.003 |
| ΔT1–T2 | 135 | 53.2 (0–200.2) | 5 | −597.4 (−887.1 to 0) | 0.051 |
| Neutralizing Abs (%Inhibition) | |||||
| T0 | 162 | 0 (0–1.89) | 13 | 0 (0–0.16) | 0.571 |
| T1 | 151 | 0.79 (0–19.76) | 6 | 99.35 (96.12–99.73) | 0.001 |
| T2 | 152 | 55.4 (23.41–83.21) | 5 | 99.64 (99.63–99.73) | <0.001 |
| ΔT0–T1 | 149 | 0.41 (0–15.33) | 6 | 97.73 (95.62–99.73) | 0.001 |
| ΔT1–T2 | 142 | 38.85 (14.31–65) | 5 | 0.02 (−0.09 to 0.3) | 0.001 |
| Interferon gamma (mIU/mL) | |||||
| T1 | 143 | 521.24 (197.09–1062.24) | 4 | 1890 (1706.46–1890) | 0.006 |
| T2 | 136 | 602.88 (189.02–1501.61) | 4 | 1573.37 (1175.31–1890) | 0.044 |
| ΔT1–T2 | 124 | 18.4 (−105.52 to 202.98) | 2 | 0 (0–0) | 0.710 |
| Inactivated vaccine | |||||
| S1/RBD IgG (BAU/mL) | |||||
| T0 | 7 | 4.58 (3.2–6.61) | 1 | 3.2 (3.2–3.2) | 0.264 |
| T1 | 19 | 1496.9 (39–2215.1) | 1 | 3840 (3840–3840) | 0.117 |
| T2 | 11 | 217.27 (32–776.11) | 1 | 690.5 (690.5–690.5) | 0.466 |
| ΔT0–T1 | 7 | 28.8 (25.39–36.8) | 1 | 3836.8 (3836.8–3836.8) | 0.127 |
| ΔT1–T2 | 10 | 0 (−1022.3 to 20) | 1 | −3149.5 (−3149.5 to 3149.5) | 0.111 |
| Neutralizing Abs (%Inhibition) | |||||
| T0 | 8 | 0 (0–5.12) | 1 | 0 (0–0) | 0.490 |
| T1 | 20 | 98.18 (9.11–99.5) | 1 | 65.43 (65.43–65.43) | 0.740 |
| T2 | 11 | 87.88 (35.31–97.7) | 1 | 99.73 (99.73–99.73) | 0.111 |
| ΔT0–T1 | 8 | 0.28 (−1.9 to 9.8) | 1 | 65.43 (65.43–65.43) | 0.120 |
| ΔT1–T2 | 10 | 0.3 (−1.75 to 24.54) | 1 | 34.3 (34.3–34.3) | 0.343 |
| Interferon gamma (mIU/mL) | |||||
| T1 | 17 | 1416.24 (675.25–1890) | 1 | 383.47 (383.47–383.47) | 0.136 |
| T2 | 11 | 957.52 (395.8–1528.06) | 1 | 1890 (1890–1890) | 0.189 |
| ΔT1–T2 | 8 | −19.26 (−833.57 to 83.41) | 1 | 1506.53 (1506.53–1506.53) | 0.121 |
T0: Before the first vaccination.
T1: Before the second vaccination.
T2: After T1 for 3 months.
For quantitative determination of anti-SARS-CoV-2 S1 (IgG), samples with a ratio of <25.6 BAU/mL were interpreted as negative. Ratios of 25.6–35.2 BAU/mL was considered borderline, and a ratio ≥35.2 BAU/mL was considered positive.
For the SARS-CoV-2 neutralizing antibody, the interpreting results were as follows: % inhibition (IH) <20: negative, % IH ≥20 to <35: borderline, % IH ≥35: positive.
For the SARS-CoV-2 interferon-gamma ELISA, the interpreting results were as follows: negative: <100 mlU/mL, borderline: 100–200 mlU/mL, positive: >200 mlU/mL.
3.7. Vaccine type and immune response
Of the 20 patients receiving inactivated vaccines, seven, one and 14 were in the HD, CKD and control groups, respectively. Antibodies were detected at a positive level (>35 BAU/mL) at T1 and increased progressively to a median of 217.27 [IQR 32–460] at T2 among patients with HD. NA levels were detected at low titers at T2 in both CKD and HD groups. All patients in the control group responded to the inactivated vaccine with an antibody titer above threshold values. Only 40 % of patients on HD presented positive cellular immunity as measured by INFγ compared with the 100 % response rate in controls (Table 2). Factors associated with seropositivity are shown in Supplementary Table 2.
3.8. Adverse events
Among vaccine recipients, mild-to-moderate pain at the injection site was the most commonly reported local reaction, which resolved within 1–2 days. Fever was the second most common symptom. The local reactions did not increase after the second dose. Fever occurred more frequently in the control group (p = 0.025) and no serious AEs were recorded (Table 7 ).
Table 7.
Side effects of the COVID-19 vaccine after the first and second doses.
| No |
Yes |
|||
|---|---|---|---|---|
| n | % | n | % | |
| First dose vaccine | ||||
| Fever | 125 | 69.44 | 55 | 30.56 |
| Numbness | 179 | 100.00 | – | |
| Headache | 164 | 91.11 | 16 | 8.89 |
| Other | 98 | 54.44 | 82 | 45.56 |
| Second dose vaccine | ||||
| Fever | 149 | 93.13 | 11 | 6.88 |
| Numbness | 158 | 99.37 | 1 | 0.63 |
| Headache | 159 | 100.00 | – | |
| Other | 130 | 81.25 | 30 | 18.75 |
Other: Soreness at the injection site, muscle ache.
4. Discussion
Patients with CKD, especially ESRD undergoing dialysis, are at a very high risk of death following COVID-19 [14], [15]. Evidence suggests that patients with CKD may have a less robust antibody response after vaccination than healthy controls [16], [17], [18], [19], [20] . Our study consisted of a diverse group of patients with CKD receiving different therapies. Our major finding was that patients with CKD, including those on maintenance HD, developed a substantial humoral response following the two vaccine doses (inactivated and ChAdOx1 nCoV-19 vaccines). Humoral seroconversion responses were maintained for as long as 12 weeks after completing the second dose, and the responses were equivalent to those of healthy individuals. However, patients with KT developed fewer humoral immune responses than those in the other groups. Immunosuppression may induce a weak anti-SARS-CoV-2 antibody response.
The immune response from inactivated whole-virus SARS-CoV-2 vaccine among patients with HD was demonstrated to be satisfactory. However, fewer patients achieved humoral immune responses compared with healthy individuals [21]. In our study, >50 % of all patients except recipients of KT experienced seroconversion after receiving the second dose of inactivated vaccines. Related studies have reported variable responses to COVID-19 vaccines among patients with CKD, with most studies reporting on mRNA vaccines [22], [23], [24], [25], [26]. However, the durability of this immune response and the extent to which it translates to protective immunity remain unclear. A systematic review of 18 studies found that the antibody response to full vaccination with two doses of COVID-19 mRNA vaccines among patients undergoing HD, CAPD and KT was lower than that in the healthy population [27]. In phase 3 trials, BNT162b2, mRNA-1273 and ChAdOx1 nCoV-19 prevented COVID-19 in 95, 94.1 and 70.4 % of participants [28], [29], [30], respectively, suggesting that the mRNA vaccines might induce protective immunity more reliably than ChAdOx1 nCoV-19. In addition, both mRNA vaccines and viral-vector vaccines induce balanced humoral and T cell immunity [31].
Our study measured cellular immunity to better explore the immunogenicity of these specific populations using IFNγ levels. We found a significant correlation between IFNγ, SARS-CoV-2-specific antibodies and NA.Cytotoxic CD8+ T cells help accelerate the clearance of many respiratory viruses [32] and are essential in reducing the risk of SARS-CoV-2. Here, we demonstrated a good T cell response among patients with CKD and those on HD, which occurred as early as after the first dose of the ChAdOx1 nCoV-19 vaccine. The level of cellular immunity in this study correlated well with anti-SARS-CoV-2 antibody and NA, as in related studies [33]. Cellular immunity was then extrapolated to a good humoral immune response.
The antibody responses and NA levels in both vaccine groups did not significantly different except in the control group at T1. After the second dose, the level of immunity was similar (Supplementary Tables 3–7). The sample size in the inactivated vaccine group was small, and the protocol for the inactivated vaccine was only 3–4 weeks apart. This implied that antibody levels in the inactivated group declined more rapidly than in the other groups and this vaccine should not be recommended among patients with CKD and those on HD. The inappropriately high level of anti-spike IgG in the control group at T1 after inactivated vaccine might have been caused by natural infection. Since anti-nucleocapsid was performed only before participants recruitment.
The new cases of COVID-19 were detected after the first doses of vaccination; more than one half of these patients were in the HD group (85.71 %). Our study suggested a more rapid vaccination response among patients with CKD and on dialysis. The results also implied that patients on HD should not be considered for a delayed second vaccination dose. To prevent new cases of COVID-19, the second dose should be scheduled early as four to eight weeks after the first dose. Most people with symptomatic SARS-CoV-2 undergo seroconversion to produce a detectable, specific antibody response in the acute phase (Table 6). However, they should be re-vaccinated because the specific IgM rises in the acute phase and the IgG peaks appear later but decline after three to four months [34], [35].
Most studies have not reported an association between antibody response and other factors, such as age or sex. Our findings showed significant associations for blood group O, which constitutes a novel finding. Related studies have revealed that blood group O is associated with less viral infection and illness severity [36], [37], [38].Blood group A was found to be associated with an increased risk of infection and mortality but a decreased risk of intubation and death [39]. The molecular mechanisms by which ABO polymorphism impacts risks of SARS-CoV2 infection might involve ABO antibodies inhibiting the interaction between angiotensin converting enzyme-2 receptor and the virus, related to the presence of the anti-A antibody [40] or anti-A isohemaglutinin titers [41]. Further studies need to confirm these findings. This study showed the effectiveness of the ChAdOx1 nCoV-19 vaccine over inactivated vaccines among patients with CKD. We found no difference in serious AEs between the two vaccine formulations, except for fever and numbness, which resolved in a few days.
Our study exhibits several strengths, including a comprehensive overview of the immunogenicity of both humoral and cellular responses to COVID-19 vaccines in a broad sample of patients with CKD. The sizes of the HD and CKD cohorts were sufficiently large for the control group to allow us to identify differences. The results presented here have a long follow-up to three months after vaccination in contrast to only <four weeks in other studies. This may have implications for treatment and policy, because the ChAdOx1 nCoV-19 vaccine remains one of the main COVID-19 vaccine used in many countries. Nevertheless, our findings were limited by the small sample size and unequal distribution of the CKD population The number of patients other than those undergoing HD was insufficient to draw a meaningful conclusion concerning the other subgroups. The loss follow-up rate was also high in the control group.
5. Conclusion
Immunity among patients on HD and those with CKD after completing two vaccinations with candidate vaccines was strong, although the responses among recipients of KT and patients on CAPD were below acceptable levels, reinforcing the idea that this population should be vaccinated as soon as possible and receive a booster dose with the same or a different vaccine platform, such as an mRNA vaccine. A timely second dose of the COVID-19 vaccine seems necessary to ensure protection of patients with kidney disease from SARS-CoV-2. Blood group O and vaccine type were associated with good immune response.
6. Ethics approval
This study protocol was reviewed and approved by the Vajira Institutional Review Board, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, approval number 94/2564.
7. Consent to participate
Written informed consent was obtained from all participants, and the experiment was performed in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Thananda Trakarnvanich reports financial support was provided by Navamindradhiraj University. Thananda Trakarnvanich reports a relationship with Navamindradhiraj University that includes: funding grants and non-financial support. None to be declared.
Acknowledgments
Acknowledgements
We thank Ms. Saowalak Saikwan for data gathering and extraction And Ms Worachanee Imjaijit for her statistical assistance. We are grateful to Ms. Oranu Teansuwan and Ms Mala Treewatchareekorn for laboratory assistance.
Author contributions
Thananda Trakarnvanich: Concept and design, acquisition, analysis, interpretation of data, drafting of the manuscript, supervision, and administrative, technical and material support. Tanun Ngamvitchukorn: Concept and design, acquisition, analysis, or interpretation of data. Uraporn Phumisantiphong: Drafting the manuscript and laboratory analysis. Kittisak Pholtawornkulchai: Concept, design and supervision. Krittima Phochanasomboon: acquisition, analysis and material support. Anan Manomaipiboon: Supervision, and administrative, technical and material support. All authors discussed the results and implications and commented on the manuscript at all stages. All authors read and approved the final manuscript.
Funding/Support
This study was supported by a grant from Navamindradhiraj University (Grant No. 67/2564.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.09.067.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
The data supporting the findings of this study are available from the corresponding author. Data supporting the findings of this study are openly available in “figshare” at http://doi.org/10.6084/m9.figshare.19552222.
References
- 1.Hilbrands L.B., Duivenvoorden R., Vart P., et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J.J., Lee T.H., Tian Y.C., et al. Immunogenicity rates after SARS-CoV-2 vaccination in people with end-stage kidney disease: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:e2131749. doi: 10.1001/jamanetworkopen.2021.31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kengibe P., Makulo J., Nlandu Y., et al. Response to single dose hepatitis B vaccine in Congolese non-HIV hemodialysis patients: a prospective observational study. PAMJ. 2019;34 doi: 10.11604/pamj.2019.34.122.19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensouna I., Caudwell V., Kubab S., et al. LSARS-CoV-2 Antibody response after a third dose of the BNT 162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal. Am J Kidney Dis. 2021;S0272-6386(21):00833–00837. doi: 10.1053/j.ajkd.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupper A, Sharon N, Finn T, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 2021:CJN.03500321. doi: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed]
- 6.Carr E.J., Kronbichler A., Graham-Brown M., et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. KI Reports. 2021;6:2292–2304. doi: 10.1016/j.ekir.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021;371:eabf4063. [DOI] [PMC free article] [PubMed]
- 8.Labriola L., Scohy A., Seghers F. A longitudinal, 3-month serologic assessment of SARS-CoV-2 infections in a Belgian haemodialysis facility. Clin Jam Soc Nephrol. 2020;16:613–661. doi: 10.2215/CJN.12490720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikizler T., Coates P., Rovin B., Ronco P. Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int. 2021;99:1275–1279. doi: 10.1016/j.kint.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torreggiani M., Blanchi S., Fois A., Fessi H., Piccoli G.B. Neutralizing SARS-CoV-2 antibody response in dialysis patients after the first dose of the BNT162b2 mRNA COVID-19 vaccine: the war is far from being won. Kidney Int. 2021;99:1494–1496. doi: 10.1016/j.kint.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raja N., Rajagopalan A., Arunachalam J., Prasath A., Durai R., Rajendran M. Humoral response to viral vector COVID-19 vaccine in hemodialysis patients. Kidney Res Clin Pract. 2022;41(3):342–350. doi: 10.23876/j.krcp.21.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y.M., Liu X.Z., Lin M.M., Zan J.C., Hu Y.T., Wang X.Q., et al. Immunosuppression impaired the immunogenicity of inactivated SARS-CoV-2 vaccine in non-dialysis kidney disease patients. J Infect. 2022;85(2):174–211. doi: 10.1016/j.jinf.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruminhent J., Setthaudom C., Kitpermkiat R., Kiertiburanakul S., Malathum K., Assanatham M., et al. Immunogenicity of ChAdOx1 nCoV-19 vaccine after a two-dose inactivated SARS-CoV-2 vaccination of dialysis patients and kidney transplant recipients. Sci Rep. 2022 Mar 4;12(1):3587. doi: 10.1038/s41598-022-07574-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taji L., Thomas D., Oliver M.J., et al. COVID-19 in patients undergoing long-term dialysis in Ontario. CMAJ. 2021 doi: 10.1503/cmaj.202601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jager K.J., Kramer A., Chesnaye N.C., et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon B., Rubey H., Treipl A., et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared to healthy controls. Nephrol Dial Transplant. 2021;36:1709–1716. doi: 10.1093/ndt/gfab179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buti M., Viladomiu L., Jardi R., et al. Long-term immunogenicity and efficacy of hepatitis B vaccine in hemodialysis patients. Am J Nephrol. 1992;12:144–147. doi: 10.1159/000168436. [DOI] [PubMed] [Google Scholar]
- 18.Ghadiani M.H., Besharati S., Mousavinasab N., Jalalzadeh M. Response rates to HB vaccine in CKD stages 3–4 and hemodialysis patients. J Res Med Sci. 2012;17:527–533. [PMC free article] [PubMed] [Google Scholar]
- 19.Vandecasteele S.J., Ombelet S., Blumental S., Peetermans W.E. The ABC of pneumococcal infections and vaccination in patients with chronic kidney disease. Clin Kidney J. 2015;8:318–324. doi: 10.1093/ckj/sfv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Y.T., Guo C.Y., Tsai M.S., et al. Poor immune response to a standard single dose non-adjuvanted vaccination against 2009 pandemic H1N1 Influenza Virus A in the adult and elder hemodialysis patients. Vaccine. 2012;30:5009–50018. doi: 10.1016/j.vaccine.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Boongird S., Chuengsaman P., Phanprasert S., et al. Anti-SARS-CoV-2 spike protein S1 receptor-binding domain antibody after vaccination with inactivated whole-virus SARS-CoV-2 in end-stage kidney disease patients: an initial report. Kidney Int. 2021;100:1136–1138. doi: 10.1016/j.kint.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Windpessl M., Bruchfeld A., Anders H.J., et al. COVID-19 vaccines and kidney disease. Nat Rev Nephrol. 2021;17:291–293. doi: 10.1038/s41581-021-00406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zitt E., Davidovic T., Schimpf J., et al. The safety and immunogenicity of the mRNA-BNT162b2 SARS-CoV-2 vaccine in hemodialysis patients. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.704773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polewska K., Tylicki P., Biedunkiewicz B., et al. Safety and tolerability of the BNT162b2 mRNA COVID-19 vaccine in dialyzed patients. COViNEPH Project Medicina (Kaunas) 2021;57:732. doi: 10.3390/medicina57070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kho M.M.L., Reinders M.E.J., Baan C.C., et al. The RECOVAC IR study: the immune response and safety of the mRNA-1273 COVID-19 vaccine in patients with chronic kidney disease, on dialysis, or living with a kidney transplant - a prospective, controlled, multicenter observational cohort by the REnal patients COVID-19 VACcination (RECOVAC) consortium COVID-19 VACcination (RECOVAC) consortium. Nephrol Dial Transplant. 2021 doi: 10.1093/ndt/gfab186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stumpf J., Siepmann T., Lindner T., et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9 doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akyol M., Çevik E., Ucku D., et al. Immunogenicity of SARS-CoV-2 mRNA vaccine in dialysis and kidney transplant patients: a systematic review. Tuberk Toraks. 2021;69:547–560. doi: 10.5578/tt.20219612. [DOI] [PubMed] [Google Scholar]
- 28.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt M.E., Varga S.M. The CD8 T cell response to respiratory virus infections. Front Immunol. 2018;9:678. doi: 10.3389/fimmu.2018.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Post N., Eddy D., Huntley C., et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS ONE. 2020;15:e0244126. doi: 10.1371/journal.pone.0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sho B., Abe K.T., Zuo M., et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–34. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed]
- 36.Ray J.G., Schull M.J., Vermeulen M.J. Association between ABO and Rh blood groups and SARS-CoV-2 infection or severe COVID-19 illness: a population-based cohort study. Ann Intern Med. 2021;174:308–315. doi: 10.7326/M20-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J., Yang Y., Huang H. Relationship between the ABO blood group and the COVID-19 susceptibility. Clin Infect Dis. 2021;73:328–331. doi: 10.1093/cid/ciaa1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kibler M., Dietrich L., Kanso M. Risk and severity of COVID-19 and ABO blood group in transcatheter aortic valve patients. J Clin Med. 2020;9:3769. doi: 10.3390/jcm9113769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zietz M., Zucker J., Tatonetti N.P. Associations between blood type and COVID-19 infection, intubation, and death. Nat Commun. 2020;11:5761. doi: 10.1038/s41467-020-19623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y, Latz CA, DeCarlo CS, Lee S, Png CYM, Kibrik P, et al. Relationship between blood type and outcomes following COVID-19 infection. Semin Vasc Surg. 2021;34(3):125–31. doi: 10.1053/j.semvascsurg.2021.05.005. [DOI] [PMC free article] [PubMed]
- 41.Franchini M., Capra F., Targher G. Relationship between ABO blood group and von Willebrand factor levels: from biology to clinical implications. Thromb J. 2007;5:14. doi: 10.1186/1477-9560-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author. Data supporting the findings of this study are openly available in “figshare” at http://doi.org/10.6084/m9.figshare.19552222.