Abstract
Background
Studies on sociodemographic disparities in Covid-19 vaccination uptake in the general population are still limited and mostly focused on older adults. This study examined sociodemographic differences in Covid-19 vaccination uptake in the total Swedish population aged 18–64 years.
Methods
National Swedish register data within the SCIFI-PEARL project were used to cross-sectionally investigate sociodemographic differences in Covid-19 vaccination among Swedish adults aged 18–64 years (n = 5,987,189) by 12 October 2021. Using logistic regression models, analyses were adjusted for sociodemographic factors, region of residence, history of Covid-19, and comorbidities. An intersectional analysis approach including several cross-classified subgroups was used to further address the complexity of sociodemographic disparities in vaccination uptake.
Findings
By 12 October 2021, 76·0% of the Swedish population 18–64 years old had received at least two doses of Covid-19 vaccine, an additional 5·5% had received only one dose, and 18·5% were non-vaccinated. Non-vaccinated individuals were, compared to vaccinated, more often younger, male, had a lower income, were not gainfully employed, and/or were born outside Sweden. The social patterning for vaccine dose two was similar, but weaker, than for dose one. After multivariable adjustments, findings remained but were attenuated indicating the need to consider different sociodemographic factors simultaneously. The intersectional analysis showed a large variation in vaccine uptake ranging from 32% to 96% in cross-classified subgroups, reflecting considerable sociodemographic heterogeneity in vaccination coverage.
Interpretation
Our study, addressing the entire Swedish population aged 18–64 years, showed broad sociodemographic disparities in Covid-19 vaccine uptake but also wide heterogeneities in coverage. The intersectional analysis approach indicates that focusing on specific sociodemographic factors in isolation and group average risks without considering the heterogeneity within such groups will risk missing the full variability of vaccine coverage.
Funding
SciLifeLab / Knut & Alice Wallenberg Foundation, Swedish Research Council, Swedish government ALF agreement, FORMAS.
1. Introduction
The pandemic spread of the novel coronavirus SARS-CoV-2 that causes Covid-19 is still a serious worldwide threat to public health. Covid-19 vaccines efficiently prevent serious Covid-19 disease, and major efforts have been made globally to develop and distribute effective vaccines. Sweden initiated its vaccination programme on 27 December 2020; and in early October 2021, 79·5% of the population aged >12 years had received at least one and 74·8% at least two doses[1]. During this period, the predominant SARS-CoV-2 variants of concern in Sweden were alfa and delta. The programme for adults was implemented in four consecutive stages, where the first three stages mainly included the older age groups, health- or elderly-care workers, and various risk groups. Stage four included individuals aged 18–59 years who had not been part of the previous stages, and by May 2021 most of the regions had initiated vaccination in stage four[2]. Within the age group 18–59 years, older individuals continued to be prioritised before younger.
Sociodemographic differences in both attitudes and actual uptake of vaccines have previously been demonstrated. Among older adults, sociodemographic disparities have been shown for the uptake of seasonal influenza[3], [4], shingles, and pneumococcal vaccination[3]. Sociodemographic differences have also been shown in the uptake of childhood vaccinations[5] and even though the general uptake of childhood vaccinations in Sweden is high[6] sociodemographic differences in childhood vaccination have been shown[7]. However, studies on Covid-19 vaccination uptake in general populations are still few and mostly focus on older adults. In the older age group, research primarily from the UK has shown that living in a deprived area was associated with lower Covid-19 vaccination uptake, and that vaccination coverage differs between ethnic groups[8], [9], [10]. Also, factors such as having a less advantaged socioeconomic position, living alone or living in a multigenerational household have been associated with lower vaccination coverage[8]. Our research group recently published a study on vaccination uptake in older adults (≥60 years) based on a large national representative sample of older Swedish adults, where a lower vaccination coverage was seen among those characterised by relatively lower age, male sex, living alone, low income, and being born in low- and middle income countries[11].
Less is known about the uptake of Covid-19 vaccination among middle-aged and younger individuals. A previous study has demonstrated a lower uptake and even larger sociodemographic differences in vaccination uptake in the younger and middle-aged population than in the older population[12]. In a recent preprint addressing a working population of 40–64 years olds in England, vaccination uptake clearly differed by occupation with lower vaccination coverage in individuals working in elementary occupations, while managers and individuals in professional and administrative occupations had a higher uptake[13]. More studies on vaccination uptake in younger and middle-aged groups in a general population are thus urgently needed to better characterise predictors of vaccine uptake. Sweden has a long tradition of different population-based registers and health registers. Importantly, every individual in Sweden is given a unique personal identity number (PIN), which can be used to accurately link data from different registers, including healthcare and sociodemographic data sources[14]. This creates a unique opportunity to study the Swedish population on an individual level using register data, which is a major advantage when analysing sociodemographic differences in vaccination coverage.
Broad vaccination coverage in all age groups is essential to reduce the risk of severe Covid-19 and consequently lower the pressure on the healthcare system. Furthermore, addressing sociodemographic differences in vaccine coverage is of great importance to reduce widening health inequalities, especially since groups with a lower vaccine uptake coincide with those most impacted by a more serious Covid-19 infection[15]. Recent research has shown that an intersectional analysis approach considering combinations of overlapping sociodemographic compositional factors rather than group average risks can contribute to a more nuanced understanding of socioeconomic disparities in health[11], [16], [17]. The aim of this study was to describe sociodemographic disparities in Covid-19 vaccination uptake in the entire Swedish population aged 18–64 years, and to further examine heterogeneity within sociodemographic groups using intersectional analysis models. We utilised data from the unique nationwide linked multi-register observational study SCIFI-PEARL (Swedish Covid-19 Investigation for Future Insights – A Population Epidemiology Approach Using Register Linkage)[18].
2. Method
2.1. Study design and study population
National Swedish register data within the SCIFI-PEARL project were utilised to cross-sectionally investigate sociodemographic disparities in Covid-19 vaccination among Swedish adults aged 18–64 years. SCIFI-PEARL is a nationwide, regularly updated, register-based study with multiple linkages of individual data using the unique PIN. The design of the SCIFI-PEARL study is described in detail elsewhere[18] and the study has successively been expanded to cover the entire Swedish population. A cohort was designed for the current, and additional objectives related to Covid-19, and it included all individuals aged 18–64 years, and who were alive and resident in Sweden on 1 January 2020 (N = 6,064,779). After exclusion of individuals who emigrated (n = 59,725) or died (n = 17,865) during follow-up, the study population included 5,987,189 individuals. At the time of the study, data availability extended to 12 October 2021 due to the data delivery processes which defined the study period.
2.2. Outcome
The primary outcome in this study was having received at least one dose of a Covid-19 vaccine by 12 October 2021. The two secondary outcomes were having received only one dose or having received at least two doses of a Covid-19 vaccine, respectively. Vaccination data was obtained by linkage to the National Vaccination Register (NVR).
2.3. Exposure and covariates
The sociodemographic data, including age, sex, income, occupational status, and country of birth, were obtained from the National Register of the Total Population (RTB) and the Longitudinal Integrated Database for Health Insurance and Labour Market Studies (LISA) from Statistics Sweden. Age was categorised into three groups: 18–34 years, 35–49 years, and 50–64 years. The household disposable income divided by the weighted number of members in the household was provided through the LISA register and dichotomised into medium-high (2nd and 3rd tertile) and low (1st tertile). Occupational status was assessed as working (employed or self-employed) or other (e.g., students, unemployed, long-term sick leave, early retirement). The country of birth was grouped into three categories based on the World Bank classification: Sweden, High-income countries (HIC) with a Gross National Income (GNI) per capita of ≥$12,696, and Low- (GNI per capita of ≤$1,045) or Middle- (GNI per capita between $1,046 and $12,695) income countries (LMIC))[19]. An intersectional approach was used to investigate heterogeneity in population groups regarding vaccine uptake. The intersectional multi-categorical variable consisted of all potential combinations of the above-mentioned sociodemographic variables (i.e., age (three categories), sex (two categories), income (two categories), country of birth (three categories), and occupational status (two categories)), resulting in 72 strata.
Covariates included having a history of Covid-19 (yes/no), prior comorbidity between 2015 and 2019, and area of residence. Covid-19 history was retrieved from SmiNet (the national register including all positive SARS-CoV-2 polymerase chain reaction (PCR) test results held by the Public Health Agency of Sweden), and from the National Patient Register (NPR) (International Classification of Diseases, 10th revision, Swedish version (ICD-10-SE) codes U07.1 and U07.2 as primary or secondary diagnosis from hospitalisations and specialist outpatient visits). Region of residence was categorised into regions with a major city (i.e., Stockholm Region, Västra Götaland Region, and Skåne Region), or not (all other regions). Information on prior comorbidities was obtained from hospitalisations and specialist outpatient visits registered in the NPR. Prior comorbidities were defined based on primary and secondary (ICD-10) diagnoses, consisting of circulatory diseases (I00-I99), respiratory diseases (J00-J99), psychiatric diseases (F20-F39), cancer (C00-C97), and diabetes (E10, E11, E13, E14).
2.4. Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
2.5. Statistical analyses
Descriptive analyses are presented as percentages for categorical variables. Differences in proportions were analysed with chi-square tests with a statistical significance level of 5%. Standardised mean differences (SMD) to evaluate differences between groups were calculated using the R package. Multivariable logistic regression was employed to obtain odds ratios (ORs) for having received at least one dose of vaccine by 12 October 2021, in several different models (see below). Separate analyses were performed for the secondary outcomes of having received only one dose or having received at least two doses of a Covid-19 vaccine, respectively. Sensitivity analyses focusing on those aged 20–64 years and excluding those last invited to vaccination (i.e., 18–19 year olds), were also performed.
Five different models were built for the analysis. Model 1 (crude model) only included age. Model 2 (crude model) included all other sociodemographic variables analysed separately. Model 3 included mutual (i.e., simulanteous) adjustment for all included sociodemographic variables in the regression model. Model 4 was model 3 with additional adjustments for history of Covid-19, prior comorbidities, and residential area. Model 5 (the intersectional approach model) included the 72 intersectional strata. Subjects with missing data on some sociodemographic characteristics (i.e., income, occupational status, or country of birth n = 9960) were not included in analyses using these variables. The discriminatory accuracy (DA) was assessed by calculating the area under the receiver operating characteristics curve (AUC) for the models. An AUC between 0·6 and 0·7 was considered weak/moderate, AUC 0·7-0·8 strong and AUC > 0·8 very strong [16], [17]. The analyses were done using statistical packages SPSS computer software 26.0, Stata 16.0 (StataCorp. TX, USA) and R (R Foundation for Statistical Computing, Vienna, Austria).
2.6. Data statement
The data in this study are pseudonymised individual level data from Swedish healthcare registers and are not publicly available according to Swedish legislation. They can be obtained from the respective Swedish public data holders on the basis of ethics approval for the research in question, subject to relevant legislation, processes and data protection.
3. Results
The study population included 5,987,189 individuals aged 18–64 years during 2021, of whom 76·0% had received at least two doses of a Covid-19 vaccine, an additional 5·5% had received only one dose, and 18·5% were non-vaccinated by 12 October 2021 (Table 1 ). The non-vaccinated individuals were, compared to those vaccinated with two doses, more often younger, male, had a lower income, were not gainfully employed, were born outside Sweden, and/or lived in a region with a major city. Comorbidity in terms of cardiovascular disease, respiratory disease, cancer, or diabetes mellitus was more prevalent among the vaccinated individuals, whereas psychiatric disease was more common among the non-vaccinated. All of these differences were statistically significant. Similar statistically significant differences, however with generally smaller differences in proportions, were seen when comparing the non-vaccinated group to individuals who had received only one dose with regard to sex, income, occupational status, country of birth and respiratory disease. However, those who had received only one dose were more often younger, had a history of Covid-19, lived in a region holding a large Swedish city, and more often had psychiatric disease compared to the non-vaccinated group (Table 1). The standardised mean differences were generally, with some exceptions, larger comparing those having received two doses and those non-vaccinated compared to those having received only one dose and those non-vaccinated (Table 1).
Table 1.
Distribution of sociodemographic factors, history of Covid-19 and comorbidities in the Swedish population aged 18–64 years, by having received one dose of Covid-19 vaccination, two doses of Covid-19 vaccination or no Covid-19 vaccine, presented as percentages and standardised mean differences (SMD).
| Characteristics | Two doses | One dose | Non-vaccinated | p-valued | p-valuee | SMDf | SMDg | Total |
|---|---|---|---|---|---|---|---|---|
| (N = 4,550,469; 76·0%) | (N = 330,233; 5·5%) | (N = 1,106,487; 18·5%) | N = 5,987,189 | |||||
| Age group | ||||||||
| 18–34 years | 31·3% | 59·3% | 50·4% | 0·475 | 0·179 | 36·4% | ||
| 35–49 years | 32·6% | 27·4% | 31·8% | <0·001 | <0·001 | 32·2% | ||
| 50–64 years | 36·1% | 13·3% | 17·7% | 31·4% | ||||
| Male | 50·0% | 52·9% | 55·5% | <0·001 | <0·001 | 0·111 | 0·052 | 51·2% |
| Incomea | ||||||||
| Medium/High | 73·4% | 55·5% | 44·0% | 0·625 | 0·230 | 67·0% | ||
| Low | 26·6% | 44·5% | 56·0% | <0·001 | <0·001 | 33·0% | ||
| Occupational statusb | ||||||||
| Working | 81·7% | 65·9% | 59·6% | 0·500 | 0·131 | 76·7% | ||
| Other | 18·3% | 34·1% | 40·4% | <0·001 | <0·001 | 23·3% | ||
| Country of birthc | ||||||||
| Sweden | 81·5% | 68·1% | 54·0% | 0·595 | 0·241 | 75·7% | ||
| HIC | 5·3% | 5·4% | 12·0% | <0·001 | <0·001 | 6·6% | ||
| LMIC | 13·2% | 26·5% | 34·0% | 17·7% | ||||
| History of Covid-19 | 15·1% | 20·4% | 15·9% | <0·001 | <0·001 | 0·022 | 0·117 | 15·6% |
| Living in a region holding a large Swedish city | 52·2% | 63·1% | 61·0% | <0·001 | <0·001 | 0·179 | 0·042 | 54·4% |
| Comorbidities (2015–2019) | ||||||||
| Cardiovascular disease | 6·7% | 3·8% | 3·8% | <0·001 | 0·93 | 0·127 | 0·000 | 6·0% |
| Respiratory disease | 8·1% | 8·7% | 7·5% | <0·001 | <0·001 | 0·021 | 0·045 | 8·0% |
| Cancer | 2·0% | 0·8% | 0·8% | <0·001 | 0·50 | 0·099 | 0·001 | 1·7% |
| Psychiatric disease | 4·3% | 6·0% | 5·5% | <0·001 | <0·001 | 0·056 | 0·020 | 4·6% |
| Diabetes | 2·0% | 1·0% | 1·0% | <0·001 | 0·64 | 0·080 | 0·001 | 1·8% |
a. Income: The household disposable income divided by the weighted number of members in the household; Medium/High: 2nd and 3rd tertile, Low: 1st tertile.
b. Occupational status: Working or Other (e.g., student, long-term sick-leave, unemployed, early retired).
c. Country of birth: Sweden, HIC: High Income Countries; LMIC: Low-or Middle Income Countries.
d. Statistically significant differences between the group having received two doses compared to those non-vaccinated (p < 0·05).
e. Statistically significant differences between the group having received one dose compared to those non-vaccinated (p < 0·05).
f. Standardised mean difference (SMD) between the group having received two doses compared to those non-vaccinated.
g. Standardised mean difference (SMD) between the group having received one dose compared to those non-vaccinated.
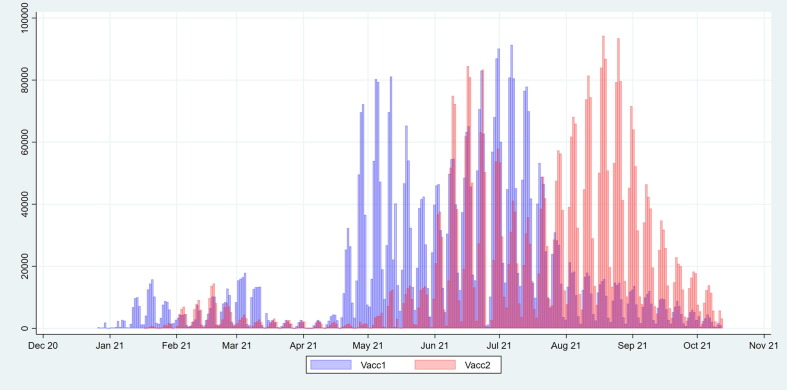
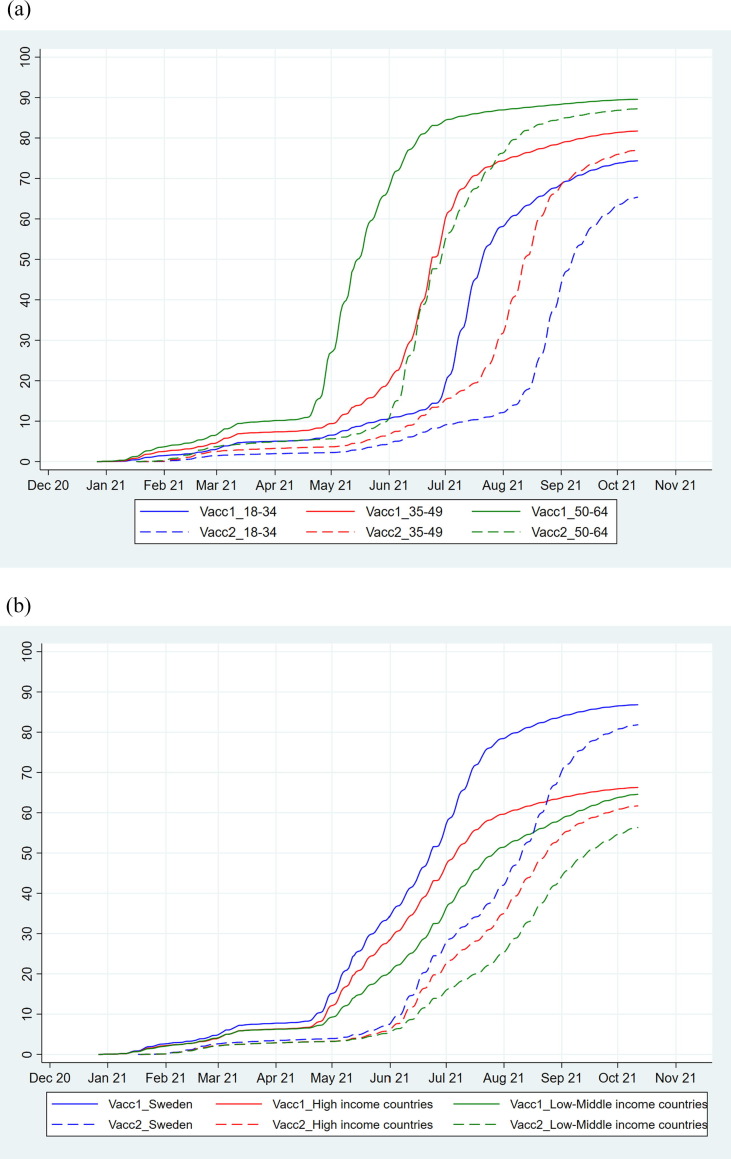
Vaccinations of risk groups started in late December 2020. Large scale first dose vaccinations in the age group < 65 years commenced in late April 2021 for those aged 60–64 years and mid-May the same year for those aged 18–59 years, with initial relative priority for the older ages within the group. Large scale second dose vaccinations started in June 2021 (Fig. 1 ). Older age groups were vaccinated before the younger age groups for both vaccine doses (Fig. 2 a). The daily number vaccinated with the first dose increased until July 2021, at which point it started to level off, and the number vaccinated with the second dose increased until late August 2021 (Fig. 1).
Fig. 1.
Daily number of individuals aged 18–64 years in the study population who have received their first (blue) and second dose (red) of Covid-19 vaccine since the start of vaccination on 27 December 2020 until 12 October 2021. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Cumulative uptake (%) of vaccination by age group (a; blue = 18–34 years; red = 35–49 years; green = 50–64 years), and by country of birth (b; blue = Sweden, red = High income countries, green = Low- or middle-income countries) in the Swedish population aged 18–64 years who received their first dose and second dose of a Covid-19 vaccine since the start of vaccination on 27 December 2020 until 12 October 2021. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Overall more women than men were vaccinated and this was most pronounced in the first phase of the vaccination program (Supplementary Fig. 1). During the initiation of large scale vaccination, coverage was lower among groups born outside Sweden, and these differences widened over time (Fig. 2b).
The percentage of individuals who had received at least one dose of a Covid-19 vaccine differed across sociodemographic groups as shown in Table 2 . The lowest uptake was seen among individuals born outside Sweden, where 66% born in HIC and 65% born in LMIC had received at least one vaccine dose compared to 87% of those born in Sweden. Lower age, male sex, having a low income, not being gainfully employed, and being born outside Sweden were factors associated with a lower vaccine uptake as well as higher ORs of non-vaccination which persisted in the fully adjusted model. For example, regarding low income, the OR for non-vaccination in model 2 were 3·30 (95% CI: 3·29-3·31) compared to 1·99 (95% CI: 1·98-2·00) in model 3, which indicates that some of the differences in the crude model may be due to other sociodemographic factors. Sensitivity analyses focusing on those aged 20–64 years and excluding individuals aged 18 and 19 years, showed a similar uptake of vaccination in the youngest age group (20–34 years) (i.e., 74% were vaccinated with at least one dose). In the fully adjusted model 4, which was mutually adjusted for all sociodemographic factors, living in a region with a major city, selected comorbidities, and having a history of Covid-19, the ORs and AUC were generally not substantially different from those in Model 3. Excluding subjects diagnosed with Covid-19 within 14 days after vaccination did not change this pattern of association (data not shown).
Table 2.
Percentage Covid-19 vaccinated among individuals in the Swedish population aged 18–64 years, and adjusted odds ratios (ORs) with 95% Confidence Intervals (CI) of non-vaccination for categories defined by sociodemographic factors i.e., age, sex, income, country of birth, and occupational status.
| Characteristics | Vaccinatedd | Model 1e | Model 2 g | Model 3i | Model 4j |
|---|---|---|---|---|---|
| % | ORf 95 %CI | ORf 95 %CI | ORf 95 %CI | ORf 95 %CI | |
| Age group | |||||
| 18–34 years | 74% | 1·00 | (· ·) | 1·00 | 1·00 |
| 35–49 years | 82% | 0·65 (0·65-0·65) | 0·66 (0·66-0·66) | 0·66 (0·66-0·66) | |
| 50–64 years | 90% | 0·34 (0·34-0·34) | 0·40 (0·40-0·40) | 0·41 (0·41-0·41) | |
| Sex | |||||
| Male | 80% | 1·00 | 1·00 | 1·00 | |
| Female | 83% | 0·81 (0·81-0·81) | 0·77 (0·77-0·78) | 0·77 (0·76-0·77) | |
| Incomea | |||||
| Medium/High | 88% | 1·00 | 1·00 | 1·00 | |
| Low | 69% | 3·30 (3·29-3·31) | 1·99 (1·98-2·00) | 2·04 (2·03-2·05) | |
| Occupational statusb | |||||
| Working | 86% | 1·00 | 1·00 | 1·00 | |
| Other | 68% | 2·82 (2·81-2·83) | 1·61 (1·60-1·61) | 1·62 (1·62-1·63) | |
| Country of birthc | |||||
| Sweden | 87% | 1·00 | 1·00 | 1·00 | |
| HIC | 66% | 3·35 (3·33-3·38) | 3·23 (3·21-3·26) | 3·10 (3·08-3·13) | |
| LMIC | 65% | 3·61 (3·59-3·63) | 2·67 (2·66-2·68) | 2·59 (2·58-2·61) | |
| AUC (ROC-curve) | 0·61 (0·61-0·61) | (· ·)h | 0·73 (0·73-0·73) | 0·74 (0·74-0·74) |
a. Income: The household disposable income divided by the weighted number of members in the household; Medium/High: 2nd and 3rd tertile, Low: 1st tertile.
b. Occupational status: Working or Other (e.g., student, long-term sick-leave, unemployed, early retired).
c. Country of birth: Sweden, HIC: High Income Countries; LMIC: Low- or Middle income countries.
d. Having received at least one dose of a Covid-19 vaccine.
e. Model 1: Only including age in the model.
f. Odds ratios (ORs) and 95% Confidence Interval (CI).
g. Model 2: Crude odds ratios models for each factor.
h. AUC Model 2: Sex, 0·53 (0·53-0·53); Income 0·65 (0·65-0·65); Country of birth 0·64 (0·64-0·64); Occupational status, 0·60 (0·60-0·60).
i. Model 3: Mutually adjusted.
j. Model 4: Model 3 + adjusted for living in region with major city, comorbidities, and having a history of Covid-19.
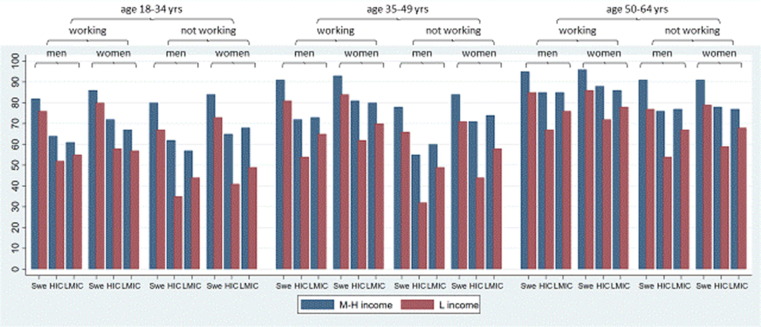
The analysis of 72 intersectional strata, using the multi-categorical variable, showed large heterogeneity in Covid-19 vaccine uptake (Fig. 3 ; Supplementary Table 1). For example, among those not gainfully employed, vaccine uptake ranged from 32% to 91% (Fig. 3). The strata with a lower vaccine uptake were generally characterised by younger age (5 of 10 strata), having a low income (10 of 10 strata), not working (8 of 10 strata), and being born outside Sweden (Table 3 , Supplementary Table 1). The two strata with the lowest uptake of vaccination included men aged 18 to 34 years or 35 to 49 years, born outside Sweden (in a high income country) who were not working and with low income. On the other hand, the strata with higher vaccine uptake were most often characterised by older age, 50–64 years (7 of 10 strata), having a medium/high income (9 of 10 strata), and being born in Sweden (8 of 10 strata). The two strata with the highest uptake of vaccination included working men or women aged 50 to 64 years born in Sweden with medium/high income. The AUC of 0·74 for Model 5 seen in Supplementary Table 1 was similar to Model 3 (AUC = 0·73) seen in Table 2 including the same sociodemographic variables.
Fig. 3.
Proportion vaccinated (with at least one dose of Covid-19 vaccine) in 72 intersectional strata defined by age groups (18–34 years, 35–49 years and 50–64 years), occupational status (working, not working), sex (men, women), country of birth (Swe: Sweden, HIC: High income countries, LMIC: Low-middle income countries) and income (L income: low income [1st tertile] red M−H income: Medium/high income [2nd and 3rd tertile] blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Percentage vaccinated with at least one dose of a Covid −19 vaccine among individuals in the Swedish population aged 18–64 years, and odds ratios (ORs) with 95% Confidence Interval (CI) for non-vaccination related to intersectional strata based on sociodemographic factors i.e., age, sex, income, country of birth, and occupational status. Only the twenty intersectional strata with the lowest and highest ORs, respectively, of non-vaccination are shown.
| Intersectional strata | N | Vaccinatedd | Model 5 |
|---|---|---|---|
| % | OR (95% CI)e | ||
| Women/Med-High inc/Work/Born Swe/Age 50–64 | 572,247 | 96% | 0·22 (0·22-0·22) |
| Men/Med-High inc/Work/Born Swe/Age 50–64 | 604,033 | 95% | 0·25 (0·25-0·26) |
| Women/Med-High inc/Work/Born Swe/Age 35–49 | 465,081 | 93% | 0·33 (0·33-0·34) |
| Women/ Med-High inc /Not work/Born Swe/Age 50–64 | 36,566 | 91% | 0·46 (0·45-0·48) |
| Men/ Med-High inc /Not work/Born Swe/Age 50–64 | 30,657 | 91% | 0·46 (0·44-0·48) |
| Men/ Med-High inc /Work/Born Swe/Age 35–49 | 521,215 | 91% | 0·47 (0·47-0·48) |
| Women/Med-High inc/Work/Born HIC/Age 50–64 | 43,815 | 88% | 0·61 (0·59-0·63) |
| Women/Med-High inc/Work/Born Swe/Age 18–34 | 384,303 | 86% | 0·74 (0·73-0·75) |
| Women/Low inc/Work/Born Swe/Age 50–64 | 65,801 | 86% | 0·76 (0·74-0·77) |
| Women/Med-High inc/Work/Born LMIC/Age 50–64 | 58,641 | 86% | 0·78 (0·76-0·80) |
| Men/Med-High inca/Workb/Born Swec/Age 18–34 | 444,329 | 82% | 1·00f |
| Men/Low inc/Work/Born HIC/Age 35–49 | 16,146 | 54% | 3·91 (3·78-4·03) |
| Men/Low inc/Not Work/Born HIC/Age 50–64 | 13,243 | 54% | 3·99 (3·85-4·13) |
| Men/Low inc/Work/Born HIC/Age 18–34 | 10,423 | 52% | 4·22 (4·06-4·39) |
| Men/Low inc/Not Work/Born LMIC/Age 35–49 | 52,123 | 49% | 4·81 (4·72-4·90) |
| Women/Low inc/Not Work/Born LMIC/Age 18–34 | 83,033 | 49% | 4·89 (4·81-4·97) |
| Men/Low inc/Not work/Born LMIC/Age 18–34 | 81,730 | 44% | 5·95 (5·86-6·05) |
| Women/Low inc/Not work/Born HIC/Age 35–49 | 12,418 | 44% | 5·98 (5·76-6·20) |
| Women/Low inc/Not work/Born HIC/Age 18–34 | 18,551 | 41% | 6·63 (6·44-6·84) |
| Men/Low inc/Not work/Born HIC/Age 18–34 | 18,341 | 35% | 8·73 (8·46-9·01) |
| Men/Low inc/Not work/Born HIC/Age 35–49 | 15,630 | 32% | 9·88 (9·55-10·23) |
a. Income: The household disposable income divided by the weighted number of members in the household; Medium/High: 2nd and 3rd tertile, Low: 1st tertile.
b. Working (Work), Not working (Not work).
c. Country of birth: Swe: Sweden; HIC: High-income country; LMIC: Low-middle income country.
d. At least one dose of vaccination.
e. Odds ratios (ORs) and 95% confidence intervals (CI).
f. Reference group.
The odds of not having received dose two among those having received dose one, was higher among younger age groups, males, low income groups, those not gainfully employed, and those born in LMIC (Supplementary Table 2). These associations persisted, but were generally reduced after adjustments. Sensitivity analyses focusing on those aged 20–64 years and excluding individuals aged 18 and 19 years, showed a similar uptake of vaccination in the youngest age group (20–34 years) (i.e., 89% were vaccinated with at least two doses). Furthermore, excluding subjects diagnosed with Covid-19 within 14 days after vaccination did not change this pattern of association (data not shown).
4. Discussion
The results from this nationwide register-based study of Swedish adults aged 18–64 years, demonstrated wide social disparities in the uptake of at least one dose of a Covid-19 vaccine, with generally lower uptake in males, in younger age groups, among individuals with a low income, among those not gainfully employed and among those born outside Sweden. A similar but less pronounced social patterning was seen for having received two doses. Analysis of intersectional strata considering overlapping sociodemographic determinants rather than average group effects showed even larger sociodemographic variation in vaccine uptake in cross-classified subgroups.
The results of our study confirms and expands the results of the few published studies and reports that investigated sociodemographic differences in the uptake of Covid-19 vaccination in a general population[8], [9], [10], [11]. Our results also align with a recent study on the English population aged > 50 years, showing that the ORs of non-vaccination were higher among men, younger individuals, in the urban population, and among ethnic minorities[20]. Similar sociodemographic disparities have been seen among the elderly concerning the uptake of seasonal influenza, shingles, and pneumococcal vaccines[3]. However, only a few previous studies have specifically explored Covid-19 vaccine uptake disparities in adults of working age. A recent study covering ages 18 years and older showed a lower uptake of Covid-19 vaccination in younger age groups, among men, lower socioeconomic position groups, and ethnic minority groups [12]. These findings are in line with the results presented in the present paper. We could also show that such differences in vaccine uptake were present for both dose one and dose two, and after mutual adjustments for different demographic factors, as well as adjustments for region, history of Covid-19, and comorbidities.
The vaccination program in Sweden was implemented in consecutive stages, with the first dose of large scale vaccinations in the age group < 65 years commencing in late April 2021. As found in the present study, older people were generally vaccinated before younger individuals. As a result, age differences seen in the present study might to some extent be a result of the role out of the vaccination program. Nevertheless, younger age is an important sociodemographic factor to consider with regard to vaccine hesitancy. As in our study, previous research has found that younger age groups tend to be more hesitant to Covid-19 vaccination than older groups[21], [22]. The lack of intention to get vaccinated may be related to the fact that younger adults are known to have a lower risk of serious illness from Covid-19 and this perception has also been linked to vaccine hesitancy[23]. Therefore, the Covid-19 vaccination program may have challenges in common with other public health behaviour efforts related to unhealthy behaviours in young age (e. g., alcohol consumption, drug use and unhealthy diet)[24]. Other possible reasons for young adults not getting vaccinated are concerns about safety aspects, the efficacy and duration of the protective effect and fear of side effects[22], [23].
Individuals born outside Sweden had the lowest Covid-19 vaccination uptake, as shown in this study for adults of working age. Other studies have similarly shown large differences in vaccination coverage across ethnic groups[8], [9], [10], [11], [20]. This difference may in part be due to socioeconomic factors, and in the present study the differences were reduced after mutual adjustments for these factors. There are also other potential explanations such as lack of institutional trust[25], fear of side effects and lack of information[26]. Furthermore, employment was associated with a higher proportion of vaccination, regarding doses one and two of a Covid-19 vaccine. Large occupational groups such as health-care workers, elderly care workers, police and security workers, and teachers have been exposed to Covid-19 through their occupation and might therefore be more prone and encouraged to be vaccinated. Another explanation could be the healthy worker effect, i.e., those employed have been shown to have a healthier behaviour and better health to be able to work[27].
Earlier studies have indicated that an intersectional analysis approach adds valuable insights into understanding sociodemographic disparities in health[16], [17]. However, it has not been common with regard to differences in the Covid-19 vaccine uptake. In our previous study on older adults (age 60 years and above), also utilising an intersectional approach to investigate sociodemographic differences in vaccination coverage, we showed substantial differences both between and within sociodemographic groups[11]. The present study found similar patterns of associations. For example, in the younger age group 18–34 years, vaccination uptake in different intersectional subgroups ranged from 35% to 86% (mean: 74%), while the uptake in men ranged from 32% to 95% (mean: 80%). Furthermore, among individuals born outside Sweden the mean vaccination uptake was lower compared to among those born in Sweden (66% and 65% if born in a HIC or LMIC respectively compared to 87% if born in Sweden) and the ten intersectional strata with the lowest vaccination uptake all encompassed subgroups born outside Sweden (range 32–54%). However, in four strata comprising individuals born outside Sweden, gainfully employed and with a medium–high income, the vaccination uptake was high among both women (88% and 86% if born in a HIC or a LMIC respectively) and men (85% both if born in a HIC or a LMIC). This indicates that focusing solely on single sociodemographic groups and group average risks, without considering the intragroup heterogeneity will tend to not reflect the full variability of the actual vaccine coverage within such groups. The findings also indicate that despite exposure to a sociodemographic factor associated with low vaccination uptake (i.e., being born outside Sweden), other protective factors (being gainfully employed and with a medium–high income) may alter the effect of this exposure. Even though the intersectional model did not improve the discriminatory accuracy compared to the mutually adjusted model holding the same sociodemographic variables, it provides a more nuanced, and alternative, picture of vaccination coverage linked to sociodemographic variables.
Aw et al suggest that vaccine hesitancy may be associated with several factors including mistrust in vaccine safety and efficacy, fear of transmission at vaccination centres, feeling healthy and not believing in the risk of developing severe illness from Covid-19 if infected, distrust in authorities, and the belief that natural immunity is better than vaccine-induced immunity[28]. Other aspects of vaccine safety, including concerns regarding the speed of vaccine development, have also been discussed in qualitative research[29]. The large differences in vaccination rates between sociodemographic groups, highlight the need for better communication of facts, improvements in outreach activities, and increased accessibility to lower barriers to health care.
The social patterning for the second vaccine dose was similar, but weaker, than for dose one. This might be expected since the threshold to take dose two probably is lower if the first dose already has been taken. The fact that sociodemographic aspects of vaccine hesitancy are important when considering adherence to the second dose of Covid-19 vaccine is in line with earlier research from England showing a higher hesitancy for receiving the second dose among foreign-born groups, among males, and among more deprived groups[20]. Sociodemographic differences in the potential acceptability of the Covid-19 vaccine have also been shown regarding taking a booster dose, with lower acceptability among lower age and lower educated groups[30]. Some potential explanations for the decision to not take the second dose might include a feeling of adequate protection with one dose, having had side-effects after the first dose, or the belief of being adequately protected due to previous Covid-19 infection combined with one dose.
This study has several strengths. By including the entire Swedish population aged 18–64 years, there should be essentially no selection bias. However, non-permanent residents (e.g., immigrants who intend to stay in Sweden for less than one year or diplomats) will not be assigned a PIN and therefore not included in the registers. The extensive study population allowed us to conduct intersectional analyses using multiple variables, which requires a large study sample. All data used are register-based and taken from high-quality, nation-wide health registers. All subjects are included with the Swedish PIN allowing essentially error-free record linkage of data from multiple sources, strengthening our study further by reducing misclassification of exposure and outcome and minimising missing data. Our vaccination data are particularly comprehensive since it is mandatory and regulated by Swedish law to report all Covid-19 vaccinations to the National Vaccination Register. We were also able to include information on several different sociodemographic characteristics. However, every year, a very small number of individuals change their PIN, most commonly due to inaccurate birthdate or inaccurate sex registered at immigration or birth. Nevertheless, Statistics Sweden and the National Board of Health and Welfare, have systems to control PINs and an individual receives a correct PIN once an incorrect PIN is identified[14]. Another limitation of the study is that our observation window might not provide individuals aged 18–19 years, who were the last offered vaccination, sufficient time to have received two doses of a vaccine during the study period. Sensitivity analyses excluding these individuals did not change the age differences seen in vaccination uptake.
5. Conclusion
In conclusion, our findings, addressing the entire Swedish population aged 18–64 years, showed a generally lower Covid-19 vaccine uptake in males, in younger age groups, among individuls with a low income, those born outside Sweden, those not gainfully employed, those resident in a region with a major city, and with a history of Covid-19, but a generally higher uptake among those with diagnosed chronic disease. The social patterning for vaccine dose two was similar, but weaker, than for dose one. The intersectional analyses revealed a pattern with wide differences in vaccination coverage across as well as within sociodemographic groups. This approach is thus useful when trying to further understand how sociodemographic factors are associated with vaccination uptake.
6. Contributors
MS: Contributed to the literature research, conceptualisation, methodology, data analyses, writing and critical revision of the manuscript. LL: Contributed to the literature research, conceptualisation, writing and critical revision of the manuscript. CN: Contributed to the conceptualisation, methodology, data analyses, and critical revision of the manuscript. HL: Contributed to data curation, software, visualisation, data analyses, and critical revision of the manuscript. AS, NH, and NN: contributed to the conceptualisation, methodology, and critical revision of the manuscript. SL: Contributed to the conceptualisation, and critical revision of the manuscript. MG: Contributed to the investigation, funding acquisition, and critical revision of the manuscript. FN: Contributed to the investigation, funding acquisition, conceptualisation, methodology, and critical revision of the manuscript. MR: Contributed to the conceptualisation, methodology, data analyses, and critical revision of the manuscript.
7. Ethics committee approval
The study has ethical approval from the Swedish Ethics Review Authority (EPM), no. 2020-01800, 2020-05829, 2021-00267, 2021-00829, 2021-02106, 2021-04098, 2022-00500-02, 2022-01207-02.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Nyberg reports prior employment at AstraZeneca until 2019, and ownership of some AstraZeneca shares. Dr. Gisslén reports personal fees (DSMB) from AstraZeneca, personal fees from Gilead, personal fees from GSK/ViiV, personal fees from MSD, other from Gilead, other from GSK/ViiV, personal fees from Biogen, personal fees from Novocure, personal fees from Amgen, personal fees from Novo Nordisk, outside the submitted work. Dr. Hammar reports ownership of AstraZeneca shares and consulting with Sobi. Dr Leach reports consulting for Scandinavian Biopharma. MD. Spetz, MD. Lundberg, Dr. Nwaru, Dr. Santosa, Dr. Ng, Dr. Li, Dr. Rosvall have nothing to disclose.
Acknowledgements
This study was made possible by funding from the SciLifeLab National COVID-19 Research Program, financed by the Knut and Alice Wallenberg Foundation, and the Swedish Research Council, the Iris Jonzén-Sandblom and Greta Jonzén Foundation and the Gothenburg Society of Medicine. The underlying SCIFI-PEARL study currently has basic funding by a grant from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement and from FORMAS (Research Council for Environment, Agricultural Sciences and Spatial Planning), a Swedish Research Council for Sustainable Development.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.09.065.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- 1.Folkhälsomyndigheten [FHM], Statistik för vaccination mot covid-19 [Statistics regarding vaccination against Covid-19]. Available online: https://www.folkhalsomyndigheten.se/smittskydd-beredskap/utbrott/aktuella-utbrott/covid-19/statistik-och-analyser/statistik-over-registrerade-vaccinationer-covid-19/. Accessed April 30 2022.
- 2.Sveriges Kommuner och Regioner [SKR]. Regionernas planering avseende vaccinering mot covid-19, delrapport 6 [The planning regarding vaccination against Covid-19 for regions, report 6]. Stockholm, Sweden, SKR; 2021. https://skr.se/download/18.5bb54e0c179a302981232fd/1621980314035/Regionernas_planer ing_%20vaccinering_covid-19_delrapport%206.pdf.
- 3.Tan P.S., Patone M., Clift A.K., Dambha-Miller H., Saatci D., Ranger T., et al. Influenza, shingles and pneumococcal vaccine uptake, offer and refusal in adult populations at high-risk for COVID-19: a UK population-based cohort study. SSRN Electronic Journal. 2021 doi: 10.2139/ssrn.3783784. [DOI] [Google Scholar]
- 4.Nagata J.M., Hernández-Ramos I., Kurup A.S., Albrecht D., Vivas-Torrealba C., Franco-Paredes C. Social determinants of health and seasonal influenza vaccination in adults ≥65 years: a systematic review of qualitative and quantitative data. BMC Public Health. 2013;13:388. doi: 10.1186/1471-2458-13-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocquier A., Ward J., Raude J., Peretti-Watel P., Verger P. Socioeconomic differences in childhood vaccination in developed countries: a systematic review of quantitative studies. Expert Rev Vaccines. 2017;16:1107–1118. doi: 10.1080/14760584.2017.1381020. [DOI] [PubMed] [Google Scholar]
- 6.Barnvaccinationsprogrammet i Sverige 2020, Årsrapport, artikelnummer 21204 [The Childhood Immunization Program in Sweden, 2020], Stockholm, Sweden, 2021. https://www.folkhalsomyndigheten.se/contentassets/7adcc634ba994601a17e7c2ac2aa3871/barnvaccinationsprogrammet-sverige-2020-arsrapport.pdf.
- 7.Wemrell M., Vicente R.P., Merlo J. Mapping sociodemographic and geographical differences in human papillomavirus non-vaccination among young girls in Sweden. Scand J Public Health. 2022 Feb;4 doi: 10.1177/14034948221075410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nafilyan V., Dolby T., Razieh C., Gaughan C.H., Morgan J., Ayoubkhani D., et al. Sociodemographic inequality in COVID-19 vaccination coverage among elderly adults in England: a national linked data study. BMJ Open. 2021;11:e053402. doi: 10.1136/bmjopen-2021-053402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry M., Akbari A., Cottrell S., Gravenor M.B., Roberts R.A., Lyons R., et al. Inequalities in coverage of COVID-19 vaccination: A population register based cross-sectional study in Wales, UK. Vaccine. 2021;39(42):6256–6261. doi: 10.1016/j.vaccine.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glampson B., Brittain J., Kaura A., Mulla A., Mercuri L., Brett S.J., et al. Assessing COVID-19 Vaccine Uptake and Effectiveness Through the North West London Vaccination Program: Retrospective Cohort Study. JMIR Public Health Surveill. 2021;7 doi: 10.2196/30010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spetz M., Lundberg L., Nwaru C., Li H., Santosa A., Leach S., et al. The social patterning of Covid-19 vaccine uptake in older adults: A register-based cross-sectional study in Sweden. Lancet Reg Health Eur. 2022;15:100331. doi: 10.1016/j.lanepe.2022.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolby T., Finning K., Baker A., Fowler-Dowd L., Khunti K., Razieh C., et al. Monitoring sociodemographic inequality in COVID-19 vaccination uptake in England: a national linked data study. J Epidemiol Community Health. 2022;76(7):646–652. doi: 10.1136/jech-2021-218415. [DOI] [PubMed] [Google Scholar]
- 13.[preprint] Nafilyan V, Dolby T, Finning K, Morgan J, Edge R, Glickman M et al. Differences in COVID-19 vaccination coverage by occupation in England: a national linked data study, medRxiv, doi: 10.1101/2021.11.10.21266124. [DOI]
- 14.Ludvigsson J.F., Otterblad-Olausson P., Pettersson B.U., Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wemrell M., Karlsson N., Perez Vicente R., Merlo J. An intersectional analysis providing more precise information on inequities in self-rated health. Int J Equity Health. 2021;20:54. doi: 10.1186/s12939-020-01368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Axelsson Fisk S., Lindström M., Perez-Vicente R., Merlo J. Understanding the complexity of socioeconomic disparities in smoking prevalence in Sweden: a cross-sectional study applying intersectionality theory. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-042323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyberg F., Franzén S., Lindh M., Vanfleteren L., Hammar N., Wettermark B., et al. Swedish Covid-19 Investigation for Future Insights - A Population Epidemiology Approach Using Register Linkage (SCIFI-PEARL) Clin Epidemiol. 2021;13:649–659. doi: 10.2147/CLEP.S312742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The World Bank. World Bank country and lending groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. Accessed April 30 2022.
- 20.Tessier E., Rai Y., Clarke E., Lakhani A., Tsang C., Makwana A., et al. Characteristics associated with COVID-19 vaccine uptake among adults aged 50 years and above in England (8 December 2020-17 May 2021): a population-level observational study. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-055278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson E., Reeve K.S., Niedzwiedz C.L., Moore J., Blake M., Green M., et al. Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain Behav Immun. 2021;94:41–50. doi: 10.1016/j.bbi.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donin G., Erfányuková A., Ivlev I. Factors Affecting Young Adults’ Decision Making to Undergo COVID-19 Vaccination: A Patient Preference Study. Vaccines (Basel) 2022;10(2):265. doi: 10.3390/vaccines10020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams S.H., Schaub J.P., Nagata J.M., Park M.J., Brindis C.D., Irwin C.E., Jr. Young Adult Perspectives on COVID-19 Vaccinations. J Adolesc Health. 2021;69(3):511–514. doi: 10.1016/j.jadohealth.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartelink V, Lager A (redaktörer). Folkhälsorapport 2019. Stockholm. Centrum för epidemiologi och samhällsmedicin, Region Stockholm; 2019. [Public Health Report 2019. Stockholm]. Available online: https://www.folkhalsorapportstockholm.se/globalassets/verksamheter/forskning-och-utveckling/centrum-for-epidemiologi-och-samhallsmedicin/folkhalsorapporten/rapporter-pdf/folkhalsorapport_191113_webb_korr.pdf.
- 25.Bagasra A.B., Doan S., Allen C.T. Racial differences in institutional trust and COVID-19 vaccine hesitancy and refusal. BMC Public Health. 2021;21:2104. doi: 10.1186/s12889-021-12195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folkhälsomyndigheten [FHM]. Acceptans för Covid-19 vaccination [Acceptance of the Covid-19 vaccination]. Stockholm, Sweden, FHM;2021. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/utbrott/aktuella-utbrott/covid-19/statistik-och-analyser/acceptans-for-vaccination-mot-covid-19/resultat-aprilmaj-2021/.
- 27.Arrighi M.H., Hertz-Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology. 1994;5(2):189–196. doi: 10.1097/00001648-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Aw J., Seng J.J.B., Seah S.S.Y., Low L.L. COVID-19 Vaccine Hesitancy-A Scoping Review of Literature in High-Income Countries. Vaccines (Basel) 2021;9(8):900. doi: 10.3390/vaccines9080900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown P., Waite F., Larkin M., Lambe S., McShane H., Pollard A.J., et al. “It seems impossible that it’s been made so quickly”: a qualitative investigation of concerns about the speed of COVID-19 vaccine development and how these may be overcome. Hum Vaccin Immunother. 2022;18(1):2004808. doi: 10.1080/21645515.2021.2004808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadete T., Batra K., Netski D.M., Antonio S., Patros M.J., Bester J.C. Assessing Acceptability of COVID-19 Vaccine Booster Dose among Adult Americans: A Cross-Sectional Study. Vaccines (Basel) 2021;9(12):1424. doi: 10.3390/vaccines9121424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.