Abstract
Purpose
This study reports on the risk of radiation-induced myelitis (RM) of the spinal cord from a large single-institutional experience with 1 to 5 fraction stereotactic body radiation therapy (SBRT) to the spine.
Methods and Materials
A retrospective review of patients who received spine SBRT to a radiation naïve level at or above the conus medullaris between 2007 and 2019 was performed. Local failure determination was based on SPIne response assessment in Neuro-Oncology criteria. RM was defined as neurologic symptoms consistent with the segment of cord irradiated in the absence of neoplastic disease recurrence and graded by Common Toxicity Criteria for Adverse Events, version 4.0. Rates of adverse events were estimated and dose-volume statistics from delivered treatment plans were extracted for the planning target volumes and spinal cord.
Results
A total of 353 lesions in 277 patients were identified that met the specified criteria, for which 270, 70, and 13 lesions received 1-, 3-, and 5-fraction treatments, respectively, with a median follow-up of 46 months (95% confidence interval [CI], 41-52 months) for all surviving patients. The median overall survival was 33.0 months (95% CI, 29-43). The median D0.03cc to the spinal cord was 11.7 Gy (interquartile range [IQR], 10.5-12.4), 16.7 Gy (IQR, 12.8-20.6), and 26.0 Gy (IQR, 24.1-28.1), for 1-, 3-, 5-fractions. Using an a/b = 2Gy for the spinal cord, the median single-fraction equivalent-dose (SFED2) was 11.7 Gy (IQR, 10.2-12.5 Gy) and the normalized biological equivalent dose (nBED2/2) was 19.9 Gy (IQR, 15.4-22.8 Gy). One patient experienced grade 2 RM after a single-fraction treatment. The cumulative probability of RM was 0.3% (95% CI, 0%-2%).
Conclusions
Spine SBRT is safe while limiting the spinal cord (as defined on treatment planning magnetic resonance imaging or computed tomography myelogram) D0.03cc to less than 14 Gy, 21.9 Gy, and 30 Gy, for 1, 3, and 5-fractions, consistent with standard guidelines.
Introduction
Stereotactic body radiation therapy (SBRT) is the delivery of a high dose of radiation therapy in one to 5 fractions, taking advantage of rapid dose fall-off to spare the surrounding normal tissue. The use of (SBRT) for management of spinal metastases may provide improvements in pain relief and local tumor control compared with conventional spine radiation therapy.1, 2, 3, 4, 5, 6 Due to proximity of the spinal cord to the treated volume and the potential severity of radiation-induced myelitis (RM), the spinal cord is the primary dose-limiting organ-at-risk (OAR). Safe delivery of spine SBRT requires the use of appropriate high-resolution planning images, prioritization of the sparing of the spinal cord in planning, and high-precision image guidance and patient repositioning technology during treatment delivery.
More than 2 decades after the introduction of spine SBRT, discussion continues as to the optimal dose-constraints to apply to the spinal cord.2,7 Because of the potential for substantial morbidity associated with RM, standard practice has been to use conservative constraints on the maximum dose received by the spinal cord in the treatment plan and reduce the dosimetric coverage of the target volume as necessary. Various recommended constraints have been published and used to inform clinical practice for patients with no history of spine radiation therapy.7, 8, 9, 10, 11, 12, 13, 14 The reported rates of RM using these constraints ranges from 1% to 5%.3,6,15 However, there are significant differences in the structure used to represent the spinal cord for treatment planning, which affects the interpretation of these outcomes. Some reports are based on reporting doses to the true spinal cord,9,11 as delineated on a coregistered magnetic resonance imaging (MRI) or computed tomography (CT) myelogram. Others use a planning-risk volume (PRV) to address concerns about inter- or intrafraction motion, either using the thecal sac as an anatomic surrogate,8,12 or adding a geometric margin to the anatomically defined spinal cord.16 The decision of which approach to take has implications for treatment planning and outcomes, as using a more generous margin to protect the spinal cord may result in reductions in the coverage of the planning target volume (PTV) for the tumor. Additional evidence demonstrating the safety of using the true spinal cord alone for treatment planning could translate to improvements in treatment planning and optimizing dose to the spinal target. In addition, there is less evidence regarding the spinal cord tolerance for fractionated SBRT. Sahgal et al,8 provided fractionation-specific recommendations, but other guidelines have relied on biological models whose applicability to SBRT remains an open question.7,17 There is continuing need for clinical data about the risk of RM and spinal cord dose constraints to inform clinical practice. In the present study, we report on the risk of RM for patients receiving spine SBRT with no history of previous radiation to the spine from a single-institutional experience.
Methods and Materials
Patient selection
After institutional review board approval (Institutional Review Board No. 19-000352-17), we reviewed consecutive adult patients (aged ≥18 years) with metastatic lesions of the spine who underwent SBRT between December 2007 and October 2019 with no previous radiation therapy or surgery to the treated area of the spine. Patients who received spine SBRT with proton therapy or treatment sites entirely below the level of the conus medullaris were excluded. We included both patients with either single spine level or multispine level treatments. Patients who received treatment for multiple spine levels in one contiguous volume were counted as a single lesion, while lesions in which treatment volumes were separated were counted as multiple lesions.
CT simulation
The standard procedure for CT simulation is dependent on the lesion location and physician preference. Typically, patients with cervical or upper thoracic lesions were immobilized with a custom head-and-shoulder-rest (Klarity Medical, Heath, OH) and a 5-point Aquaplast mask (Orfit Industries, Wijnegem, BE), and BlueBAG BodyFIX vacuum cushions (Elekta, Stockholm, Sweden) for lower thoracic and lumbar sites. The planning CT was acquired with 1-mm slice thickness (1.25 mm in-plane resolution). After the CT simulation, MRI was acquired in treatment position, unless the patient was ineligible for MRI. In those cases, a CT-myelogram was acquired.
Treatment planning
Clinical target volume (CTV) and OAR delineation and prescription dose were determined by the treating physician. Our institutional practice for spine SBRT often uses a 2 clinical target volume delineation technique18: at the discretion of the treating physician, a simultaneous integrated boost was included to a high-risk CTV (CTV_high) was created from the gross tumor volume using a margin of 0 to 2 mm. All PTVs were a 0 to 3 mm isometric expansion of the corresponding CTVs, consistent with the Radiation Therapy Oncology Group (RTOG) guidelines based on the tumor location within the spinal segments.19 The prescription for single fraction treatments was typically 18 to 24 Gy to the high-risk gross tumor volume (14-18 Gy to the low-risk PTV), 30 to 36 Gy for 3-fractions (with 21-24 Gy to the low-risk PTV), and 50 Gy for 5 fractions (40 Gy to the low-risk PTV). The dose was prescribed volumetrically. If an MRI study was acquired in treatment position, the spinal cord was delineated from a T2-weighted sequence; otherwise, the CT myelogram was used. The spinal cord contour was extended at least 5 mm superior and inferior to the PTV. Other OAR structures were segmented using the planning CT according to expert consensus guidelines.
Treatment delivery used static-gantry intensity modulated radiation therapy was used until volumetric-modulated arc therapy was implemented. The primary objective during optimization was to spare the spinal cord. The spinal cord was delineated on MRI or CT-myelogram without adding any geometric or anatomic margin. The minimum dose received by the 0.03 cc of the spinal cord receiving the highest dose (D0.03 cc) was constrained to be less than 14 Gy, 21.9 Gy, and 30 Gy for 1-, 3-, and 5-fraction treatments, respectively. In addition, no more than 10% of the cord volume could get more than 10 Gy, 18 Gy, or 23 Gy. The planning objective for the targets was to cover at least 80% of the low-risk PTV and 90% of the low-risk CTV with the low-risk prescription dose, and at least 90% of the high-risk PTV covered by the simultaneous integrated boost dose. The prioritization of the spinal cord maximum dose meant that reduced target coverage would be accepted for cases in which the spinal cord was near the target. Other OARs were spared according to international guidelines standards for SBRT treatments, primarily TG-1019. Dose calculation was performed using the AAA algorithm with heterogeneity corrections based on the Hounsfield units of the planning CT.
Treatment delivery
After the patient was positioned and immobilized on the treatment table of the linear accelerator (Clinac 2100eX, 2100iX, or TrueBeam, Varian Medical Systems, Palo Alto, CA), planar imaging was acquired to reposition the patient in better alignment with the planning CT and to verify the correct level of the spine was being treated using an orthogonal pair of kV-radiographs. Final patient positioning was performed using a CBCT image and a 6 degree-of-freedom treatment table. Patient positioning was monitored at multiple intervals during treatment using kV-radiographs acquired with the On-Board Imaging system or ExacTrac (BrainLab AG, Munich, Germany). If the x-ray monitoring indicated potential intrafraction patient motion, the treatment delivery was paused while a CBCT was acquired and used for repositioning the patient.
Data collection and clinical endpoints
Patient characteristics, treatment characteristics, and oncologic outcomes were collected retrospectively. Local tumor control was based on the SPIne response assessment in Neuro-Oncology consensus criteria,20 defined as evidence of disease progression at the site of SBRT seen on imaging (MRI, positron emission tomography, or single photon-emission planar). All radiation treatment plans, pretreatment imaging, and post treatment imaging were reviewed. RM was defined as neurologic symptoms consistent with the segment of spinal cord irradiated in the absence of neoplastic disease recurrence,21 and graded with Common Terminology Criteria for Adverse Events version 4.0.22 Time to development of RM was measured from final radiation fraction to first clinical documentation of RM.
Analysis and review
Patient and treatment characteristics were summarized as frequency and percentages for discrete variables and as median and range for continuous variables. Summaries are reported including all 353 treated lesions and by fractionation grouping. The Kaplan-Meier method was used to estimate overall survival, and the cumulative incidence method was used to estimate local failure and RM considering death as a competing risk.
Before the analysis, the spinal cord contour used for treatment planning was reviewed for accuracy by a radiation oncologist or medical physicist with adjustment of any deviations. For select DVH-statistics for the spinal cord, the SFED23 and normalized biological-equivalent dose (nBED, with a reference dose of 2 Gy per fraction)24 were computed using an α/β ratio of 2 Gy for the spinal cord. In addition, the shortest distance (in 3-dimensions) from the surface of the spinal cord structure to the surface of both PTV structures was computed by a custom script written using the application programing interface (API) for the treatment planning system.25 The treatment plans were grouped by proximity of the spinal cord to PTV_high and PTV_low in 1 mm increments (<1 mm, 1-2 mm, etc), and the mean value and interquartile range (IQR) were computed for select DVH-statistics for the high- and low-risk PTVs. A comparison of the mean values of the selected DVH-statistics for both the 1 to 2 mm group and the 2 to 3 mm group with the <1 mm group was performed. An alpha-level of 0.05 was set for all tests of statistical significance.
Results
Patient characteristics
A total of 353 treated lesions in 277 patients were identified that met the specified criteria; the characteristics of the patients and lesions receiving these treatments are shown in Table 1. The median observation time for surviving patients was 46 months (95% confidence interval [CI], 41-52 months). For the overall cohort, the patients were predominantly male (75%) with a median age of 67 years (range, 20-89 years).
Table 1.
Patient characteristics
| No. of fractions | All | 1 | 3 | 5 |
|---|---|---|---|---|
| No. of lesions | 353 | 270 (76%) | 70 (20%) | 13 (4%) |
| Median age, y (range) | 67.2 (20-89) | 67.9 (30-86) | 65.0 (20-87) | 61.8 (21-89) |
| Sex | ||||
| Male | 265 (75%) | 211 (78%) | 43 (61%) | 11 (85%) |
| Female | 88 (25%) | 59 (22%) | 27 (39%) | 2 (15%) |
| ECOG performance status | ||||
| 0 | 213 (60%) | 166 (61%) | 42 (60%) | 5 (38%) |
| 1 | 107 (30%) | 77 (29%) | 24 (34%) | 6 (46%) |
| 2 | 30 (8%) | 24 (9%) | 4 (6%) | 2 (15%) |
| 3 | 2 (1%) | 2 (1%) | 0 (0%) | 0 (0%) |
| 4 | 1 (0%) | 1 (0%) | 0 (0%) | 0 (0%) |
| Bilsky grade | ||||
| 0 | 268 (76%) | 217 (80%) | 41 (59%) | 10 (77%) |
| 1 | 52 (15%) | 38 (14%) | 14 (20%) | 0 (0%) |
| 2 | 26 (7%) | 10 (4%) | 13 (19%) | 3 (23%) |
| 3 | 7 (2%) | 5 (2%) | 2 (3%) | 0 (0%) |
| SINS category | ||||
| ≤6 | 214 (61%) | 173 (64%) | 34 (49%) | 7 (54%) |
| ≥7 | 139 (39%) | 97 (36%) | 36 (51%) | 6 (46%) |
| Location of treatment | ||||
| Cervical | 70 (19%) | 55 (20%) | 12 (17%) | 3 (23%) |
| Cervical + thoracic | 3 (1%) | 1 (0%) | 2 (3%) | 0 (0%) |
| Thoracic | 259 (71%) | 192 (71%) | 49 (70%) | 8 (62%) |
| Thoracic + lumbar | 2 (1%) | 2 (1%) | 0 (0%) | 0 (0%) |
| Lumbar | 29 (8%) | 20 (20%) | 7 (7%) | 2 (15%) |
| No. of lesions treated | ||||
| 1 | 268 (76%) | 216 (80%) | 44 (63%) | 8 (62%) |
| 2 | 53 (15%) | 36 (13%) | 15 (21%) | 2 (15%) |
| 3 | 27 (8%) | 17 (6%) | 7 (10%) | 3 (23%) |
| 4 | 5 (1%) | 1 (0%) | 4 (6%) | 0 (0%) |
| Primary tumor | ||||
| Prostate | 150 (42%) | 136 (50%) | 14 (20%) | 0 (0%) |
| Kidney | 56 (16%) | 40 (15%) | 14 (20%) | 2 (15%) |
| Endometrial | 29 (8%) | 24 (9%) | 5 (7%) | 0 (0%) |
| Sarcoma | 28 (8%) | 13 (5%) | 7 (10%) | 8 (62%) |
| Lung | 27 (8%) | 16 (6%) | 11 (16%) | 0 (0%) |
| Other | 63 (18%) | 41 (15%) | 19 (27%) | 3 (23%) |
| Histology | ||||
| Adenocarcinoma | 179 (51%) | 153 (57%) | 26 (37%) | 0 (0%) |
| Other | 174 (49%) | 117 (43%) | 44 (63%) | 13 (100%) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; SINS = spinal instability neoplastic score.
The characteristics of the treatments are shown in Table 2. Single-fraction treatments were delivered to 270 of the lesions (76%), while 70 lesions received 3-fraction treatments (20%), and 13 lesions received 5-fraction treatment (4%). The median prescribed dose to the high-risk PTV was 20 Gy (range, 16-24 Gy), 30 Gy (range, 30-39 Gy), and 50 Gy (range, 45-50 Gy) for 1-, 3-, and 5-fraction treatments, respectively, and the median dose prescribed to the low-risk PTV was 16 Gy (range, 14-18 Gy), 24 Gy (range, 21-30 Gy), and 40 Gy (range, 35-40 Gy).
Table 2.
Treatment characteristics
| No. of fractions | All | 1 | 3 | 5 |
|---|---|---|---|---|
| PTV_high | ||||
| Present | 336 (95%) | 261 (97%) | 62 (89%) | 13 (100%) |
| Prescribed dose, Gy, median (range) | 20 (16-24) | 30 (30-39) | 50 (45-50) | |
| Volume, cc, mean (range) | 26.0 (0.5-166.1) | 20.2 (0.5-166.1) | 38.4 (0.8-357) | 33.3 (1.6-103.1) |
| Max dose, Gy, mean (STD) | 25.1 (3.3) | 36.6 (4.2) | 58.3 (4.0) | |
| Mean dose, Gy, mean (STD) | 22.8 (2.7) | 32.9 (3.5) | 52.5 (2.0) | |
| Min dose, Gy, mean (STD) | 15.2 (3.8) | 19.5 (6.3) | 34.0 (9.2) | |
| D80%, mean (STD) | 102.6% (4.9) | 102.9% (4.4%) | 101.6% (6.6) | 102.1% (2.3) |
| PTV_low | ||||
| Prescribed dose, Gy, median (range) | 16 (14-18) | 24 (21-30) | 40 (35-40) | |
| Volume, cc, mean (range) | 72.6 (5.3-631) | 64.2 (5.3-631) | 80.8 (9.3-452) | 58.3 (1.6-662) |
| Median dose, Gy, mean (STD) | 19.7 (2.1) | 29.0 (2.3) | 45.2 (2.3) | |
| Mean dose, Gy, mean (STD) | 19.5 (2.3) | 28.8 (2.3) | 45.1 (2.2) | |
| Min dose, Gy, mean (STD) | 10.5 (2.3) | 14.1 (4.7) | 18.7 (5.4) | |
| D90%, mean (STD) | 102.6% (5.9) | 102.8% (5.7) | 102.2% (6.4) | 99.8% (6.8%) |
| Spinal cord | ||||
| Volume, cc, mean (STD) | 6.2 (4.7) | 5.9 (4.2) | 7.1 (6.2) | 6.3 (3.9) |
| Distance from PTV_high, cm, mean (range) | 0.3 (0-1.6) | 0.3 (0-1.5) | 0.2 (0-1.6) | 0.3 (0-1.5) |
| D0.03 cc, Gy, median (IQR) | 11.7 (10.5-12.4) | 16.7 (12.8-20.6) | 26.0 (24.1-28.1) | |
| D0.1cc, Gy, median (IQR) | 11.0 (9.9-11.6) | 16.0 (12.0-19.7) | 24.5 (23.3-28.6) | |
| D1cc, Gy, median (IQR) | 8.4 (6.6-12.1) | 13.8 (9.3-16.1) | 20.2 (17.4-22.2) | |
| D2cc, Gy, median (IQR) | 7.1 (5.2-8.0) | 11.0 (7.5-14.1) | 16.3 (11.8-20.4) | |
| Spinal cord, SFED2 | ||||
| D0.03cc, Gy, median (IQR) | 11.7 (10.2-12.5) | |||
| D0.1cc, Gy, median (IQR) | 11.0 (9.6-11.7) | |||
| D1cc, Gy, median (IQR) | 8.5 (7.5-9.2) | |||
| D2cc, Gy, median (IQR) | 7.1 (5.1-8.1) | |||
| Spinal cord, nBED2/2 | ||||
| D0.03cc, Gy, median (IQR) | 39.9 (31.0-45.5) | |||
| D0.1cc, Gy, median (IQR) | 35.5 (27.8 – 39.9) | |||
| D1cc, Gy, median (IQR) | 22.3 (17.7 - 25.9) | |||
| D2cc, Gy, median (IQR) | 16.2 (9.1 – 20.6) |
Abbreviations: D80% = minimum dose delivered to 80% of the PTV; D90% = minimum dose delivered to 90% of the PTV; IQR = interquartile range; PTV_high = high-risk planning-target volume; Max = maximum; Min = minimum; nBED2 = normalized biological equivalent dose using a reference dose of 2 Gy; PTV_low = low-risk planning target volume; SFED2/2 = single-fraction equivalent dose using a reference dose of 2 Gy and an α/β ratio of 2 Gy for the spinal cord; STD = standard deviation.
Patient outcomes
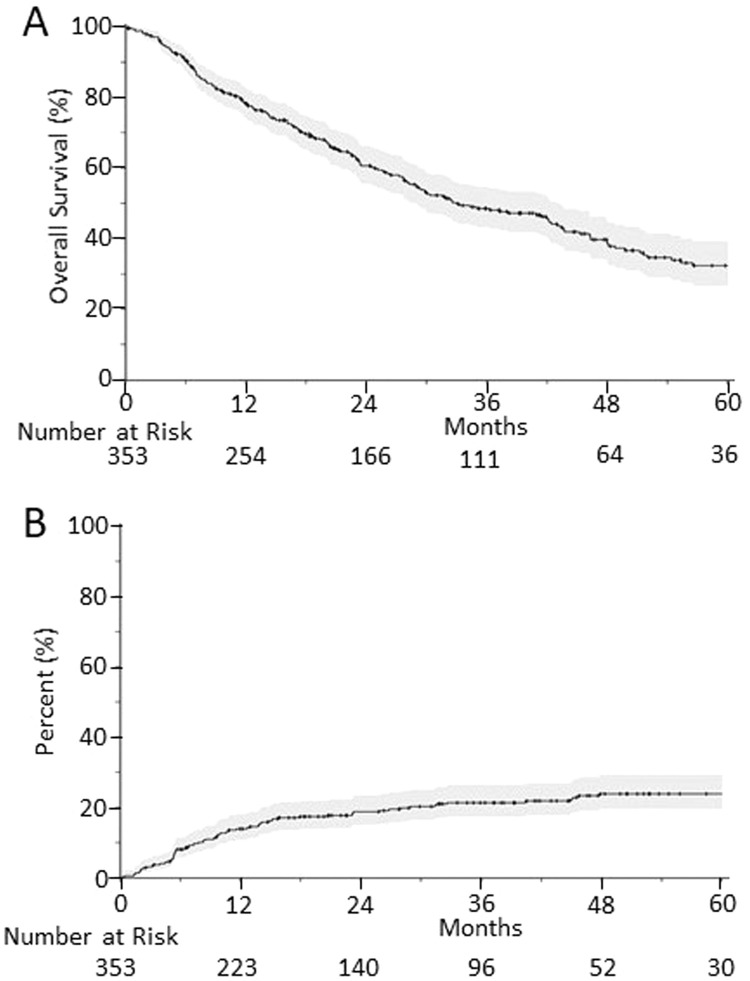
The Kaplan-Meier estimate of the overall survival is shown in Fig. 1A. The median overall survival was 33.0 months (95% CI, 28.9%-43.0%). The 1-, 2-, and 5-year overall survival was 78.3% (95% CI, 74.0%-82.8%), 60.6% (95% CI, 55.5%-66.3%), and 32.4% (26.6%-39.4%), respectively. The cumulative incidence of local failure with death as a competing risk is shown in Fig. 1B, and the 1-, 2-, and 5-year cumulative incidence of local failure with death as a cumulative risk is 13.9% (95% CI, 10.7%-18.1%), 18.8% (95% CI, 15.0%-23.5%), and 23.9% (95% CI, 19.5%-29.3%).
Fig. 1.
Kaplan-Meier estimated overall survival for all patients receiving spine stereotactic body radiation therapy (upper), and cumulative incidence of local failure with death as a competing risk (lower). The shaded area representing the 95% confidence interval of the estimates.
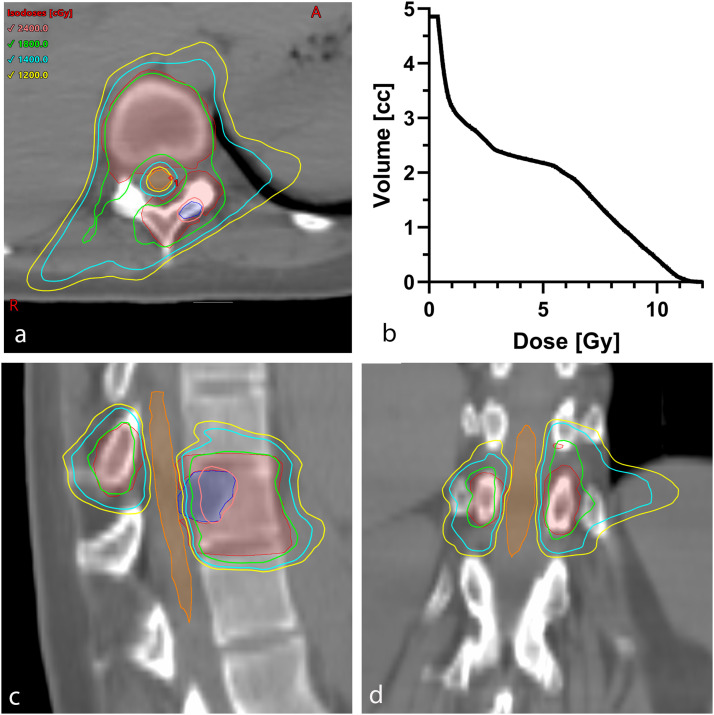
There was a single case of RM reported among these patients, after a single-fraction treatment. A 30-year-old woman with newly diagnosed metastatic angiosarcoma with symptomatic spine metastases at T10-T11 and L4 underwent a course of spine SBRT to both areas, each receiving a single fraction of SBRT with a prescribed dose of 18 Gy to low-risk PTV with a simultaneous integrated boost to 24 Gy to high-risk PTV. Details of the patient's T10-T11 treatment plan are shown in Fig. 2. The spinal cord dose met the specified constraints for this treatment plan: the maximum dose to the spinal cord was 11.8 Gy (nBED2/2 = 44.6 Gy), D0.03cc = 11.1 Gy (nBED2/2 = 37.8 Gy, respectively), and V10Gy = 6.9%. At the 5-month follow-up visit, the patient reported intermittent numbness and tingling in the lower thoracic/upper lumbar region for a 1- to 2-month duration. There was no associated weakness. A diagnosis of RM was made clinically and was graded as grade 2 per with Common Terminology Criteria for Adverse Events, version 4.0. Unfortunately, the patient was lost to follow-up approximately 1 month after the diagnosis of RM. Based on this single reported case, the cumulative probability of RM is 0.3% (95% CI, 0%-2.1%). In addition, there were 3 reports of neuropathy associated with injury to the peripheral nerve system: the risk of neuropathy after 6 months was 1.1% (95% CI, 0.4%-3.0%).
Fig. 2.
Radiation therapy treatment plan for the radiation-induced myelitis case after single-fraction stereotactic body radiation therapy to T10 and T11, prescribed 18 Gy with a simultaneous integrated boost to 24 Gy. (A), (C), (D) Dose distributions, with the low-risk planning target volume shown in red, high-risk planning target volume in blue, and spinal cord in orange. (B) Cumulative dose-volume histogram for the spinal cord.
Dosimetric results
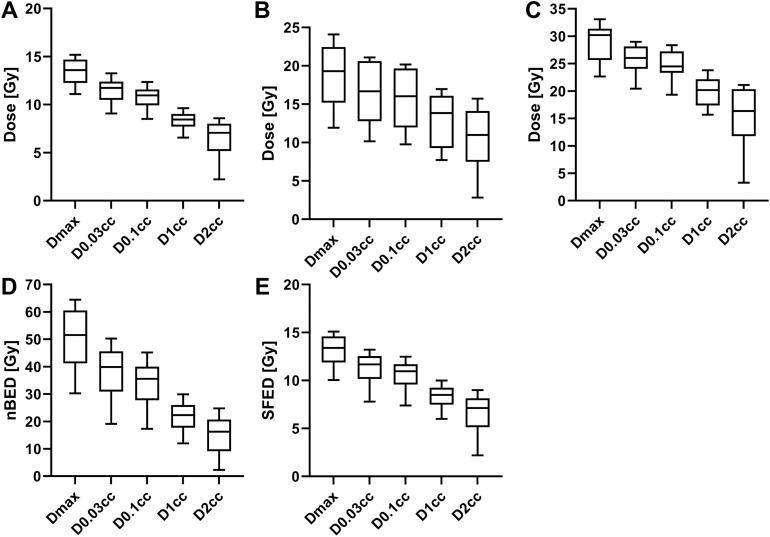
The dose-volume statistics for the spinal cord are shown in Table 2 and the distributions are represented in Fig. 3. The median D0.03cc to the spinal cord was 11.7 Gy (IQR, 10.5-12.4 Gy), 16.7 Gy (IQR, 12.8-20.6 Gy), and 26.0 Gy (IQR, 24.1-29.2 Gy) for the 1-, 3-, and 5-fraction plans, respectively. The D0.03cc objective for the given fractionation was met in 348 of 353 cases (98.5%). Using an a/b-ratio of 2 Gy, the median SFED2 was 11.7 Gy (IQR, 10.2-12.5 Gy) and nBED2/2 was 39.9 Gy (IQR, 31.0-45.5 Gy) for the entire cohort. Coverage statistics for PTV_high and PTV_low are shown Table 3, grouped by the shortest distance between the respective PTV and spinal cord. For both PTVs, the mean Dmin was significantly lower (P <.05) for the <1 mm group than the 1 to 2 mm and 2 to 3 mm groups. Likewise, for the high-risk PTV, the mean D90% was significantly different for the <1 mm group compared with the other groups.
Fig. 3.
Select dose-volume statistics for the spinal cord doses for (A) single-fraction, (B) 3-fraction, (C) 5-fraction spine stereotactic body radiation therapy, and (D) the normalized biological equivalent doses (nBED2/2) and (E) the single-fraction equivalent doses (SFED2) for all treatment plans in the cohort. The box-and-whisker plots show the median, inner quartiles, and 10 to 90 percentile ranges.
Table 3.
Planning target volume coverage based on distance from spinal cord
| Distance from cord | <1 mm | 1-2 mm | 2-3 mm | |
|---|---|---|---|---|
| PTV_High | Dmin [%] | 54.4 ± 1.5 | 64.8 ±1.6* | 74.8 ± 2.2* |
| D90% [%] | 96.3 ±1.0 | 98.8 ± 5.6* | 100.4 ± 0.5* | |
| D80% [%] | 101.3 ±7.5 | 102.4 ± 3.2 | 103.2 ± 0.3* | |
| PTV_Low | Dmin [%] | 57.0 ± 0.9 | 72.6 ± 1.7* | 77.9 ± 3.9* |
| D90% [%] | 102.3 ± 0.4 | 103.2 ± 4.8 | 104.4 ± 0.7* | |
| D80% [%] | 106.7 ± 0.3 | 106.8 ± 0.5 | 109.2 ± 1.6 |
Abbreviations: D80% = minimum dose delivered to 80% of the PTV; D90% = minimum dose delivered to 90% of the PTV; Dmin = minimum dose received by the PTV; PTV_high = high-risk planning-target volume; PTV_low = low-risk planning target volume.
Statistically significant differences between the mean of either the 1 to 2 mm or 2 to 3 mm group and the mean of the <1 mm.
Discussion
We evaluated the rate of RM after single and multifraction spine SBRT in a cohort of 353 lesions treated in 277 patients and identified one case of RM in a 30-year-old female. Due to the low incidence of RM related to spine SBRT reported in the literature, precise estimates of RM risk are not available.7,17 However, the reported risk of less than 1% is consistent with the estimates from recent reviews,5,7,13 phase 2 results reported for RTOG 0631,19 and other large studies.6,11,15 This agreement may be unsurprising, as the spinal cord dose constraints reported both here and in these other studies are consistent with the widely used guidelines, thus providing further evidence of the safety associated with those limits. In addition, the observed risk of neuropathy is consistent with the experience reported by Stubblefield et al.26
There is interest in determining specific dose-limits that ensure safe treatment delivery, potentially to allow increased dose to the tumor. However, the instance of RM in this study occurred at doses below the median dose-volume values for the single-fraction cohort. Although the dose to the spinal cord satisfied the published constraints, those guidelines were intended for reporting of the dose to either the thecal sac or to the cord with a geometric expansion of 1.5 to 2 mm, and the use of these constraints to the spinal cord alone does have potential to introduce uncertainties that could cause the dose received by the spinal cord to exceed the constraints, such as variation in imaging parameters or registration of the MRI or CT myelogram, and physiological motion of the spinal cord. Many of the other cases reported in the literature also occurred for patients whose spinal cord doses were well below the typical recommended constraints.11,24,27 Whether these RM incidents reflect a variation in radiation tolerance among the patients or the consequences of undetected inter- or intrafraction spinal cord motion, it indicates that the published guidelines are sufficient to keep the risk of radiation injury to the spinal cord low, but not zero.
It is important to note that MR imaging (or CT myelography) was acquired in treatment position with the immobilization to aid in target and OAR delineation. This may limit generalizability to other practices for which MRI or CT myelograms in treatment position are not readily available. To reduce the risk of RM, some centers use a PRV for treatment planning and dose-reporting purposes. Different alternatives to using the true spinal cord have been discussed, including using an anatomic surrogate like the thecal sac27 or adding a geometric margin to the true spinal cord.16 Each of these approaches has consequences for how patients are treated as the fundamental planning challenge for spine SBRT is balancing of target coverage with spinal cord sparing. In our practice, the primary treatment planning objective was meeting the recommended spinal cord constraints for the spinal cord. Coupled with our frequent intrafraction verification imaging of the patient during treatment, this has demonstrated to be generally safe.
This prioritization of the spinal cord means that the dose coverage of the target volumes was affected by the proximity of the spinal cord to the tumor and may negatively affect local control. Using the results from Table 3, we can estimate how the use of a 2-mm margin would affect the high-risk PTV coverage. For example, if the true spinal cord was 2 to 3 mm from the PTV and a 2-mm PRV is added, we would expect that, on average, then the minimum dose to the high-risk PTV would be reduced from 75% to 54% of prescription dose. For a single-fraction treatment with an 24 Gy prescribed dose to the high-risk volume, this would correspond to a reduction of Dmin from a BED of 50 Gy to 30 Gy (using a/b value of 10 Gy for tumor), which would be below the threshold of 33.4 Gy BED for the high-risk target Dmin corresponding significantly higher rates of local control reported by Bishop et al.28 Given the widely established record of safety of spine SBRT using the true spinal cord contour for planning, the decision whether to use a spinal cord PRV must be weighed among the potential risks associated with reduced dose to the tumor and institutional spine SBRT practice, including patient immobilization, availability of MR or CT myelography, treatment planning techniques, and image guidance and patient position monitoring techniques. Further investigation into dosimetric predictors of local control would help inform the discussion of the risks and benefits of these approaches.
Novelties of our study include it being the largest single-institution spine SBRT experience with multiple fractionation schemes known to the authors and providing important support for the recommended dose-volume constraints for multiple fractions. Additionally, we report a robust experience demonstrating the low risk of RM when treatments are optimized on the spinal cord without the use of a PRV. Finally, we apply a novel approach to analyzing the relationship between proximity of the spinal cord to the treatment volumes, which addresses the potential effect of PRV spinal cord structure on PTV coverage. The spinal cord dose constraints, treatment planning and delivery techniques conform to the widely accepted guidelines and standard practice, meaning that the results and conclusions drawn can be broadly applied.
There are limitations to our study. First, it was limited by its retrospective nature, including potential selection bias and a heterogeneous patient cohort. Second, treatment decisions were made by the treating radiation oncologist, resulting in heterogenous dose-fractionation schemes, which may have been chosen due to tumor location and expected spinal cord dose. Third, with regards to RM, one case was identified and made clinically by the treating radiation oncologist, but, unfortunately, the patient was lost to follow-up and the complete clinical course is not known. Additionally, other patients were lost to follow-up or passed away within 3 to 6 months of treatment, potentially underestimating the true rate.
Conclusion
These results provide further evidence that spine SBRT can be delivered safely by limiting spinal cord D0.03 cc to be less than 11.7 Gy (IQR, 10.5-12.4 Gy), 16.7 Gy (IQR, 12.8-20.6 Gy), and 26.0 Gy (IQR, 24.1-29.2 Gy) for the 1-, 3-, and 5-fractions, consistent with current guidelines,
Footnotes
Sources of support: This work had no specific funding.
Disclosures: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Research data are not available at this time.
References
- 1.Garg AK, Shiu AS, Yang J, et al. Phase 1/2 trial of single: Session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer. 2012;118:5069–5077. doi: 10.1002/cncr.27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glicksman RM, Tjong MC, WFP Neves-Junior, et al. Stereotactic ablative radiotherapy for the management of spinal metastases: A review. JAMA Oncol. 2020;6:567–577. doi: 10.1001/jamaoncol.2019.5351. [DOI] [PubMed] [Google Scholar]
- 3.Sahgal A, Myrehaug SD, Siva S, et al. Stereotactic body radiotherapy (SBRT) versus conventional palliative radiotherapy (CRT) for patients with painful spinal metastases: A Phase 3, randomized controlled trial. Lancet Oncol. 2021;22:1023–1033. doi: 10.1016/S1470-2045(21)00196-0. [DOI] [PubMed] [Google Scholar]
- 4.Sprave T, Verma V, Förster R, et al. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol. 2018;128:274–282. doi: 10.1016/j.radonc.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Husain ZA, Sahgal A, De Salles A, et al. Stereotactic body radiotherapy for de novo spinal metastases: Systematic review: International Stereotactic Radiosurgery Society practice guidelines. J Neurosurg Spine. 2017;27:295–302. doi: 10.3171/2017.1.SPINE16684. [DOI] [PubMed] [Google Scholar]
- 6.Yamada Y, Katsoulakis E, Laufer I, et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus. 2017;42:E6. doi: 10.3171/2016.9.FOCUS16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahgal A, Chang JH, Ma L, et al. Spinal cord dose tolerance to stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2021;110:124–136. doi: 10.1016/j.ijrobp.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85:341–347. doi: 10.1016/j.ijrobp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 10.Kim DN, Medin PM, Timmerman RD. Emphasis on repair, not just avoidance of injury, facilitates prudent stereotactic ablative radiotherapy. Semin Radiat Oncol. 2017;27:378–392. doi: 10.1016/j.semradonc.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Katsoulakis E, Jackson A, Cox B, Lovelock M, Yamada Y. A detailed dosimetric analysis of spinal cord tolerance in high-dose spine radiosurgery. Int J Radiat Oncol Biol Phys. 2017;99:598–607. doi: 10.1016/j.ijrobp.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs IC, Patil C, Gerszten PC, Adler JR, Jr, Burton SA. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64(Suppl 2):A67–A72. doi: 10.1227/01.NEU.0000341628.98141.B6. [DOI] [PubMed] [Google Scholar]
- 13.Grimm J, Sahgal A, Soltys SG, et al. Estimated risk level of unified stereotactic body radiation therapy dose tolerance limits for spinal cord. Semin Radiat Oncol. 2016;26:165–171. doi: 10.1016/j.semradonc.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryu S, Jin JY, Jin R, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109:628–636. doi: 10.1002/cncr.22442. [DOI] [PubMed] [Google Scholar]
- 15.Kelley KD, Racareanu R, Sison CP, et al. Outcomes in the radiosurgical management of metastatic spine disease. Adv Radiat Oncol. 2019;4:283–293. doi: 10.1016/j.adro.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oztek MA, Mayr NA, Mossa-Basha M, et al. The dancing cord: Inherent spinal cord motion and its effect on cord dose in spine stereotactic body radiation therapy. Neurosurgery. 2020;87:1157–1166. doi: 10.1093/neuros/nyaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. int J Radiat Oncol Biol Phys. 2010;76(Suppl 3):S42–S49. doi: 10.1016/j.ijrobp.2009.04.095. [DOI] [PubMed] [Google Scholar]
- 18.Cox BW, Spratt DE, Lovelock M, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e597–e605. doi: 10.1016/j.ijrobp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1-3) spine metastases: Phase 2 results. Pract Radiat Oncol. 2014;4:76–81. doi: 10.1016/j.prro.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thibault I, Chang EL, Sheehan J, et al. Response assessment after stereotactic body radiotherapy for spinal metastasis: A report from the SPIne response assessment in Neuro-Oncology (SPINO) group. Lancet Oncol. 2015;16:e595–e603. doi: 10.1016/S1470-2045(15)00166-7. [DOI] [PubMed] [Google Scholar]
- 21.Wong CS, Fehlings MG, Sahgal A. Pathobiology of radiation myelopathy and strategies to mitigate injury. Spinal Cord. 2015;53:574–580. doi: 10.1038/sc.2015.43. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health, National Cancer Institute . US Department of Health and Human Services; Washington, DC: 2009. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. [Google Scholar]
- 23.Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: Useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 24.Sahgal A, Ma L, Gibbs I, et al. Spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:548–553. doi: 10.1016/j.ijrobp.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Breen WG, Jeans EB, Gergelis KR, et al. Ablative radiotherapy for ultracentral lung cancers: Dosimetric, geometric, and volumetric predictors of outcomes and toxicity. Radiother Oncol. 2021;158:246–252. doi: 10.1016/j.radonc.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Stubblefield MD, Ibanez K, Riedel ER, et al. Peripheral nervous system injury after high-dose single-fraction image-guided stereotactic radiosurgery for spine tumors. Neurosurg Focus. 2017;42:E12. doi: 10.3171/2016.11.FOCUS16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbs IC, Kamnerdsupaphon P, Ryu M-R, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185–190. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Bishop AJ, Tao R, Rebueno NC, et al. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys. 2015;92:1016–1026. doi: 10.1016/j.ijrobp.2015.03.037. [DOI] [PubMed] [Google Scholar]