Abstract
Rationale
To describe cardiopulmonary function during exercise 12 months after hospital discharge for coronavirus disease 2019 (COVID-19), assess the change from 3 to 12 months, and compare the results with matched controls without COVID-19.
Methods
In this prospective, longitudinal, multicentre cohort study, hospitalised COVID-19 patients were examined using a cardiopulmonary exercise test (CPET) 3 and 12 months after discharge. At 3 months, 180 performed a successful CPET, and 177 did so at 12 months (mean age 59.3 years, 85 females). The COVID-19 patients were compared with controls without COVID-19 matched for age, sex, body mass index and comorbidity. Main outcome was peak oxygen uptake (V′O2 peak).
Results
Exercise intolerance (V′O2 peak <80% predicted) was observed in 23% of patients at 12 months, related to circulatory (28%), ventilatory (17%) and other limitations including deconditioning and dysfunctional breathing (55%). Estimated mean difference between 3 and 12 months showed significant increases in V′O2 peak % pred (5.0 percentage points (pp), 95% CI 3.1–6.9 pp; p<0.001), V′O2 peak·kg−1 % pred (3.4 pp, 95% CI 1.6–5.1 pp; p<0.001) and oxygen pulse % pred (4.6 pp, 95% CI 2.5–6.8 pp; p<0.001). V′O2 peak was 2440 mL·min−1 in COVID-19 patients compared to 2972 mL·min−1 in matched controls.
Conclusions
1 year after hospital discharge for COVID-19, the majority (77%), had normal exercise capacity. Only every fourth had exercise intolerance and in these circulatory limiting factors were more common than ventilator factors. Deconditioning was common. V′O2 peak and oxygen pulse improved significantly from 3 months.
Short abstract
Exercise capacity improves in COVID-19 patients from 3 to 12 months after hospitalisation, and the majority have normal exercise capacity (77%). Circulatory limitations are more common than ventilatory limitation after COVID-19. Deconditioning is common. https://bit.ly/3DlPxcG
Introduction
Severe coronavirus disease 2019 (COVID-19) may be followed by organ dysfunction and persisting symptoms [1, 2]. In hospitalised patients, the lung has been the organ primarily affected by COVID-19 infection, and consequently, respiratory symptoms and exercise intolerance are prevalent [3, 4]. Dyspnoea is the most frequently reported respiratory symptom after COVID-19, affecting approximately half of the patients 3 months after hospitalisation for COVID-19 [5].
The cardiopulmonary exercise test (CPET) provides an integrated assessment of the cardiorespiratory system and is considered the gold standard for evaluating exercise capacity and dyspnoea on exertion. Hence, in patients who continue to experience dyspnoea after COVID-19, CPET is a valuable tool. Deconditioning has been considered the main limiting factor of exercise capacity 3 months after COVID-19, followed by circulatory and ventilatory limitations [5–7]. However, most studies have a short time interval between COVID-19 diagnosis and follow-up, usually 3–6 months [6, 7], which may not be long enough for pulmonary structural changes and exercise abnormalities to resolve. Whether or not these limitations to exercise persist 1 year after COVID-19 infection is still unknown.
In a prospective study of patients hospitalised for COVID-19, we aimed to 1) determine cardiopulmonary exercise capacity at 12 months, including the impact of persisting dyspnoea and treatment in the intensive care unit (ICU); 2) assess the change in cardiopulmonary exercise capacity from 3 to 12 months; and 3) compare the results from the post-COVID-19 population with a matched control group without a history of COVID-19.
We hypothesised that exercise capacity would improve from 3 to 12 months after discharge.
Methods
Study design and variables
The present study was a substudy of all patients undergoing CPET at 3 and/or 12 months in a prospective observational study of patients hospitalised for COVID-19 in Norway, the Patient-Reported Outcomes and Lung Function after Hospitalization for COVID-19 (PROLUN) study. The main study included participants aged ≥18 years with a discharge diagnosis of COVID-19 before 1 June 2020 from six hospitals in different parts of Norway. The patients were invited to follow-up visits 3 and 12 months after discharge, with pulmonary function, dyspnoea and computed tomography findings as primary outcomes [5, 8]. The study was registered as ClinicalTrials.gov with identifier number NCT04535154.
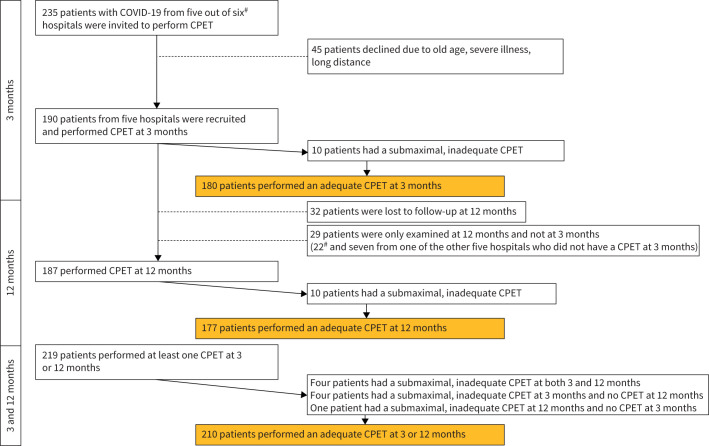
Among the 264 PROLUN patients providing consent, 256 attended at least one of the visits.
In the present substudy, CPET was performed in 190 patients at 3 months, and in 187 at 12 months (figure 1). One of the centres performed CPET only at 12 months (n=22). All patients with valid CPET at either 3 or 12 months (n=210) were included in the analyses (figure 1).
FIGURE 1.
Flow chart of the study population. COVID-19: coronavirus disease 2019; CPET: cardiopulmonary exercise testing. #: 22 patients from one hospital were only examined at 12 months.
Informed consent was obtained from all participants. The regional ethics committee, South-Eastern Norway (identifier 125384) and data protection officers at the participating hospitals provided ethical approval.
Comorbidity was based on both medical records and self-report, and included a previous diagnosis of COPD, myocardial infarction, heart failure, cerebral vascular accident or peripheral vascular disease.
Obesity was defined as body mass index (BMI) >30 kg·m−2. The World Health Organization Ordinal Scale for Clinical Improvement was used to score the severity of COVID-19 infection [9].
Dyspnoea and pulmonary function tests
The modified Medical Research Council (mMRC) scale (grades 0–4) was used to classify self-reported dyspnoea [10]; mMRC 0 was defined as no dyspnoea.
Spirometry, body plethysmography and diffusing capacity of the lung for carbon monoxide (DLCO) were performed (Jaeger Master Screen PFT; Vyaire Medical, Germany) according to guidelines, using Global Lung Function Initiative reference values [11–13].
CPET
Stepwise incremental treadmill exercise according to a modified Bruce protocol was applied for CPET (Vyntus CPX; Vyaire Medical), which included continuous measurement of ECG and pulse oximetry (SpO2). Mouthpiece and nose clip were used for breath-by-breath measurements of ventilation (V′E), oxygen consumption (V′O2) and expired carbon dioxide (V′CO2). The Borg CR-10 scale was used for the assessment of perceived exertion and dyspnoea [14]. V′O2·kg−1, oxygen pulse (V′O2 peak/heart rate), respiratory exchange ratio (RER), V′E/V′CO2 slope and ventilatory equivalents were calculated. Ventilatory efficiency was assessed using the V′E/V′CO2 slope up to the ventilatory compensation point and by nadir ventilatory equivalent for carbon dioxide (V′E/V′CO2 nadir). Breathing reserve was calculated as (1−V′E/maximal voluntary ventilation (MVV))×100%, using an estimate of forced expiratory volume in 1 s (FEV1)×40 for MVV [15]. The anaerobic threshold was assessed using the V-slope method [16]. Post-exercise capillary blood samples were collected from the fingertip within 1 min and analysed for lactate, pH and carbon dioxide tension (ABL 800 Flex; Radiometer Medical, Denmark). Norwegian reference values, from a healthy population, were used to calculate CPET values relative to expected for age and sex (% predicted) [17], except for V′E/V′CO2 slope and V′E/V′CO2 nadir [18]. The prediction equation for V′O2 peak (mL·min−1) [17] was used for assessment of exercise intolerance and V′O2 at the anaerobic threshold V′O2max % pred. Exercise intolerance was defined as V′O2 peak <80% pred. Ventilatory limitation to exercise was defined when breathing reserve was <15% [15]. The Wassermann flowchart was used to define circulatory limitation in participants when it led to a circulatory category [16], including ECG changes consistent with ischaemia or arrhythmia. Deconditioning was defined as V′O2 peak <80% pred with normal breathing reserve and no evidence of cardiocirculatory pathology (assessed by ECG, V′E/V′CO2 slope, and oxygen-pulse curve) with normal or low V′O2 peak at anaerobic threshold.
Ventilatory inefficiency was defined as V′E/V′CO2 and/or V′E/V′CO2 nadir z-score >1.645 [18]. Dysfunctional breathing was determined by random swings in ventilation due to chaotic changes in tidal volume and respiratory frequency, accompanied by hypocapnia and respiratory alkalosis. CPET was considered submaximal, and thus inconclusive and invalid, when exercise was restricted by non-cardiopulmonary factors, including back or leg pain, in patients with RER <1.0 and lactate <3.0 mmol·L−1.
Matched controls (HUNT4 HOPE)
The matched controls were recruited from HUNT4 HOPE, part of the large population-based Norwegian study HUNT (the Trøndelag Health Study), where CPET and echocardiography were performed in 2461 participants between 2017 and 2019 [19]. After matching individually for comorbidity and sex, matching at group level was done for age, BMI and blood pressure. The HUNT4 HOPE CPET treadmill protocol increased inclination and/or speed every minute until voluntary exhaustion. Continuous gas analysis was performed with the MetaLyzer II (Cortex Biophysik, Leipzig, Germany) mixing chamber system with patients wearing an oro-nasal mask.
In total 177 patients and 207 controls were included in the analysis.
Statistical methods
Data are presented as mean±sd, median (interquartile range (IQR)) or frequency (%), as appropriate. Normality of data and residuals were checked by inspection of histograms and QQ-plots and Shapiro–Wilk or Anderson–Darling tests.
The change in outcome variables from 3 to 12 months and potential interactions with ICU stay or dyspnoea were analysed using linear mixed models (LMMs). A subject-specific random intercept accounted for within-subject correlations. Models with and without interaction between ICU stay or dyspnoea and the categorical time variable (3 and 12 months) were fitted. Since the interaction effect was not statistically significant, results for the effect of time on ICU stay or dyspnoea from main effect models are presented. All models included sex, comorbidity (present or not present), BMI and age, all measured at 3 months, as additional covariates, and a fixed effect for the hospitals to adjust for a potential centre effect. To explore other potential predictors of change in the outcome variables, LMMs including interactions of time with obesity, comorbidity, age and sex, in addition to ICU stay and dyspnoea, were fitted similarly. The lmer function and the models in the lme4 package were fitted in R version 3.4.4 [20, 21].
A subset of CPET variables were compared between the patients with COVID-19 and the controls using multiple regression analysis, adjusting for age, sex, BMI, resting systolic blood pressure, COPD, diabetes, previous heart failure and previous myocardial infarction. After matching for comorbidity and sex, matching on group level was done for age, BMI and blood pressure. Because of the partly individual matching of controls (Methods section), LMMs were first fitted to account for potential within-pair correlations. Because these correlations were very small, we used ordinary regression models. For the compared CPET variables, the normality assumption for the residuals was considered reasonable. Other assumptions for regression analyses were checked by correlations between the variables, variance inflation factor and inspection of plots of residuals versus predicted and found to be satisfactory.
The main study, PROLUN, was an observational study with the prevalence of reduced lung function after hospitalisation and interstitial lung findings after 3 and 12 months as primary outcomes. There were no a priori sample size calculations for these outcomes, and the study included all eligible patients in the six hospitals until 1 June 2020.
p<0.01 was considered statistically significant, to give some protection against false positive results.
Results
Study population characteristics
The 12-month visit was completed at a median (IQR) of 376 (309–472) days after discharge from the hospital. The mean±sd age was 58.1±13.8 years, 41% (n=85) were female and mean±sd BMI was 28.5±4.8 kg·m−2. The patients were hospitalised for a median of 6 (3–11) days; 41 (20%) patients were treated in an ICU for a median of 10 (4–15) days, and 27 (13%) were intubated and mechanically ventilated for median 10 (7–15) days (supplementary table S1). Comorbidity at baseline was present in 26 (13%) patients and obesity in 59 (29%) patients. Figure 2 summarises the main findings of the study. Supplementary table S1 summarises the descriptive data of the study population.
FIGURE 2.
Cardiopulmonary exercise capacity and limitations 1 year after coronavirus disease 2019. PROLUN: Patient-Reported Outcomes and Lung Function after Hospitalization for COVID-19; ICU: intensive care unit; V′O2 peak: peak oxygen uptake.
At 12 months, 41 (22%) patients had supervised rehabilitation. The majority attended in-patient rehabilitation (n=27); fewer attended community-based (n=8) and outpatient (n=6) rehabilitation.
The patients lost to follow-up were slightly older, had a higher degree of obesity, were female, fewer were born in Norway, and they had lower V′O2 peak. They had similar rates of ICU admission, comorbidity and dyspnoea.
Descriptive results
Dyspnoea
mMRC was ≥1 in 86 (47%) patients at 12 months compared with 89 (51%) patients at 3 months (supplementary table S1).
Pulmonary function tests at 12 months
Mean±sd FEV1 was 94±15% pred, forced vital capacity (FVC) was 97±13% pred, total lung capacity (TLC) was 97±17% pred and DLCO was 92±17% pred. Results below the lower limit of normal (z-score <−1.645) were observed in 12 (7%) patients for FEV1, 14 (8%) for FVC and 25 (15%) for DLCO. V′O2 peak % pred correlated with TLC % pred (r=0.38, p<0.001), but not with FEV1 % pred (r=0.01, p=0.94) or DLCO % pred (r=0.01, p=0.95).
Cardiopulmonary exercise test at 12 months
Observed CPET variables at 12 months are presented in table 1.
TABLE 1.
Estimated changes in cardiopulmonary exercise testing variables in coronavirus disease 2019 (COVID-19) patients from 3 to 12 months in estimated values from linear mixed models and observed values at 3 and 12 months
| Patients | 3 months | Patients | 12 months | Change from 3 to 12 months, estimate (95% CI) | p-value | |
| Performance | ||||||
| V′O2 peak, mL·min−1 | 180 | 2306±797 | 177 | 2451±776 | 93 (40–144) | <0.001 |
| V′O2 peak, % pred | 180 | 87±19 | 177 | 92±20 | 5.0 (3.1–6.9) | <0.001 |
| V′O2 peak·kg−1, mL·kg−1·min−1 | 180 | 27±9 | 177 | 29±8 | 0.7 (0.1–1.3) | 0.03 |
| V′O2 peak·kg−1, % pred | 180 | 82±19 | 177 | 86±21 | 3.4 (1.6–5.1) | <0.001 |
| Perceived dyspnoea Borg CR-10 at max. load | 175 | 8±2 | 175 | 9±2 | 0.1 (−0.2–0.4) | 0.39 |
| Ventilation | ||||||
| V′E at max. load, L·min−1 | 180 | 82±30 | 177 | 88±29 | 2.8 (−0.3–6.2) | 0.08 |
| Breathing reserve, % | 180 | 20±20 | 177 | 19±19 | −0.4 (−3.3–2.5) | 0.80 |
| Circulation | ||||||
| Heart rate at max. load, beats·min−1 | 180 | 153±22 | 177 | 155±22 | 0.0 (−2.5–2.3) | 0.95 |
| Heart rate at max. load, % pred | 180 | 90±11 | 177 | 92±10 | 0.5 (−1.0–1.8) | 0.49 |
| Systolic BP at max. load, mmHg | 170 | 191±35 | 164 | 190±32 | −3.8 (−10.7–3.2) | 0.31 |
| Diastolic BP at max. load, mmHg | 170 | 84±18 | 164 | 83±18 | −0.5 (−3.7–2.8) | 0.78 |
| Oxygen pulse at max. load, mL·stroke−1 | 180 | 15±4 | 177 | 16±4 | 0.6 (0.3–0.9) | <0.001 |
| Oxygen pulse at max. load, % pred | 180 | 98±20 | 176 | 102±22 | 4.6 (2.5–6.8) | <0.001 |
| Gas exchange | ||||||
| V′E/V′CO2 slope | 180 | 29±6 | 177 | 29±5 | −0.1 (−0.8–0.7) | 0.88 |
| V′E/V′CO2 nadir | 180 | 29±4 | 177 | 29±4 | −0.1 (−0.5–0.4) | 0.77 |
| RER at max. load | 180 | 1.06±0.10 | 177 | 1.07±0.10 | −0.01 (−0.03–0.01) | 0.28 |
| PETCO2 at AT, kPa | 178 | 4.7±0.6 | 173 | 4.8±0.5 | 0.03 (−0.04–0.11) | 0.39 |
| PCO2 at max. load, kPa | 164 | 4.6±0.6 | 142 | 4.8±0.6 | 0.2 (0.1–0.3) | <0.001 |
| Anaerobic threshold | ||||||
| V′O2 at AT, mL·min−1 (V-slope) | 174 | 1339±423 | 170 | 1526±555 | 53 (8–97) | 0.02 |
| V′O2 at AT, % pred V′O2max | 174 | 52±12 | 170 | 58±18 | 2.9 (1.3–4.6) | <0.001 |
| Lactate at max. load, mmol·L−1 | 160 | 8.2±3.7 | 162 | 9.2±3.8 | 0.7 (0.2–1.2) | 0.003 |
Data are presented as n or mean±sd, unless otherwise stated. The results are adjusted for centre, intensive care unit stay, age, sex, body mass index and comorbidity at 3 months. 95% confidence intervals and p-values are found by bootstrapping. The results are given from models with main effects only, as the interaction effects were not significant. V′O2: oxygen uptake; max.: maximum; V′E: expired ventilation; BP: blood pressure; V′CO2: carbon dioxide output; RER: respiratory exchange ratio; PETCO2: end-tidal carbon dioxide pressure; AT: anaerobic threshold; PCO2: partial pressure of carbon dioxide.
Exercise limiting factors
V′O2 peak <80% pred was observed in 40 (23%) patients. The exercise-limiting factors were circulatory limitations in 11 (28%), ventilatory limitations in seven (17%) and other factors in 22 (55%). Among the 22 patients with other limiting factors, three satisfied our definition of dysfunctional breathing, and 19 satisfied the definition of deconditioning.
Ventilatory inefficiency
Ventilatory inefficiency was observed in 30 (17%) patients and was related to ventilatory factors (n=6), circulatory factors (n=10) and dysfunctional breathing (n=13). The cause of ventilatory inefficiency could not be established in one participant. Patients with ventilatory inefficiency had lower mean±sd V′O2 peak % pred (74±19% versus 97±17%, p<0.001), end-tidal carbon dioxide pressure at maximal exercise (4.1±0.4 versus 4.7±0.5 kPa, p<0.001) and lactate (6.9±3.6 versus 9.7±3.7 mmol·L−1, p<0.001) compared to those with normal ventilatory efficiency. Among 27 patients with ventilatory inefficiency, 17 (63%) reported dyspnoea by mMRC. Among 85 patients reporting dyspnoea, 17 (20%) had ventilatory inefficiency.
Changes from 3 to 12 months and determinants of change
Exercise intolerance was observed in 23% at 12 months, compared to 34% at 3 months. V′O2 peak, oxygen pulse, lactate and partial pressure of carbon dioxide, as well as V′O2 at anaerobic threshold (V′O2max) % pred, were significantly higher at 12 months compared to 3 months after hospital discharge (table 1). Estimated mean increases in V′O2 peak % pred and V′O2·kg−1 % pred were 5.0 percentage points (pp) (95% CI 3.1–6.9 pp) and 3.4 pp (95% CI 1.6–5.1 pp), respectively (table 1).
There was little or no evidence of any interactions between time and age, sex, obesity and comorbidity (figure 3c and d, supplementary tables S2 and S3).
FIGURE 3.
Peak oxygen uptake (V′O2 peak) % predicted and oxygen pulse % pred, and expired ventilation (V′E)/carbon dioxide output (V′CO2) slope according to a) dyspnoea, b) intensive care unit (ICU) status, c) obesity and d) comorbidity status at 3 and 12 months.
SpO2 was 98±1% at rest and 95±4% at maximal load at 12 months. Desaturation (defined as SpO2 desaturation >5 pp) was not observed during CPET at 12 months compared to in 34 (23%) patients at 3 months.
Impact of dyspnoea or ICU treatment on cardiopulmonary function
Patients reporting dyspnoea at 3 months were more likely to be female, had a higher BMI and more comorbidity compared to patients without dyspnoea, but there were no differences in pulmonary function or number treated with noninvasive ventilation or mechanical ventilator (supplementary table S1). Patients reporting dyspnoea had lower V′O2 peak and higher V′E/V′CO2 slope at 12 months compared to those with dyspnoea (table 2, figure 3b). However, the changes in CPET variables from 3 to 12 months were the same for patients with and without dyspnoea (table 2, figure 3a).
TABLE 2.
Estimated effect of dyspnoea and intensive care unit (ICU) stay on cardiopulmonary exercise testing variables from linear mixed models (n=210)
| Dyspnoea versus no dyspnoea | ICU versus no ICU | |||
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Performance | ||||
| V′O2 peak, mL·min−1 | −172 (−322– −16) | 0.031 | −283 (−459– −105) | 0.001 |
| V′O2 peak, % pred | −6.6 (−11.9– −1.0) | 0.022 | −10.1 (−15.9– −4.2) | <0.001 |
| V′O2 peak·kg−1, mL·kg−1·min−1 | −2.7 (−4.5– −0.9) | 0.003 | −3.0 (−5.0– −1.0) | 0.004 |
| V′O2 peak·kg−1, % pred | −8.6 (−13.5– −3.4) | 0.001 | −8.2 (−13.8– −2.8) | 0.003 |
| Perceived dyspnoea Borg CR-10 at max. load | 0.4 (−0.1–0.9) | 0.133 | −0.1 (−0.6–0.5) | 0.895 |
| Ventilation | ||||
| V′E at max. load, L·min−1 | −1.8 (−8.2–5.0) | 0.620 | −4.9 (−13.7–4.0) | 0.280 |
| Breathing reserve, % | 1.4 (−4.3–7.4) | 0.634 | 5.3 (−1.2–12.0) | 0.109 |
| Circulation | ||||
| Heart rate at max. load, beats·min−1 | −5.5 (−10.1– −0.7) | 0.020 | −4.4 (−10.5–1.4) | 0.134 |
| Heart rate at max. load, % pred | −3.4 (−6.1– −0.5) | 0.016 | −2.8 (−6.3–0.6) | 0.110 |
| Systolic BP at max. load, mmHg | −12.3 (−21.5– −3.5) | 0.008 | −5.9 (−16.6–4.5) | 0.258 |
| Diastolic BP at max. load, mmHg | −6 (−11.0–−1.3) | 0.014 | 2.4 (−2.5–7.3) | 0.335 |
| Oxygen pulse at max. load, mL·stroke−1 | −0.7 (−1.5–0.2) | 0.150 | −1.4 (−2.4– −0.5) | 0.006 |
| Oxygen pulse at max. load, % of predicted | −4.4 (−9.6–1.2) | 0.127 | −7.6 (−13.1– −2.1) | 0.009 |
| Gas exchange | ||||
| V′E/V′CO2 slope | 2.1 (0.9–3.3) | 0.001 | 0.9 (−0.9–2.9) | 0.340 |
| V′E/V′CO2 nadir | 1.0 (0.1–1.9) | 0.029 | 0.7 (−0.3–1.7) | 0.190 |
| RER at max. load | −0.02 (−0.05–0.01) | 0.143 | 0.01 (−0.02–0.04) | 0.484 |
| PETCO2 at AT, kPa | −0.12 (−0.26–0.02) | 0.095 | 0.08 (−0.06–0.22) | 0.255 |
| PCO2 at max. load, kPa | −0.1 (−0.3–0.1) | 0.392 | 0.0 (−0.2–0.2) | 0.900 |
| Anaerobic threshold | ||||
| V′O2 at AT, mL·min−1 (V-slope) | −40 (−136–66) | 0.513 | −34 (−134–68) | 0.502 |
| V′O2 at AT, % pred V′O2max | −1.8 (−5.0–1.9) | 0.380 | −1.4 (−4.7–1.9) | 0.389 |
| Lactate at max. load, mmol·L−1 | −0.5 (−1.5–0.5) | 0.286 | −0.1 (−1.4–1.2) | 0.842 |
The results are given from models with main effects only, as the interaction effects between dyspnoea or ICU stay and time were not significant (p-values ranged from 0.077 to 0.970 for dyspnoea and from 0.062 to 0.997 for ICU). The results are adjusted for centre and for age, sex, body mass index and comorbidity at 3 months. 95% confidence intervals and p-values are found by bootstrapping. V′O2: oxygen uptake; max.: maximum; V′E: expired ventilation; BP: blood pressure; V′CO2: carbon dioxide output; RER: respiratory exchange ratio; PETCO2: end-tidal carbon dioxide pressure; AT: anaerobic threshold; PCO2: partial pressure of carbon dioxide.
Patients admitted to an ICU at the index hospitalisation had lower V′O2 peak and oxygen pulse compared to patients not treated in an ICU (table 2, figure 3b). However, the changes in CPET variables from 3 to 12 months were the same for patients with and without ICU treatment (table 2, figure 3b).
Comparison between COVID-19 patients and matched control group
At 12 months, the COVID-19 patients had lower V′O2 peak and V′O2 peak·kg−1 than matched controls (table 3). Maximal heart rate, breathing frequency and V′E were lower in the COVID-19 patients compared to the matched controls (table 3).
TABLE 3.
Cardiopulmonary exercise testing variables compared between controls and coronavirus disease 2019 patients at 12 months’ follow-up
| Control | Patient | Patient versus control, estimate (95% CI) | p-value | |
| Subjects | 207¶ | 177+ | 380§ | |
| Performance | ||||
| V′O2 peak, mL·min−1 | 2952±944 | 2451±776 | −529 (−638– −421) | <0.001 |
| V′O2 peak·kg−1, mL·kg−1·min−1 | 34.9±10.3 | 28.6±8.4 | −6.4 (−7.6– −5.2) | <0.001 |
| Perceived dyspnoea Borg CR-10 at max. load# | 8.9±1.8 | 8.5±2.0 | −0.4 (−0.8–0.0) | 0.040 |
| Ventilation | ||||
| V′E at max. load, L·min−1 | 102.7±31.0 | 87.5±29.3 | −16.9 (−21.0– −12.8) | <0.001 |
| Breathing frequency at max. load, breaths·min−1 | 43.5±7.5 | 39.0±7.8 | −4.6 (−6.1– −3.1) | <0.001 |
| Circulation | ||||
| Heart rate at max. load, beats·min−1 | 172.0±17.0 | 155.3±21.9 | −16.7 (−19.8– −13.5) | <0.001 |
| Gas exchange | ||||
| RER at max. load | 1.10±0.06 | 1.07±0.10 | −0.03 (−0.05– −0.02) | <0.001 |
Data are presented as n or mean±sd, unless otherwise stated. Results from multiple linear regression, adjusted for age, sex, body mass index, systolic blood pressure, COPD, diabetes, myocardial infarction, and congestive heart failure. V′O2 peak: peak oxygen uptake; max.: maximum; V′E: expired ventilation; RER: respiratory exchange ratio. #: the Borg score from controls (HUNT4 HOPE) was a scale graded 6–20, which was converted to the Borg CR-10 scale used in the present study (https://borgperception.se/); ¶: perceived dyspnoea Borg CR-10 at max. load n=203, heart rate at max. load n=205; +: perceived dyspnoea Borg CR-10 at max. load n=175; §: perceived dyspnoea Borg CR-10 at max. load n=374, heart rate at max. load n=378.
Mean RER at maximal load was 1.10 for the controls and 1.07 for the patients, which was a significant difference in the adjusted analysis (supplementary table S4). However, there was only little evidence of differences in CPET variables between controls and patients, when RER in patients was dichotomised to ≥1.10 or <1.10 (supplementary table S4).
Discussion
The main findings in this study were that the majority of COVID-19 patients had normal exercise capacity at 12 months; exercise intolerance was reduced; and V′O2 peak and oxygen pulse improved from 3 to 12 months after hospitalisation. The frequency of ventilatory limitation was low at 12 months. Patients with dyspnoea or ICU treatment had lower values of V′O2 peak at 12 months, but similar improvement from 3 to 12 months, compared to patients without dyspnoea or ICU treatment. The study patients had lower V′O2 peak at 12 months compared to matched controls.
Exercise capacity and limitations
Exercise capacity improved from 3 to 12 months after hospitalisation, and the increase in V′O2 peak was considered sufficient to have a positive impact on activities of daily living. At 12 months, the majority had regained normal exercise capacity and the prevalence of exercise intolerance was reduced to every fourth patient.
Circulatory limitations were more frequent than ventilatory limitations in patients with exercise intolerance. Mean values of pulmonary function tests were within normal limits at 12 months, few had abnormal values. Except for TLC, there were no correlations between V′O2 peak and pulmonary function tests, which support that exercise capacity for most patients is limited by factors other than the lungs.
The majority of patients with exercise intolerance were limited by other than circulatory and ventilatory factors. This group included patients with deconditioning and dysfunctional breathing, but other virus-induced limitations may also have been present. Our study was limited to noninvasive methods; thus, we cannot explain all aspects of the mechanisms interfering with exercise capacity. However, deconditioning due to inactivity seems to be the most prevalent exercise limitation. Naeije and Caravita [22] grouped together 581 COVID-19 patients from 11 studies and found a CPET profile of deconditioning in the recovery phase of an acute inflammatory process.
As stated by the Fick equation (V′O2 peak = cardiac output × arteriovenous oxygen difference), a low V′O2 peak may be related to either reduced cardiac output or reduced peripheral oxygen extraction. Both these mechanisms may apply in patients with deconditioning [23, 24]. Furthermore, reduced peripheral oxygen extraction has been shown in COVID-19 patients with small-fibre neuropathy, complicating evaluation of exercise limitation even more [25, 26].
Dysfunctional breathing with large disharmonic variations in tidal volume and respiratory frequency, accompanied by hypocapnia and respiratory acidosis was limiting exercise capacity in a few patients. Similar dysfunctional breathing patterns have also been observed in other studies [27, 28].
Dyspnoea
Dyspnoea was reported by half of the patients, consistent with findings in other studies [29]. Among patients with dyspnoea, there were more females, more obesity and more comorbidity compared to patients without dyspnoea. Patients with dyspnoea had lower V′O2 peak·kg−1 % pred compared to those without dyspnoea. However, in the patients reporting dyspnoea, few had circulatory or ventilatory limitations. This is similar to observations in a CPET study of COVID-19 patients with prominent dyspnoea, where only mild physiological abnormalities were found [30].
Patients with dyspnoea had reduced ventilatory efficiency, with dysfunctional breathing as the most frequent cause. Although ventilatory inefficiency and hyperventilation may account for some of the reported dyspnoea in our study, only one-fifth of the patients with dyspnoea showed ventilatory inefficiency. Perceived dyspnoea is often multifactorial [31], complicating the interpretation of this symptom. Given the magnitude of the COVID-19 pandemic, it will be essential to differentiate symptoms caused by COVID-19 from dyspnoea due to other aetiologies.
ICU treatment
Patients treated in an ICU had the same improvement in V′O2 peak and oxygen pulse from 3 to 12 months compared to patients without ICU treatment. However, they still had lower V′O2 peak despite more frequent rehabilitation.
Patients and matched controls
Even though the patients in our study improved their exercise capacity from 3 to 12 months, it was still not normalised compared to the matched controls. Maximal heart rate and ventilation were lower among the COVID-19 patients compared to matched controls, indicating slightly submaximal performance. This could have influenced the comparison between patients and matched controls, but subgroup analyses show that patients with RER ≥1.1 or <1.1 both have lower V′O2 peak compared to the matched controls.
Limitations
As all study patients were hospitalised in the first phase of the pandemic, when vaccines were not available, our results may not apply to a vaccinated population. The study was performed in hospitalised patients during acute COVID-19 infection and the results may not apply to the subjects with long COVID who were not hospitalised.
Unlike the COVID-19 patients, the controls have not been hospitalised. However, the only purpose of the controls is to account for pre-existing comorbidity when evaluating if the patients have recovered their expected exercise capacity. Timely change in exercise capacity cannot be compared, as the controls only had one assessment.
CPET was performed using different equipment and protocol in the COVID-19 population and the matched HUNT control group. There have been reports of higher V′O2 peak in the HUNT fitness population compared to other population cohorts and difference between patients and other controls might have been smaller [17, 19].
CPET was performed on a treadmill, which gives 5–10% higher V′O2 peak compared to a cycle ergometer. Cardiac output was not measured during exercise, and muscle biopsies were not performed; thus, evaluation of deconditioning is hampered with some uncertainty.
The study's strength is the inclusion of most patients hospitalised for COVID-19 in the study's catchment areas in Norway at the beginning of the pandemic, representing an unselected, and thus representative, hospital population.
Conclusions
Exercise capacity was normal in 77% of the patients 1 year after hospital discharge for COVID-19. In patients with exercise intolerance, circulatory limitation to exercise was more common than ventilatory limitation. Deconditioning seemed to be the most prevalent exercise limitation, but other unknown mechanisms may have contributed to exercise intolerance. V′O2 peak and oxygen pulse improved significantly from 3 to 12 months, but V′O2 peak was lower compared to matched controls. Even though patients with dyspnoea or ICU treatment had lower V′O2 peak at 1 year, they still had similar improvement from 3 months, compared to patients without dyspnoea or ICU treatment.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00745-2022.Supplement (181.9KB, pdf)
Shareable PDF
Acknowledgements
The Trøndelag Health Study (HUNT) is a collaboration between HUNT Research Centre (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology NTNU), Trøndelag County Council, Central Norway Regional Health Authority and the Norwegian Institute of Public Health.
Footnotes
Conflict of interest: C.B. Ingul has received lecture fees from Bayer AS, unrelated to the current study. I. Skjørten has provided lectures for doctors’ education paid by Norwegian Directorate of Health and Norwegian Medical Association. G. Einvik has received research grants from AstraZeneca to perform the current study. A. Edvardsen has received payment or honoraria for lectures, presentations or educational events from GlaxoSmithKline and Chiesi. K. Stavem has received consulting fees from UCB Pharma and MSD, unrelated to the present study. All other authors have nothing to disclose.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01739-2022
Support statement: This work was supported by the National Association for Heart and Lung Diseases, Akershus University Hospital and Norwegian Health Association. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control . SARS-CoV-2 Variants of Concern as of 9 June 2022. www.ecdc.europa.eu/en/covid-19/variants-concern Date last accessed: 29 June 2022.
- 3.Carfi A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clavario P, De Marzo V, Lotti R, et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int J Cardiol 2021; 340: 113–118. doi: 10.1016/j.ijcard.2021.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skjørten I, Ankerstjerne OAW, Trebinjac D, et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J 2021; 58: 2100996. doi: 10.1183/13993003.00996-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jahn K, Sava M, Sommer G, et al. Exercise capacity impairment after COVID-19 pneumonia is mainly caused by deconditioning. Eur Respir J 2022; 59: 2101136. doi: 10.1183/13993003.01136-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassar MP, Tunnicliffe EM, Petousi N, et al. Symptom persistence despite improvement in cardiopulmonary health – insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine 2021; 41: 101159. doi: 10.1016/j.eclinm.2021.101159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J 2021; 57: 2003448. doi: 10.1183/13993003.03448-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20: e192–e197. doi: 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher CM, Elmes PC, Fairbairn AS, et al. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959; 2: 257–266. doi: 10.1136/bmj.2.5147.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017; 50; 1700010. doi: 10.1183/13993003.00010-2017 [DOI] [PubMed] [Google Scholar]
- 13.Hall GL, Filipow N, Ruppel G, et al. Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J 2021; 57: 2000289. doi: 10.1183/13993003.00289-2020 [DOI] [PubMed] [Google Scholar]
- 14.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381. [PubMed] [Google Scholar]
- 15.Radtke T, Crook S, Kaltsakas G, et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev 2019; 28: 180101. doi: 10.1183/16000617.0101-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sietsema KE, Sue DY, Stringer WW, et al. Wasserman & Whipp's Principles of Exercise Testing and Interpretation. 6th Edn. Dordrecht, Wolters Kluwer, 2021. [Google Scholar]
- 17.Edvardsen E, Hansen BH, Holme IM, et al. Reference values for cardiorespiratory response and fitness on the treadmill in a 20- to 85-year-old population. Chest 2013; 144: 241–248. doi: 10.1378/chest.12-1458 [DOI] [PubMed] [Google Scholar]
- 18.Sun XG, Hansen JE, Garatachea N, et al. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med 2002; 166: 1443–1448. doi: 10.1164/rccm.2202033 [DOI] [PubMed] [Google Scholar]
- 19.Letnes JM, Dalen H, Aspenes ST, et al. Age-related change in peak oxygen uptake and change of cardiovascular risk factors. The HUNT Study. Prog Cardiovasc Dis 2020; 63: 730–737. doi: 10.1016/j.pcad.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 20.Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Software 2015; 67: 1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 21.R Core Team . R: a Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing, 2018. www.R-project.org/ [Google Scholar]
- 22.Naeije R, Caravita S. Phenotyping long COVID. Eur Respir J 2021; 58: 2101763. doi: 10.1183/13993003.01763-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrick-Ranson G, Hastings JL, Bhella PS, et al. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol 2014; 116: 736–745. doi: 10.1152/japplphysiol.00342.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh I, Joseph P, Heerdt PM, et al. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest 2022; 161: 54–63. doi: 10.1016/j.chest.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balbi P, Saltalamacchia A, Lullo F, et al. Peripheral neuropathy in patients recovering from severe COVID-19: a case series. Medicina 2022; 58: 523. doi: 10.3390/medicina58040523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shouman K, Vanichkachorn G, Cheshire WP, et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res 2021; 31: 385–394. doi: 10.1007/s10286-021-00803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motiejunaite J, Balagny P, Arnoult F, et al. Hyperventilation: a possible explanation for long-lasting exercise intolerance in mild COVID-19 survivors? Front Physiol 2021; 11: 614590. doi: 10.3389/fphys.2020.614590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taverne J, Salvator H, Leboulch C, et al. High incidence of hyperventilation syndrome after COVID-19. J Thorac Dis 2021; 13: 3918–3922. doi: 10.21037/jtd-20-2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eberst G, Claudé F, Laurent L, et al. Result of one-year, prospective follow-up of intensive care unit survivors after SARS-CoV-2 pneumonia. Ann Intensive Care 2022; 12: 23. doi: 10.1186/s13613-022-00997-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alba GA, Ziehr DR, Rouvina JN, et al. Exercise performance in patients with post-acute sequelae of SARS-CoV-2 infection compared to patients with unexplained dyspnea. EClinicalMedicine 2021; 39: 101066. doi: 10.1016/j.eclinm.2021.101066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012; 185: 435–452. doi: 10.1164/rccm.201111-2042ST [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00745-2022.Supplement (181.9KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00745-2022.Shareable (340.4KB, pdf)