Abstract
The combined role of inflammatory markers [including neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), monocyte/lymphocyte ratio (MLR), and systemic immune-inflammation index (SII)] and PET/CT metabolic parameters [including maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), metabolic tumor volume (MTV), and TLG (total lesion glycolysis)] at baseline in evaluating the binary stage [extensive-stage disease (ED) and limited-stage disease (LD)] of small cell lung cancer (SCLC) is unclear. In this study, we verified that high metabolic parameters and inflammatory markers were related to the binary stage of SCLC patients, respectively (p < 0.05). High inflammatory markers were also associated with high MTV and TLG in patients with SCLC (p < 0.005). Moreover, the incidences of co-high metabolic parameters and inflammatory markers were higher in ED-SCLC (p < 0.05) than those in LD-SCLC. Univariate logistic regression analysis demonstrated that Co-high MTV/NLR, Co-high MTV/MLR, Co-high MTV/SII, Co-high TLG/NLR, Co-high TLG/MLR, and Co-high TLG/SII were significantly related to the binary stage of SCLC patients (p = 0.00). However, only Co-high MTV/MLR was identified as an independent predictor for ED-SCLC (odds ratio: 8.67, 95% confidence interval CI: 3.51–21.42, p = 0.000). Our results suggest that co-high metabolic parameters and inflammatory markers could be of help for predicting ED-SCLC at baseline. Together, these preliminary findings may provide new ideas for more accurate staging of SCLC.
Keywords: SCLC, inflammatory markers, metabolic parameters, PET/CT, MTV/MLR
Introduction
Lung cancer is one of the main causes of cancer-related death in the world (1, 2). According to pathology, lung cancer is mainly divided into adenocarcinoma, squamous cell carcinoma, small cell lung cancer (SCLC), and so on. Among them, SCLC accounts for about 15%, with the characteristics of early metastasis, easy recurrence, and low 5-year survival rate (as low as 5%–10%) (2). According to a binary stage method in most of the articles, SCLC is classified into limited-stage disease (LD-SCLC) confined to the ipsilateral hemithorax and extensive-stage disease (ED-SCLC) spread beyond the ipsilateral hemithorax, the former including contralateral mediastinal and ipsilateral supraclavicular lymph nodal metastases and the latter including hematogenous metastases and malignant pleural or pericardial effusion (3). The management of different stages are completely different in SCLC patients (4). Chemoradiotherapy is the standard treatment of LD-SCLC patients. While a proposed treatment program for ED-SCLC is systemic chemotherapy, which could offer rapid responses and the best palliation. The median survival times of LD-SCLC and ED-SCLC are only 15–20 months and 8–13 months (4), respectively. Therefore, correct staging is pivotal regarding the selection of appropriate and effective treatment strategies for individual patients with SCLC.
18F-fluorodeoxyglucose (18F-FDG) positron-emission tomography (PET)/computed tomography (CT), providing both functional and morphological data, is a systemic non-invasive imaging technique and used in tumor staging, treatment responses and recurrence diagnosis (5, 6). Maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) are used as semi-quantitative parameters of PET/CT, which reflect the local metabolism and the biological aggressiveness of tumors (7). However, the use of PET/CT is still controversial since some previous studies showed that false-positive results affected stage for SCLC patients by using PET/CT (5). Hence, the metabolic parameters via PET/CT are not sufficient to evaluate the binary stage of SCLC. The FDG uptake in lesions is affected by many different factors including infection and inflammation (8). The SCLC patients with normal blood counts are advised to conduct a bone marrow biopsy in order to exclude bone marrow involvement (4). Recent studies have confirmed that inflammation plays significant roles in tumor microenvironment, where it influences tumor development, progression, and treatment response (9). Meanwhile, a growing body of evidence indicated that increased levels of serum inflammatory markers such as neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), monocyte/lymphocyte ratio (MLR), and systemic immune-inflammation index (SII) correlated with the stage of malignancies [e.g., non-small cell lung cancer (NSCLC) (10), renal cell carcinoma (11), and colon cancer (12)]. Drawing on the above discoveries, the combined evaluation of metabolic parameters and inflammatory markers may be highly effective in detecting binary stage for SCLC at baseline.
However, to our knowledge, there are rare studies on the correlation between PET/CT semi-quantitative parameters and inflammatory markers in detecting the binary stage of SCLC. Therefore, the purpose of this study was to evaluate the relationship between inflammatory markers (NLR, PLR, MLR, and SII) in peripheral blood and semi-quantitative parameters (SUVmax, SUVmean, MTV, and TLG) via PET/CT and their combined role on detecting ED-SCLC.
Materials and methods
Subjects
All patients with SCLC underwent PET/CT scanning between January 2016 and June 2019 at the First Affiliated Hospital of Nanjing Medical University. Clinical data such as gender, age, smoking history, and hematological parameters [e.g., neutrophil (N), monocyte (M), lymphocyte (L), and platelet (P) counts] closest to the day of PET/CT scanning were collected. The Department of Clinical Laboratory of the First Affiliated Hospital of Nanjing Medical University performed the data analyses. Inflammatory markers based on N, M, L, and P—NLR, MLR, PLR, and SII—were calculated using the formula N/L, M/L, P/L, and P×N/L, respectively. The inclusion criteria were as follows (1): diagnosed with SCLC by surgical or biopsy specimens (2); did not undergo any treatment before PET/CT scanning and inflammatory marker measurement (3); PET/CT scanning performed within 1 week after inflammatory marker measurement (4); diagnosis of pleural effusion, pericardial effusion, lymph node, and distant organ metastasis by pathological examination and imaging examination such as contrast enhanced CT (CECT), PET/CT, and magnetic resonance imaging (MRI); and (5) without other tumors and without other diseases that alter hematological parameters. The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
18F-FDG PET/CT scanning
18F-FDG PET/CT (Biograph 16HR; Siemens, Germany) examinations were acquired after fasting for 6 h and 60–75 min after intravenous injection of 18F-FDG (3.70–5.55 MBq/kg weight). The blood glucose level was below 120 mg/dl in all included patients before tracer injection. All patients had normal tidal breathing during PET and CT scans. Patients underwent low-dose CT scans (120–140 kV, 65 mA and 5.0 mm slice), followed by PET scans with six to eight bed positions (2 min per bed positions) per patient based on the height. The PET images were reconstructed with attenuation corrected CT using the ordered subset expectation maximization (OSEM) algorithm. Then, all dates were transferred in DICOM format to the Beth Israel PET/CT viewer plugin for FIJI and displayed as axial, coronal, and sagittal images. The SUVmax, SUVmean, MTV, and TLG of all lesions were delineated semiautomatically by the Beth Israel PET/CT viewer plugin for FIJI (http://sourceforge.net/projects/bifijiplugins/) (ImageJ distribution) (13).
Statistical analysis
Data were analyzed using SPSS 25.0 software (SPSS, IL, USA). Data in accordance with normal distribution were expressed as the mean ± standard deviation (SD) values, while non-normal distribution data were expressed as median (inter-quartile interval). Statistical differences between groups were assessed by t-test or Mann–Whitney U test. t-test was performed for data in accordance with normal distribution, while non-normal distribution data were analyzed by Mann–Whitney U test. A Chi-square test was performed for rate comparisons. Receiver operating characteristic (ROC) curve analysis was performed to find optimal cutoff values of NLR, MLR, SII, MTV, and TLG to predict ED-SCLC. The area under curve (AUC) was calculated as a measure of the accuracy of the test. Logistic regression analysis was used to assay the association of patients’ clinical features, inflammatory markers, and metabolic parameters in detecting ED-SCLC. p-value < 0.05 was considered to be statistically significant.
Results
Patients’ characteristics
A total of 119 patients met the inclusion criteria and were enrolled in our study. Of these 119 patients, the median age was 64 (range: 25–91) years; 105 (64.6%) were male; 14 were female; 92 had a history of smoking; 47 SCLC patients were diagnosed with ED-SCLC, which spread beyond the ipsilateral hemithorax ( Table 1 ). Median SUVmax, SUVmean, MTV, and TLG values for all lesions and median NLR, PLR, MLR, and SII values of the patients are shown in Table 1 .
Table 1.
Patient characteristics.
| Number (n = 119) | Value | |
|---|---|---|
| Gender | ||
| Male | 105 (88.2%) | |
| Female | 14 (11.8%) | |
| Age | ||
| ≤64 | 59 (49.6%) | |
| >64 | 60 (50.4%) | |
| Smoking | ||
| Yes | 92 (77.3%) | |
| No | 27 (22.7%) | |
| Tumor Stage | ||
| LD-SCLC | 72 (60.5%) | |
| ED-SCLC | 47 (39.5%) | |
| Inflammatory markers | ||
| NLR | 2.63 (0.30, 17.86) | |
| PLR | 132.49 (48.70, 561.19) | |
| MLR | 0.28 (0.04, 2.49) | |
| SII | 541.01 (47.88, 2,410.20) | |
| Metabolic parameters | ||
| SUVmax | 12.78 (5.39, 47.34) | |
| SUVmean | 6.87 (3.17, 21.49) | |
| MTV | 65.58 (2.95, 1,208.91) | |
| TLG | 468.25 (19.77, 6,965.86) | |
Correlation of inflammatory markers with clinical features and binary stage of SCLC
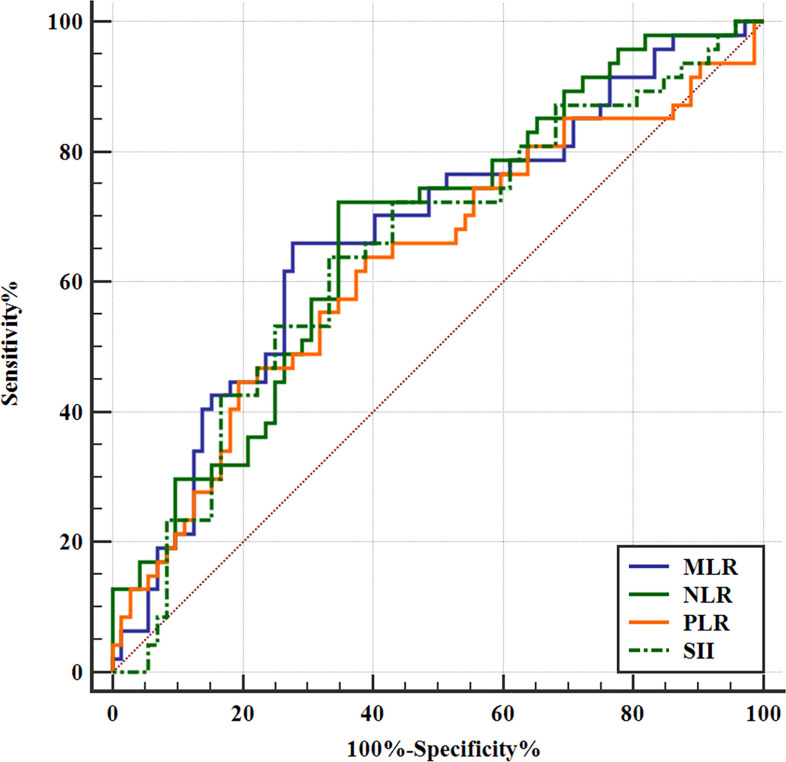
In patients with SCLC, NLR, PLR, MLR, and SII were significantly higher in ED-SCLC than LD-SCLC (p > 0.05, Table 2 ). Furthermore, we found that MLR is higher in patients older than 64 years. NLR, MLR, and SII are higher in male patients than female patients. NLR and SII are higher in patients with smoking than those without smoking ( Table 2 ). With a cutoff value of 2.64, 170.67, 0.31, and 583.1, high NLR, PLR, MLR, and SII could respectively predict ED-SCLC (p < 0.05, Figure 1 ).
Table 2.
Analysis of inflammatory markers in patients with SCLC (n = 119).
| NLR | p | PLR | p | MLR | p | SII | p | |
|---|---|---|---|---|---|---|---|---|
| Age | 0.992 | 0.103 | 0.019 | 0.116 | ||||
| ≤64 | 2.48 (1.00, 8.69) | 143.59 (54.40, 390.00) | 0.26 (0.04, 2.06) | 580.32 (136.54, 2410.20) | ||||
| >64 | 2.87 (0.30, 17.86) | 117.91 (48.70, 561.19) | 0.35 (0.08, 2.49) | 496.51 (47.88, 2201.47) | ||||
| Gender | 0.005 | 0.062 | 0.007 | 0.007 | ||||
| Male | 2.86 (2.01, 3.61) | 132.71 (105.73, 168.22) | 0.28 (0.22, 0.42) | 565.83 (403.96, 802.21) | ||||
| Female | 1.72 (0.66, 14.68) | 99.35 (48.70, 441.18) | 0.21 (0.04, 0.47) | 316.10 (123.22, 2201.47) | ||||
| Smoking | 0.036 | 0.125 | 0.079 | 0.025 | ||||
| Yes | 2.78 (0.30, 16.40) | 138.88 (54.40, 561.19) | 0.29 (0.08, 2.49) | 595.21 (47.88, 2410.20) | ||||
| No | 1.96 (0.66,17.86) | 113.66 (48.70,366.97) | 0.25 (0.04, 1.86) | 425.75 (123.22, 1988.99) | ||||
| Tumor Stage | 0.001 | 0.019 | 0.002 | 0.007 | ||||
| LD-SCLC | 2.23 (0.30, 6.87) | 123.11 (48.70, 390.00) | 0.26 (0.04, 2.06) | 460.84 (47.88, 2410.20) | ||||
| ED-SCLC | 3.17 (1.00, 17.86) | 149.33 (54.40, 561.19) | 0.37 (0.08, 2.49) | 737.47 (136.54, 2201.47) |
Figure 1.
Receiver operating characteristic (ROC) curves of inflammatory markers for predicting binary stage of SCLC. NLR, PLR, MLR, and SII could predict the binary stage of SCLC. The ROC curve analysis of the NLR to predict ED-SCLC. With an NLR of 2.64 as the threshold, the sensitivity and specificity in the prediction of ED-SCLC were 72.34% and 65.28%, respectively. The AUC was 0.672 (95% confidence interval [CI]: 0.580–0.756; p = 0.0006). The ROC curve analysis of the PLR to predict ED-SCLC. With an PLR of 170.67 as the threshold, the sensitivity and specificity for the prediction of ED-SCLC were 44.68% and 80.56%, respectively. The AUC was 0.628 (95% CI: 0.535–0.715; p = 0.0178). The ROC curve analysis of the MLR to predict ED-SCLC. With an MLR of 0.31 as the threshold, the sensitivity and specificity for the prediction of ED-SCLC were 65.96% and 70.83%, respectively. The AUC was 0.669 (95% CI: 0.577–0.753; p = 0.0010). The ROC curve analysis of the SII to predict ED-SCLC. With an SII of 583.1 as the threshold, the sensitivity and specificity for the prediction of ED-SCLC were 63.83% and 66.67%, respectively. The AUC was 0.646 (95% CI: 0.553–0.731; p = 0.0055).
Correlation of metabolic parameters of SCLC with clinical features and binary stage of SCLC
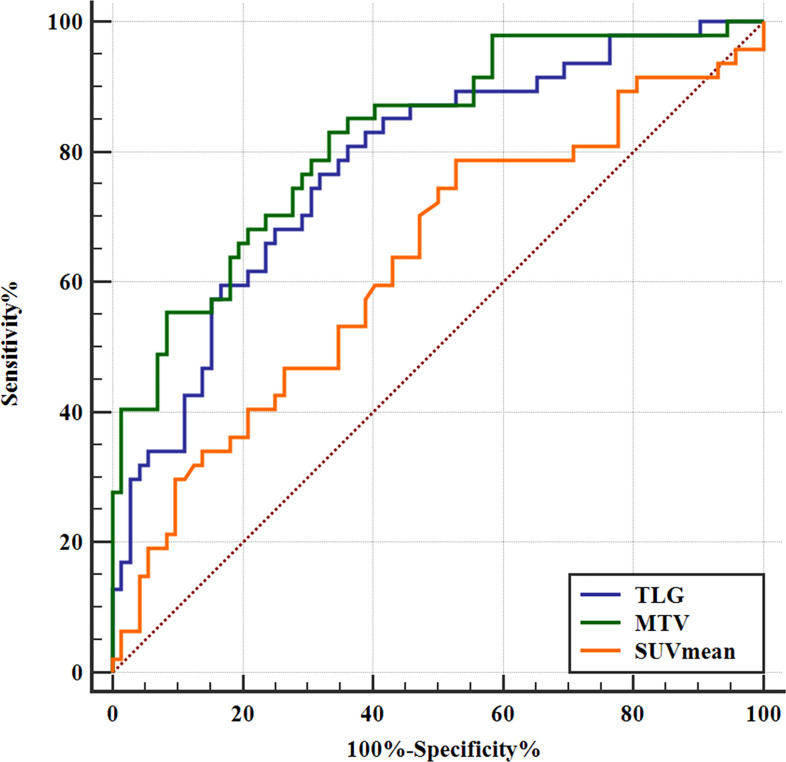
In patients with SCLC, SUVmean, MTV, and TLG were higher in ED-SCLC compared to those in LD-SCLC, respectively (p > 0.05), whereas SUVmax in patients with ED and those with LD were not significantly different ( Table 3 ). With a cutoff value of 7.69, 61.36, and 405.85, high MTV and TLG could separately predict ED-SCLC (p < 0.05, Figure 2 ), while ROC analysis revealed that SUVmax could not predict ED-SCLC (p = 0.123 and 0.087). There were no significant differences in SUVmax, SUVmean, MTV, and TLG observed in SCLC patients with different age and smoking ( Table 3 ).
Table 3.
Analysis of metabolic parameters of SCLC on PET/CT scanning (n = 119).
| SUVmax | p | SUVmean | p | MTV (cm3) | p | TLG (g) | p | |
| Age | 0.987 | 0.782 | 0.564 | 0.644 | ||||
| ≤64 | 12.83 (5.39, 47.34) | 6.84 (3.33, 21.49) | 71.28 (4.29, 1,208.91) | 476.35 (28.85, 5,530.39) | ||||
| >64 | 12.78 (5.85, 25.53) | 7.02 (3.17,20.88) | 64.34 (2.95, 1,091.43) | 461.58 (19.77, 6,965.86) | ||||
| Gender | 0.817 | 0.332 | 0.040 | 0.096 | ||||
| Male | 12.78 (5.39, 47.34) | 6.86 (3.17, 21.49) | 71.28 (2.95, 1,208.91) | 482.41 (19.77, 6,965.86) | ||||
| Female | 12.88 (7.06, 21.20) | 7.42 (3.90,13.28) | 31.92 (7.24, 120.97) | 237.66 (34.54, 1,606.65) | ||||
| Smoking | 0.172 | 0.133 | 0.211 | 0.198 | ||||
| Yes | 13.02 (5.39, 47.34) | 7.07 (3.17, 21.49) | 72.13 (2.95, 1,208.91) | 487.74 (19.77, 6,965.86) | ||||
| No | 11.91 (6.12, 25.53) | 6.24 (3.49, 11.94) | 41.58 (7.24, 1,091.43) | 317.70 (34.54, 5,556.47) | ||||
| Tumor Stage | 0.788 | 0.018 | 0.000 | 0.000 | ||||
| LD-SCLC | 12.78 (5.85, 26.12) | 7.42 (3.32, 14.88) | 38.93 (2.95, 299.21) | 290.37 (19.77, 4,267.74) | ||||
| ED-SCLC | 12.83 (5.39, 47.34) | 6.43 (3.17, 36.25) | 161.81 (7.58, 1208.91) | 1126.64 (43.66, 6,965.86) |
Figure 2.
Receiver operating characteristic (ROC) curves of SUVmean, MTV, and TLG for predicting binary stage of SCLC. The SUVmean, MTV, and TLG could predict tumor stage. The ROC curve analysis of the SUVmean to predict ED-SCLC. With an SUVmean of 7.69 as the threshold, the sensitivity and specificity in the prediction of ED-SCLC were 78.72% and 47.22%, respectively. The AUC was 0.628 (95% CI: 0.535–0.715; p = 0.0166). The ROC curve analysis of the MTV to predict ED-SCLC. With an MTV of 61.36 as the threshold, the sensitivity and specificity in the prediction of ED-SCLC were 82.98% and 66.67%, respectively. The AUC was 0.823 (95% CI: 0.742–0.887; p < 0.0001). The ROC curve analysis of the TLG to predict ED-SCLC. With a TLG of 405.85 as the threshold, the sensitivity and specificity for the prediction of ED-SCLC were 80.85% and 63.89%, respectively. The AUC was 0.779 (95% CI: 0.694–0.850; p < 0.0001).
Correlation of high metabolic parameters of SCLC with increased inflammatory markers
All SCLC patients were divided into low-MTV (or low-TLG) and high-MTV (or high-TLG) groups by cutoff values of 61.36 (or 405.85) (the cutoff value predicting ED-SCLC). The results showed that the NLR, PLR, MLR, and SII were higher in the high-MTV or high-TLG patients than in the low-MTV or low-TLG patients, respectively (p < 0.05, Table 4 ).
Table 4.
Correlation of inflammatory markers with different MTV or TLG levels of SCLC.
| MTV (cm3) | p | TLG (g) | p | |||
|---|---|---|---|---|---|---|
| ≤61.36 (56) | >61.36 (63) | ≤405.85 (55) | >405.85 (64) | |||
| NLR | 2.13 (0.30, 6.31) | 3.13 (1.00, 17.86) | 0.000 | 2.14 (0.30, 8.69) | 3.12 (1.00, 17.86) | 0.001 |
| PLR | 118.22 (48.70, 384.62) | 164.71 (154.40, 561.19) | 0.001 | 120.30 (48.70, 384.62) | 164.30 (54.40, 561.19) | 0.001 |
| MLR | 0.25 (0.08, 0.81) | 0.34 (0.04, 2.49) | 0.008 | 0.26 (0.08, 0.81) | 0.34 (0.04, 2.49) | 0.019 |
| SII | 438.80 (47.88, 2233.15) | 750.17 (136.54, 2410.20) | 0.000 | 443.40 (47.88, 2233.15) | 743.82 (136.54, 2410.20) | 0.000 |
Correlation of co-high metabolic parameters/inflammatory markers with binary stage of SCLC
Patients with SCLC were grouped into co-low MTV/NLR or co-low MTV/MLR or co-low MTV/SII, low MTV/high NLR or low MTV/high MLR or low MTV/high SII, high MTV/low NLR or high SUVmax/low MLR, and co-high MTV/NLR or co-high MTV/MLR groups, respectively, based on the corresponding cutoff values (the cutoff value predicting ED-SCLC). TLG was the same as above. The results showed that the incidences of Co-high MTV/NLR, Co-high MTV/PLR, Co-high MTV/MLR, Co-high MTV/SII, Co-high TLG/NLR, Co-high TLG/PLR, Co-high TLG/MLR, and Co-high TLG/SII were higher in ED-SCLC patients than those in LD-SCLC, respectively (p = 0.000, Table 5 ). The incidences of Co-high MTV/NLR, Co-high MTV/MLR, Co-high MTV/SII, Co-high TLG/NLR, Co-high TLG/MLR, and Co-high TLG/SII were higher in male patients than those in female patients, respectively ( Table 5 ). The incidences of Co-high MTV/MLR and Co-high TLG/MLR were higher in patients older than 64 years. However, the MTV and TLG of all lesions and NLR, PLR, MLR, or SII status did not exhibit a significant relationship with smoking.
Table 5.
Relationship of metabolic parameters and inflammatory markers with binary stage of SCLC.
| Tumor Stage | Gender | |||||
|---|---|---|---|---|---|---|
| LD-SCLC | ED-SCLC | p | Female | Male | p | |
| Co-low MTV/NLR | 37 | 2 | 0.000 | 9 | 30 | 0.013 |
| Low MTV/High NLR | 11 | 6 | 0 | 17 | ||
| High MTV/Low NLR | 10 | 11 | 3 | 18 | ||
| Co-high MTV/NLR | 14 | 28 | 2 | 40 | ||
| Co-low MTV/PLR | 44 | 6 | 0.000 | 9 | 41 | 0.207 |
| Low MTV/High PLR | 4 | 2 | 0 | 6 | ||
| High MTV/Low PLR | 14 | 20 | 2 | 32 | ||
| Co-high MTV/PLR | 10 | 19 | 3 | 26 | ||
| Co-low MTV/MLR | 36 | 3 | 0.000 | 9 | 30 | 0.007 |
| Low MTV/High MLR | 12 | 5 | 0 | 17 | ||
| High MTV/Low MLR | 15 | 13 | 4 | 24 | ||
| Co-high MTV/MLR | 9 | 26 | 1 | 34 | ||
| Co-low MTV/SII | 39 | 4 | 0.000 | 9 | 34 | 0.035 |
| Low MTV/High SII | 9 | 4 | 0 | 13 | ||
| High MTV/Low SII | 9 | 13 | 3 | 19 | ||
| Co-high MTV/SII | 15 | 26 | 2 | 39 | ||
| Co-low TLG/NLR | 36 | 2 | 0.000 | 9 | 29 | 0.012 |
| Low TLG/High NLR | 10 | 7 | 0 | 17 | ||
| High TLG/Low NLR | 11 | 11 | 3 | 19 | ||
| Co-high TLG/NLR | 15 | 27 | 2 | 40 | ||
| Co-low TLG/PLR | 43 | 6 | 0.000 | 9 | 40 | 0.183 |
| Low TLG/High PLR | 3 | 3 | 0 | 6 | ||
| High TLG/Low PLR | 15 | 20 | 2 | 33 | ||
| Co-high TLG/PLR | 11 | 18 | 3 | 26 | ||
| Co-low TLG/MLR | 35 | 3 | 0.000 | 9 | 29 | 0.007 |
| Low TLG/High MLR | 11 | 6 | 0 | 17 | ||
| High TLG/Low MLR | 16 | 13 | 4 | 25 | ||
| Co-high TLG/MLR | 10 | 25 | 1 | 34 | ||
| Co-low TLG/SII | 38 | 4 | 0.000 | 9 | 33 | 0.033 |
| Low TLG/High SII | 8 | 5 | 0 | 13 | ||
| High TLG/Low SII | 10 | 13 | 3 | 20 | ||
| Co-high TLG/SII | 16 | 25 | 2 | 39 | ||
Univariate analysis revealed that Co-high MTV/NLR (p = 0.000), Co-high MTV/NLR (p = 0.001), Co-high MTV/MLR (p = 0.000), Co-high MTV/SII (p = 0.000), Co-high TLG/NLR (p = 0.000), Co-high TLG/PLR (p = 0.005), Co-high TLG/MLR (p = 0.000), Co-high TLG/SII (p = 0.001), and smoking were related to the binary stage of SCLC ( Table 6 ). Multivariate analysis further revealed that only Co-high MTV/MLR [odds ratio (OR): 8.67, 95% CI: 3.51–21.42, p = 0.000] was an independent predictor for ED-SCLC ( Table 6 ). However, the gender and age did not exhibit a significant relationship with the binary stage of SCLC ( Table 6 ).
Table 6.
Univariate and multivariate logistic regression analysis of potential relationships between patients’ characteristics and binary stage of SCLC.
| Univariatep-value | Multivariatep-value | OR | 95% CI for OR | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender | 0.057 | 0.184 | 4.50 | 0.96 | 21.12 |
| Age | 0.910 | 0.314 | 1.04 | 0.50 | 2.12 |
| Smoking | 0.042 | 0.163 | 2.81 | 1.04 | 7.62 |
| Co-high MTV/NLR | 0.000 | 0.241 | 6.11 | 2.68 | 13.93 |
| Co-high MTV/PLR | 0.001 | 0.416 | 4.21 | 1.73 | 10.21 |
| Co-high MTV/MLR | 0.000 | 0.000 | 8.67 | 3.51 | 21.42 |
| Co-high MTV/SII | 0.000 | 0.270 | 4.71 | 2.10 | 10.56 |
| Co-high TLG/NLR | 0.000 | 0.437 | 5.13 | 2.28 | 11.54 |
| Co-high TLG/PLR | 0.005 | 0.615 | 3.44 | 1.44 | 8.22 |
| Co-high TLG/MLR | 0.000 | 0.405 | 7.05 | 2.92 | 16.99 |
| Co-high TLG/SII | 0.001 | 0.432 | 3.98 | 1.79 | 8.84 |
Discussion
In this study, our results revealed that the baseline inflammatory markers (NLR, MLR, PLR, and SII) and metabolic parameters (MTV and TLG) were significantly correlated with the binary stage of SCLC. In addition, hematological parameters (NLR, MLR, PLR, and SII) were significantly associated with MTV and TLG in SCLC patients. More importantly, co-high semi-quantitative parameters (MTV and TLG) and hematological parameters (NLR, MLR, PLR, and SII) were significantly related to ED-SCLC, but only Co-high MTV/MLR was identified as an independent predictor for ED-SCLC.
Growing evidence has demonstrated that inflammatory markers (NLR, PLR, MLR, and SII) in peripheral blood have been suggested to be correlated with the stage of different tumors, such as NSCLC (10), renal cell carcinoma (11), and colon cancer (12). Oner et al. (11) demonstrated that NLR and LMR predicted the late stage in renal cell carcinoma. In a study of colon cancer and NSCLC, Uludag et al. (12) and Goksel et al. (10) showed that NLR and PLR were significantly higher in late stage than those in early stage. In accordance with the previous studies, our study suggested that hematological parameters (NLR, PLR, MLR, and SII) were correlated with the binary stage in patients with SCLC. High NLR, MLR, and SII can be caused by increased neutrophils and monocytes or/and decreased lymphocytes in peripheral blood. Inflammation cells are known to be considered as part of the tumor microenvironment and promote development (14). Monocytes or neutrophils could directly form complexes with tumor cells and mediate migration in blood vessel (15). The complexes help metastatic seeds escape immune surveillance, while lymphocytes prevent the development of cancer by secreting protective inflammatory factors (16). Platelets also secrete inflammatory factors (e.g., vascular endothelial growth factor, VEGF) to facilitate tumor angiogenesis and metastasis (17). All the above theories suggested that baseline NLR, MLR, PLR, and SII might predict ED SCLC.
SUVmax reflects the maximum value of tumor metabolism. However, MTV and TLG are calculated based on the volume of interest (VOI); thus, they may be better to reflect tumor metabolism and burden. In the previous study, 18F-FDG PET/CT is used as a reliable molecular imaging method for staging patients with SCLC (5). In the present study, we also explored the application of semi-quantitative parameters via 18F-FDG PET/CT to assess the binary stage in patients with SCLC. Our study demonstrated that SUVmean, MTV, and TLG were related to the binary stage of SCLC patients, but SUVmax was not. Apostolova et al. (18) reported that MTV and SUVmax were associated with stage in patients with NSCLC. In another study, Dolan et al. (19) demonstrated that an elevated TLG was correlated with TNM stage of NSCLC. In addition, Hu et al. (20) found that MTV and TLG were related to the stage in patients with adenocarcinoma, and only MTV was associated with stage in patients with squamous cell carcinoma. Based on the above findings, the relationships between different metabolic parameters and the stage of lung cancer were not identical, but they could be used to evaluate the tumor stage for lung cancer. Although the pathological type and tumor stage in the present study are different from the previous studies, our results also supported this.
In addition, with the increase of MTV and TLG of SCLC patients, hematological parameters of NLR, PLR, MLR, and SII were elevated. A previous study reported that there were positive correlations between NLR and metabolic parameters (SUVmax, SUVmean, MTV, TLG, whole-body MTV, and TLG) via PET/CT in patients with SCLC (21). However, the PLR, MLR, and SII were respectively associated with MTV and TLG of SCLC in our study, which have not been reported before. To our knowledge, the underlying mechanism of relationship between inflammatory markers and metabolic parameters is undergoing investigation. A similar relationship between metabolic parameters and inflammatory markers was demonstrated in other cancers including colorectal cancer (22) and NSCLC (10). Xu et al. (22) suggested that SUVmax, MTV, and TLG were significantly associated with LMR and NLR. In a study of NSCLC, Goksel et al. (10) reported that MTV and TLG were positively related to NLR and PLR. These relationships between semi-parameters and hematological parameters in patients with different malignancies may be explained by certain opinions. On the one hand, inflammatory cells infiltrate primary tumors, resulting in the increase of 18F-FDG uptake (23). On the other hand, the hypoxia promotes the secretion of VEGF by inflammatory cells, resulting in tumor angiogenesis and increase of 18F-FDG uptake within tumor (24). The local tumor metabolism may have resulted from tumor metabolic itself and inflammatory cells (25). Interestingly, in the present study, we observed that the co-high MTV (or TLG) and inflammatory markers (NLR, PLR, MLR, and SII) were associated with ED-SCLC, but only co-high MTV/MLR was considered as an independent predictor for ED-SCLC. MTV represents the metabolic tumor volume of all lesions, and MLR reflects the host’s systemic inflammatory response. These findings mean that co-high MTV/MLR might be not only more accurate, but also effective for detecting ED-SCLC in the present study. Therefore, the correlation between metabolic parameters via 18F-FDG PET/CT and inflammatory markers needs further research. In a word, our results preliminarily demonstrated the synergistic effect of tumor metabolic activity with inflammatory markers in assessing the binary stage of SCLC.
SCLC has a close association with smoking, which is considered a factor in the development of SCLC (26, 27). Smoking has been proven to associate with inflammatory markers such as NLR, eosinophil-to-lymphocyte ratio (ELR), and lymphocyte-to-monocyte ratio (LMR) (28). In this study, although there was no significant correlation between inflammatory markers of NLR, PLR, MLR, and SII or metabolic parameters with smoking status, the incidences of Co-high MTV/MLR, Co-high MTV/SII, Co-high TLG/MLR, and Co-high TLG/SII were higher in smokers than nonsmokers. Furthermore, smoking was not an independent predictor for the binary stage of SCLC, which is due to only few patients being never-smokers in this study. However, understanding the association between smoking, inflammation markers, tumor metabolism, and binary stage in SCLC patients needs additional research in the future.
There are some limitations in this study. Firstly, as a retrospective study, there are some limitations such as a great gender ratio difference, the lack of a control matched group, and the absence of a rigorous control of the inflammation-related lung diseases (e.g., obstructive pneumonia, interstitial pneumonia, and chronic obstructive pulmonary disease). Secondly, the sample size was relatively small and all patients were only from a single center. A multi-center prospective study with a larger sample size should be carried out in the future.
Conclusion
In conclusion, our results demonstrate that the baseline inflammatory markers (NLR, MLR, and SII) and tumor metabolic parameters are associated with the binary stage in patients with SCLC. Moreover, the co-high MTV/MLR based on metabolic tumor volume and systemic inflammatory response could be of help for predicting the ED-SCLC. However, further investigation needs to evaluate the combined role of inflammatory markers and tumor metabolic parameters via PET/CT in detecting ED-SCLC at baseline.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JS, YH, YL, DL, TL, and YXH directly participated in the planning, execution or analysis of the study. JS, YH, and TL designed the study and interpreted the results. DL and YL contributed to collection of clinical dates. JS and YH drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Jiangsu Provincial Health and Family Planning Commission Foundation (H2018029) and Research Project of Jiangsu Cancer Hospital (No. ZJ202122). These funders did participate in design, data collection, and analysis of the present study and have no role in the decision to publish the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2. Kalemkerian GP. Small cell lung cancer. Semin Respir Crit Care Med (2016) 37(5):783–96. doi: 10.1055/s-0036-1592116 [DOI] [PubMed] [Google Scholar]
- 3. Kalemkerian GP, Loo BW, Akerley W, Attia A, Bassetti M, Boumber Y, et al. NCCN guidelines insights: Small cell lung cancer, version 2. 2018. J Natl Compr Canc Netw (2018) 16(10):1171–82. doi: 10.6004/jnccn.2018.0079 [DOI] [PubMed] [Google Scholar]
- 4. Dingemans AC, Fruh M, Ardizzoni A, Besse B, Faivre-Finn C, Hendriks LE, et al. Small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up(). Ann Oncol (2021) 32(7):839–53. doi: 10.1016/j.annonc.2021.03.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martucci F, Pascale M, Valli MC, Pesce GA, Froesch P, Giovanella L, et al. Impact of (18)F-FDG PET/CT in staging patients with small cell lung cancer: A systematic review and meta-analysis. Front Med (Lausanne) (2019) 6:336. doi: 10.3389/fmed.2019.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farsad M. FDG PET/CT in the staging of lung cancer. Curr Radiopharm (2020) 13(3):195–203. doi: 10.2174/1874471013666191223153755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Han R, Wang Q, Zheng J, Lin C, Lu C, et al. Biological significance of (18)F-FDG PET/CT maximum standard uptake value for predicting EGFR mutation status in non-small cell lung cancer patients. Int J Gen Med (2021) 14:347–56. doi: 10.2147/IJGM.S287506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature (2021) 593(7858):282–8. doi: 10.1038/s41586-021-03442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goksel S, Cengiz A, Ozturk H, Yurekli Y. Prognostic impact of the (18)F-fluorodeoxyglucose positron-emission tomography/computed tomography metabolic parameters and correlation with hematological inflammatory markers in lung cancer. J Cancer Res Ther (2021) 17(4):925–30. doi: 10.4103/jcrt.JCRT_1046_20 [DOI] [PubMed] [Google Scholar]
- 11. Oner I, Sackan F, Ozdemir D. Evaluation of the preoperative haematological parameters predicting the high T-stage and fuhrman grade in renal cell carcinoma. J Coll Physicians Surg Pak (2022) 32(4):461–6. doi: 10.29271/jcpsp.2022.04.461 [DOI] [PubMed] [Google Scholar]
- 12. Uludag SS, Sanli AN, Zengin AK, Ozcelik MF. Systemic inflammatory biomarkers as surrogate markers for stage in colon cancer. Am Surg (2022) 88(6):1256–62. doi: 10.1177/0003134821995059 [DOI] [PubMed] [Google Scholar]
- 13. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods (2012) 9(7):676–82. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalafati L, Mitroulis I, Verginis P, Chavakis T, Kourtzelis I. Neutrophils as orchestrators in tumor development and metastasis formation. Front Oncol (2020) 10:581457. doi: 10.3389/fonc.2020.581457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao W, Wang P, Jia H, Chen M, Gu X, Liu M, et al. Lymphocyte count or percentage: which can better predict the prognosis of advanced cancer patients following palliative care? BMC Cancer (2017) 17(1):514. doi: 10.1186/s12885-017-3498-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Best MG, Wesseling P, Wurdinger T. Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res (2018) 78(13):3407–12. doi: 10.1158/0008-5472.CAN-18-0887 [DOI] [PubMed] [Google Scholar]
- 18. Apostolova I, Ego K, Steffen IG, Buchert R, Wertzel H, Achenbach HJ, et al. The asphericity of the metabolic tumour volume in NSCLC: correlation with histopathology and molecular markers. Eur J Nucl Med Mol Imaging. (2016) 43(13):2360–73. doi: 10.1007/s00259-016-3452-z [DOI] [PubMed] [Google Scholar]
- 19. Dolan RD, Maclay JD, Abbass T, Colville D, Buali F, MacLeod N, et al. The relationship between (18)F-FDG-PETCT-derived tumour metabolic activity, nutritional risk, body composition, systemic inflammation and survival in patients with lung cancer. Sci Rep (2020) 10(1):20819. doi: 10.1038/s41598-020-77269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu WD, Wang HC, Wang YB, Cui LL, Chen XH. Correlation study on 18F-FDG PET/CT metabolic characteristics of primary lesion with clinical stage in lung cancer. Q J Nucl Med Mol Imaging. (2021) 65(2):172–7. doi: 10.23736/S1824-4785.19.03146-7 [DOI] [PubMed] [Google Scholar]
- 21. Mirili C, Guney IB, Paydas S, Seydaoglu G, Kapukaya TK, Ogul A, et al. Prognostic significance of neutrophil/lymphocyte ratio (NLR) and correlation with PET-CT metabolic parameters in small cell lung cancer (SCLC). Int J Clin Oncol (2019) 24(2):168–78. doi: 10.1007/s10147-018-1338-8 [DOI] [PubMed] [Google Scholar]
- 22. Xu J, Li Y, Hu S, Lu L, Gao Z, Yuan H. The significant value of predicting prognosis in patients with colorectal cancer using (18)F-FDG PET metabolic parameters of primary tumors and hematological parameters. Ann Nucl Med (2019) 33(1):32–8. doi: 10.1007/s12149-018-1299-z [DOI] [PubMed] [Google Scholar]
- 23. Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med (1992) 33(11):1972–80. [PubMed] [Google Scholar]
- 24. Kwilas AR, Donahue RN, Tsang KY, Hodge JW. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron (2015) 2(1):e677. doi: 10.14800/ccm.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du S, Sun H, Gao S, Xin J, Lu Z. Metabolic parameters with different thresholds for evaluating tumor recurrence and their correlations with hematological parameters in locally advanced squamous cell cervical carcinoma: an observational (18)F-FDG PET/CT study. Quant Imaging Med Surg (2019) 9(3):440–52. doi: 10.21037/qims.2019.02.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamilton G, Rath B. Smoking, inflammation and small cell lung cancer: recent developments. Wien Med Wochenschr (2015) 165(19-20):379–86. doi: 10.1007/s10354-015-0381-6 [DOI] [PubMed] [Google Scholar]
- 27. Schuller HM. The impact of smoking and the influence of other factors on lung cancer. Expert Rev Respir Med (2019) 13(8):761–9. doi: 10.1080/17476348.2019.1645010 [DOI] [PubMed] [Google Scholar]
- 28. Cekici Y, Yilmaz M, Secen O. New inflammatory indicators: association of high eosinophil-to-lymphocyte ratio and low lymphocyte-to-monocyte ratio with smoking. J Int Med Res (2019) 47(9):4292–303. doi: 10.1177/0300060519862077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.