Abstract
Temperature is an important environmental factor affecting plant anthocyanin synthesis. High temperatures are associated with decreased anthocyanin pigmentation in chrysanthemum. To reveal the effects of high temperature on anthocyanin biosynthesis in chrysanthemum, ray florets of the heat-sensitive cultivar “Nannong Ziyunying” (ZYY) were subjected to RNA sequencing. A total of 18,286 unigenes were differentially expressed between the control and treatment groups. Functional annotation and enrichment analyses of these unigenes revealed that the heat shock response and flavonoid pathways were significantly enriched, suggesting that the expression of these genes in response to high temperature is associated with the fading of chrysanthemum flower color. In addition, genes related to anthocyanin synthesis and heat shock response were differentially expressed under high-temperature stress. Finally, to further investigate the molecular mechanism of discoloration under high-temperature stress and facilitate the use of marker-assisted breeding for developing novel heat-tolerant cultivars, these results were used to mine candidate genes by analyzing changes in their transcription levels in chrysanthemum.
Keywords: chrysanthemum, anthocyanin biosynthesis, high temperature, flower discoloration, RNA-Seq
Introduction
With the intensification of global warming, high-temperature stress has become a major environmental factor limiting the normal growth and development of plants. Elevated temperatures affect a series of physiological, biochemical, and developmental processes in plants, such as photosynthesis and respiration, pollen activity, membrane stability, and enzyme activity, which in turn affect yield and quality (Atkin and Tjoelker, 2003; Nagar et al., 2015; Dusenge et al., 2019; Zhu et al., 2021). Temperature tolerance varies greatly among different plants. For instance, the rise in global temperatures has produced little effect on plants native to the tropics and subtropics; meanwhile, chrysanthemum is a cold-tolerant but heat-labile ornamental plant that is greatly affected by high temperatures (Gins et al., 2019). In general, high-temperature stress leads to delay in flowering, fading of flower color, and variation in flower type in chrysanthemums. Among these, flower color is one of the most important ornamental characteristics, and anthocyanins are the key component for flower color. Temperature-mediated regulation of flower color and the flavonoid pathway has been extensively studied (Nozaki and Fukai, 2008; Van Der Ploeg and Heuvelink, 2015). However, only a few studies have explored the regulation of chrysanthemum discoloration under high-temperature stress.
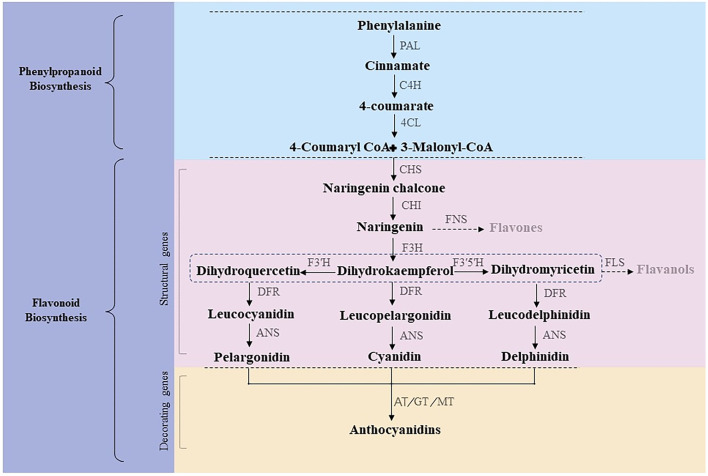
Anthocyanins are naturally synthesized water-soluble pigments present in various plant tissues and organs, such as roots, stems, leaves, flowers, fruits, and seeds. The anthocyanin synthetic pathway, or the phenylalanine pathway, is divided into three stages (Figure 1) (Winkel-Shirley, 2002). The first stage involves a phenylalanine precursor, which is oxidized by phenylalanine ammonia lyase (PAL), cinnamate 4-hydroxylase (C4H), and 4-coumaroyl CoA enzyme (4CL) to produce 4-coumaroyl-CoA. At the second stage, 4-coumaroyl-CoA is catalyzed by chalcone synthase (CHS) to produce chalcone, which is isomerized by chalcone isomerase (CHI) to form colorless trihydroxyflavonol. Finally, flavanone 3-hydroxylase (F3H), flavonoid-3',5'-hydroxylase (F3'5'H), and flavonoid-3'-hydroxylase (F3'H) catalyze the formation of colorless dihydroflavonols. At the third stage, colorless dihydroflavonols are further reduced to leucoanthocyanidins by dihydroflavonol 4-reductase (DFR) and then converted to anthocyanins by anthocyanin synthase (ANS) (Hichri et al., 2011; Zhang et al., 2016). Subsequently, glycosyltransferase (GT), methyltransferase (MT), and acyltransferase (AT) catalyze the formation of cornflower (cyanidin, Cy), geranium (pelargonidin, Pg), and delphinium (delphinidin, Dp) pigments (Caputi et al., 2012; Zhao et al., 2012; Bontpart et al., 2015). Of note, chrysanthemums lack the delphinium synthetic pathway; thus, there are no blue chrysanthemums in nature (Noda et al., 2017). Structural genes encoding CHS, CHI, DFR, F3H, ANS, and 3GT involved in anthocyanin synthesis have been verified in several species (Shi et al., 2015; Sun et al., 2016; Li et al., 2020).
Figure 1.
Anthocyanin synthetic pathway.
In recent years, several transcription factors involved in anthocyanin biosynthesis have been elucidated. For instance, MYB, bHLH, WD40, MADS-box and bZIP transcription factors are related to flower color. Structural genes involved in flavonoid pathways are primarily regulated by the conserved MYB–bHLH–WD40 (MBW) complex (Nabavi et al., 2020), which directly regulate flavonoid metabolism in plants through the transcriptional activation or repression of related structural genes. Among these, the transcription factor R2R3–MYB, which is the core component of the MBW protein complex, is mainly responsible for regulating flavonoid metabolism in plants (Xu et al., 2015). The MADS box proteins are a large family of transcription factors (TF) that control various developmental processes, not only the MADS box gene family has been involved in floral organogenesis according to the ABCDE model. However, MADS box TFs also regulate anthocyanin biosynthesis (Li et al., 2016; Qi et al., 2022). Further, bZIP transcription factors are involved in many signaling pathways, such as light signaling, environmental stress responses, and biological processes of plant growth and development (Chinnusamy et al., 2004; Golldack et al., 2014; An et al., 2019). HY5 is an important bZIP transcription involved in anthocyanin synthesis. It affects anthocyanin synthesis and metabolism by regulating the promoter activity of anthocyanin synthetic structural genes, including CHS, CHI, F3H, F3'H, DFR, and ANS (Zhang et al., 2011). Additionally, anthocyanin biosynthesis is affected by environmental factors, such as light and temperature. Temperature is crucial for anthocyanin metabolism. As such, the red color of maple leaves fades from the cooler northern regions to the warmer southern regions of the United States. This is because higher temperatures improve respiration, promote sugar consumption, and inhibit anthocyanin accumulation, leading to leaf color fading in plants (Deal et al., 1990). Furthermore, temperature affects the transcriptional expression of various genes in plants. Anthocyanin accumulation in marigold leaves is related to temperature, and accumulated temperatures are negatively correlated with marigold anthocyanin content (Armitage and Carlson, 1981). Under low temperatures, anthocyanin synthesis-related genes are induced and activated, elevating anthocyanin content in plants. In apple fruit, GT activity is significantly increased at low temperatures, increasing anthocyanin content beyond the normal value (Ubi et al., 2006). However, under high temperature, genes related to anthocyanin synthesis are inhibited, thereby reducing anthocyanin content (Islam et al., 2005). In plum fruits, over 60–70% anthocyanins are degraded under high temperature (Niu et al., 2017). Recently, a novel atypical subgroup 7 (SG7) R2R3–MYB transcription factor, CmMYB012, was discovered in chrysanthemum, which blocks anthocyanin biosynthesis by inhibiting CmCHS, CmDFR, CmANS, and CmUFGT expression under high-temperature stress (Zhou et al., 2021). Chrysanthemums can grow normally at 18–25°C; above 32°C, however, growth and development are delayed. In the temperature-sensitive chrysanthemum cultivars “Nannong Sichengdian,” “Nannong Ziyunying,” and “Nannong Zizhu,” flower color fades and ornamental quality declines under heat stress (Qiu, 2018). Abiotic stresses affect anthocyanin synthesis through several pathways. To date, the molecular mechanisms of chrysanthemum anthocyanin synthesis and flower discoloration in high-temperature environments remain unclear and warrant further exploration.
When plants are subjected to high-temperature stress, a series of heat shock response (HSR) pathways are activated, including the antioxidant protection system, thermoprotectant synthesis, membrane lipid peroxidation, heat shock protein expression, hormone regulation and signaling, and transcriptional regulation (Hasanuzzaman et al., 2013). Heat shock transcription factors (HSFs) and heat shock proteins (HSPs) play central roles in high-temperature stress response and acquired thermotolerance in plants (Zhang et al., 2015). HSFs act as key regulators of heat stress response. Specifically, they serve as transcriptional activators of HSPs, which are the terminal components of signal transduction pathways and mediate the expression of HSPs (Qu et al., 2013). HsfA1s are key transcriptional regulators in HSR (Ohama et al., 2017). Moreover, HSPs play pivotal roles in heat tolerance and are essential for the normal growth and development of plants under high temperatures. HSPs function as molecular chaperones and are classified into five families based on their molecular weight: HSP100, HSP90, HSP70, HSP60, and small HSPs (sHSPs) (Whitley et al., 1999; Tsan and Gao, 2004). Ca2+ and reactive oxygen species (ROS) are involved in signaling pathways that connect heat stress sensors and transcriptional regulators (Kotak et al., 2007). As one of the first signals in HSR, Ca2+ influx increases the strength of response (Liu et al., 2005). This alters plasma membrane (PM) fluidity, which in turn regulates cyclic nucleotide-gated calcium channels (CNGCs), allowing Ca2+ to enter the cytoplasm. CNGCs activate HSR by regulating the transition of cAMP and cGMP. As a Ca2+ signaling transmitter, CaM3 interacts with phosphorylated genes in response to heat stress (Finka et al., 2012; Tunc-Ozdemir et al., 2013). Additionally, nitric oxide (NO) has been reported to improve plant heat tolerance. In particular, NO acts as a signal upstream of CAM3 under heat stress (Rai et al., 2020). Furthermore, oxidative stress induced by HSR acts as secondary stress, which mediates ROS generation and causes molecular and cellular damage (Qu et al., 2013). The generated ROS are sensed by HSFs, which activate mitogen-activated protein kinase (MAPK) signaling, ultimately leading to the synthesis of antioxidant enzymes (SOD, POD, APX, CAT, and GPX) (Martindale and Holbrook, 2002). The MAPK cascade is an important signal transduction pathway in plants, being closely related to growth, development, and stress response (Kumar et al., 2020).
Here, using RNA sequencing (RNA-Seq), we found that anthocyanin synthesis-related genes, including CHS, CHI, DFR, F3H, ANS, 3GT, and F3'H, were significantly downregulated by heat in the temperature-sensitive cultivar “Nannong Ziyunying.” Moreover, MYB, bHLH, WD40, and bZIP transcription factors that regulate flavonoid metabolism and genes related to heat stress response-related processes, such as Ca2+ signaling, MAPK cascade, HSF and HSP function, and ROS generation, were differentially expressed between heat-treated and untreated plants. Overall, the present study aimed to explore molecular mechanisms underlying anthocyanin synthesis under high-temperature stress.
Materials and methods
Plant material and growth conditions
The cut-flower chrysanthemum cultivars “Nanong Ziyunying” and “Nannong Zizhu” were obtained from the China Chrysanthemum Germplasm Resources Preservation Center of Nanjing Agricultural University. Cuttings were rooted in trays for 15 days, transplanted into pots, grown for a month at a suitable temperature (day/night = 24°C/18°C), and cultured under a 16/8 h light/dark cycle and 70% relative humidity until the bud period. Thereafter, two groups of plants were transferred to separate light incubators with different environments: control group (CK) (8/16 h light/dark cycle with day and night temperature of 22°C) and high-temperature treatment group (8/16 h light/dark cycle with day/night temperatures of 38°C/22°C). Both groups of plants were watered every 5 days during the treatment period. The two cultivars with different treatments were sampled at the bud, early bloom, and bloom stages at the same period. Three biological and three technical replicates were collected for each sample and stored in liquid nitrogen for subsequent high-throughput sequencing assays.
Determination of anthocyanin type and content
Determination of anthocyanin type
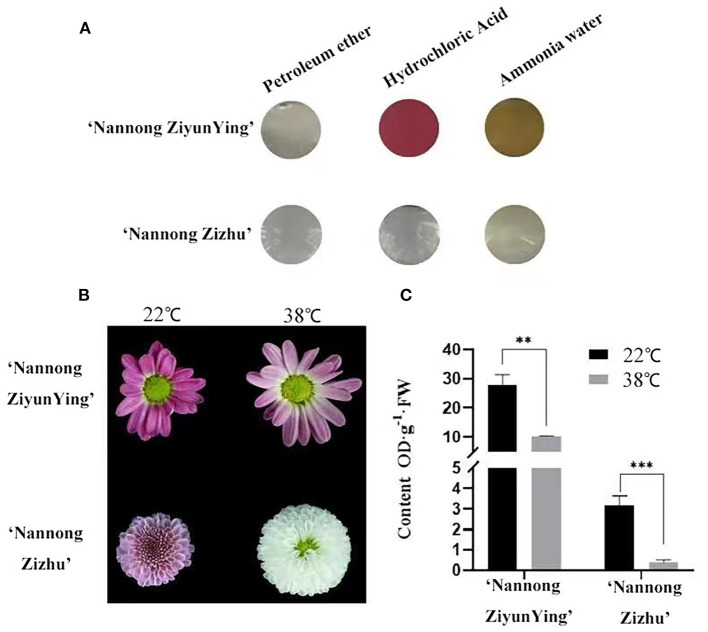
Qualitative analysis was performed to determine the anthocyanin type in high-temperature-treated cultivars. Briefly, the ray florets of “Nannong Ziyunying” and “Nannong Zizhu” were collected; each fresh sample weighed approximately 0.1 g. Then, 5 mL of petroleum ether, 5 mL of 10% hydrochloric acid, and 5 mL of 30% ammonia water were added to the samples. Subsequently, the samples were rapidly ground to a slurry, and the supernatant was collected after centrifugation (at 5,000 rpm for 10 min) for analysis. The type of anthocyanin component was determined based on color change.
Determination of anthocyanin content
Anthocyanin content of high-temperature-treated cultivars was determined as follows. At the flowering stage, ray florets of both cultivars from different treatment groups were collected. Next, 0.2 g of each sample was weighed and cryogenically ground to a powder. Then, 5 mL of solvent (methanol:water:formic acid:trifluoroacetic acid = 70:27:2:1) was added to each sample, and the extracts were shaken thoroughly. After 24 h, the samples were centrifuged (at 5,000 rpm for 10 min), and the supernatant was collected for analysis. Absorbance was measured at 530 and 657 nm using a spectrophotometer (Puana/T6 New Century). Each sample was analyzed three times.
Anthocyanin content was calculated according to the following formula:
Total RNA extraction, library construction, and RNA-Seq
Total RNA was extracted using RNA isoPlus (TaKaRa, Japan). The quality of extracted RNA was determined using agarose gel electrophoresis, and RNA quality and concentration were confirmed using a spectrophotometer (ND-1,000; NanoDrop, Wilmington, DE). High-throughput sequencing was performed using the BGISEQ-500 platform.
The basic steps and data processing methods for transcriptome sequencing were as follows. First, total RNA was processed for mRNA enrichment and rRNA depletion. DNA/RNA hybrid strands were selectively digested using RNase H, the remaining DNA probe was digested with DNase I, and desired RNA was obtained after purification. Next, RNA was fragmented in a specific buffer and then reverse transcribed with N6 random primers to synthesize double-stranded DNA; ends of the DNA fragments were blunted, modified, and PCR-amplified with specific primers. The obtained PCR products were thermally denatured into single strands and circularized with bridge primers to obtain a single-stranded circular DNA library for sequencing. Finally, high-quality clean reads were obtained after filtering the raw reads. Clean reads were assembled using Trinity and TGICL to remove redundancy and splicing, and the final unigenes were obtained by matching with the reference genome. Functional annotation and simple sequence repeat (SSR) detection of unigenes, simultaneous calculation of differentially expressed genes (DEGs) for each sample, and in-depth cluster and functional enrichment analyses were performed as described below.
DEG analysis, gene function annotation, GO and KEGG enrichment
BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to search the assembled unigenes against the NCBI NR (non-redundant protein sequences), Pfam (protein families), Swiss-Prot (a manually annotated and reviewed protein sequences), Kyoto Encyclopedia of Genes and Genomes (KEGG), Cluster of Orthologous Groups (COG), NCBI NT (nucleotide sequences), Gene Ontology (GO), and Eukaryotic Orthologous Groups (KOG) functional databases. The GO functions of all unigenes were classified using WEGO. According to the KEGG annotation information, metabolic pathways of the unigenes were explored. DEGs were detected using the PossionDis method. The Q-value was used to correct for the significant p-values obtained in original hypothesis tests. To minimize the false-positive rate, a Q-value<0.01 and |log2 fold change|>1 were set as the thresholds for filtering DEGs. A log2 fold change>0 indicated an upregulated gene; otherwise, it was considered downregulated.
Quantitative real-time PCR
Light Cycler 480 was used for qRT-PCR on DEGs to verify the reliability of the transcriptomic data. The specific primers used for qRT-PCR were designed using Primer Premier 5 (Table 1). Three technical and three biological replicates were set for each test sample. The reactions were performed using SYBR Premix Ex TaqTM (TaKaRa, Japan) according to the manufacturer's instructions. CmEF1α was selected as the reference gene. Relative gene expression was assessed using the 2−ΔΔCT method.
Table 1.
Primers used in the study.
| Primer name | Sequence (5'-3') |
|---|---|
| qCmCHS-F | ATCATCCAATGATGGTGCCATATAGGC |
| qCmCHS-R | GACCGCTACACCTCCTAATTGTGTACT |
| qCmCHI-F | ATACCTGAAGCACCAATCGCAGT |
| qCmCHI-R | ATTTTCCTCTAGTTGAAAAAGCC |
| qCmF3H-F | CCAACATTCATCATTCATCTCCCCAGA |
| qCmF3H-R | ACTTGACAACAACCACTTTCAGGGAGG |
| qCmDFR-F | CGGAGAAAGCAGCATGGAAA |
| qCmDFR-R | GGGAACGAGGGACTGATAAATG |
| qCmANS-F | ATCAACTACTACCCAAAATGCCC |
| qCmANS-R | CCTAACCTTCTCCTTATTCACAA |
| qCm3GT-F | GGAGAAATGGAAGATAAACCGAA |
| qCm3GT-R | CGCCGAATAAAGGAAATCCTAAG |
| qCmEF1α-F | GTACCCTGGGCACCACAAGT |
| qCmEF1α-R | CTACCAACGGCCTGCAAATC |
| qCmHY5-F | GGCTGACAAAGAAAACAAACGGT |
| qCmHY5-R | CGTTTTGCAGTGTGGACAAACGC |
Results
Phenotypic analysis of “Nannong Ziyunying” and “Nannong Zizhu” under high-temperature stress
The chrysanthemum cultivars “Nannong Ziyunying” and “Nannong Zizhu” were easily affected, and their flower color changed under high-temperature stress. We analyzed the anthocyanin types of the two cultivars and found that the pigments were anthocyanins and flavonoids but not carotenoids (Figure 2A). Thus, we hypothesized that temperature affects anthocyanin synthesis during flowering in “Nannong Ziyunying” and “Nannong Zizhu” subjected to heat stress. In addition, the flower color of both cultivars faded following high-temperature treatment (Figure 2B). To explore the correlation between flower color and anthocyanin content, we measured anthocyanin content in treated and untreated fresh ray florets. The total anthocyanin content in the control and treatment groups was, respectively 27.89 and 10.19 OD·g−1·FW for “Nanong Ziyunying” and respectively, 3.16 and 0.38 OD·g−1·FW for “Nannong Zizhu.” Thus, in both cultivars, the total anthocyanin content of ray florets was significantly reduced after high-temperature treatment (Figure 2C).
Figure 2.
Qualitative analysis of anthocyanin types and content in two cut chrysanthemum cultivars. (A) Calibration of anthocyanin components. (B) Comparison of high-temperature-treated and untreated flower color phenotypes. (C) Anthocyanin content of high-temperature treated and untreated cultivars. Error bars represent standard deviations from three biological replicates. Values are presented as mean ± SE (n = 3). **Significant at P < 0.01 and ***extremely significant at P < 0.001, as determined by Student's t-test.
Transcriptome analysis after high-temperature treatment
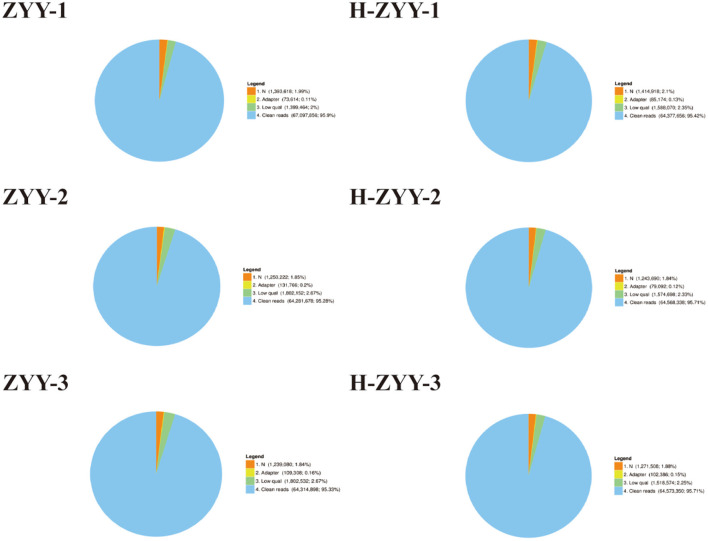
The heat-sensitive cultivar “Nanong Ziyunying” was selected as the material for transcriptome analysis. Fresh ray florets were subjected to high-temperature and normal conditions, and high-quality RNA was extracted. Three biological replicates were used per treatment group. The control groups were designated ZYY-1, ZYY-2, and ZYY-3, and the high-temperature treatment groups were designated H-ZYY-1, H-ZYY-2, and H-ZYY-3. After sequencing, raw data were filtered and processed for statistical analysis (Table 2). Overall, the six databases contained a low proportion of low-quality bases (quality<20) and the sequencing quality was good (Figure 3).
Table 2.
Filtered read quality statistics.
| Sample | Total raw reads (M) | Total clean reads (M) | Total clean bases (Gb) | Clean reads Q20 (%) | Clean reads Q30 (%) | Clean read ratio (%) |
|---|---|---|---|---|---|---|
| ZYY-1 | 69.96 | 67.1 | 6.71 | 98.22 | 92.16 | 95.9 |
| ZYY-2 | 67.47 | 64.28 | 6.43 | 98.18 | 92.1 | 95.28 |
| ZYY-3 | 67.47 | 64.31 | 6.43 | 98.15 | 91.94 | 95.33 |
| H-ZYY-1 | 67.47 | 64.38 | 6.44 | 98.22 | 92.2 | 95.42 |
| H-ZYY-2 | 67.47 | 64.57 | 6.46 | 98.17 | 92.02 | 95.71 |
| H-ZYY-3 | 67.47 | 64.57 | 6.46 | 98.19 | 92.06 | 95.71 |
Figure 3.
Comparison of raw reads in six RNA libraries. “Clean reads” represent the elimination of reads containing adaptor; “N” represents reads accounting for over 10%; “low quality” represents remaining reads after the number of bases with quality value (Q) ≤ 5 is over 50% of all reads. Numbers in brackets indicate the percentage of reads of each type.
A total data of 38.93 GB of data were generated from the BGISEQ-500 platform. After assembly and de-redundancy, 151,614 unigenes were obtained. The total length, average length, N50, and GC content were 154,407,019 bp, 1,018 bp, 1,561, and 39.36%, respectively (Table 3). The obtained unigenes were annotated against the seven functional databases using BLAST, and respectively 93,977 (NR: 61.98%), 56,053 (NT: 36.97%), 63,543 (Swiss-Prot: 41.91%), 72,078 (KOG: 47.54%), 69,096 (KEGG: 45.57%), 73,203 (GO: 48.28%), and 63,728 (Pfam: 42.03%) unigenes were functionally annotated. The transdecoder detected 72,964 coding sequences and 20,880 SSRs distributed in 17,391 unigenes and predicted 2,742 unigenes encoding transcription factors.
Table 3.
Unigene quality index statistics.
| Sample | Total number | Total length | Mean length | N50 | N70 | N90 | GC (%) |
|---|---|---|---|---|---|---|---|
| ZYY-1 | 98,773 | 77,777,880 | 787 | 1,176 | 718 | 337 | 39.58 |
| ZYY-2 | 92,109 | 72,159,678 | 783 | 1,170 | 716 | 336 | 39.7 |
| ZYY-3 | 97,167 | 75,886,636 | 780 | 1,163 | 712 | 334 | 39.59 |
| H-ZYY-1 | 82,749 | 70,451,968 | 851 | 1,257 | 799 | 376 | 39.84 |
| H-ZYY-2 | 82,070 | 69787,861 | 850 | 1264 | 794 | 374 | 39.9 |
| H-ZYY-3 | 83,300 | 70,866,818 | 850 | 1,263 | 793 | 375 | 39.82 |
| All-unigene | 151,614 | 154,407,019 | 1,018 | 1,561 | 1,012 | 463 | 39.36 |
Analysis of DEGs and functional enrichment after high temperature treatment
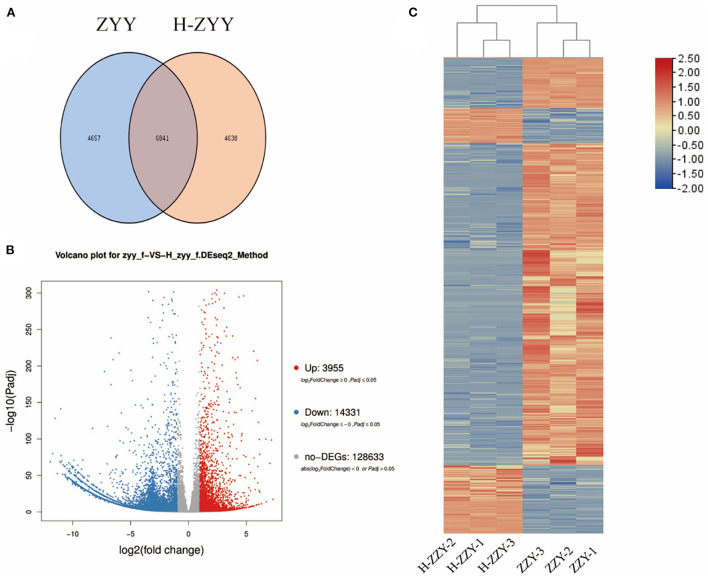
As shown in Figure 4, Venn diagrams of transcripts illustrated 6,841 co-expressed genes, 4,657 ZYY-specific, and 4638 H-ZYY-specific genes between the control (ZYY) and treated (H-ZYY) groups (Figure 4A). Analysis of transcriptome data revealed 18,286 DEGs between ZYY and H-ZYY, of which 14,331 unigenes were downregulated and 3,955 were upregulated in the treatment group (Figure 4B). Additionally, hierarchical clustering of DEGs was observed between ZYY (ZYY-1, ZYY-2, and ZYY-3) and H-ZYY (H-ZYY-1, H-ZYY-2, and H-ZYY-3) (Figure 4C).
Figure 4.
Analysis of differentially expressed genes. (A) Venn diagram. (B) Volcano plot. (C) Heatmap.
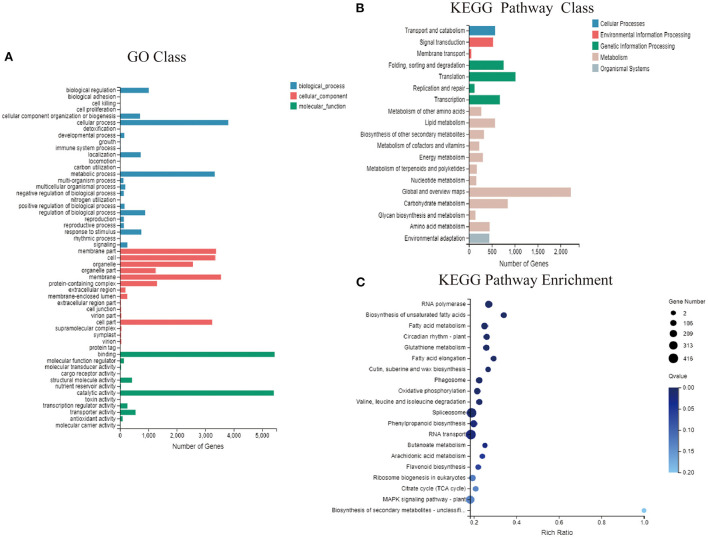
The functional classification of GO annotations mainly included three categories, namely “molecular function” “cellular component” and “biological processes” corresponding to respectively, 15, 15, and 9 specific functions (Figure 5A). Among these, respectively 5,447 and 5,415 unigenes annotated to “molecular function” mainly focused on “catalytic activity” and “binding.” Moreover, 3,341 unigenes annotated to “biological processes” mainly focused on “cellular and metabolic processes.” Among unigenes annotated to “cell components,” 3,355, 3,241, 3,550, and 3,374 focused on “cell” “cell part” “membrane” and “membrane part,” respectively. Gene-related KEGG pathways were divided into five branches: “cellular processes” “environmental information processing” “genetic information processing” “metabolism” and “organismal systems” (Figure 5B). Most of the unigenes were functionally annotated to “metabolic pathways” which were divided into 11 sub-categories, with 2,224 genes in the global and overview maps and 847 genes in the carbohydrate metabolism category. In addition, unigenes classified into “genetic information processing” pathways were divided into four sub-categories focusing on “translation” and “folding, sorting, and degradation,” with respectively, 1,015 and 757 genes. Functional enrichment analysis revealed abundant unigenes in “RNA transport” “spliceosome” and “plant MPAK signaling” pathways. Of the 416, 408, and 306 DEGs, 63 were enriched in “flavonoid biosynthesis” which is associated with flower color (Figure 5C).
Figure 5.
GO and KEGG functional enrichment analysis. (A) Functional distribution of GO annotations. (B) KEGG function distribution statistics. (C) KEGG function-enriched bubble chart.
RNA-Seq database mining of DEGs in response to high-temperature treatment
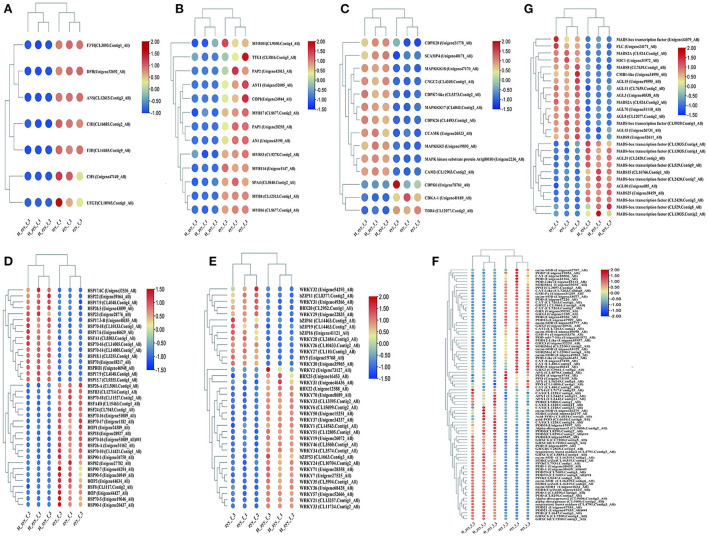
DEGs between the ZYY and H-ZYY groups were compared with the gene function annotation information, and the transcription factors and genes potentially involved in response to high-temperature treatment were extracted from the transcriptome database. Since the flower color of “Nannong Ziyunying” changed after high-temperature treatment, we performed differential analysis of genes related to flower color metabolism. The structural genes CHS (Unigene47149_All), CHI (CL16685.Contig2_All), DFR (Unigene32692_All), F3H (CL14465.Contig9_All), ANS (CL12615.Contig3_All), 3GT (CL10945.Contig1_All), and F3'H (CL3093Contig1_All) were downregulated (Figure 6A). The MBW transcription factors, which are associated with flower color, were analyzed. Of these, nine MYB transcription factors; the bHLH transcription factor AN1 (Unigene43198_All); and three WD40 transcription factors, namely TTG1 (CL5816.Contig5_All), COP1 (Unigene24944_All), and SPA4 (CL5840.Contig2_All), were downregulated (Figure 6B). The expression levels of MADS-box transcription factors also changed in the two phenotypes of ray florets after high-temperature treatment. Among them, the expression of AGL31 (CL2420.Contig3_All), MADS15 (CL16766.Contig1_All), and MADS25 (Unigene38459_All) etc., were upregulated, while CMB1-like (Unigene34995_All), AGL15 (Unigene19595_All), AGL3 (Unigene40538_All), SOC1 (Unigene31972_All), FLC (Unigene24171_All), AGL15 (Unigene26731_All), and MADS8 (Unigene52611_All), were significantly downregulated (Figure 6G). Furthermore, signaling molecules, such as Ca2+ and ROS, accumulate in plants in response to high-temperature stress. Ca2+ is one of the primary and essential signals for heat shock response, and it activates MAPK to transmit signals to the nucleus (Ohama et al., 2017). Our analysis revealed that MAPKKK17 (CL6043.Contig2_All), MAPKKK5 (Unigene19893_All), the MAPK kinase substrate protein At1g80180 (Unigene2236_All), and MAPKKK18 (Unigene47170_All) in the MAPK cascade were upregulated following high-temperature treatment. Likewise, the intracellular Ca2+ signaling pathway genes CAM3 (CL12903.Contig2_All), CCAMK (Unigene26523_All), SCAMP4 (Unigene40171_All), and CNGC2 (CL4349.Contig1_Al) were upregulated, whereas CDKA-1 (Unigene41169_All) and TDR4 (CL12077.Contig2_All) were downregulated (Figure 6C) after high-temperature treatment. Stress induces ROS production via both enzymatic and non-enzymatic pathways and is associated with multiple stages of plant development and growth. Our analysis revealed that several genes encoding antioxidant enzymes, such as SOD[Mn] (CL2200.Contig2_All), Cu/Zn-SOD (Unigene49263_Al), POD (Unigene40341_All), POD-like (Unigene36451_All), POD16 (Unigene47909_All), POD7 (Unigene19853_All), CAT (Unigene27470_All), CAT2 (CL7263.Contig1_All), APX (CL16249.Contig4_All), PPO (CL3897.Contig2_All), GRX2 (CL9661.Contig1_All), and GRX3 (Unigene32220_All) were downregulated under high-temperature stress. However, Cu/zn-SOD (CL16319.Contig1_All), POD31 (Unigene8545_All), POD3 (CL8290.Contig7_All), POD-1 (Unigene38435_All), PPO (CL7934.Contig1_All), CAT (CL1228.Contig24_All), GRXC6 (CL7200.Contig1_All), APX2 (CL2442.Contig11_All) were upregulated, indicating heat enhanced ROS metabolism (Figure 6F).
Figure 6.
Mining of major pathways in response to high-temperature stress. (A) Anthocyanin metabolic pathway. (B) MBW transcription factors. (C) Ca2+ channels and Ca2+ signaling. (D) HSFs and HSPs. (E) Stress-responsive transcription factors. (F) ROS response pathway. (G) MADS-box transcription factors.
HSFs and HSPs are involved in various biological processes, particularly in response to heat stress. Specifically, the heat shock transcription factor (Hsf) HsfA1 (CL8503.Contig1_All) was upregulated, while HSF8 (CL1117.Contig2_All), HSFB3 (CL12714.Contig1_All), HSFA4D (CL15463.Contig1_All), and HSF24 (CL7043.Contig1_All) were downregulated. In addition, 16 HSPs were upregulated and 16 were downregulated after high-temperature treatment. Among these, HSP70 (Unigene18217_All) was significantly upregulated, whereas HSP90 (Unigene31147_All) was significantly downregulated (Figure 6D). In addition to HSFs and HSPs, transcription factors, such as B-box (BBX), bZIP, DREB, and WRKY, were differentially expressed. In particular, the BBX transcription factors BBX22 (Unigene12588_All) and BBX25 (Unigene16453_All) were upregulated, while BBX20 (CL2952.Contig1_All) was downregulated. Among bZIP transcription factors, bZIP53 (CL1663.Contig3_All), bZIP61 (CL14463.Contig5_All), bZIP19 (CL14463.Contig7_All), bZIP11 (CL8377.Contig2_All), and bZIP16 (Unigene41121_All) were upregulated, while HY5 (Unigene19768_All) was significantly downregulated. DREB is an important stress-responsive transcription factor in plants, which can specifically bind cis-acting DRE/CRT elements to induce the expression of stress tolerance genes (Luo et al., 2015). Our analysis revealed that DREB3 (CL11734.Contig2_All), DREB1F (CL3574.Contig1_All), DREB1A (CL3994.Contig1_All), DREB1B (Unigene68428_All), and DREB1D (Unigene34237_All) were upregulated in response to high temperature. In addition, 22 WRKY transcription factors were differentially expressed, of which 15 were upregulated and 7 were downregulated. Specifically, WRKY2 (Unigene73127_All) was significantly upregulated, while WRKY3 (CL110.Contig3_All) was significantly downregulated (Figure 6E).
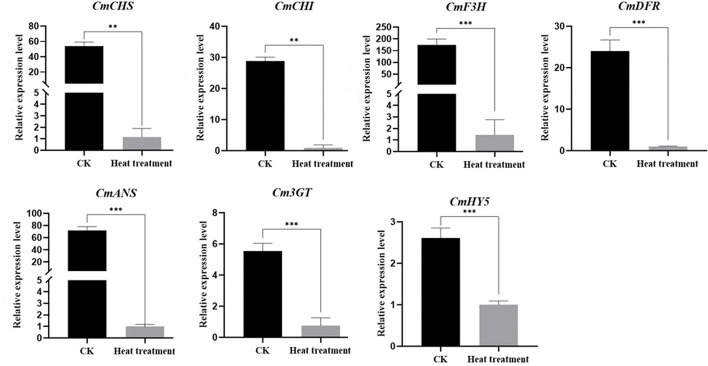
qRT-PCR validation of flower color-related DEGs
To validate the expression of differential genes in the RNA-Seq database, we selected seven DEGs, namely CmCHS, CmCHI, CmDFR, CmF3H, CmANS, Cm3GT, and CmHY5, which are structural anthocyanin synthetic genes and transcription factors, for qRT-PCR analysis. The results of qRT-PCR were consistent with those of RNA-Seq, and all seven genes were significantly downregulated after high-temperature treatment (Figure 7).
Figure 7.
Verification of the relative expression of anthocyanin metabolism gene following high-temperature treatment. Values are presented as mean ± SE (n = 3). **Significant at P < 0.01 and ***significant at P < 0.001, as determined by Student's t-test.
Discussion
How does chrysanthemum respond to high-temperature stress?
Through transcriptome analysis, changes in flower color following high-temperature treatment of the heat-sensitive cultivar “Nannong Ziyunying” at the molecular level were revealed. Our analysis revealed that HsfA1 (CL8503.Contig1_All) was significantly upregulated after high-temperature treatment. HsfA1 is a key regulator of response to high-temperature stress (El-Shershaby et al., 2019). For instance, in tomato, HsfA1 serves a unique function of regulating plant thermotolerance as a major transcription factor in response to heat stress, and hsfa1 mutants exhibit a heat-sensitive phenotype (Mishra et al., 2002). In addition, HSP70 (Unigene18217_All) expression was significantly upregulated after high-temperature treatment. HSP70 is the main and highly conserved protein activated by stress (Usman et al., 2017), and the role of HSP70 in relation to heat tolerance has been reported in several crops, such as soybean (Glycine max) (Ortiz and Cardemil, 2001), wheat (Triticum aestivum) (Duan et al., 2011), chili pepper (Capsicum annuum) (Usman et al., 2015), chrysanthemum (Chrysanthemum morifolium Ramat) (Song et al., 2014), and creeping bentgrass (Agrostis palustris) (Ye et al., 2012). The same results were obtained in our experiments, indicating that HSFs and HSPs respond to high-temperature stress in chrysanthemum.
In addition, high-temperature stress promotes the fluidity of cell membrane, which is related to Ca2+ channels in the cell membrane structure. Genes involved in Ca2+ signaling are activated under high-temperature stress, and heat shock signals are transmitted from the outside to inside of cells through CNGCs, which are implicated in the regulation of Ca2+ signaling (Finka et al., 2012). In particular, CNGC2 and CNGC4 are associated with high-temperature stress response (Gao et al., 2012). Our analysis revealed that CNGC2 (CL4349.Contig1_All) was upregulated after high-temperature treatment, suggesting that Ca2+ signaling is activated under heat stress in chrysanthemum. In the Ca2+ signaling pathway, Ca2+ ions combine with calmodulin (CaM) to promote the activity of calcium-dependent protein kinases (CDPKs) and MAPKs, which transmit heat stress signals to the nucleus and regulate the expression of thermostable genes (Awasthi et al., 2015). According to our transcriptomic data, MAPKs (Unigene2236_All) and CAM3 (CL12903.Contig2_All) were upregulated after high-temperature treatment. In addition, high-temperature stress induces ROS production and accumulation in plants, promoting antioxidant enzyme activity. In the present study, the expression levels of SOD, POD, CAT, GRX, PPO, and APX were significantly altered (Figure 6F). Increased activity of these antioxidant enzymes is beneficial for scavenging different types of ROS [e.g., singlet oxygen (1O2), superoxide (), hydrogen peroxide (H2O2), and hydroxyl radicals (OH−)] generated by high-temperature stress and enhancing the heat tolerance of plants (Medina et al., 2021). In addition, polyphenol oxidase (PPO) and peroxidase (POD) have been implicated in the anthocyanin degradation pathway for regulating flower and fruit color (Oren-Shamir, 2009; Zhang et al., 2019). ROS metabolism, similar to Ca2+ signaling pathway, mediates heat response and regulates immune activity in plants. Furthermore, ROS promote the expression of heat shock transcription factors; however, excess ROS accumulation leads to the generation of large amounts of NO, which induces the expression of CaM3 and promotes the transcription of downstream HsfA1 and related HSP genes (Ohama et al., 2017; Zhu et al., 2021). Furthermore, NO possesses ROS scavenging activity, and it has been reported to improve the antioxidant and thermal tolerance of Arabidopsis (Wang et al., 2014) and chrysanthemum (Yang et al., 2011). Typically, when plants are subjected to high-temperature stress, a series of transcriptional regulatory cascades are activated to respond to heat shock. HSF and HSP expression, Ca2+ signaling activation, and ROS production are the three major regulatory networks involved in HSR (Ohama et al., 2017). Our analysis of transcriptomic data revealed that genes and enzymes associated with these three pathways produced a transcriptional regulatory response in chrysanthemum; therefore, these components may also be the major regulators of HSR in chrysanthemum. However, the precise responses of these pathways to high temperature and mechanisms underlying the regulation of flower color in chrysanthemum remain unclear.
What is the link between anthocyanin synthesis and high temperature stress?
Anthocyanin biosynthesis is regulated by an array of enzymes. Genes encoding these enzymes, namely CHS, CHI, DFR, F3H, ANS, 3GT, and F3'H, are called structural genes related to anthocyanin metabolism (Willits et al., 2005; Zhang et al., 2014). Our transcriptome analysis revealed that the expression of anthocyanin synthesis-related genes was significantly downregulated (Figures 6A, 7), consistent with qRT-PCR results, and the total anthocyanin content also decreased under high-temperature stress. In addition, CmHY5 (Unigene19768_All), a bZIP transcription factor, was significantly downregulated. HY5 is known to regulate flower color in apples, and its association with BBXs, COP1, and MYBs has been demonstrated in rice (Luo et al., 2018), Arabidopsis (Gangappa et al., 2013), and apple (Liu et al., 2019). Here, we used qRT-PCR to verify HY5 downregulation and to further investigate the molecular mechanism underlying the regulation of flower color under high-temperature stress. In plants, structural genes involved in anthocyanin biosynthesis are primarily regulated by a class of conserved MBW complexes (Jin et al., 2016; Goswami et al., 2018). Among these, the MYB and bHLH transcription factors play major regulatory roles. Specifically, the MBW protein complex directly regulates anthocyanin content in plants by activating or inhibiting the transcription of structural genes related to anthocyanin synthesis (Nabavi et al., 2020). In our analysis, eight MYB transcription factors were downregulated (Figure 6B). These results are consistent with reports that high-temperature stress inhibited the expression of MdMYB4, MdMYB16, MdMYB17, and MdMYB114 and affected the synthesis of anthocyanin in apples (Lin-Wang et al., 2011). MpMYBS3 is highly expressed in response to low-temperature signals (Dou et al., 2016), and our analysis showed that CmMYBS3 (CL9278.Contig2_All) was downregulated under high-temperature stress; hence, CmMYBS3 may be involved in high-temperature stress response. PpMYB114 promotes the expression of PpUFGT involved in anthocyanin metabolism (Ni et al., 2019). In the present study, CmMYB114 (Unigene1147_All) and Cm3GT (CL8501.Contig3_All) were downregulated after high-temperature treatment, indicating that a similar regulatory network may exist in chrysanthemum. In Petunia hybrida, PhPAP1 positively regulated the anthocyanin structural gene PhCHS, and the overexpression of PhPAP1 and PhCHS led to abundant anthocyanin accumulation after 12 h of UV-A irradiation (Matoušek et al., 2006). Moreover, in the ethylene-insensitive Arabidopsis mutant ein2-1, abundant sucrose-induced anthocyanins were accumulated at high temperature through elevated PAP1 transcription (Kwon et al., 2011). These results are consistent with CmPAP1 (Unigene28293_All) downregulation after high-temperature treatment in our experiment. In chrysanthemum, a large amount of ethylene may be synthesized under high-temperature stress, which downregulates downstream CmPAP1 expression and hinders anthocyanin accumulation. Furthermore, PAP1 and PAP2 can bind the COP1/SPA complex and positively control anthocyanin accumulation in Arabidopsis (Maier et al., 2013). Our data showed that PAP2 (Unigene42013_Al), COP1 (Unigene24944_All), and SPA4 (CL5840.Contig2_All) were downregulated, indicating a similar regulatory mechanism in chrysanthemum. ANT1 can form a complex with EGL3, GL3, and TTG1 to positively regulate anthocyanin metabolism (Petroni and Tonelli, 2011), which is consistent observation that ANT1 (Unigene52085_All) was downregulated in chrysanthemum at high temperature.
Furthermore, bHLH transcription factors affect flower color. In particular, PhAN1 can interact with MYB and WD to positively regulate anthocyanin synthesis and activate various signaling pathways through interaction with MYB transcription factors (Quattrocchio et al., 2006; Petroni and Tonelli, 2011). In Arabidopsis thaliana, the WD protein TTG1 acts in concert with the GL3, EGL3, or TT8 proteins as well as the R2R3–MYB transcription factors PAP1, PAP2, MYB113, and MYB114 (Zhang et al., 2003; Zimmermann et al., 2004; Gonzalez et al., 2008). In Freesia hybrida Klatt, FhTTG1 encodes the WD40 protein, which forms the MBW complex and regulates anthocyanin or procyanidin biosynthesis, the specificity of which depends, in part, on the interacting proteins (Yamagishi et al., 2010; Verweij et al., 2016; Qi et al., 2022). Our data showed that the bHLH transcription factor AN1 (Unigene52085_All) and the WD40 protein TTG1 (CL5816.Contig5_All) were downregulated; these proteins may interact with MYB transcription factors to form the MBW complex and reduce anthocyanin synthesis under high-temperature stress. In addition, MADS-box transcription factors have been found to be involved in anthocyanin biosynthesis in a variety of plants, such as apple (Onik et al., 2018), strawberry (Lu et al., 2018), and sweet cherry (Qi et al., 2020). Recently, studies have shown that ScAG and ScAGL11 as negative regulators of anthocyanin biosynthesis in Senecio cruentus ray florets, which influence bicolor pattern formation (Qi et al., 2022). In our results, CMB1-like (Unigene34995_All), AGL15 (Unigene19595_All), AGL3 (Unigene40538_All), and SOC1 (Unigene31972_All) were significantly downregulated after high temperature stress. It is speculated that MADS-box transcription factors are involved in anthocyanin biosynthesis in chrysanthemum, but the specific molecular mechanism needs to be further studied.
In summary, chrysanthemum response to high temperature may involve the following three pathways: (1) extracellular Ca2+ influx promotes Ca2+ channels and signaling responses, which in turn promotes the expression of downstream MAPKs and transmits the high-temperature signal to the nucleus; (2) ROS production and accumulation of leads to a series of complex heat stress response pathways, such as HSF and HSP synthesis and NO accumulation, which in turn activates the plant's own immune stress response; and (3) structural genes involved in anthocyanin synthesis are downregulated, thereby inhibiting anthocyanin production and leading to flower discoloration under high temperature in chrysanthemum. These structural genes are regulated by different MBW protein complexes and other transcription factors that affect anthocyanin biosynthetic pathways in plants. However, although we assessed the expression of genes related to plant response to high temperature and anthocyanin metabolism through transcriptome analysis, response to heat stress and regulation of anthocyanin metabolism in plants involve complex regulatory networks. The links among these genes and their respective molecular functions remain clear and warrant further exploration.
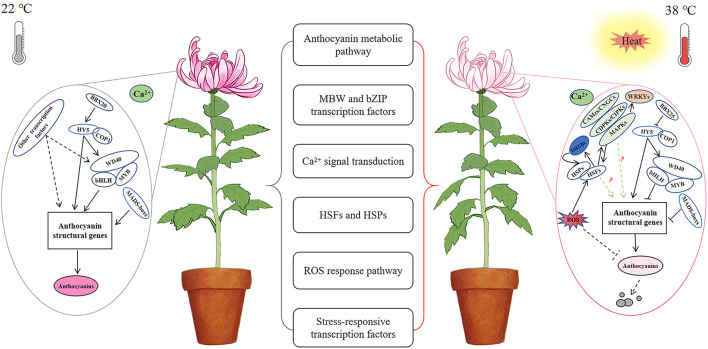
Conclusion
The discoloration phenotype of chrysanthemums under high-temperature stress is directly related to the inhibition of anthocyanin synthesis. However, multiple pathways may be involved in response to high-temperature stress, indicating the presence of a complex molecular regulatory network involving anthocyanin metabolism in chrysanthemums (Figure 8). Overall, our preliminary transcriptomic analysis revealed the regulation of flower color, as affected by high-temperature stress, although the precise molecular mechanism should be further studied.
Figure 8.
A possible regulatory network of chrysanthemum flower color under high temperature stress. Anthocyanin biosynthesis was normal at 22°C; anthocyanin biosynthesis was inhibited and accelerated degradation under high temperature stress at 38°C.
Data availability statement
The datasets generated for this study can be found in the NCBI sequence reads archive (SRA) database under BioProject No. PRJNA859415.
Author contributions
JJ designed the experiments. XH and JQ performed the experiments. ZS and XH analyzed the data. ZS, XH, and GW wrote the manuscript. L-jZ, SC, WF, and FC discussed the results and commented on the manuscript. L-jZ and ZS provided the funds. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the Hainan Provincial Natural Science Foundation of China (322QN340), the special research fund for doctoral students of Sanya Yazhou Bay Science and Technology City (HSPHDSRF-2022-07-002), and the Guiding Fund Key Projects for Sanya Institute of Nanjing Agricultural University (NAUSY-ZD03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- An J. P., Wang X. F., Zhang X. W., Bi S. Q., You C. X., Hao Y. J. (2019). MdBBX22 regulates UV-B-induced anthocyanin biosynthesis through regulating the function of MdHY5 and is targeted by MdBT2 for 26S proteasome-mediated degradation. Plant Biotechnol. J. 17, 2231. 10.1111/pbi.13196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage A., Carlson W. (1981). The effect of quantum flux density, day and night temperature and phosphorus and potassium status on anthocyanin and chlorophyll content in marigold leaves [Tagetes patula]. J. Am. Soc. Horticultural Sci. 106, 639–642. 10.21273/JASHS.106.5.639 [DOI] [Google Scholar]
- Atkin O. K., Tjoelker M. G. (2003). Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 8, 343–351. 10.1016/S1360-1385(03)00136-5 [DOI] [PubMed] [Google Scholar]
- Awasthi R., Bhandari K., Nayyar H. (2015). Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 3, 11. 10.3389/fenvs.2015.00011 [DOI] [Google Scholar]
- Bontpart T., Cheynier V., Ageorges A., Terrier N. (2015). BAHD or SCPL acyltransferase? What a dilemma for acylation in the world of plant phenolic compounds. New Phytol. 208, 695–707. 10.1111/nph.13498 [DOI] [PubMed] [Google Scholar]
- Caputi L., Malnoy M., Goremykin V., Nikiforova S., Martens S. (2012). A genome-wide phylogenetic reconstruction of family 1 UDP-glycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land. Plant J. 69, 1030–1042. 10.1111/j.1365-313X.2011.04853.x [DOI] [PubMed] [Google Scholar]
- Chinnusamy V., Schumaker K., Zhu J. K. (2004). Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J. Exp. Bot. 55, 225–236. 10.1093/jxb/erh005 [DOI] [PubMed] [Google Scholar]
- Deal D., Raulston J., Hinesley L. (1990). Leaf color retention, dark respiration, and growth of red-leafed Japanese maples under high night temperatures. J. Am. Soc. Hortic. Sci. 115, 135–140. 10.21273/JASHS.115.1.135 [DOI] [Google Scholar]
- Dou T. X., Hu C. H., Sun X. X., Shao X. H., Wu J H., Ding L. J., et al. (2016). MpMYBS3 as a crucial transcription factor of cold signaling confers the cold tolerance of banana. PCTOC. 125, 93–106. 10.1007/s11240-015-0932-y [DOI] [Google Scholar]
- Duan Y. H., Guo J., Ding K., Wang S. J., Zhang H., Dai X. W., et al. (2011). Characterization of a wheat HSP70 gene and its expression in response to stripe rust infection and abiotic stresses. Mol. Biol. Rpt. 38, 301–307. 10.1007/s11033-010-0108-0 [DOI] [PubMed] [Google Scholar]
- Dusenge M. E., Duarte A. G., Way D. A. (2019). Plant carbon metabolism and climate change: elevated CO 2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 221, 32–49. 10.1111/nph.15283 [DOI] [PubMed] [Google Scholar]
- El-Shershaby A., Ullrich S., Simm S., Scharf K.-D., Schleiff E., Fragkostefanakis S. (2019). Functional diversification of tomato HsfA1 factors is based on DNA binding domain properties. Gene 714, 143985. 10.1016/j.gene.2019.143985 [DOI] [PubMed] [Google Scholar]
- Finka A., Cuendet A. F. H., Maathuis F. J., Saidi Y., Goloubinoff P. (2012). Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24, 3333–3348. 10.1105/tpc.112.095844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa S. N., Crocco C. D., Johansson H., Datta S., Hettiarachchi C., Holm M., et al. (2013). The Arabidopsis B-BOX protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. The Plant Cell. 25, 1243–1257. 10.1105/tpc.113.109751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Han X., Wu J., Zheng S., Shang Z., Sun D., et al. (2012). A heat-activated calcium-permeable channel–arabidopsis cyclic nucleotide-gated ion channel 6–is involved in heat shock responses. Plant J. 70, 1056–1069. 10.1111/j.1365-313X.2012.04969.x [DOI] [PubMed] [Google Scholar]
- Gins M., Gins V., Kononkov P., Baikov A., Pivovarov V., Fotev Y. V., et al. (2019). The effect of low positive temperature on the content of low-molecular weight antioxidants in the organs of a vegetable chrysanthemum. Russ. Agric. Sci. 45, 434–438. 10.3103/S1068367419050070 [DOI] [Google Scholar]
- Golldack D., Li C., Mohan H., Probst N. (2014). Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front. Plant Sci. 5, 151. 10.3389/fpls.2014.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Zhao M., Leavitt J. M., Lloyd A. M. (2008). Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in arabidopsis seedlings. Plant J. 53, 814–827. 10.1111/j.1365-313X.2007.03373.x [DOI] [PubMed] [Google Scholar]
- Goswami G., Nath U. K., Park J.-I., Hossain M. R., Biswas M. K., Kim H.-T., et al. (2018). Transcriptional regulation of anthocyanin biosynthesis in a high-anthocyanin resynthesized Brassica napus cultivar. J. Biol. Res. Thessaloniki 25, 1–15. 10.1186/s40709-018-0090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M., Nahar K., Alam M. M., Roychowdhury R., Fujita M. (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 14, 9643–9684. 10.3390/ijms14059643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hichri I., Barrieu F., Bogs J., Kappel C., Delrot S., Lauvergeat V. (2011). Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 62, 2465–2483. 10.1093/jxb/erq442 [DOI] [PubMed] [Google Scholar]
- Islam M. S., Jalaluddin M., Garner J. O., Yoshimoto M., Yamakawa O. (2005). Artificial shading and temperature influence on anthocyanin compositions in sweetpotato leaves. HortScience 40, 176–180. 10.21273/HORTSCI.40.1.176 [DOI] [Google Scholar]
- Jin X., Huang H., Wang L., Sun Y., Dai S. (2016). Transcriptomics and metabolite analysis reveals the molecular mechanism of anthocyanin biosynthesis branch pathway in different Senecio cruentus cultivars. Front. Plant Sci. 7, 1307. 10.3389/fpls.2016.01307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak S., Larkindale J., Lee U., von Koskull-Döring P., Vierling E., Scharf K.-D. (2007). Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 10, 310–316. 10.1016/j.pbi.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Kumar R. R., Arora K., Goswami S., Sakhare A., Singh B., Chinnusamy V., et al. (2020). MAPK enzymes: a ROS activated signaling sensors involved in modulating heat stress response, tolerance and grain stability of wheat under heat stress. 3 Biotech 10, 1–11. 10.1007/s13205-020-02377-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y., Oh J. E., Noh H., Hong S.-W., Bhoo S. H., Lee H. (2011). The ethylene signaling pathway has a negative impact on sucrose-induced anthocyanin accumulation in arabidopsis. J. Plant Res. 124, 193–200. 10.1007/s10265-010-0354-1 [DOI] [PubMed] [Google Scholar]
- Li C., Wang Y., Xu L., Nie S., Chen Y., Liang D., et al. (2016). Genome-wide characterization of the MADS-box gene family in radish (Raphanus sativus L.) and assessment of its roles in flowering and floral organogenesis. Front. Plant Sci. 7, 1390. 10.3389/fpls.2016.01390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Ye J., Li H., Shi Q. (2020). Characterization of metabolites and transcripts involved in flower pigmentation in primula vulgaris. Front. Plant Sci. 11, 572517. 10.3389/fpls.2020.572517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Wang K., Micheletti D., Palmer J., Volz R., Lozano L., Espley R., et al. (2011). High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant Cell Environ. 34, 1176–1190. 10.1111/j.1365-3040.2011.02316.x [DOI] [PubMed] [Google Scholar]
- Liu H.T., Sun D.Y., Zhou R.G. (2005). Ca2+ and AtCaM3 are involved in the expression of heat shock protein gene in arabidopsis. Plant Cell Environ. 28, 1276–1284. 10.1111/j.1365-3040.2005.01365.x19211698 [DOI] [Google Scholar]
- Liu W., Wang Y., Sun J., Jiang H., Xu H., Wang N., et al. (2019). MdMYBDL1 employed by MdHY5 increases anthocyanin accumulation via repression of MdMYB16/308 in apple. Plant Sci. 283, 32–40. 10.1016/j.plantsci.2019.01.016 [DOI] [PubMed] [Google Scholar]
- Lu W., Chen J., Ren X., Yuan J., Han X., Mao L., et al. (2018). One novel strawberry MADS-box transcription factor FaMADS1a acts as a negative regulator in fruit ripening. Sci. Hortic. 227, 124–131. 10.1016/j.scienta.2017.09.042 [DOI] [Google Scholar]
- Luo C., Xiao G., Li Q. (2015). Research advance of the transcription factors related to stress resistances in rice. Guangxi Zhiwu/Guihaia 35, 942–947. [Google Scholar]
- Luo Y., Xie M., Zhang C., Wang W., Zhu J., Wan X., et al. (2018). Function analysis of rice zinc finger protein gene OsBBX22 in response to heat stress. Genomics Appl Biol. 37, 836–844. 10.13417/j.gab.037.000836 [DOI] [Google Scholar]
- Maier A., Schrader A., Kokkelink L., Falke C., Welter B., Iniesto E., et al. (2013). Light and the E3 ubiquitin ligase COP 1/SPA control the protein stability of the MYB transcription factors PAP 1 and PAP 2 involved in anthocyanin accumulation in arabidopsis. Plant J. 74, 638–651. 10.1111/tpj.12153 [DOI] [PubMed] [Google Scholar]
- Martindale J. L., Holbrook N. J. (2002). Cellular response to oxidative stress: signaling for suicide and survival. J. Cell. Physiol. 192, 1–15. 10.1002/jcp.10119 [DOI] [PubMed] [Google Scholar]
- Matoušek J., Vrba L., Škopek J., Orctová L., Pešina K., Heyerick A., et al. (2006). Sequence analysis of a “true” chalcone synthase (chs _H1) oligofamily from hop (Humulus lupulus L.) and PAP1 activation of chs _H1 in heterologous systems. J. Agric. Food Chem. 54, 7606–7615. 10.1021/jf061785g [DOI] [PubMed] [Google Scholar]
- Medina E., Kim S.-H., Yun M., Choi W.-G. (2021). Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants 10, 371. 10.3390/plants10020371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S. K., Tripp J., Winkelhaus S., Tschiersch B., Theres K., Nover L., et al. (2002). In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev. 16, 1555–1567. 10.1101/gad.228802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S. M., Šamec D., Tomczyk M., Milella L., Russo D., Habtemariam S., et al. (2020). Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 38, 107316. 10.1016/j.biotechadv.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Nagar S., Singh V., Arora A., Dhakar R., Ramakrishnan S. (2015). Assessment of terminal heat tolerance ability of wheat genotypes based on physiological traits using multivariate analysis. Acta physiologiae plantarum 37, 1–9. 10.1007/s11738-015-2017-2 [DOI] [Google Scholar]
- Ni J., Bai S., Zhao Y., Qian M., Tao R., Yin L., et al. (2019). Ethylene response factors Pp4ERF24 and Pp12ERF96 regulate blue light-induced anthocyanin biosynthesis in ‘Red Zaosu’pear fruits by interacting with MYB114. Plant Mol. Biol. 99, 67–78. 10.1007/s11103-018-0802-1 [DOI] [PubMed] [Google Scholar]
- Niu J., Zhang G., Zhang W., Goltsev V., Sun S., Wang J., et al. (2017). Anthocyanin concentration depends on the counterbalance between its synthesis and degradation in plum fruit at high temperature. Sci. Rep. 7, 1–16. 10.1038/s41598-017-07896-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda N., Yoshioka S., Kishimoto S., Nakayama M., Douzono M., Tanaka Y., et al. (2017). Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism. Sci. Adv. 3, 7. 10.1126/sciadv.1602785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki K., Fukai S. (2008). Effects of high temperature on floral development and flowering in spray chrysanthemum. J. Appl. Hortic. 10, 8–14. 10.37855/jah.2008.v10i01.02 [DOI] [Google Scholar]
- Ohama N., Sato H., Shinozaki K., Yamaguchi-Shinozaki K. (2017). Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 22, 53–65. 10.1016/j.tplants.2016.08.015 [DOI] [PubMed] [Google Scholar]
- Onik J. C., Hu X., Lin Q., Wang Z. (2018). Comparative transcriptomic profiling to understand pre-and post-ripening hormonal regulations and anthocyanin biosynthesis in early ripening apple fruit. Molecules 23, 1908. 10.3390/molecules23081908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren-Shamir M. (2009). Does anthocyanin degradation play a significant role in determining pigment concentration in plants? Plant Sci. 177, 310–316. 10.1016/j.plantsci.2009.06.015 [DOI] [Google Scholar]
- Ortiz C., Cardemil L. (2001). Heat-shock responses in two leguminous plants: A comparative study. J. Expt. Bot. 52, 1711–1719. 10.1093/jxb/52.361.1711 [DOI] [PubMed] [Google Scholar]
- Petroni K., Tonelli C. (2011). Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 181, 219–229. 10.1016/j.plantsci.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Qi F., Liu Y., Luo Y., Cui Y., Lu C., Li H., et al. (2022). Functional analysis of the ScAG and ScAGL11 MADS-box transcription factors for anthocyanin biosynthesis and bicolour pattern formation in senecio cruentus ray florets. Hortic. Res. 9, uhac071. 10.1093/hr/uhac071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Liu C., Song L., Li M. (2020). PaMADS7, a MADS-box transcription factor, regulates sweet cherry fruit ripening and softening. Plant Sci. 301, 110634. 10.1016/j.plantsci.2020.110634 [DOI] [PubMed] [Google Scholar]
- Qiu J. (2018). Evaluation of character stability of spray cut chrysanthemum under temperature stress. Nanjing Agricultural University. [Google Scholar]
- Qu A.-L., Ding Y.-F., Jiang Q., Zhu C. (2013). Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 432, 203–207. 10.1016/j.bbrc.2013.01.104 [DOI] [PubMed] [Google Scholar]
- Quattrocchio F., Verweij W., Kroon A., Spelt C., Mol J., Koes R. (2006). PH4 of Petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. Plant Cell 18, 1274–1291. 10.1105/tpc.105.034041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K. K., Pandey N., Rai S. P. (2020). Salicylic acid and nitric oxide signaling in plant heat stress. Physiol. Plant. 168, 241–255. 10.1111/ppl.12958 [DOI] [PubMed] [Google Scholar]
- Shi Q., Zhou L., Wang Y., Li K., Zheng B., Miao K. (2015). Transcriptomic analysis of Paeonia delavayi wild population flowers to identify differentially expressed genes involved in purple-red and yellow petal pigmentation. PLoS ONE 10, e0135038. 10.1371/journal.pone.0135038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A., Zhu X., Chen F., Gao H., Jiang J., Chen S. (2014). A chrysanthemum heat shock protein confers tolerance to abiotic stress. Int. J. Mol. Sci. 15, 5063–5078. 10.3390/ijms15035063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Fan X., Zhang Y., Jiang J., Sun H., Liu C. (2016). Transcriptome analysis of genes involved in anthocyanins biosynthesis and transport in berries of black and white spine grapes (Vitis davidii). Hereditas 153, 1–22. 10.1186/s41065-016-0021-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M.-F., Gao B. (2004). Heat shock protein and innate immunity. Cell. Mol. Immunol. 1, 274–279. [PubMed] [Google Scholar]
- Tunc-Ozdemir M., Tang C., Ishka M. R., Brown E., Groves N. R., Myers C. T., et al. (2013). A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 161, 1010–1020. 10.1104/pp.112.206888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubi B. E., Honda C., Bessho H., Kondo S., Wada M., Kobayashi S., et al. (2006). Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature. Plant Sci. 170, 571–578. 10.1016/j.plantsci.2005.10.009 [DOI] [Google Scholar]
- Usman M. G., Rafii M. Y., Ismail M. R., Malek M. A., Latif M. A. (2015). Expression of target gene Hsp70 and membrane stability determine heat tolerance in chili pepper. J. Am. Soc. Hortic. Sci. 140, 144–150. 10.21273/JASHS.140.2.144 [DOI] [Google Scholar]
- Usman M. G., Rafii M. Y., Martini M. Y., Yusuff O. A., Ismail M. R., Miah G. (2017). Molecular analysis of Hsp70 mechanisms in plants and their function in response to stress. Biotechnol. Genet. Eng. Rev. 33, 26–39. 10.1080/02648725.2017.1340546 [DOI] [PubMed] [Google Scholar]
- Van Der Ploeg A., Heuvelink E. (2015). The influence of temperature on growth and development of chrysanthemum cultivars. J. Hortic. Sci. Biotechnol. 81, 174–182. 10.1080/14620316.2006.11512047 [DOI] [Google Scholar]
- Verweij W., Spelt C. E., Bliek M., de Vries M., Wit N., Faraco M., et al. (2016). Functionally similar WRKY proteins regulate vacuolar acidification in petunia and hair development in Arabidopsis. Plant Cell 28, 786–803. 10.1105/tpc.15.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Guo Y., Jia L., Chu H., Zhou S., Chen K., et al. (2014). Hydrogen peroxide acts upstream of nitric oxide in the heat shock pathway in arabidopsis seedlings. Plant Physiol. 164, 2184–2196. 10.1104/pp.113.229369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley D., Goldberg S. P., Jordan W. D. (1999). Heat shock proteins: a review of the molecular chaperones. J. Vasc. Surg. 29, 748–751. 10.1016/S0741-5214(99)70329-0 [DOI] [PubMed] [Google Scholar]
- Willits M. G., Kramer C. M., Prata R. T., De Luca V., Potter B. G., Steffens J. C., et al. (2005). Utilization of the genetic resources of wild species to create a nontransgenic high flavonoid tomato. J. Agric. Food Chem. 53, 1231–1236. 10.1021/jf049355i [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. (2002). Molecular genetics and control of anthocyanin expression. 37, 75–94. 10.1016/S0065-2296(02)37044-7 [DOI] [Google Scholar]
- Xu W., Dubos C., Lepiniec L. (2015). Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends Plant Sci. 20, 176–185. 10.1016/j.tplants.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Yamagishi M., Shimoyamada Y., Nakatsuka T., Masuda K. (2010). Two R2R3-MYB genes, homologs of petunia AN2, regulate anthocyanin biosyntheses in flower tepals, tepal spots and leaves of Asiatic hybrid lily. Plant Cell Physiol. 51, 463–474. 10.1093/pcp/pcq011 [DOI] [PubMed] [Google Scholar]
- Yang W., Sun Y., Chen S., Jiang J., Chen F., Fang W., et al. (2011). The effect of exogenously applied nitric oxide on photosynthesis and antioxidant activity in heat stressed chrysanthemum. Biol. Plant. 55, 737–740. 10.1007/s10535-011-0178-4 [DOI] [Google Scholar]
- Ye S., Yu S., Shu L., Wu J., Wu A., Luo L. (2012). Expression profile analysis of 9 heat shock protein genes throughout the life cycle and under abiotic stress in rice. Chin. Sci. Bull. 57, 336–343. 10.1007/s11434-011-4863-7 [DOI] [Google Scholar]
- Zhang F., Gonzalez A., Zhao M., Payne C. T., Lloyd A. (2003). A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development. 130, 4859–69. 10.1242/dev.00681 [DOI] [PubMed] [Google Scholar]
- Zhang H., He H., Wang X., Wang X., Yang X., Li L., et al. (2011). Genome-wide mapping of the HY5-mediated genenetworks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J. 65, 346–358. 10.1111/j.1365-313X.2010.04426.x [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu B., Li J., Zhang L., Wang Y., Zheng H., et al. (2015). Hsf and Hsp gene families in Populus: genome-wide identification, organization and correlated expression during development and in stress responses. BMC Genom. 16, 1–19. 10.1186/s12864-015-1398-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang L., Zeng X., Chen R., Yang S., Pan S. (2019). Comparative transcriptome analysis reveals fruit discoloration mechanisms in postharvest strawberries in response to high ambient temperature. Food Chemistry: X. 2, 100025. 10.1016/j.fochx.2019.100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Butelli E., Martin C. (2014). Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 19, 81–90. 10.1016/j.pbi.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chu G., Hu Z., Gao Q., Cui B., Tian S., et al. (2016). Genetically engineered anthocyanin pathway for high health-promoting pigment production in eggplant. Mol. Breeding 36, 1–14. 10.1007/s11032-016-0454-2 [DOI] [Google Scholar]
- Zhao D., Tao J., Han C., Ge J. (2012). Flower color diversity revealed by differential expression of flavonoid biosynthetic genes and flavonoid accumulation in herbaceous peony (Paeonia lactiflora Pall.). Mol. Biol. Rep. 39, 11263–11275. 10.1007/s11033-012-2036-7 [DOI] [PubMed] [Google Scholar]
- Zhou L.-J., Geng Z., Wang Y., Wang Y., Liu S., Chen C., et al. (2021). A novel transcription factor CmMYB012 inhibits flavone and anthocyanin biosynthesis in response to high temperatures in chrysanthemum. Horticulture Res. 8, 248. 10.1038/s41438-021-00675-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Fonseca De Lima C. F., De Smet I. (2021). The heat is on: how crop growth, development, and yield respond to high temperature. J. Exp. Bot. 72, 7359–7373. 10.1093/jxb/erab308 [DOI] [PubMed] [Google Scholar]
- Zimmermann I. M., Heim M. A., Weisshaar B., Uhrig J. F. (2004). Comprehensive identification of arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J. 40, 22–34. 10.1111/j.1365-313X.2004.02183.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study can be found in the NCBI sequence reads archive (SRA) database under BioProject No. PRJNA859415.