Highlights
-
•
Measles spread by children socializing and playing around watering points in Semuto Subcounty.
-
•
Older children (age 5–9 years) were affected more than younger children (age 1–4 years) by measles.
-
•
This measles outbreak occurred in a vaccinated community with only a single dose of measles vaccine.
-
•
The findings support the need for a second dose of the measles vaccine in routine vaccination.
Keywords: Measles, Disease outbreaks, Risk factors, Vaccine coverage, Case–control studies, Uganda
Abstract
Background
Semuto Subcounty reported rubella/measles outbreaks in January 2020 and June–August 2021. This study investigated the outbreak in 2021 to determine the scope, and the factors associated with transmission.
Methods
A probable case was defined as a resident of Semuto Subcounty with acute onset of fever and a generalized maculopapular rash with either cough/cold or red eyes from 1 June to 31 August 2021. A confirmed case was defined as a probable case with a blood sample positive for measles-specific IgM. A village-matched case–control study was conducted with 30 cases and 122 controls (1:4 ratio). A control was defined as an individual aged 6 months–9 years, sampled at random, with no signs or symptoms of measles from 1 June to 31 August 2021, residing in the same village as the matched case. Adjusted Mantel–Haenszel odds ratios (ORMH) and confidence intervals (CIs) were calculated.
Results
Of the 30 cases (27 probable and three confirmed), 16 (53%) were male. The subcounty attack rate (AR) was 3.2/1000. Children aged 5–9 years were the most affected (AR 5.0/1000). Twenty-two (79%) cases and 116 (97%) controls had ever received measles vaccine (ORMH 0.13, 95% CI 0.03–0.52). Interaction with symptomatic persons at water collection points (ORMH 4.4, 95% CI 1.6–12) and playing at community playgrounds (ORMH 4.2, 95% CI 1.7–11) increased the odds of infection.
Conclusions
Socializing/congregating at water collection points and community playgrounds facilitated the transmission of measles in this outbreak.
Background
Measles is an acute, highly infectious, vaccine-preventable viral disease for which humans are the only reservoir. It can be transmitted through the air or from direct contact with an infected person (Bloch et al., 1985; Griffin, 1995; WHO, 2019a). The incubation period for measles is approximately 14 days, with a range of 7–21 days (Lessler et al., 2009; Heymann, 2015; WHO, 2019a). Worldwide, measles is one of the top five causes of vaccine-preventable morbidity and mortality (CDC, 2006). Before the measles vaccine was introduced in 1963, major measles epidemics occurred almost every 2–3 years globally, causing an estimated 2.6 million deaths each year (WHO, 2019a). However, despite the availability of a safe and effective vaccine, more than 140,000 people – mainly children aged <5 years – died from measles in 2018 (WHO, 2019a).
Nearly 17,500 cases of measles were recorded in Africa from January to March 2022, representing a 400% increase compared with the same period in 2021. Twenty African countries reported measles outbreaks in the first quarter of 2022, which was eight more than in the same period in 2021 (WHO, 2022). Inequalities in accessing vaccines, and disruptions caused by the coronavirus disease 2019 (COVID-19) pandemic, including a huge strain on health system capacity, impaired routine immunization services in most African countries, and forced the suspension of vaccination drives in 2020 and 2021. Only six countries in Africa attained 95% vaccine coverage (VC) for the first dose of measles vaccine in 2019, and only three countries met that target in 2020 (WHO, 2022).
At present, children in Uganda receive a single dose of measles–rubella (MR) containing vaccine at 9 months of age as part of the routine vaccination schedule (MOH, 2019a). From 2010 to 2020, the VC of single-dose measles-containing vaccine improved from 73% to 95% in Uganda (WHO, 2021). Despite this VC, of all the vaccine-preventable diseases, measles outbreaks are still the most common outbreaks reported to the Ministry of Health (MoH) in Uganda, with 89 (66%) districts reporting measles outbreaks between 2018 and 2020 (ARA, unpublished data; MoH 2019b, MoH 2019c). Due to the continuing outbreaks, a nationwide MR vaccination campaign targeting children aged 9 months–15 years was conducted in Uganda in October 2019 (MoH, 2019b,c). In order to strengthen the achievements of the campaign (ARA, unpublished data), the MoH and its partners continue to intensify routine vaccination, and are working towards making a second dose of measles vaccine available in October 2022 (WHO, 2004, 2019b; Anonymous, 2020; CDC, 2021b; MoH, 2022b). The second dose of the vaccine is intended to reduce the risk of measles, increase protection against measles, and save the country from repetitive and costly mass measles vaccinations (WHO, 2019b). The first dose of the measles vaccine will continue to be given at 9 months of age, and the second dose will be given at 18 months of age (WHO, 2019b).
The mass MR campaign in Uganda between 15 and 22 October 2019 was the largest vaccination campaign in the history of the country (WHO, 2019b). During that mass MR campaign, a total of 19,476,110 (108%) children were vaccinated against measles and rubella out of the targeted 18,100,000 children aged 9 months–15 years. As a result of the mass MR vaccination campaign, most of the measles isolation wards in the country returned to a measles-free status, with a 71% reduction in the number of clinically suspected cases through weekly surveillance reports (WHO, 2019b). In the week of 4–10 November 2019, only 212 suspected cases of measles and rubella were reported following the vaccination campaign, compared with 733 during the week of 1–7 April 2019 (WHO, 2019b).
In January 2020, shortly after the mass MR campaign, a laboratory-confirmed rubella outbreak was reported in Semuto Subcounty, Nakaseke District, Uganda, primarily among vaccinated children (Kyamwine, 2020). Blood samples were collected by the Expanded Program on Immunization (EPI) focal person of Semuto Subcounty (a diploma nurse) from five suspected cases of measles in August 2021. In accordance with the guidelines of the World Health Organization (WHO), the EPI focal person transported these samples to the Uganda National Expanded Program on Immunization (UNEPI) Laboratory at the Uganda Virus Research Institute (UVRI) for laboratory confirmation. On 25 August 2021, the MoH was notified (through e-mail and telephone) by the UVRI of three blood samples that tested positive for measles-specific IgM from patients in Semuto Subcounty. This study investigated the measles outbreak to determine the scope, assess factors associated with transmission, estimate VC and vaccine effectiveness (VE), and recommend evidence-based control measures.
Methods
Outbreak area
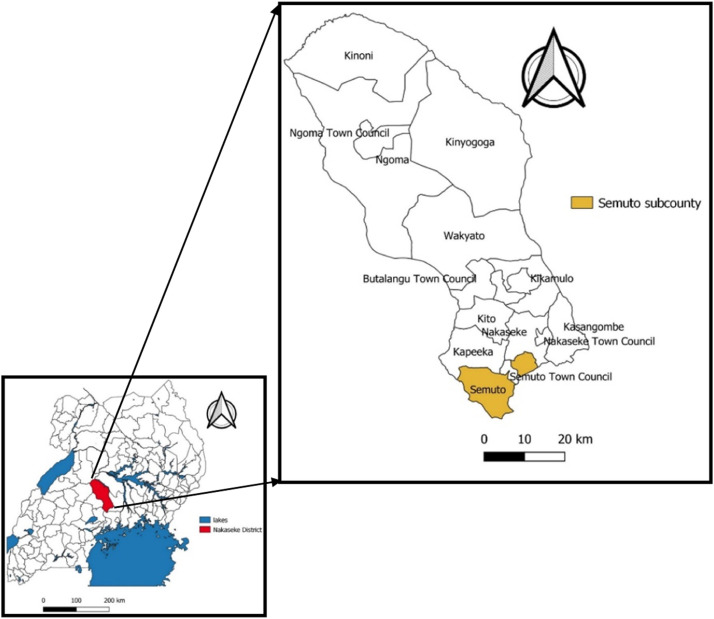
Nakaseke District is made up of 10 subcounties and five town councils (NDLG, 2022). The present study was conducted between 29 August and 11 September 2021 in Semuto Subcounty, which is located in the south of Nakaseke District in the central region of Uganda (Figure 1). Semuto Subcounty is made up six parishes (Segalye, Kikandwa, Kirema, Kikyusa, Migyingye and Kisega), and crop farming is the major economic activity of its inhabitants. There are four health centres (HCs) in Semuto Subcounty; two are government-owned (Kalege HC II and Kikandwa HC II) and two are privately owned (Kirema HC III and Bukatira HC II). All four HCs provide outpatient care services alone, including immunization. These HCs receive vaccines primarily from Nakaseke District vaccine store, and sometimes from other HCs in the district. Through the EPI cold chain, which is a system of storage and distribution of vaccines at specified temperatures of +2°C to +8°C, these vaccines are transported from the manufacturer to the national medical stores and then to Nakaseke District vaccine store (MoH, 2022a).
Figure 1.
Location of Semuto Subcounty, Nakaseke District, Uganda.
In August 2021, the administrative routine measles VC in Nakaseke District was 79%, which is lower than the recommended VC of 95% needed to achieve herd immunity against measles (Macmillan, 2021). The administrative measles VC in Semuto Subcounty in the same time period was 24% (UBOS, 2016; MoH, 2022c).
Case definition and finding
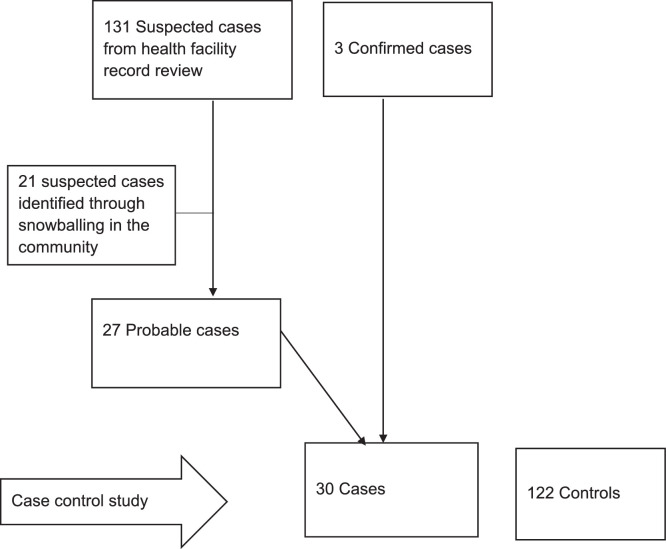
Using the Uganda national technical guidelines for integrated disease surveillance and response, a suspected case was defined as a resident of Semuto Subcounty with acute onset of fever and at least one of the following symptoms – cough, cold, red eyes or a generalized maculopapular skin rash – from 1 June to 31 August 2021. A probable case was defined as a suspected case with generalized maculopapular skin rash and at least one of the other suspected case symptoms. A confirmed case was a suspected or probable case with a positive measles-specific IgM test (MoH, 2021).
Suspected cases were listed by reviewing outpatient medical records in all four HCs in Semuto Subcounty. The parents/guardians of all suspected cases were interviewed to document their detailed clinical history, and reclassify them as probable cases or not probable cases (Figure 2). To find additional cases, members of households with suspected or probable cases were asked about other children with similar or measles-like symptoms (snowballing approach). Using an electronic standardized case investigation form, data on the case's demographics, clinical information, vaccination status and exposure history were collected. Laboratory confirmation was conducted by the UNEPI Laboratory at UVRI using the recommended WHO procedures (WHO, 2007).
Figure 2.
Study participants, Semuto Subcounty, Nakaseke District, Uganda, June–August 2021.
Descriptive epidemiology
Attack rates (ARs) were calculated by person and place, using the Uganda Bureau of Standards 2021 projected population of children in Semuto Subcounty as the denominator (UBOS, 2016). An epidemic curve was constructed to assess the time distribution of measles cases.
Hypothesis generation
Eleven hypothesis-generating interviews were conducted using a standardized measles case investigation form. Parents/guardians of cases were asked about potential risk factors for measles transmission occurring between 7 and 21 days before symptom onset. These included attending social gatherings, attending places of worship and visiting HCs. Parents/guardians were also asked about receipt of vitamin A supplementation in the 6 months before symptom onset, and the case's immunization status before symptom onset. Evidence of vaccination included child health cards or, if the health card was missing, parent/guardian's recall; the authors attempted to confirm the latter by asking for details of the site and age at which the child received the measles vaccine. Additional risk factors assessed included visiting community playgrounds and water collection points, attending medical camps, congestion levels in the household, being in contact with a symptomatic patient, and having received a visitor in the household. Hypotheses were generated about exposures based on findings from the descriptive epidemiological analysis and hypothesis-generating interviews.
Case–control investigation
A village-matched case–control investigation was conducted in the three affected parishes (Segalye, Kirema, and Kikandwa) of Semuto Subcounty to test the hypotheses. Controls were children aged 6 months–9 years, as all cases were in this age range. As all the cases were minors, parents/guardians completed a pre-tested WHO-validated electronic questionnaire. Only probable or confirmed cases were included as cases in the case–control study. A control was defined as any person aged 6 months–9 years without signs and symptoms of measles from 1 June to 31 August 2021, residing in any of the three affected parishes of Semuto Subcounty. Cases and controls were selected in a ratio of 1:4, with two additional controls identified (total of 30 cases and 122 controls). Simple random sampling was used to select controls from the same village as cases. The sampling frames were the village health team household lists. Epi Info 7.2.4.0 was used for analysis. Adjusted Mantel–Haenszel odds ratios (ORMH) (GoA, 2017) and their corresponding 95% confidence intervals (CIs) (Fleiss et al., 2013) were used to assess factors associated with measles infection. An additional analysis was conducted using a common reference group for factors that were significantly associated with the measles outbreak.
Vaccine coverage
VC for one dose of measles vaccine was estimated using the percentage of controls that had a history of measles vaccination in the case–control investigation.
Vaccine effectiveness
VE of the measles vaccine was calculated using:
VE =1-ORMH (Weinberg and Szilagyi, 2010),
where ORMH was associated with having received at least one dose of the measles vaccine from the case–control investigation.
Results
Descriptive epidemiology
In total, 30 cases (27 probable and three confirmed) were identified, 16 (53%) of whom were male. There were no deaths. The overall subcounty AR was 3.2/1000. The most affected parish was Segalye (AR 9.5/1000), followed by Kikandwa (AR 7.4/1000) and Kirema (AR 3.0/1000). The age range of the cases was 6 months–9 years. The most affected age group was 5–9 years (AR 5.0/1000), followed by 1–4 years (AR 2.6/1000) (Table 1). The AR was similar in males (3.3/1000) and females (3.2/1000).
Table 1.
Measles attack rates (ARs) by parish of residence, age group and sex during a measles outbreak in Semuto Subcounty, Nakaseke District, Uganda, June–August 2021.
| Characteristic/variable | Population at risk (n)a | Number of probable and confirmed cases (n=30) | AR/1000 | |
|---|---|---|---|---|
| Subcounty | ||||
| Semuto | 9267 | 30 | 3.2 | |
| Parish | ||||
| Segalye | 2009 | 19 | 9.5 | |
| Kikandwa | 809 | 6 | 7.4 | |
| Kirema | 1667 | 5 | 3.0 | |
| Kikyusa | 1809 | 0 | 0 | |
| Migyingye | 1869 | 0 | 0 | |
| Kisega | 1104 | 0 | 0 | |
| Age | ||||
| 6 months–<1 year | 1200 | 2 | 1.7 | |
| 1–4 years | 5077 | 13 | 2.6 | |
| 5–9 years | 2990 | 15 | 5.0 | |
| Sex | ||||
| Male | 4827 | 16 | 3.3 | |
| Female | 4440 | 14 | 3.2 | |
As only children aged ≥6 months and <10 years were affected, the total population at risk was calculated as that of children aged 6 months–<10 years.
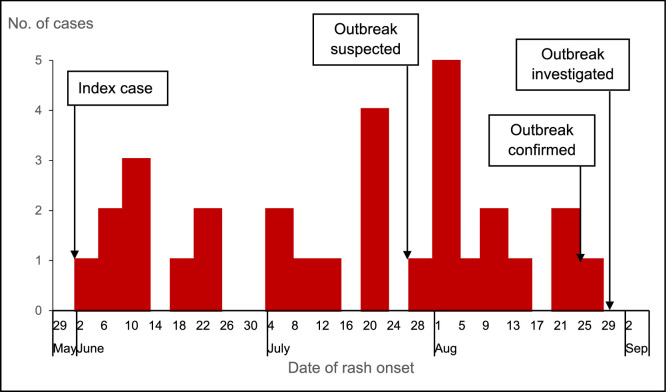
All (100%) cases presented with fever, a generalized rash and red eyes; 27 (90%) cases had cough and cold. In addition, 10 (33%) cases had pneumonia, eight (27%) cases had oral and throat sores, and two (6.7%) cases had otitis media, all of which can be considered complications of measles. The epidemic curve (Figure 3) showed a propagated measles outbreak lasting approximately 88 days. The index case was identified on 2 June 2021 in Kirema Parish. The index case was an unvaccinated male child aged 2 years with a normal nutrition status and no history of vitamin A supplementation in the 6 months preceding the infection. The outbreak was not suspected until 28 July 2021, when health workers at Kalege HC II received reports of multiple children with measles-like symptoms in the community. The outbreak was confirmed on 25 August 2021, which was the same day that the last case developed symptoms. Investigations started on 29 August 2021.
Figure 3.
Distribution of measles cases by date of rash onset, Semuto Subcounty, Nakaseke District, Uganda, June–August 2021.
Hypothesis-generating findings
Of the 11 cases interviewed, eight (73%) had visited a water collection point during the exposure period, and three (27%) had played at community playgrounds. Three (27%) cases had not received vitamin A supplementation in the 6 months preceding the outbreak, and two (18%) cases were unvaccinated. No other exposures were reported. All exposures that were reported by at least two cases were considered as potential exposures for inclusion in the case–control study. Therefore, a visit to a water collection point, vitamin A supplementation, playing at a community playground, and being unvaccinated were considered as possible factors associated with the outbreak.
Case–control investigation findings
Nine (30%) cases and 12 (10%) controls met a symptomatic person at a water collection point (ORMH 4.4, 95% CI 1.6–12). Twenty-one (70%) cases and 46 (38%) controls played at community playgrounds (ORMH 4.2, 95% CI 1.7–11) (Table 2). Meeting a symptomatic person (OR 6.8, 95% CI 1.5–31) and playing at a playground (OR 5.3, 95% CI 1.8–16) were both independently associated with increased odds of infection (Table 3). The combination of these two exposures had an additive effect on the odds of infection (OR 13.6, 95% CI 2.9–63).
Table 2.
Factors associated with the measles outbreak in Semuto Subcounty, Nakaseke District, Uganda, June–August 2021.
| Risk factor | Cases (n=30) |
Controls (n=122) |
ORMH (95% CI) | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Measles vaccination | 22a | 79 | 116b | 97 | 0.13 (0.03–0.52)c |
| Received vitamin A in last 6 months | 15 | 50 | 13d | 45 | 2.5 (0.77–8.0) |
| Visited health facility | 6 | 20 | 20e | 17 | 1.3 (0.47–3.6) |
| Travelled to a different area during the exposure period | 1f | 3 | 9 | 7 | 0.44 (0.05–3.6) |
| Visited water collection point | 22 | 73 | 82 | 67 | 1.4 (0.55–3.3) |
| Played at water collection point | 15 | 50 | 68e | 56 | 0.8 (0.35–1.8) |
| Long lines at water collection point | 10 | 33 | 33 | 27 | 1.4 (0.59–3.3) |
| Met a symptomatic person at a water collection point | 9 | 30 | 13 | 11 | 4.4 (1.6–12)c |
| Played at community playground | 21 | 70 | 46 | 38 | 4.2 (1.7–11)c |
ORMH, adjusted Mantel–Haenszel odds ratio; CI, confidence interval.
Among 28 cases responding.
Among 120 controls responding.
Significant association (P<0.05).
Among 29 controls responding.
Among 121 controls responding.
Among 29 cases responding.
Table 3.
Common reference group analysis of factors associated with the measles outbreak in Semuto Subcounty, Nakaseke District, Uganda, June–August 2021.
| Met symptomatic person at water point | Played at playground | Cases (n=30) |
Controls (n=122) |
OR (95% CI) |
P-value | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| - | - | 5 | 7 | 68 | 93 | 1.0 | ||
| + | - | 4 | 33 | 8 | 67 | 6.8 (1.5–31) | 0.024 | |
| - | + | 16 | 28 | 41 | 72 | 5.3 (1.8–16) | 0.003 | |
| + | + | 5 | 50 | 5 | 50 | 13.6 (2.9–63) | 0.001 | |
OR, odds ratio; CI, confidence interval.
Vaccine coverage and vaccine effectiveness
VC was estimated to be 97% (95% CI 92–99%) among controls aged ≥9 months–9 years. Twenty-two (79%) cases and 116 (97%) controls had a history of measles vaccination (ORMH 0.13, 95% CI 0.03–0.52), so VE was estimated to be 87% (95% CI 48–97%). Additional analysis showed that the vaccine reduced the odds of infection significantly among children aged 1–4 years (ORMH 0.01, 95% CI 0.001–0.18), but the difference was not significant in children aged 5–9 years (ORMH 3.1, 95% CI 0.16–59) (Table 4).
Table 4.
Age-group-specific impact of measles vaccine among children aged 1–9 years in Semuto Subcounty, Nakaseke District, Uganda, June–August 2021.
| Characteristic | Cases |
Controls |
OR (95% CI) | P-value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| 1–4 years | 13 | 62 | ||||
| Vaccinated | 7 | 10 | 62 | 90 | 0.01 (0.001-0.18) | 0.002a |
| Not vaccinated | 6 | 100 | 0 | 0 | ||
| 5–9 years | 18 | 58 | ||||
| Vaccinated | 18 | 22 | 54 | 78 | 3.1 (0.16–59) | 0.46 |
| Not vaccinated | 0 | 0 | 4 | 100 | ||
OR, odds ratio; CI, confidence interval.
Significant association (P<0.05).
Discussion
The measles outbreak in Semuto Subcounty in 2021 represented the second measles or rubella outbreak in 2 years following a mass MR vaccination campaign in this area. Vaccination was associated with reduced odds of infection. The outbreak was propagated by children socializing and congregating at community water collection points and playgrounds. The high VC and relatively high VE are within the norms for a single dose of measles vaccine, and almost certainly reduced community susceptibility to infection.
A history of measles vaccination was protective in this outbreak. However, ARs were twice as high in children aged 5–9 years as in children aged 1–4 years. This may be due to waning vaccine-induced immunity in older children, for whom vaccination is further in the past than for children aged 1–4 years (Yang et al., 2020). This phenomenon has been described in outbreaks of measles in India, Germany and Switzerland, in which the AR in vaccinated children increased with age despite equal immunization levels across age groups (Sharma et al., 2004; Richard et al., 2008; Wichmann et al., 2009); further study of this potential waning immunity and its role in outbreaks is warranted. While school-aged children may have more opportunities for exposure that could result in increased ARs in this age group, this outbreak occurred during a period of school closure in Uganda due to the COVID-19 pandemic (MoES, 2021), and thus this was not a factor in this outbreak. The comparatively lower protection offered by a single dose of measles vaccine led to the recommendation by WHO to add a second dose of measles vaccine into the routine vaccination schedule (WHO, 2009). Many developed countries, such as Finland, Hungry, the USA, Canada, Oman and the UK, eliminated measles by introducing and ensuring sustained high VC with two doses of measles vaccine (Gay, 2000). Based on the WHO recommendations and on observations of potential waning immunity after a single dose of MR vaccine in Uganda, UNEPI introduced a second dose of measles vaccine for older children into the national routine immunization schedule starting in October 2022 ( WHO, 2004, 2019b; Anonymous, 2020; CDC, 2021b; MoH, 2022b). Although the number of annual measles outbreaks in Uganda declined after the mass vaccination campaign in 2019, it is expected that the introduction of the second measles vaccine dose in the routine vaccination schedule will increase VE of the measles vaccine nationally and will reduce the number of outbreaks further (ARA, unpublished data).
Socializing and congregating at water collection points and community playgrounds were associated with increased odds of infection, and the impact of both exposures was additive, suggesting independent risks associated with each exposure. Other studies have also identified socializing and congregating as factors that facilitate the transmission of measles (Marin et al., 2006; Jin et al., 2011; Majwala et al., 2018). In rural Uganda, water collection points are typically areas where young children play with each other while they wait for their mothers or older siblings to collect water for domestic use. If a child at a water collection point is ill, other children can be put at risk of contracting infection. Another measles outbreak investigation in Uganda also identified interactions at water collection points as a risk factor for measles transmission (Majwala et al., 2018). In this setting, it is important to educate parents to recognize possible signs and symptoms of measles, and to keep children who are experiencing consistent symptoms at home in order to reduce the risk of propagating measles outbreaks.
VE of a single dose of measles vaccine in this outbreak was below the recommended ≥93% (CDC, 2021a). While studies have shown that measles outbreaks can occur in communities with >95% VC and with documented VE >85% (Markowitz et al., 1989; Marin et al., 2006), most measles outbreaks occur in settings with VC <95%, VE <85% or both ( Belda et al., 2017; Mohammed and Alemu, 2017; Majwala et al., 2018; Nsubuga et al., 2018). The relatively high VC and VE in this investigation could explain the small size and limited spread of this outbreak (Yeung et al., 2005). The lack of severe illness or deaths among patients in this study may also be reflected in the high vaccination rates; studies have shown that when measles occurs in immunized individuals, the illness is less severe (Aaby et al., 1986; Akramuzzaman et al., 2002).
Limitations of the study
This investigation had some limitations. The lack of sufficient cases and controls to be included in the multi-variate regression analysis model rendered it impossible to conduct a more detailed analysis (multi-variate regression analysis) to control for confounding. Vaccination status was, in some cases, based on parent's/guardian's recall, which may have led to recall bias, leading to overestimation or underestimation of VE and VC. Additionally, age is likely to be associated with social activity, which may have confounded the association between age and odds of infection.
In this investigation, it was assumed that the controls were representative of the general population, and the proportion of controls vaccinated was used to estimate VC instead of the standard WHO community survey method. This may have overestimated VC. It was not possible not to triangulate the administrative measles VC with the estimated measles VC (proportion of controls vaccinated) used to calculate VC in this study as vaccination records in some of the HCs were not up to date. This resulted in a low records-based administrative VC of 24% (UBOS, 2016; MoH, 2022c), compared with the calculated VC of 97%. Finally, the authors did not ascertain the history of measles infection outside the study exposure and outbreak period as a source of measles immunity among the controls, which could have biased the calculation of VE. If immunity in vaccinated controls is due, in part, to a previous measles infection outside the current infection, this could have artificially increased the estimated VE. Alternatively, if unvaccinated controls were previously affected more than the vaccinated population, this could have reduced estimated VE (Velicko et al., 2008).
Conclusion
This community measles outbreak affected children aged 6 months–9 years in Segalye, Kikandwa and Kirema Parishes, Semuto Subcounty from 2 June to 28 August 2021. Socializing and congregating at water collection points and community playgrounds were associated with this measles outbreak. Measles vaccination was protective against measles infection. It is recommended that the MoH team should develop information, education, and communication materials and messages with a specific focus on the risks associated with socializing with children who are ill with measles-like symptoms. It is also recommended that Nakaseke District Health Team should conduct a mass community measles vaccination (or revaccination) campaign for all children aged 6 months–9 years in Semuto Subcounty in order to capture unvaccinated children in the area, and provide a second dose for those who may have received one dose. Parents and guardians are urged to recognize, isolate and keep children with measles-like signs and symptoms at home. Children who had not received the measles vaccines were referred to nearby HCs, where they received their vaccines.
Acknowledgments
Acknowledgements
The authors wish to thank Nakaseke District leadership, including the District Health Team who spearheaded the district outbreak response efforts; and Mr. Yawe Moses, the Nakaseke District Biostatistician/Surveillance focal person and acting District Health Officer of Nakaseke District at the time for coordinating the investigation activities. The authors also wish to thank Ms. Namisango Christine, a Diploma Nurse at Kalege HC III and EPI focal person of Semuto Subcounty at the time, for her vigilance in identifying the measles cases and her involvement in the investigation.
Conflict of interest statement
None declared.
Funding
The outbreak investigation was supported by the President's Emergency Plan for AIDS Relief through US CDC Cooperative Agreement Number GH001353–01 through Makerere University School of Public Health to the Uganda Public Health Fellowship Programme, MoH. The contents of this article are exclusively the responsibility of the authors, and do not essentially represent the official views of the US CDC, Makerere University School of Public Health, or the MoH.
Ethical approval and consent to participate
The MoH gave the directive and approval to investigate this outbreak. In agreement with the International Guidelines for Ethical Review of Epidemiological Studies by the Council for International Organizations of Medical Sciences (1991) and the Office of the Associate Director for Science, CDC/Uganda, it was determined that this activity was not human subject research and that its primary intent was public health practice or disease control activity (specifically, epidemic or endemic disease control activity). This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy. All experimental protocols were approved by the US CDC human subjects review board and the MoH, and have been performed in accordance with the Declaration of Helsinki. Verbal informed consent was obtained from the participants before the start of each interview. Verbal informed consent was obtained from parents/guardians on behalf of all the children before the start of each interview as they were aged <10 years.
Availability of data and materials
The datasets upon which the findings are based belong to the Uganda Public Health Fellowship Programme. For confidentiality reasons, the datasets are not publicly available. However, the data sets can be made available upon reasonable request from the corresponding author, with permission from the Uganda Public Health Fellowship Programme.
Author contributions
EJN took the lead in conceptualization of the study idea, data analysis, and writing and editing the manuscript. JM and JN were involved in conceptualization of the study idea, and writing and editing the manuscript. DK, FN, IBK, LB, BK, ARA and JH were involved in conceptualization of the study idea, and writing, editing and reviewing the manuscript. All authors read and approved the final manuscript.
References
- Aaby P, Bukh J, Leerhøy J, Lisse IM, Mordhorst CH, Pedersen IR. Vaccinated children get milder measles infection: a community study from Guinea-Bissau. J Infect Dis. 1986;154:858–863. doi: 10.1093/infdis/154.5.858. [DOI] [PubMed] [Google Scholar]
- Akramuzzaman SM, Cutts FT, Hossain MJ, Wahedi OK, Nahar N, Islam D, et al. Measles vaccine effectiveness and risk factors for measles in Dhaka. Bangladesh. Bull World Health Organ. 2002;80:776–782. [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Gov't moves to introduce second dose of measles vaccine. The Independent, 10 December 2020. Available at: https://www.independent.co.ug/govt-moves-to-introduce-second-dose-of-measles-vaccine/(accessed 14 September 2022).
- Belda K, Tegegne AA, Mersha AM, Bayenessagne MG, Hussein I, Bezabeh B. Measles outbreak investigation in Guji zone of Oromia Region, Ethiopia. Pan Afr Med J. 2017;27(Suppl. 2):9. doi: 10.11604/pamj.supp.2017.27.2.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch AB, Orenstein WA, Ewing WM, Spain WH, Mallison GF, Herrmann KL, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics. 1985;75:676–683. [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA: 2006. Vaccine preventable deaths and the Global Immunization Vision and Strategy, 2006–2015. [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA: 2021. Vaccines and preventable diseases: measles, mumps, and rubella (MMR) vaccination: what everyone should know. [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA: 2021. Who should get MMR vaccine? Children. [Google Scholar]
- Fleiss JL, Levin B, Paik MC. John Wiley & Sons; New York: 2013. Statistical methods for rates and proportions. [Google Scholar]
- Gay NJ. Eliminating measles – no quick fix. Bull World Health Organ. 2000;78:949. [PMC free article] [PubMed] [Google Scholar]
- GoA. Foodborne disease outbreak toolkit: analysis of case control studies. Canberra: Government of Australia; 2017.
- Griffin D. Immune responses during measles virus infection. Curr Top Microbiol Immunol. 1995;191:117–134. doi: 10.1007/978-3-642-78621-1_8. [DOI] [PubMed] [Google Scholar]
- Heymann DL. American Public Health Association; Washington, DC: 2015. Control of communicable diseases manual. [Google Scholar]
- Jin Y, Ma H, Zhang L, He H, Yisimaer M, Chen M, et al. Measles outbreak on a college campus transmitted through internet cafés. J Infect Dis. 2011;204:S471–S475. doi: 10.1093/infdis/jir069. [DOI] [PubMed] [Google Scholar]
- Kyamwine IB, Kamulegeya J, Kwesiga B, Ario AR, Harris J. January 2020Uganda National Institute of Pubic Health Quarterly Epidemiological Bulletin; Nakaseke District, Uganda: 2020. Rubella outbreak among vaccinated children propagated by attending school X; p. 5. April to June. [Google Scholar]
- Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan C. Herd immunity: will we ever get there? Yale Medicine; 2021. Available at: https://www.yalemedicine.org/news/herd-immunity (accessed 14 September 2022).
- Majwala RK, Nakiire L, Kadobera D, Ario AR, Kusiima J, Atuhairwe JA, et al. Measles outbreak propagated by children congregating at water collection points in Mayuge District, eastern Uganda, July–October, 2016. BMC Infect Dis. 2018;18:412. doi: 10.1186/s12879-018-3304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M, Nguyen HQ, Langidrik JR, Edwards R, Briand K, Papania MJ, Seward JF, et al. Measles transmission and vaccine effectiveness during a large outbreak on a densely populated island: implications for vaccination policy. Clin Infect Dis. 2006;42:315–319. doi: 10.1086/498902. [DOI] [PubMed] [Google Scholar]
- Markowitz LE, Preblud SR, Orenstein WA, Rovira EZ, Adams NC, Hawkins CE, et al. Patterns of transmission in measles outbreaks in the United States, 1985–1986. N Engl J Med. 1989;320:75–81. doi: 10.1056/NEJM198901123200202. [DOI] [PubMed] [Google Scholar]
- MoES . Ministry of Education and Sports, Republic of Uganda; Kampala: 2021. Uganda COVID-19 Education Response (GPE) Project (P174033): labour management plan (LMP) [Google Scholar]
- MoH. Uganda's electronic health information system: DHIS2. Kampala: Ministry of Health.
- MoH . Ministry of Health; Kampala: 2019. Immunisation guidelines by the Uganda National Expanded Programme on Immunisation (UNEPI) [Google Scholar]
- MoH . Ministry of Health; Kampala: 2019. Uganda launches national measles–rubella and polio immunization campaign. [Google Scholar]
- MoH . Ministry of Health; Kampala: 2019. Update on the national measles–rubella and polio immunization campaign 2019. [Google Scholar]
- MoH . Ministry of Health; Kampala: 2021. National technical guidelines for integrated disease surveillance and response. [Google Scholar]
- MoH . Ministry of Health; Kampala: 2022. Immunization in practice and the new routine immunization schedule: a training guide for operational level health workers. [Google Scholar]
- MoH . Ministry of Health; Kampala: 2022. Plan of action for measles-rubella vaccine second dose introduction into the routine immunization program in Uganda. [Google Scholar]
- Mohammed Y, Alemu A. Measles outbreak investigation and response in Jarar Zone of Ethiopian Somali Regional State, Eastern Ethiopia. Int J Microbiol Res. 2017;8:86–91. [Google Scholar]
- NDLG . Nakaseke District Local Government; 2022. Nakaseke District Local Government website.http://www.nakaseke.go.ug/ Available at. accessed 14 September 2022. [Google Scholar]
- Nsubuga F, Bulage L, Ampeire I, Matovu JK, Kasasa S, Tanifum P, et al. Factors contributing to measles transmission during an outbreak in Kamwenge District, Western Uganda, April to August 2015. BMC Infect Dis. 2018;18:21. doi: 10.1186/s12879-017-2941-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J, Masserey-Spicher V, Santibanez S, Mankertz A. Measles outbreak in Switzerland – an update relevant for the European football championship (EURO 2008) Euro Surveill. 2008;13 E070726.1. [PubMed] [Google Scholar]
- Sharma MK, Bhatia V, Swami H. Outbreak of measles amongst vaccinated children in a slum of Chandigarh. Indian J Med Sci. 2004;58:47–53. [PubMed] [Google Scholar]
- UBOS . Uganda Bureau of Statistics; Kampala: 2016. National Population and Housing Census 2014 – main report. [Google Scholar]
- Velicko I, Müller LL, Pebody R, Gergonne B, Aidyralieva C, Kostiuchenko N, et al. Nationwide measles epidemic in Ukraine: the effect of low vaccine effectiveness. Vaccine. 2008;26:6980–6985. doi: 10.1016/j.vaccine.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Weinberg GA, Szilagyi PG. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis. 2010;201:1607–1610. doi: 10.1086/652404. [DOI] [PubMed] [Google Scholar]
- WHO . World Helath Organization; Geneva: 2004. M-M-R® II (measles, mumps, and rubella virus vaccine live) [Google Scholar]
- WHO . World Health Organization; Geneva: 2007. Manual for the laboratory diagnosis of measles and rubella virus infection. [Google Scholar]
- WHO Measles vaccines: WHO position paper. Wkly Epidem Rec Relevé épldim bebd. 2009;84:349–360. [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2019. Measles fact sheets.https://www.who.int/news-room/fact-sheets/detail/measles Available at. accessed 25 August 2021. [Google Scholar]
- WHO . World Health Organization; Geneva: 2019. Statement from Uganda's Minister of Health on the National Measles–Rubella and Polio Immunisation Campaign 2019. [Google Scholar]
- WHO . World Health Organization; Geneva: 2021. Uganda: measles vaccination coverage. [Google Scholar]
- WHO . World Health Organization; Geneva: 2022. Vaccine-preventable disease outbreaks on the rise in Africa. [Google Scholar]
- Wichmann O, Siedler A, Sagebiel D, Hellenbrand W, Santibanez S, Mankertz A, et al. Further efforts needed to achieve measles elimination in Germany: results of an outbreak investigation. Bull World Health Organ. 2009;87:108–115. doi: 10.2471/BLT.07.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Grenfell BT, Mina MJ. Waning immunity and re-emergence of measles and mumps in the vaccine era. Curr Opin Virol. 2020;40:48–54. doi: 10.1016/j.coviro.2020.05.009. [DOI] [PubMed] [Google Scholar]
- Yeung LF, Lurie P, Dayan G, Eduardo E, Britz PH, Redd SB, et al. A limited measles outbreak in a highly vaccinated US boarding school. Pediatrics. 2005;116:1287–1291. doi: 10.1542/peds.2004-2718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets upon which the findings are based belong to the Uganda Public Health Fellowship Programme. For confidentiality reasons, the datasets are not publicly available. However, the data sets can be made available upon reasonable request from the corresponding author, with permission from the Uganda Public Health Fellowship Programme.