Abstract
Strains of methicillin-resistant Staphylococcus aureus (MRSA) have become the most important causative agents of hospital-acquired diseases worldwide. The genetic determinant of resistance, mecA, is not a gene native to S. aureus but was acquired from an extraspecies source by an unknown mechanism. We recently identified a close homologue of this gene in isolates of Staphylococcus sciuri, a taxonomically primitive staphylococcal species recovered most frequently from rodents and primitive mammals. In spite of the close sequence similarity between the mecA homologue of S. sciuri and the antibiotic resistance determinant mecA of S. aureus, S. sciuri strains were found to be uniformly susceptible to β-lactam antibiotics. In an attempt to activate the apparently “silent” mecA gene of S. sciuri, a methicillin-resistant derivative, K1M200 (for which the MIC of methicillin is 200 μg/ml), was obtained through stepwise exposure of the parental strain S. sciuri K1 (methicillin MIC of 4 μg/ml) to increasing concentrations of methicillin. DNA sequencing of the mecA homologue from K1M200 revealed the introduction of a point mutation into the −10 consensus of the promoter: the replacement of a thymine residue at nucleotide 1577 in the susceptible strain K1 by adenine in the resistant strain K1M200, which was accompanied by a drastic increase in transcription rate and the appearance of a new protein that reacted with monoclonal antibody prepared against the penicillin-binding protein 2A (PBP2A), i.e., the gene product of S. aureus mecA. Transduction of mecA from K1M200 (cloned into a plasmid vector) into a methicillin-susceptible S. aureus mutant resulted in a significant increase of methicillin resistance (from a methicillin MIC of 4 μg/ml to 12 and up to 50 μg/ml), the appearance of a low-affinity PBP detectable by the fluorographic assay, and the production of a protein that reacted in a Western blot with monoclonal antibody to PBP2A. Antibiotic resistance and the protein products disappeared upon removal of the plasmid-borne mecA homologue. The observations support the proposition that the mecA homologue ubiquitous in the antibiotic-susceptible animal species S. sciuri may be an evolutionary precursor of the methicillin resistance gene mecA of the pathogenic strains of MRSA.
The emergence and worldwide spread of methicillin-resistant Staphylococcus aureus (MRSA) between the early 1960s and the late 1990s have begun to pose serious threats to the chemotherapy of staphylococcal diseases worldwide. The genetic determinant of methicillin resistance in MRSA is the acquired gene mecA, which encodes the low-affinity penicillin-binding protein 2A (PBP2A), which, according to current theory, can function as a surrogate transpeptidase in the presence of high concentrations of β-lactam antibiotics that inactivate the four high-affinity PBPs native to S. aureus (5). The mecA gene and the associated large (40- to 60-kb) mec element (9, 10, 13, 15, 21, 27) are not native to S. aureus but were acquired from an extraspecies source by an unknown mechanism (3, 18). The nature of the extraspecies source, i.e., the evolutionary origin of mecA and the formation of the mec element, has remained largely a matter of speculation (1, 8, 11, 20, 26).
In a recent effort to track the evolutionary origin of mecA, we used a DNA probe internal to this gene in S. aureus to screen bacterial isolates belonging to 13 different staphylococcal species for bacteria that would give a positive signal with this DNA probe under hybridization conditions of high stringency. This effort has led to the identification of a close homologue of the S. aureus mecA gene in Staphylococcus sciuri, a species considered taxonomically the most primitive among staphylococci and found mainly in rodents and primitive mammals (4). Each one of 134 independent and genetically diverse S. sciuri isolates was found to carry the mecA homologue (4), which, similarly to mecA of S. aureus, encoded a protein with a putative transglycosylase and transpeptidase domain, the latter showing the conserved motifs and linear structure typical of the penicillin-binding domain of bacterial transpeptidases (30, 31). Overall similarity was 88% on the amino acid level, while even closer similarity (91% identity) was demonstrated within the transpeptidase domains of the mecA genes of S. aureus and S. sciuri.
In methicillin-resistant strains of S. aureus, the mecA gene provides a unique and broad range of resistance to all β-lactam antibiotics. Surprisingly, strains of S. sciuri carrying the structurally similar mecA homologue were found to be uniformly susceptible to β-lactam antibiotics, including even penicillin. The contrast between the striking structural similarity of the S. sciuri mecA homologue to the mecA gene of S. aureus and the complete lack of associated antibiotic resistance in the case of S. sciuri prompted us to explore possible structural changes in the S. sciuri mecA homologue and its transcription in β-lactam-resistant mutants isolated in the laboratory. The observations described in this communication suggest that the antibiotic pressure selects for a unique structural change in the regulatory sequence of the mecA homologue, converting it to an antibiotic resistance determinant capable of expressing the resistant phenotype even in the genetic background of S. aureus.
MATERIALS AND METHODS
Bacterial strain, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. Tryptic soy broth (TSB; Difco Laboratories, Detroit, Mich.) was employed to grow staphylococcal isolates, and Luria-Bertani medium (Difco) was used to propagate Escherichia coli DH5α; ampicillin (100 μg/ml) and chloramphenicol (20 μg/ml) were added to media to ensure maintenance of the plasmids in E. coli and staphylococci, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Origin or reference |
|---|---|---|
| Strains | ||
| Escherichia coli DH5α | recA endA1 gyrA96 thi-1 hsdR17 supE44 relA1 φ80dlacZΔM15 | Bethesda Research Laboratories |
| Staphylococcus | ||
| K1 | Staphylococcus sciuri ATCC 29062, methicillin susceptible | 4 |
| K1M200 | Methicillin-step-selected mutant of K1 | This study |
| RN4220 | Restriction-negative mutant of strain 8325-4 | R. Novick |
| COL | Homogeneous Mcr | Rockefeller University collection |
| RUSA4 | COL Ω551 (mecA::Tn551) Emr heterogeneous Mcs | 7 |
| SWTD10 | RUSA4/pSTSW-6 | This study |
| SWTD11 | RUSA4/pSTSW-8 | This study |
| SWTD22 | RUSA4/pSTSW-2C | This study |
| SWET28 | RN4220/pSTSW-6 | This study |
| SWET30 | RN4220/pSTSW-8 | This study |
| SWET21 | RN4220/pSTSW-2C | This study |
| SWET33 | RN4220/pLCSW-1 | This study |
| SWET34 | RN4220/pLCSW-2 | This study |
| SWET55 | RN4220/pLCSW-5 | This study |
| MGPET1 | RN4220/pLC4, negative control strain for assay of promoter activity | 19 |
| MGPET2 | RN4220/pro9/10, positive control strain for assay of promoter activity | 19 |
| Plasmids | ||
| pSPT181C | Shuttle vector Ampr Cmr Tcr, added 1.2-kb cat fragment in plasmid pSPT181 | This study |
| pSTSW-2C | pSPT181C/3,737-bp PCR product of S. aureus mecA region | This study |
| pSTSW-6 | pSPT181C/3,460-bp PCR product of S. sciuri K1 mecA region | This study |
| pSTSW-8 | pSPT181C/3,460-bp PCR product of S. sciuri K1M200 mecA region | This study |
| pLC4 | Ampr Cmr promoterless xylE gene | 24 |
| pro9/10 | pLC4/967-bp promoter region of pbp2 | 19 |
| pLCSW-1 | pLC4/514-bp putative promoter region of K1 mecA | This study |
| pLCSW-2 | pLC4/514-bp putative promoter region of K1M200 mecA | This study |
| pLCSW-5 | pLC4/545-bp putative promoter region of COL mecA | This study |
PAP and susceptibility test.
Population analysis profiles (PAPs) were determined by spreading aliquots of overnight cultures at various dilutions onto tryptic soy agar plates containing increasing concentrations of antibiotics. The number of CFU was determined after 48 h of incubation at 37°C or at 30°C for the strains containing thermosensitive plasmids (6). Susceptibility tests were done with paper disks (31) for the following antibiotics (micrograms per disk): ampicillin (10), nafcillin (1), oxacillin (1), cefotaxime (10), vancomycin (30), teicoplanin (30), tetracycline (30), and erythromycin (15).
DNA methods.
All routine DNA manipulations were performed essentially as described in the work of Sambrook et al. (22) and Ausubel et al. (2). Introduction of shuttle plasmids into S. aureus by electroporation and transduction was described previously (17, 31). DNA sequences were determined by the dideoxy chain termination method (23) with an automated DNA sequencing system (model 377; Perkin-Elmer Applied Biosystems Inc., Foster City, Calif.) at The Rockefeller University Sequencing Facility. Nucleotide and derived amino acid sequences were analyzed with the GCG program (Genetics Computer Group, Inc., Madison, Wis.) and DNAStar software (Lasergene, Madison, Wis.).
PCR.
PCR amplification of DNA was performed as described previously (31, 32). High-fidelity PCR with the GeneAmp XL PCR kit (Perkin-Elmer Cetus, Branchburg, N.J.), which includes rTth DNA polymerase XL, was used to reduce sequencing error.
RNA preparation and Northern blot analysis.
Northern blotting was performed as previously described (33, 34). The RNA preparation was extracted by use of the FastRNA isolation kit (Bio 101, Vista, Calif.) according to the recommendations of the manufacturer. The PCR-generated DNA probes were radiolabeled with [α-32P]dCTP (Amersham Life Science Inc., Arlington Heights, Ill.) by the random prime method using the Ready to Go labeling kit (Pharmacia, Piscataway, N.J.) and hybridized under high-stringency conditions.
Primer extension analysis.
The 5′ ends of transcripts of the S. sciuri mecA homologues from strains K1 and K1M200 were determined by primer extension with the oligonucleotide MAK1PE, TTCAATGGCATCAATTGTTTCG, complementary to the DNA sequence in strain K1 between nucleotides (nt) 1730 and 1751 (32). Primer labeling with [γ-32P]ATP, reverse transcription, and primer extension were described previously (34). For each primer extension, 1 to 50 μg of RNA was used. In primer extension experiments, the products of sequencing reactions initiated by the same primer were loaded in parallel lanes on the same gel.
Promoter fusions.
The following primers were used to amplify DNA fragments encompassing the region upstream of the S. sciuri mecA homologues from strains K1 and K1M200: (i) K1MABI1N, GAAGGATCCTATAGCACCTAACACAG, representing the sequence between nt 1166 and 1191 in strain K1, and (ii) K1MAPHIII, CGAAGCTTACAATCACGATGGCGATGA, the complementary sequence between nt 1654 and 1680 (32). The DNA segment representing the promoter of the mecA gene of S. aureus was amplified using the following primers: (iii) K8MAPBI, CCAGGATCCATTTGTCGGAATGCCTTAA (corresponding to the sequence between nt 2213 and 2240 in strain K8), and (iv) primer K8MAPHIII, CACAAGCTTCTATTAAAATAAGTGGAAC (complementary to the sequence between nt 2713 and 2758) (32). The PCR products were cloned into plasmid pLC4 to generate recombinant plasmids pLCSW-1 (carrying the promoter for mecA from strain K1), pLCSW-2 (carrying the promoter for mecA from strain K1M200), and pLCSW-5 (carrying the S. aureus mecA promoter). The plasmids were next introduced into strain RN4220 by electroporation to yield strains SWET33, SWET34, and SWET55, representing strains that carried the promoter regions of mecA from strains K1 and K1M200 and S. aureus, respectively. Catechol 2,3-dioxygenase activity was used to quantitate promoter activity using the assay of Sheehan et al. (24), and crude enzyme extracts were prepared as described previously (19). The reaction mixture, consisting of 100 mM potassium phosphate buffer (pH 7.5), 0.2 mM catechol, and 100 to 300 μl of crude extract, was incubated at 37°C for 30 min, and optical density readings were taken at 375 nm at 5-min intervals. One milliunit of activity was defined as that leading to the formation of 1 nmol of 2-hydroxymuconic semialdehyde per min. Specific activity was calculated in milliunits per milligram of protein. Protein concentration was measured using the Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, Calif.). The RN4220 strain containing pLC4 (MGPET1) and pro9/10 (MGPET2) were used as the negative and positive controls, respectively.
Introduction of the S. sciuri mecA homologues into S. aureus mutant RUSA4.
The 3,460-bp regions of the mecA homologues from strains K1 and K1M200 were PCR amplified with primers K1MABI1N (GAAGGATCCTATAGCACCTAACACAG; sequence between nt 1166 and 1191) and K1MABI2 (TATGGATCCTACAGATTTGCCTGCATG; complementary sequence between nt 4602 and 4626). The amplified sequences were ligated with shuttle plasmid vector pSPT181C to form pSTSW-6 and pSTSW-8. The recombinant plasmids were introduced into strain RN4220 by electroporation and then transduced into S. aureus mutant RUSA4 by phage 80α to yield the transductants SWTD10 and SWTD11. RUSA4 is a derivative of the highly and homogeneously MRSA strain COL in which resistance was inactivated by a Tn551 insert in the resident mecA gene (7, 14). As a control, the plasmid pSTSW-2C, which carries a 3,737-bp segment of the S. aureus mecA region (corresponding to sequence between nt 1603 and 5340 in strain K8), was introduced into RUSA4 to give transductant SWTD22.
Membrane purification and analysis of PBPs.
Membrane proteins were prepared from bacterial cultures of the late exponential stage (25). Forty or eighty micrograms of each protein extract was labeled with [3H]benzylpenicillin N-ethyl-piperidin (NEP) salt (87.4 mCi/mg; Merck, Rahway, N.J.) for 10 min at 30°C after preincubation with nafcillin (20 μg/ml) for 10 min at 30°C, in order to block the appearance of all but the low-affinity PBPs in the fluorogram. The labeling reaction was stopped by addition of an excess of unlabeled benzylpenicillin. Separation of proteins was performed on 8% acrylamide gels at the constant current of 20 mA essentially according to the method of Laemmli (12). Following the separation, the gel was stained with Coomassie blue, and PBPs were detected on the dried gels by fluorography (25).
Analysis of PBP2A and PBP2A-like protein by Western blotting.
The amount of membrane protein in each sample was essentially based on the protein concentration measured by use of the Bio-Rad DC protein assay kit and further confirmed with a Coomassie blue-stained gel. Electrophoresis was performed with the same procedure as PBP analysis. Proteins for immunoblotting were transferred to a Hybond ECL nitrocellulose membrane, and Western blotting was developed using the ECL Western blotting analysis system (Amersham Pharmacia Biotech UK Ltd., Little Chalfont, England) according to the manufacturer. A monoclonal antibody against PBP2A of S. aureus (Eli Lilly & Co., Indianapolis, Ind.) was used at a concentration of 1:10,000 as the primary antibody. The secondary antibody was peroxidase-labeled anti-rabbit antibody included in the kit. To block the nonspecific signal of protein A, 3 μg of ChromPure human immunoglobulin G, Fc fragment (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.), per ml was added during the blotting procedure with the primary antibody.
RESULTS
Isolation of a β-lactam antibiotic-resistant step mutant of S. sciuri.
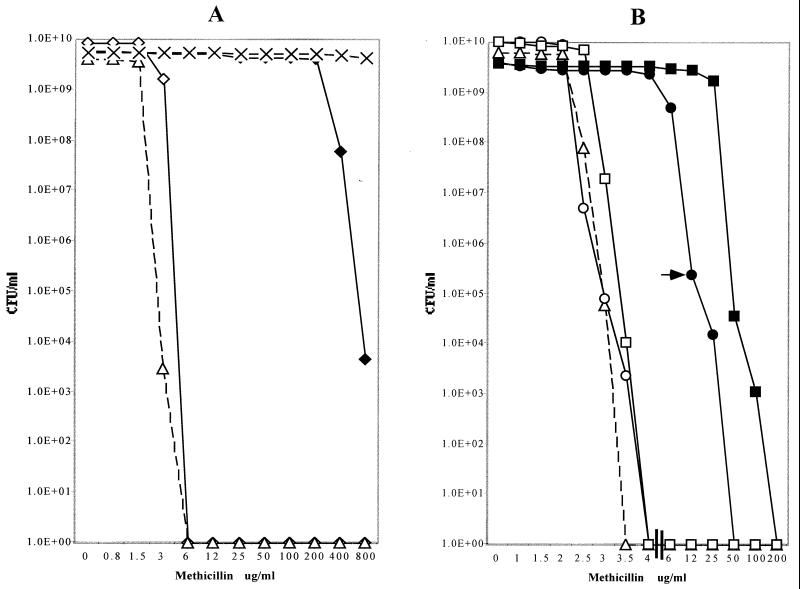
The β-lactam antibiotic-susceptible strain S. sciuri K1 (methicillin MIC of 4 μg/ml) (4, 30) was used as the parental strain to generate the highly methicillin-resistant derivative K1M200. A culture of strain K1 was incubated in growth medium (TSB) containing 4 μg of methicillin per ml until the appearance of a turbid culture that was used as the inoculum for TSB containing 8 μg of the antibiotic per ml. Stepwise exposure to gradually increasing concentrations of methicillin continued up to the isolation of the resistant culture K1M200, which was capable of growing in TSB containing 200 μg of the antibiotic per ml.
Strain K1M200 exhibited homogeneous methicillin resistance, the MIC of methicillin being more than 200 μg/ml (Fig. 1A), and was also resistant to other β-lactam antibiotics such as penicillin G, nafcillin, cefotaxime, and oxacillin. The MICs for the parental strain K1 have increased in mutant K1M200 from 0.1 to 50 μg/ml (penicillin), from 0.75 to 100 μg/ml (nafcillin), from 1 to 200 μg/ml (oxacillin), and from 6 to 400 μg/ml (cefotaxime). Both strains K1 and K1M200 were fully susceptible to vancomycin, teicoplanin, tetracycline, erythromycin, and kanamycin. The antibiotic-resistant phenotype of K1M200 was stable in response to serial culturing in the absence of antibiotic.
FIG. 1.
Methicillin susceptibility profiles of drug-susceptible and drug-resistant isolates of S. sciuri and expression of methicillin resistance in transductants of S. aureus carrying mecA homologues from S. sciuri. Bacterial strains were grown and tested for their methicillin resistance phenotype by plating different dilutions of the cultures on agar containing increasing concentrations of methicillin, as described for PAPs in Materials and Methods. (A) MRSA strain COL (dashed lines and X's) and its methicillin-susceptible insertional mutant derivative RUSA4 with the mecA gene inactivated by Tn551 (open triangles and dashed lines) and S. sciuri strain K1 (open diamonds and solid lines) and K1M200 (closed diamonds and solid lines). (B) The PAPs of transductants of RUSA4 carrying various mecA homologues from S. sciuri: transductant SWTD10 with the mecA homologue from strain K1 (open triangles and dashed lines); SWTD11 carrying the activated mecA homologue from strain K1M200 (solid circles and solid lines); SWTD11S1, a subpopulation of transductant SWTD11 exhibiting an increased resistance level and picked from the agar plate, as indicated by the arrow (solid squares and solid lines); and cultures of SWTD11 (open circles) and SWTD11S1 (open squares), after curing of the mecA-carrying plasmids from the bacteria.
Comparison of the DNA sequences of the mecA homologues carried by S. sciuri strains K1 and K1M200.
The 2,605-bp mecA region corresponding to the sequence between nt 1166 and 3771 (including 474 bp upstream and 131 bp downstream of the mecA gene) was PCR amplified from strains K1 and K1M200 using primers K1MABI1N (AGCTGCTATAGCACCTAACACAG) and CP2F3C (AATATATGGAGCATGGTATTTCTATGCAG). Comparison of the DNA sequences of PCR products identified only one difference: a single point mutation that replaced the thymine residue at nt 1577 in the promoter region of strain K1 with an adenine in strain K1M200.
Transcriptional analysis of the mecA homologues in strains K1 and K1M200.
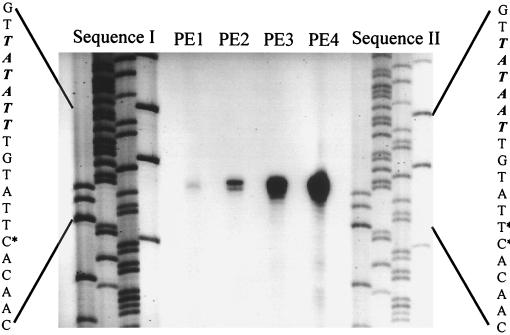
Primer extension analysis using 50 μg of RNA template from strain K1 identified only a weak signal for the primer extension product located at the position corresponding to the nt 1585 cytosine (lane PE1 in Fig. 2), suggesting that this residue is the transcriptional start (+1) of the mecA homologue in strain K1. Two reverse transcriptase (RT) products were generated with RNA from strain K1M200, and these were located at the positions corresponding to the nt 1584 thymine and the nt 1585 cytosine residues, respectively (lane PE2 in Fig. 2). The signal at nt 1584 was much stronger than that at nt 1585, and the amount of RT product produced from 1 μg of K1M200 RNA template was much larger than that generated from 50 μg of RNA from strain K1; one may roughly estimate that the transcription rate of the mecA homologue resident in K1M200 was at least a hundredfold higher than that in strain K1 (lanes PE2 to PE4 in Fig. 2). Based on the +1 site of the strain K1 mecA homologue, the nucleotides TATATT (nt 1573 to 1578) should be the −10 consensus of the promoter for the mecA homologue of strain K1. In K1M200, the −10 consensus sequence was changed from TATATT to TATAAT due to the point mutation that resulted in the replacement of thymine with adenine at nt 1577 and a greatly increased rate of transcription of mecA.
FIG. 2.
Mapping of the 5′ ends of the S. sciuri mecA transcript by primer extension analysis. Total RNAs from strains K1 and K1M200 were hybridized with an oligonucleotide (MAK1PE) complementary to the mecA mRNA of strain K1 and extended by RT. The precise base mapping was done by comparing the migration of the primer extension (PE) product with a parallel sequencing reaction primed by an identical oligonucleotide. The sequence encompassing the initiation start is enlarged on the left and the right of the mecA sequences of strains K1 and K1M200. The sequences of the coding strands are shown, and the −10 consensus sequences are indicated by italics. The initiation starts (+1) for mecA are indicated by asterisks. The left side of the figure shows the mecA sequences of strain K1 obtained using plasmid pSTSW-6 as template. The right side of the figure shows the mecA sequences of strain K1M200 obtained using plasmid pSTSW-8 as template. The primer extension products (PE1 through PE4) from left to right are RT cDNAs generated from 50 μg of K1 RNA and 1, 5, and 25 μg of RNAs from strain K1M200, respectively.
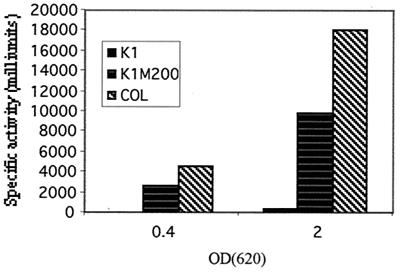
Determination of the specific activity of the catechol 2,3-deoxygenase produced by the reporter gene indicated that the promoters of strains K1 and K1M200 and the S. aureus strain COL carried by the electrotransformants SWET33, SWET34, and SWET55, respectively, generated 330, 9,900, and 18,000 U of enzyme activities at the stationary phase of growth, respectively. No enzyme activity was detectable in early-exponential-growth-phase cultures of SWET33 carrying the promoter of strain K1, while SWET34 and SWET55 carrying the promoters of strain K1M200 and the S. aureus strain COL produced 2,600 and 4,500 activity units of catechol 2,3-deoxygenase, respectively, in cultures of comparable cell densities in the early exponential phase of growth (Fig. 3).
FIG. 3.
Increased transcription of the mecA homologue in the drug-resistant strain K1M200. The rate of transcription of mecA was determined in constructs containing promoter regions from the S. sciuri strains K1 and K1M200 and from the MRSA strain COL (left to right, respectively). Crude enzyme extracts were prepared from the cultures at the early exponential growth phase (optical density at 620 nm = 0.4) and the stationary growth phase (optical density at 620 nm = 2.0). Specific enzyme activity is expressed in milliunits per milligram of cellular protein. The control strain with the vector pLC4 gave no XylE activity.
Northern blot analysis with a probe internal to the S. sciuri mecA gene (nt 3121 to 3613) showed that a band with a molecular size of 2 kb was detectable in 10 μg of total RNA from strain K1M200, which corresponded in size to the transcript of the S. sciuri mecA gene; no comparable signal was detectable with strain K1 (data not shown).
Expression of the methicillin-resistant phenotype in S. aureus from the mecA gene of the antibiotic-resistant strain K1M200 of S. sciuri
The mecA genes from S. sciuri strains K1 and K1M200 were introduced into the background of the S. aureus strain RUSA4, a derivative of the highly methicillin-resistant strain COL in which the resident S. aureus mecA gene was insertionally inactivated by Tn551 (7, 14). Figure 1B shows the antibiotic susceptibility profiles of transductant SWTD10 (carrying mecA derived from the methicillin-susceptible S. sciuri strain K1) and transductant SWTD11 (carrying mecA with the promoter mutation derived from the methicillin-resistant S. sciuri mutant K1M200). Also shown in Fig. 1B are the antibiotic susceptibility profiles of several control strains. It may be seen that the introduction of mecA from strain K1M200 was able to confer a significant degree of methicillin resistance on the S. aureus strain used as the transductional recipient. Removal of the plasmid-borne gene resulted in the complete disappearance of resistance. No increase in the methicillin MIC for the recipient strain RUSA4 was detected in transductant SWTD10 carrying the mecA gene derived from the methicillin-susceptible S. sciuri strain K1.
Production of a PBP2A-like protein in S. aureus carrying the S. sciuri mecA homologue.
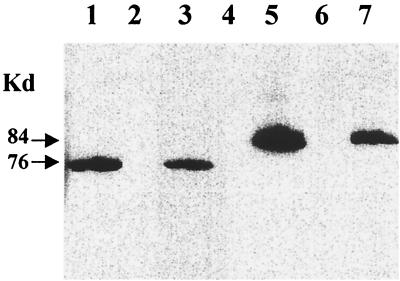
To examine the expression of the S. sciuri mecA homologues introduced into S. aureus by transduction, membrane proteins of the transductants were analyzed by Western blotting and by the PBP fluorographic assay. As controls, membrane proteins from the S. aureus strain COL, its insertional mutant RUSA4, and the transductant SWTD22 were used. A single protein band reacting with the monoclonal antibody against PBP2A was detected in each one of the membrane protein preparations from S. aureus strain COL, transductant SWTD22 (carrying the S. aureus mecA gene), and transductant SWTD11 (carrying the S. sciuri mecA gene from strain K1M200) and also in membrane protein preparations from S. sciuri strain K1M200 (Fig. 4). As estimated from the intensity of labeling, the amount of protein produced by K1M200 was about half of that detectable in the S. aureus strain COL. No protein reacting with the anti-PBP2A monoclonal antibody was detected in the membrane protein preparations from S. sciuri strain K1, the S. aureus mecA insertional mutant RUSA4, and the transductant SWTD10, which carried mecA from the drug-susceptible S. sciuri strain K1.
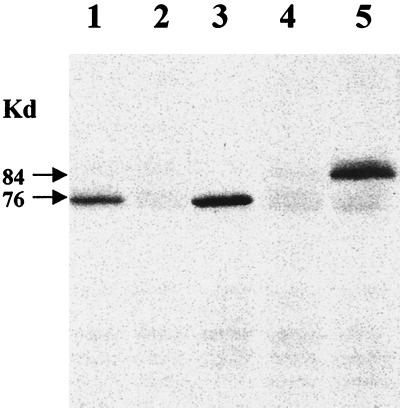
FIG. 4.
Detection by Western blotting of a PBP2A-like protein encoded by the mecA homologue of S. sciuri. Membrane protein extracts were produced and tested by Western blotting for the production of protein that reacts with monoclonal antibody prepared against PBP2A, the gene product of the antibiotic resistance gene of S. aureus. The amount of membrane protein used was 30 μg in lanes 1 through 5 and 60 μg in lanes 6 and 7. Lane 1, MRSA strain COL; lane 2, S. aureus mutant RUSA4; lane 3, transductant SWTD22; lane 4, transductant SWTD10; lane 5, transductant SWTD11; lane 6, S. sciuri strain K1; lane 7, S. sciuri strain K1M200.
The membrane protein preparations were also tested by the fluorographic assay to detect low-affinity PBPs under the conditions of a competition assay. Protein preparations from the same constructs that reacted with the monoclonal antibody in the Western blot assay also showed the presence of a low-affinity PBP in the fluorographic assay which had the same apparent molecular size as the protein identified by Western blotting (Fig. 5). Interestingly, both the antibody-reactive protein band and the low-affinity PBP band produced by transductant SWTD11 migrated slower than PBP2A detected by these two assays in the S. aureus strain COL and transductant SWTD22.
FIG. 5.
Detection by the fluorographic penicillin-binding assay of a PBP2A-like protein encoded by the mecA homologue of S. sciuri. Membrane proteins prepared as described in Materials and Methods were tested under conditions of a competition assay for the presence of a low-affinity PBP using the fluorographic assay. Lanes 1 to 5 contain 30 μg of membrane proteins each prepared from the MRSA strain COL (lane 1), mutant RUSA4 (lane 2), transductant SWTD22 carrying a plasmid-borne copy of the S. aureus mecA gene (lane 3), transductant SWTD10 carrying the mecA homologue from S. sciuri strain K1 (lane 4), and transductant SWTD11 carrying the activated mecA homologue of S. sciuri strain K1M200 (lane 5). The membrane preparations were preincubated with nafcillin for 10 min, followed by an additional incubation with [3H]benzylpenicillin, and processed for fluorography as described in Materials and Methods.
DISCUSSION
The studies described here were designed to probe the similarities and contrasts that exist between the mecA homologues carried by two staphylococcal species, S. aureus and S. sciuri. In S. aureus, the mecA gene is acquired from an unknown extraspecies source: it is present only in methicillin-resistant strains, providing these bacteria with blanket resistance against the most important class of antimicrobial agents—the family of β-lactam antibiotics. In S. sciuri, a close structural homologue of the S. aureus mecA gene appears to be a domestic gene present in each one of the large number of independent isolates examined. Yet, in contrast to the case of S. aureus, S. sciuri strains carrying the mecA gene homologue are uniformly susceptible to all β-lactam antibiotics (4). S. sciuri is a staphylococcal species taxonomically remote from S. aureus, and it is unlikely that these bacteria have often been exposed to β-lactam antibiotics in their natural habitat, which is the skin of rodents and primitive mammals (4). In an attempt to probe a possible recruitment of the mecA homologue of S. sciuri as part of a drug resistance mechanism, we tested the effect of selective antibiotic pressure applied to S. sciuri in the laboratory on the structure and expression of the mecA homologue.
The results of these experiments were quite striking. Comparison of the sequence of the S. sciuri mecA homologue of the drug-susceptible strain S. sciuri K1 to that of the laboratory-selected methicillin-resistant derivative K1M200 identified a single point mutation introduced into the promoter region of the mecA gene of the resistant strain. The replacement of the thymine residue with an adenine at nt 1577 changed the sequence TATATT to TATAAT, which was accompanied by a striking increase in the rate of transcription of the mecA gene and the appearance in the resistant cells of a protein product that reacted with a monoclonal antibody prepared against the S. aureus gene product PBP2A. The monoclonal antibody has specifically recognized the 38-amino-acid peptide encoded by the DNA sequence between nt 115 and 174 close to the amino terminus of the S. aureus mecA gene (29). These findings strongly suggest that the mechanism of β-lactam resistance generated by antibiotic selection in the laboratory mutant S. sciuri K1M200 involves, as a genetic determinant of drug resistance, the mecA homologue resident in this bacterium. The critical event for the recruitment of this gene to be part of the mechanism of drug resistance appears to be the generation of a more efficient promoter.
In order to further test the relationship between the methicillin-resistant phenotype and the structure of the S. sciuri mecA homologues, the mecA gene of the drug-susceptible parental strain S. sciuri K1 and the mecA gene of the drug-resistant mutant strain K1M200 were ligated into plasmid vectors which were then introduced by transduction into the background of S. aureus. The S. aureus strain selected for this purpose was a methicillin-susceptible derivative of a highly and homogeneously resistant MRSA strain, COL, in which the resident mecA gene was inactivated by a Tn551 insert (14). Previous studies have shown that the genetic background of this strain allows optimal expression of the resistant phenotype (7).
These transduction experiments produced several observations indicating that the mecA homologue from the antibiotic-resistant strain of S. sciuri can also generate a drug-resistant phenotype in the heterologous background of S. aureus and that the mechanism of this resistance is similar to the one operating in MRSA strains, namely, it confers a broad range of resistance to β-lactam antibiotics and is associated with the production of a PBP2A-like protein.
Introduction of the mecA homologue from the drug-resistant strain K1M200 into S. aureus strain RUSA4 resulted in the increase of the methicillin MIC from 4 μg/ml for the recipient strain to 12 μg/ml for the transductant SWTD11. In addition, the methicillin MIC for a subpopulation of the same transductant (SWTD11S1) increased even more, to 50 μg/ml. Upon removal of the plasmid-borne mecA gene, the MICs of the cured bacteria were reduced back to the level of susceptibility of the recipient strain, indicating that the drug-resistant phenotype depended on the presence of the S. sciuri mecA homologue introduced into the S. aureus cells. No increase in the MIC was detected for transductants that received the mecA homologue derived from the drug-susceptible strain S. sciuri K1.
Yet another similar feature of the drug-resistant phenotype in MRSA strains and in the S. sciuri strain K1M200 as well as its transductant derivative in S. aureus was the broad range of resistance to structurally different β-lactam antibiotics.
Transductants that received the activated mecA homologue from the drug-resistant mutant K1M200 (but not transductants that received the “silent” mecA homologue from the drug-susceptible strain K1) produced a single low-affinity PBP detectable both by fluorography run under conditions of a competition assay and by Western blotting (Fig. 4 and 5). These observations confirm and extend the validity of the conclusions derived from similar experiments done in the S. sciuri background: it seems that the S. sciuri mecA homologue encodes a protein that is similar both in antigenicity and in penicillin-binding properties to PBP2A, i.e., the gene product of the S. aureus methicillin resistance determinant.
Interestingly, the molecular size of this PBP2-like protein was greater than what would be predicted from the size of the gene and also greater than the size of PBP2A encoded by the S. aureus mecA gene. Similar apparent molecular size differences have already been observed in mutant proteins, and they may be related to altered detergent binding properties or incomplete denaturation under the conditions of the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (16, 28).
Together, the observations described in this communication provide experimental evidence that the mecA homologue which is a native gene in S. sciuri with an as yet undefined domestic function can be recruited to become an antibiotic resistance determinant under conditions of drug selection. The critical alteration that makes the silent mecA homologue of a drug-susceptible S. sciuri strain an effective drug resistance determinant appears to be the replacement of a single nucleotide within the promoter sequence, which results in a drastic increase in the rate of transcription of the gene into a protein that closely resembles the gene product of the S. aureus mecA determinant.
The additional observation, namely, that the activated form of the S. sciuri mecA gene can replace the S. aureus mecA determinant producing a PBP2A-like protein and providing a significant level of broad-range β-lactam resistance to S. aureus, supports the proposal that the mecA homologue ubiquitous in the animal species of S. sciuri may be the evolutionary precursor of the methicillin resistance determinant of MRSA.
ACKNOWLEDGMENTS
Partial support for these investigations came from a grant from the U.S. Public Health Service (1 RO1 AI45738) and from the Lounsbery Foundation.
The help of Isabel Couto (ITQB/UNL) in the isolation of strain K1M200 is gratefully acknowledged. Monoclonal antibody prepared against PBP2A of S. aureus was provided by Paul Skatrud of Eli Lilly & Co.
REFERENCES
- 1.Archer G L, Niemeyer D M. Origin and evolution of DNA associated with resistance to methicillin in staphylococci. Trends Microbiol. 1994;2:343–352. doi: 10.1016/0966-842x(94)90608-4. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. New York, N.Y: Greene Publishing Associates and John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 3.Beck W D, Berger-Bachi B, Kayser F H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986;165:373–378. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couto I, de Lencastre H, Severina E, Kloos W, Webster J, Santos Sanches I, Tomasz A. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microb Drug Resist. 1996;2:377–391. doi: 10.1089/mdr.1996.2.377. [DOI] [PubMed] [Google Scholar]
- 5.de Jonge B L M, Tomasz A. Abnormal peptidoglycan produced in a methicillin-resistant strain of Staphylococcus aureus grown in the presence of methicillin: functional role for penicillin-binding protein 2A in cell wall synthesis. Antimicrob Agents Chemother. 1993;37:342–346. doi: 10.1128/aac.37.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lencastre H, Figueiredo A, Urban K, Rahal J, Tomasz A. Multiple mechanisms of methicillin resistance and improved methods for detection in clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:632–639. doi: 10.1128/aac.35.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lencastre H, Tomasz A. Reassessment of the number of auxiliary genes essential for expression of high-level methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:2590–2598. doi: 10.1128/aac.38.11.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Kharroubi A, Jacques P, Piras G, Van Beeumen J, Coyette J, Ghuysen J M. The Enterococcus hirae R40 penicillin-binding protein 5 and the methicillin-resistant Staphylococcus aureus penicillin-binding protein 2′ are similar. Biochem J. 1991;280:463–469. [PMC free article] [PubMed] [Google Scholar]
- 9.Fontana R. Penicillin-binding proteins and the intrinsic resistance to β-lactams in Gram-positive cocci. J Antimicrob Chemother. 1985;16:412–416. doi: 10.1093/jac/16.4.412. [DOI] [PubMed] [Google Scholar]
- 10.Hartman B J, Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986;29:85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiramatsu K. Molecular evolution of MRSA. Microbiol Immunol. 1995;39:531–543. doi: 10.1111/j.1348-0421.1995.tb02239.x. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Matsuhashi M, Song M D, Ishino F, Wachi M, Doi M, Inoue M, Ubukata K, Yamashita N, Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to β-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986;167:975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews P, Tomasz A. Insertional inactivation of the mec gene in a transposon mutant of a methicillin-resistant clinical isolate of Staphylococcus aureus. Antimicrob Agents Chemother. 1990;34:1777–1779. doi: 10.1128/aac.34.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews P R, Reed K C, Stewart P R. The cloning of chromosomal DNA associated with methicillin and other resistances in Staphylococcus aureus. J Gen Microbiol. 1987;133:1919–1929. doi: 10.1099/00221287-133-7-1919. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen T B, Reynolds J A. Measurements of molecular weights by gel electrophoresis. Methods Enzymol. 1978;48:3–10. doi: 10.1016/s0076-6879(78)48003-6. [DOI] [PubMed] [Google Scholar]
- 17.Oshida T, Tomasz A. Isolation and characterization of a Tn551-autolysis mutant of Staphylococcus aureus. J Bacteriol. 1992;174:4952–4959. doi: 10.1128/jb.174.15.4952-4959.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattee P A. Staphylococcus aureus. Genet Maps. 1990;5:22–27. [Google Scholar]
- 19.Pinho G M, de Lencastre H, Tomasz A. Transcriptional analysis of the Staphylococcus aureus penicillin binding protein 2 gene. J Bacteriol. 1998;180:6077–6081. doi: 10.1128/jb.180.23.6077-6081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piras G, Raze D, El Kharroubi A, Hastir D, Englebert S, Coyette J, Ghuysen J M. Cloning and sequencing of the low-affinity penicillin-binding protein 3r-encoding gene of Enterococcus hirae S185: modular design and structural organization of the protein. J Bacteriol. 1993;175:2844–2852. doi: 10.1128/jb.175.10.2844-2852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds P E, Brown D F. Penicillin-binding protein of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985;192:28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheehan B J, Foster T J, Dorman C J, Park S, Stewart G S. Osmotic and growth-phase dependent regulation of the eta gene of Staphylococcus aureus: a role for DNA supercoiling. Mol Gen Genet. 1992;232:49–57. doi: 10.1007/BF00299136. [DOI] [PubMed] [Google Scholar]
- 25.Sieradzki K, Pinho M G, Tomasz A. Inactivated pbp4 in highly glycopeptide-resistant laboratory mutants of Staphylococcus aureus. J Biol Chem. 1999;274:18942–18946. doi: 10.1074/jbc.274.27.18942. [DOI] [PubMed] [Google Scholar]
- 26.Song D M, Wachi M, Doi M, Ishino F, Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987;221:167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- 27.Tesch W, Strassle A, Berger-Bachi B, O'Hara D, Reynolds P, Kayser F H. Cloning and expression of methicillin resistance from Staphylococcus epidermidis in Staphylococcus carnosus. Antimicrob Agents Chemother. 1988;32:1494–1499. doi: 10.1128/aac.32.10.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber W, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 29.Wu C Y E, Hoskins J, Blaszczak L C, Preston D A, Skatrud P L. Construction of a water-soluble form of penicillin-binding protein 2a from a methicillin-resistant Staphylococcus aureus isolate. Antimicrob Agents Chemother. 1992;36:533–539. doi: 10.1128/aac.36.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S, Piscitelli C, de Lencastre H, Tomasz A. Tracking the evolutionary origin of the methicillin resistance gene: cloning and sequencing of a homologue of mecA from a methicillin susceptible strain of Staphylococcus sciuri. Microb Drug Resist. 1996;2:435–441. doi: 10.1089/mdr.1996.2.435. [DOI] [PubMed] [Google Scholar]
- 31.Wu S, de Lencastre H, Sali A, Tomasz A. A phosphoglucomutase-like gene essential for the optimal expression of methicillin resistance in Staphylococcus aureus. Microb Drug Resist. 1996;2:277–286. doi: 10.1089/mdr.1996.2.277. [DOI] [PubMed] [Google Scholar]
- 32.Wu S, de Lencastre H, Tomasz A. Genetic organization of the mecA region in methicillin-susceptible and methicillin-resistant strains of Staphylococcus sciuri. J Bacteriol. 1998;180:236–242. doi: 10.1128/jb.180.2.236-242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu S W, de Lencastre H. Mrp—a new auxiliary gene essential for optimal expression of methicillin resistance in Staphylococcus aureus. Microb Drug Resist. 1999;5:9–18. doi: 10.1089/mdr.1999.5.9. [DOI] [PubMed] [Google Scholar]
- 34.Wu S W, de Lencastre H, Tomasz A. The Staphylococcus aureus transposon Tn551: complete nucleotide sequence and transcriptional analysis of the expression of the erythromycin resistance gene. Microb Drug Resist. 1999;5:1–7. doi: 10.1089/mdr.1999.5.1. [DOI] [PubMed] [Google Scholar]