Abstract
Background
Breast cancer surgery is associated with considerable acute post-surgical pain and restricted mobility. Various regional and neuraxial anesthesia techniques have been used to alleviate post-mastectomy pain. Ultrasound-guided serratus anterior plane block (SAPB) has been considered a simple and safe technique. This randomized control study was performed to compare the efficacy of SAPB with the thoracic paravertebral block (TPVB) for postoperative analgesia after breast cancer surgery.
Methods
A total of 40 adult ASA physical status I - II female patients undergoing radical mastectomy were randomly allocated into two groups to receive either ultrasound-guided TPVB or SAPB with 0.4 mL.kg-1 0.5% ropivacaine, 30 min before surgery. All patients received standardized general anesthesia for surgery. Injection diclofenac and tramadol were used for postoperative rescue analgesia. The time to first rescue analgesia, total analgesic consumption in the first 24 hours, postoperative pain scores, and any adverse effects were recorded.
Results
The time to first rescue analgesia was significantly longer in the SAPB group (255.3 ± 47.8 min) as compared with the TPVB group (146.8 ± 30.4 min) (p < 0.001). Total diclofenac consumption in 24 hours was also less in the SAPB group (138.8 ± 44.0 mg vs 210.0 ± 39.2 mg in SAPB and TPVB group respectively, p < 0.001). Postoperative pain scores were significantly lower in the SAPB group as compared with TPVB group (p < 0.05). The incidence of PONV was also less in the SAPB group (p = 0.028). No block-related adverse effects were reported.
Conclusion
We found that the serratus anterior plane block was more effective than the thoracic paravertebral block for postoperative analgesia after breast cancer surgery.
Keywords: Analgesia, Nerve block, Radical mastectomy, Ultrasound interventional
Introduction
Modified radical mastectomy, a common surgical procedure in breast cancer patients, is associated with considerable acute postoperative pain and restricted shoulder mobility leading to delayed hospital discharge.1 Adequate post-surgical pain management is important for the early mobilization and long-term well-being of these patients. Various regional anesthesia techniques have been used to provide postoperative analgesia in this group of patients including intercostal plane block,2 local anesthetic infiltration,3 brachial plexus block,4 thoracic epidural,5 thoracic paravertebral block (TPVB),6, 7, 8 and thoracic wall blocks.9, 10, 11, 12 Among these, TPVB is the most commonly used technique for controlling post-mastectomy pain but carries a high failure rate (6 - 10%) and the risk of hypotension, pneumothorax, and vascular puncture.8
Recently, thoracic wall blocks (Pecs I and II) have been evolved as less invasive alternatives to the paravertebral block for providing extended postoperative analgesia after breast surgery.9, 10, 11 The serratus anterior plane block (SAPB) is a newer interfacial plane block of the chest wall and appears to be safe and easy to perform as serratus muscle is superficial and easily identifiable under ultrasound.12 Blanco et al.13 suggested that SAPB may target the thoracic nerves more selectively than pectoral blocks and provides predictable and effective anesthesia to the anterolateral chest wall. However, literature is scarce about the efficacy of SAPB for postoperative analgesia after breast cancer surgery. Therefore, the present study has been planned to compare the efficacy of ultrasound-guided SAPB with TPVB for the management of postoperative pain after total mastectomy and axillary clearance surgery in breast cancer patients. The primary objective of the study was to evaluate the duration of postoperative analgesia, and the secondary objectives were to observe the pain scores and rescue analgesic consumption for 24 hours postoperatively.
Methods
This randomized control trial was carried out after obtaining approval from the institutional ethics committee (reference number NK/1585/MD/10559-60) and written informed consent from the patients. The study was registered with the clinical trial registry (REF/2017/01/013128) and adheres to the applicable CONSORT guidelines. A total of 40 ASA (American Society of Anesthesiologists) physical status I - II female patients in the age group of 18 - 65 years, scheduled to undergo total mastectomy with axillary clearance under general anesthesia were included. The patients who had local infection at the block site, coagulopathy, morbid obesity (BMI > 40 kg.m-2), allergy to local anesthetics, decreased pulmonary reserve, uncontrolled hypertension or ischemic heart disease, renal dysfunction, and pre-existing neurological deficits and psychiatric illness were excluded. The patients were kept fasting overnight and pre-medicated with tablet alprazolam 0.25 mg and tablet ranitidine 150 mg orally the night before and two hours prior to surgery. They have explained the numeric rating scale (NRS, 0 - 10, where 0 stands for no pain and 10 stands for worst imaginable pain) for postoperative pain assessment.
The patients were randomly allocated into two groups using computer-generated random numbers which were kept in sealed opaque envelopes numbered sequentially and opened just before administration of the block. Group 1 patients received thoracic paravertebral block at T4 level (TPVB group) while group 2 patients received serratus anterior plane block at the level of the 5th intercostal space (SAPB group). The blocks were given 30 minutes before surgery by an experienced anesthesiologist (having experience of administration of more than 20 blocks each with the same technique) in the pre-operative room under all aseptic precautions and vital parameters monitoring. The anesthesiologist who performed the blocks did not participate in further management of the patients and data collection.
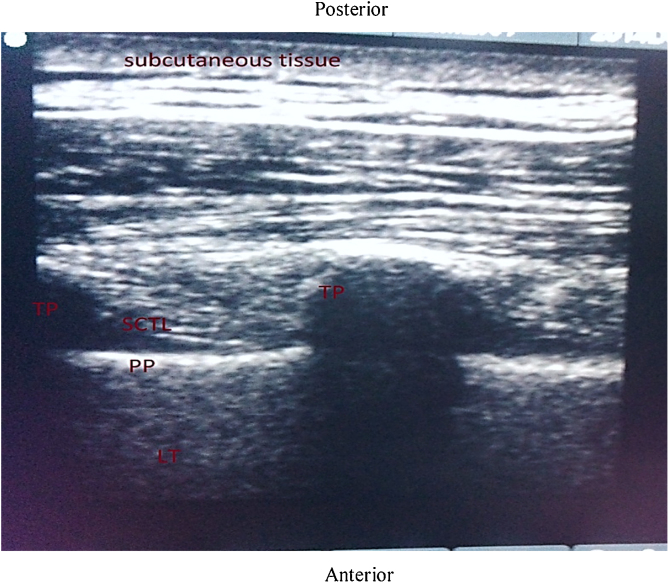
In TPVB group, the block was performed by placing the patient in a sitting position. The spinous processes of C7 to T6 vertebrae were marked with a permanent skin marker. Both cervical and thoracic paravertebral areas were prepared with 5% povidone-iodine solution and covered with sterile drapes. After covering the USG cable with a sterile ultrasound probe cover, a linear high frequency (5-10 MHz) ultrasound probe (Sonosite, Inc. Bothell. WA 98021 USA) was placed vertically 2.5 cm away from the midline in the sagittal paramedian plane at T4 level on the side of surgery. At this level, the probe was moved laterally and obliquely, until the typical double layer of the internal intercostal membrane (IIM), the transverse process, superior costotransverse ligament, and the pleura were visualized in one image (Fig. 1). After infiltrating the skin with 3 - 5 mL of 2% lignocaine, a 22-G, 80-mm SonoPlex needle was inserted in-plane from caudal to cranial direction. Once the needle tip had reached in between the pleura and costotransverse ligament, 0.4 mL.kg-1 of 0.5% ropivacaine was administered after negative aspiration. During the administration of the local anesthetic downward displacement of the pleura was observed.
Figure 1.
Ultrasound image of thoracic paravertebral space showing the transverse process (TP), parietal pleura (PP), and superior costotransverse ligament (SCTL).
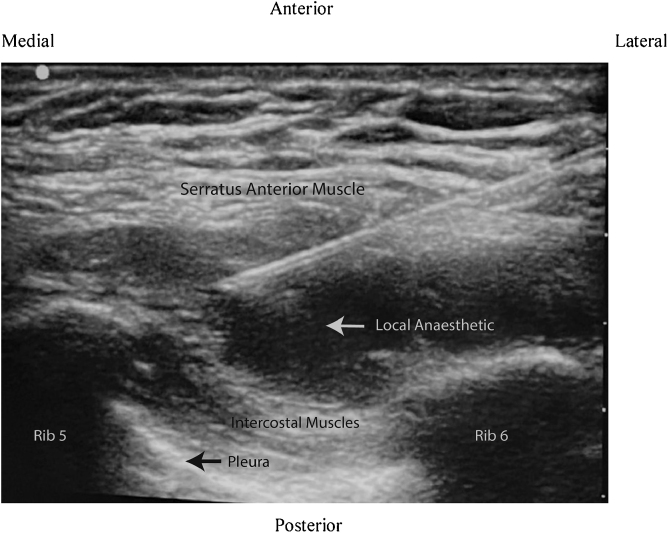
In the SAPB group, patients were placed in a lateral position with the operating side up and the arm abducted. Under all antiseptic precautions, a linear high-frequency ultrasound probe was placed vertically in the mid-axillary line at the level of the 5thintercostal space. At this level, one can identify subcutaneous tissue and serratus anterior muscle in the superficial plane, the intercostal muscles (external, internal, and innermost) in the intermediate plane and ribs, pleura, and lung in the deep plane. The skin puncture site was infiltrated with 2% lignocaine and a 22-G, 50-mm SonoPlex needle was inserted from caudal to the cranial direction in an in-plane approach until the tip was placed between serratus anterior muscle and intercostal muscles. After negative aspiration, 0.4 mL.kg-1 of 0.5% ropivacaine was deposited in this space (Fig. 2).
Figure 2.
Ultrasound image of serratus anterior plane block (echogenic needle is placed below the serratus anterior muscle between 5th and 6th rib to deposit local anesthetic).
The patients were monitored continuously for heart rate and oxygen saturation, and the non-invasive blood pressure was recorded at 5-min intervals. The sensory level of the block was assessed by an anesthesiologist blinded to the group allocation, with pinprick sensation every 5 minutes in each dermatomal distribution from T1 to T6 and the number of dermatomes having less sensation as compared with the opposite side was noted. If pinprick sensation did not decrease in any segment until 30 minutes after the block, then it was considered as a block failure. These patients were managed with additional (2 µg.kg-1 during induction plus according to the requirement) doses of fentanyl for perioperative analgesia. Any block-related complications like hypotension, vascular puncture, Horner’s syndrome, and pneumothorax, were recorded.
The patients were shifted to the operating room 30 minutes after the procedure. Anesthesia was induced with fentanyl 1 µg.kg-1 followed by propofol 2 - 3 mg.kg-1 until the loss of verbal response. Atracurium 0.5 mg.kg-1 was used to facilitate tracheal intubation. Anesthesia was maintained with 60% nitrous oxide in oxygen and isoflurane (MAC 1 - 1.3). The patient’s lungs were ventilated with positive pressure ventilation to maintain end-tidal carbon dioxide (EtCO2) between 32 - 36 mmHg. The patients received a continuous infusion of normal saline at the rate of 5 - 8 mL.kg-1.h-1. An increase in mean arterial pressure (MAP) > 25% of baseline was considered as inadequate analgesia and 1 µg.kg-1 fentanyl was administered intravenously. Any episode of hypotension (MAP < 25% of baseline) was treated with infusion of normal saline, and if required injection mephentermine in titrated doses. All patients received ondansetron 0.1 mg.kg-1 30 minutes before the end of surgery to prevent postoperative nausea and vomiting. After completion of surgery residual neuromuscular blockade was reversed with intravenous neostigmine 50 µg.kg-1 and glycopyrrolate 10 µg.kg-1 and the endotracheal tube was removed once they were fully awake and breathing adequately.
The patients were monitored for 24 hours after surgery in the post-anesthesia care room. The vital parameters and pain scores (NRS) were recorded at 0, ½, 1, 2, 4, 6, 8, 12, and 24 hours by an investigator blinded to the group allocation. Patients with NRS more than 3 or those demanding analgesics received diclofenac sodium 75 mg intravenously. If NRS was remained high 30 minutes after administration of diclofenac, then injection tramadol 1 mg.kg-1 was given. Time for first rescue analgesia administration (i.e., the time from administration of block to the first demand of diclofenac) and the total analgesic consumption in 24 hours were recorded. Postoperative nausea and vomiting (PONV) were assessed using a 4-point scale system (0 = no PONV, 1 = mild nausea, 2 = severe nausea, 3 = vomiting), and antiemetic ondansetron (4 mg) was given if the PONV score was > 1. Any other adverse effects like hypotension, respiratory depression, shivering and urinary retention were also recorded.
Statistical analysis
Statistical analysis was performed using SPSS version 22 (Statistical Packages for the Social Sciences, Chicago, IL). The normality of quantitative data was checked by measures of the Kolmogorov-Smirnov test of normality. Continuous and quantitative variables were expressed in mean and standard deviation when normally distributed, and as median and interquartile ranges when non-normally distributed. Categorical variables were expressed in absolute and relative frequencies. Normally distributed data were compared using unpaired t-test and for non-normally distributed data Mann-Whitney U test was used. The Chi-square test or Fisher’s exact test was applied for categorical data. Intragroup comparison of hemodynamic variables from baseline was done by repeated-measures ANOVA followed by Student’s t-test. Post-hoc analysis with Bonferroni correction was applied for multiple comparisons. The Kaplan-Meier survival curves were drawn with the time to first analgesic administration in the postoperative period, being considered as the event and log-rank analysis was performed for comparison between the groups. A p value < 0.05 was considered statistically significant.
The sample size was calculated based on a previous study by Kulhari et al,10 an increase in the duration of postoperative analgesia by 30 minutes with SAPB was considered a clinically relevant difference. For a significance level of 0.05 and power of 0.8, at least 18 patients in each group were needed. Therefore, 40 patients were included in the study.
Results
The groups were comparable regarding the patients’ age, weight, height, and ASA physical status (Table 1). There was no significant difference between the two groups in the duration of surgery and the total volume of local anesthetic used for the blocks. Although more patients in the SAPB group had sensory spread at the level of T1, T2, and T6 as compared to the TPVB group, it was not statistically significant (Table 2). One patient in the SAPB group had no sensory deficit and considered as block failure.
Table 1.
Demographic data.
| Parameters | TPVB group (n = 20) | SAPB group (n = 20) |
|---|---|---|
| Age (yr) | 48.2 ± 9.8 | 50.8 ± 9.5 |
| Weight (kg) | 59.8 ± 11.6 | 62.3 ± 9.6 |
| Height (cm) | 157.9 ± 4.4 | 157.5 ± 4.4 |
| ASA 1 : 2 (n) | 16:4 | 15:5 |
| Duration of surgery (min) | 71.3 ± 14.6 | 76.5 ± 15.7 |
| Drug (LA) volume (mL) | 23.7 ± 3.7 | 23.6 ± 3.9 |
Values are expressed as mean ± SD or number (n) of patients.
Table 2.
Dermatomal spread of sensory block.
| Dermatomal level | TPVB group (n = 20) | SAPB group (n = 20) | p-value |
|---|---|---|---|
| T1 | 0 | 4 (20%) | - |
| T2 | 4 (20%) | 6 (30%) | 0.465 |
| T3 | 20 (100%) | 19 (95%) | 1.000 |
| T4 | 20 (100%) | 19 (95%) | 1.000 |
| T5 | 20 (100%) | 19 (95%) | 1.000 |
| T6 | 14 (70%) | 17 (85%) | 0.451 |
Values are presented as the number (%) of patients.
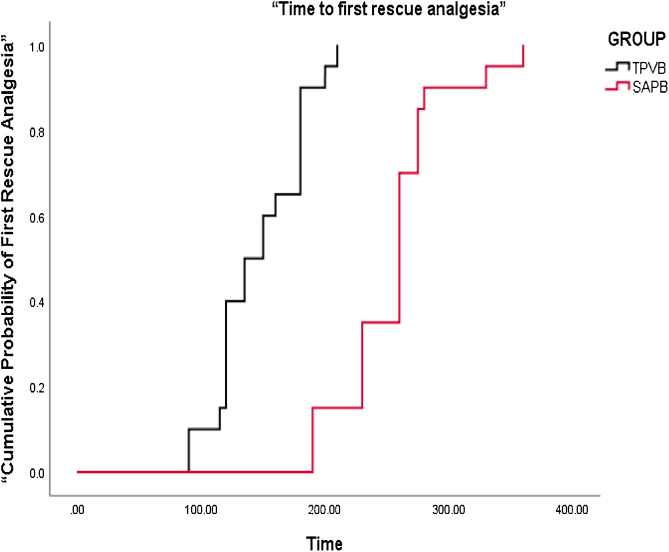
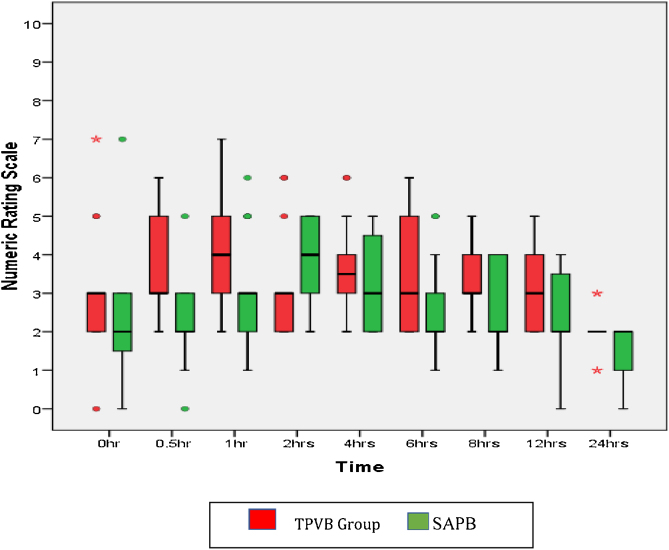
The Kaplan-Meier survival curves for the comparative cumulative probability of first recue analgesic requirement in the postoperative period are shown in Fig. 3. Time to first rescue analgesia (duration of analgesia) was significantly longer in the SAPB group (255.3 ± 47.8 min) as compared with the TPVB group (146.8 ± 30.4 min) with a p value of < 0.001. The diclofenac consumption in the first 24 hours after surgery was significantly less in the SAPB group as compared to the TPVB group (p < 0.001) while tramadol requirement was comparable in both the groups (Table 3). Post-operative pain scores were significantly lower in the SAPB group as compared to the TPVB group (p < 0.05) during the first 2 hours and then at 6, 8, and 24 hours after surgery (Fig. 4). The intraoperative and postoperative hemodynamic variables were comparable in both groups except just after induction of anesthesia when MAP in the TPVB group was lower as compared to the SAPB group (p < 0.039). No episode of hypotension or bradycardia was reported in any group of patients. The incidence of PONV was significantly less in the SAPB group (p = 0.028). Eight patients in the TPVB group had PONV (grade > 2) and all received antiemetics whereas two patients in the SAPB group had PONV and only one of them required antiemetics. No block- related adverse effects were reported in any group of patients.
Figure 3.
Kaplan-Meier survival curves for the cumulative probability of first recue analgesic requirement in the postoperative period in both the groups (log rank analysis for comparison between groups shows p < 0.001).
Table 3.
Duration of analgesia and rescue analgesic requirement.
| Parameters | TPVB group (n = 20) | SAPB group (n = 20) | p-value |
|---|---|---|---|
| Time to first rescue Analgesia (min) | 146.75 ± 30.361 | 255.26 ± 47.798 | < 0.001 |
| Total Diclofenac (mg) | 210.00 ± 39.236 | 138.75 ± 44.036 | < 0.001 |
| Total Tramadol (mg) | 92.86 ± 18.898 | 98 ± 12.46 | 0.626 |
Values are expressed as mean ± SD.
Figure 4.
Numeric rating scale (NRS) scores for 24 hours after surgery (median, IQR).
p values: ½ h < 0.001, 1 h = 0.007, 2 h = 0.040, 4 h = 0.167, 6 = 0.040, 8 h = 0.021, 12 h = 0.080, 24 h = 0.032.
Discussion
In this randomized control study, we found that the patients who received SAPB had a longer duration of postoperative analgesia as compared to the patients who received TPVB. The SAPB group patients also showed lower postoperative pain scores and demanded less rescue analgesia in comparison to the TPVB group. The postoperative tramadol consumption was low in both groups.
Both SAPB and PVPB are attractive regional anesthesia options for providing postoperative analgesia after a radical mastectomy. Although the analgesic efficacy of TPVB versus placebo is well established in various thoracic surgeries, it carries theoretical risks of hypotension and pneumothorax, and not all providers have expertise and are comfortable with the technique.8 The SAPB has recently gained popularity because of its relative safety, and the ease with which it is learnt and performed.12 SAPB is usually performed at the level of 5th or 6th rib in midaxillary line via deposition of local anesthetic superficial or deep to the serratus anterior muscle.13 Although both superficial and deep approaches have been found equally effective for providing postoperative analgesia after breast surgery, Abdulla et al.14 reported that deep SAPB is more advantageous from a surgical point of view than superficial SAPB. Also, this avoids the possibility of transitory palsy of the long thoracic nerve leading to a winged scapula that can be mistaken with a surgical lesion of this nerve.
SAPB has been used effectively for breast cancer surgery as well as video-assisted trans-thoracic surgery.15, 16 A recent meta-analysis17 has shown that SAPB reduced postoperative pain scores, decreased opioid consumption in the first 24 hours after surgery, and prolonged time to first analgesia request as well as reduced the incidence of PONV and pruritus as compared with non-block care in breast and thoracic surgery patients. The block appeared safe with no study reporting any block-related complications.17 The preoperative administration of SAPB also improved the quality of recovery and patient satisfaction following breast cancer surgery.18
However, the efficacy of SAPB in comparison with TPVB is not well established. Our results are supported by a recent study,19 which also found SAPB superior to TPVB in terms of the delayed requirement for the first rescue analgesia and 24 hours reduced analgesic consumption in patients undergoing breast cancer surgery. However, in a previous study, Hetta et al.20 found SAPB inferior to the TPVB in terms of duration of postoperative analgesia and rescue analgesic requirements. This may be due to multiple injection technique used in the TPVB group (at T2, T4, and T6 levels) in their study.20
SAPB targets the lateral cutaneous branches of the intercostal nerves as they traverse between the fascial planes and provide extensive anesthesia of the anterolateral chest wall.21 Although TPVB targets the spinal nerves directly, the spread of local anesthetic is not predictable, it may spread either laterally to block the intercostal nerves or medially into the epidural space through the intervertebral foramina.22 A single level TPVB can block one to four dermatomes only. Therefore, a single-level injection of TPVB may not be enough to produce sufficient analgesia after extensive breast cancer surgeries.
In the present study, the duration of analgesia was 255.3 ± 47.8 minutes in SAPB group, which is comparable to the previous reports. Blanco et al.13 demonstrated 386 (±160) minutes paresthesia in 3 - 5 dermatomes after administration of deep SAPB with 0.4 mg.kg-1 local anesthetic in volunteers. Rahimzadeh et al.23 reported the time to first rescue analgesia as 323.5 ± 49.7 minutes after SAPB in patients undergoing breast cancer surgery. In a recent meta-analysis, Chong et al.17 have also found the time to first analgesic request in SAPB as 379.2 minutes. However, a longer duration of postoperative analgesia (> 12 hours) has been reported after SAPB in patients undergoing partial or simple mastectomy.24, 25 This may be because the axillary dissection usually requires analgesia up to T1 level while the spread of local anesthetic in the present study was mainly from T2 to T6 segments. Therefore, the pain caused by the axillary dissection might not be effectively controlled by the block. We used about 23 mL local anesthetic while up to 30 - 40 mL of local anesthetic has been used in previous studies.17, 24
Our success rate was 95% in the SAPB group and 100% in the TPVB group. We used echogenic needles to perform the blocks, as the use of echogenic needles under real-time ultrasound provides better visualization of the needle tip concerning the nearby structures and spread of local anesthetics, thus avoids complications. None of our patients had any block- related complications. The low incidence of PONV in patients receiving SAPB might be due to better pain relief (lower pain scores). Previous studies also reported a lower incidence of PONV in patients receiving SAPB.17
The main limitation of our study is that the subjects and the anesthesiologist performing the block were not blinded to the group assignment, though the investigator who collected the data was not aware of the group allocation of the patients. In addition, we followed up the patients for only 24 hours after surgery. We did not assess the effect of the block on early hospital discharge and the incidence of chronic pain. Another limitation of this study is that it is a single-center study. Therefore, further multicenter studies are required to generalize our results.
In conclusion, we found SAPB superior to TPVB in terms of prolonged duration of postoperative analgesia and reduced rescue analgesic requirement after radical mastectomy in breast cancer patients. Therefore, SAPB may be a viable alternative to the TPVB, which is technically more challenging and have a higher potential for adverse effects. Further studies are required to compare the efficacy and safety of SAPB with other chest wall blocks. As SAPB usually provides 4 - 6 hours of postoperative analgesia in these patients, the role of additives can also be assessed.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Woodworth G.E., Ivie R.M.J., Nelson S.M., et al. Perioperative Breast Analgesia: A Qualitative Review of Anatomy and Regional Techniques. Reg Anesth Pain Med. 2017;42:609–631. doi: 10.1097/AAP.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 2.Elvir-Lazo O.L., White P.F. The role of multimodal analgesia in pain management after ambulatory surgery. Curr Opin Anaesthesiol. 2010;23:697–703. doi: 10.1097/ACO.0b013e32833fad0a. [DOI] [PubMed] [Google Scholar]
- 3.Bansal P., Saxena K.N., Taneja B., et al. A comparative randomized study of paravertebral block versus wound infiltration of bupivacaine in modified radical mastectomy. J Anaesthesiol Clin Pharmacol. 2012;28:76–80. doi: 10.4103/0970-9185.92449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fassoulaki A. Brachial plexus block for pain relief after modified radical mastectomy. Anesth Analg. 1982;61:986–987. [PubMed] [Google Scholar]
- 5.Manion S.C., Brennan T.J. Thoracic epidural analgesia and acute pain management. Anesthesiology. 2011;115:181–188. doi: 10.1097/ALN.0b013e318220847c. [DOI] [PubMed] [Google Scholar]
- 6.Riain S.C.Ó., Donnell B.O., Cuffe T., et al. Thoracic paravertebral block using real-time ultrasound guidance. Anesth Analg. 2010;110:248–251. doi: 10.1213/ANE.0b013e3181c35906. [DOI] [PubMed] [Google Scholar]
- 7.Klein S.M., Bergh A., Steele S.M., et al. Thoracic paravertebral block for breast surgery. Anesth Analg. 2000;90:1402–1405. doi: 10.1097/00000539-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Lönnqvist P.A., MacKenzie J., Soni A.K., et al. Paravertebral blockade: Failure rate and complications. Anaesthesia. 1995;50:813–815. doi: 10.1111/j.1365-2044.1995.tb06148.x. [DOI] [PubMed] [Google Scholar]
- 9.Blanco R. The ‘pecs block’: a novel technique for providing analgesia after breast surgery. Anaesthesia. 2011;66:847–848. doi: 10.1111/j.1365-2044.2011.06838.x. [DOI] [PubMed] [Google Scholar]
- 10.Kulhari S., Bharti N., Bala I., et al. Efficacy of pectoral nerve block versus thoracic paravertebral block for postoperative analgesia after radical mastectomy: a randomized controlled trial. Br J Anaesth. 2016;117:382–386. doi: 10.1093/bja/aew223. [DOI] [PubMed] [Google Scholar]
- 11.Wahba S.S., Kamal S.M. Thoracic paravertebral block versus pectoral nerve block for analgesia after breast surgery. Egypt J Anaesth. 2014;30:129–135. [Google Scholar]
- 12.De la Torre P.A., García P.D., Alvarez S.L., et al. A novel ultrasound-guided block: a promising alternative for breast analgesia. Aesthet Surg J. 2014;34:198–200. doi: 10.1177/1090820X13515902. [DOI] [PubMed] [Google Scholar]
- 13.Blanco R., Parras T., McDonnell J.G., et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68:1107–1113. doi: 10.1111/anae.12344. [DOI] [PubMed] [Google Scholar]
- 14.Abdallah F.W., Cil T., MacLean D., et al. Too deep or not too deep? A propensity-matched comparison of the analgesic effects of a superficial versus deep serratus fascial plane block for ambulatory breast cancer surgery. Reg Anesth Pain Med. 2018;43:480–487. doi: 10.1097/AAP.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 15.Mazzinari G., Rovira L., Casasempere A., et al. Interfacial block at the serratus muscle plane versus conventional analgesia in breast surgery: a randomized controlled trial. Reg Anesth Pain Med. 2019;44:52–58. doi: 10.1136/rapm-2018-000004. [DOI] [PubMed] [Google Scholar]
- 16.Kim D.-H., Oh Y.J., Lee J.G., et al. Efficacy of ultrasound-guided serratus plane block on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: a randomized, triple-blind, placebo-controlled study. Anesth Analg. 2018;126:1353–1361. doi: 10.1213/ANE.0000000000002779. [DOI] [PubMed] [Google Scholar]
- 17.Chong M., Berbenetz N., Kumar K., et al. The serratus plane block for postoperative analgesia in breast and thoracic surgery: a systematic review and meta-analysis. Reg Anesth pain med. 2019;44:1066–1074. doi: 10.1136/rapm-2019-100982. [DOI] [PubMed] [Google Scholar]
- 18.Yao Y., Li J., Hu H., et al. Ultrasound-guided serratus plane block enhances pain relief and quality of recovery after breast cancer surgery: A randomised controlled trial. Eur J Anaesthesiol. 2019;36:436–441. doi: 10.1097/EJA.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 19.Amin S.R.M., Abdelrahman E.A., Afify E.E.S., et al. Ultrasound-guided serratus anterior plane block versus thoracic paravertebral block for postmastectomy analgesia. Benha Med J. 2018;35:429–436. [Google Scholar]
- 20.Hetta D.F., Rezk K.M. Pectoralis-serratus interfascial plane block vs thoracic paravertebral block for a unilateral radical mastectomy with axillary evacuation. J Clin Anesth. 2016;34:91–97. doi: 10.1016/j.jclinane.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Mayes J., Davison E., Panahi P., et al. An anatomical evaluation of the serratus anterior plane block. Anaesthesia. 2016;71:1064–1069. doi: 10.1111/anae.13549. [DOI] [PubMed] [Google Scholar]
- 22.Cowie B., McGlade D., Ivanusic J., et al. Ultrasound-Guided thoracic paravertebral blockade: a cadaveric study. Anesth Analg. 2010;110:1735–1739. doi: 10.1213/ANE.0b013e3181dd58b0. [DOI] [PubMed] [Google Scholar]
- 23.Rahimzadeh P., Imani F., Faiz S.H.R., et al. Impact of the ultrasound-guided serratus anterior plane block on post-mastectomy pain: a randomised clinical study. Turk J Anaesth Reanim. 2018;46:388–392. doi: 10.5152/TJAR.2018.86719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohgoshi Y., Yokozuka M., Terajima K. Serratus-Anterior plane block for breast surgery. Masui. 2015;64:610–614. [PubMed] [Google Scholar]
- 25.Shokri H., Kasem A.A. Efficacy of postsurgical ultrasound-guided serratus intercostal plane block and wound infiltration on postoperative analgesia after female breast surgeries. A comparative study. Egypt J Anaesth. 2017;33:35–40. [Google Scholar]