Abstract
Objective
There is an evidence gap regarding the predictive accuracy of the triglyceride-glucose (TyG) index for long-term major adverse cardiovascular events (MACEs) in individuals with high cardiovascular risk. The aim of this investigation was to evaluate the predictive value of the TyG index for long-term MACEs in patients at high cardiovascular risk.
Methods
In total, 483 patients with high cardiovascular risk were included in this analysis. The study population was separated into 2 groups depending on the occurrence of long-term MACEs. The independent predictors of long-term MACEs in patients with high cardiovascular risk were investigated. The long-term prognostic value of the TyG index in these patients was evaluated in terms of MACEs.
Results
Age, male sex, diabetes mellitus, and the TyG index were demonstrated to be independent predictors of long-term MACE occurrence in patients with high cardiovascular risk. The TyG index was independently related to long-term MACEs in patients with high cardiovascular risk (hazard ratio, 1.003; 95% confidence interval [CI], 1.001–1.006; p=0.011). The receiver operating characteristic curve revealed that the optimum value of the TyG index to predict long-term MACEs in the overall study cohort was >9.68, with 65% sensitivity and 63% specificity (area under the curve, 0.71; 95% CI, 0.65–0.77; p<0.001).
Conclusion
The TyG index was demonstrated to be an independent predictor of long-term MACE occurrence in patients with high cardiovascular risk who had not been previously diagnosed with cardiovascular disease.
Keywords: Triglyceride, Glucose, Index, Cardiovascular risk, Cardiovascular diseases
INTRODUCTION
Atherosclerotic cardiovascular disease (ASCVD) is the major cause of morbidity and mortality in modern society, emphasizing the need for its early detection and prevention.1 In current practice, available risk stratification models rely mostly on traditional cardiovascular risk parameters to estimate an individual’s risk of ASCVD.2 However, some cardiovascular risk factors are potentially modifiable, and a prior study has revealed that insulin resistance (IR) is closely associated with new ASCVD events both in individuals with and without diabetes.3
The triglyceride-glucose (TyG) index is the natural logarithm of the product of the fasting triglyceride (TG) and fasting blood glucose (FBG) levels divided by 2. Large-scale epidemiological studies have found that the TyG index might be utilized as a simple and reliable surrogate indicator of IR.4 Studies have shown that the TyG index is associated with metabolic syndrome, stroke, and poorer outcomes in patients with chronic coronary syndrome.5,6,7 Furthermore, a prior study found that this index might predict major adverse cardiovascular events (MACEs) in individuals with acute coronary syndrome.8 Nevertheless, no evidence is available on the predictive accuracy of the TyG index for long-term MACEs in individuals with high cardiovascular risk. Therefore, this study aimed to contribute to the literature regarding the use of the TyG index in a primary prevention setting. The main goal of the investigation was to explore the predictive relevance of the TyG index for long-term MACEs in patients with high cardiovascular risk. We hypothesized that the TyG index would be an independent predictor of long-term MACE occurrence in patients with high cardiovascular risk.
MATERIALS AND METHODS
1. Subjects
This is a retrospective chart review study. The medical files of patients who were evaluated at outpatient visits at a tertiary center were reviewed retrospectively. In this analysis, only individuals with a 10-year ASCVD risk score of ≥7.5%, who were stratified as having high cardiovascular risk, were included.9 ASCVD risk was calculated according to a previously proposed risk calculator.9 Patients aged <18 and >80 years old, as well as those with prior ASCVD, stroke, active infection, severe liver or renal illness, excessive obesity (body mass index [BMI] >45 kg/m2), auto-immune disease, thyroid disease, or cancer were excluded. Furthermore, individuals with incomplete health records were excluded from the final analysis. The hospital database was used to collect laboratory data and demographic information for all patients. Anthropometric data were used to calculate BMI. Blood pressure measurements were also performed to detect individuals with hypertension (HT). HT was defined as a systolic blood pressure (SBP) of >140 mmHg, diastolic blood pressure (DBP) of >90 mmHg, reported use of anti-hypertensive medications, or a history of HT. Participants were defined as having diabetes if their FBG level was >126 mg/dL, their hemoglobin A1c (HbA1c) level was >6.5%, or if they reported use of hypoglycemic medications or a history of diabetes. The standard biplane Simpson method was used to calculate the left ventricular ejection fraction (LVEF).10 The simplified biplane area-length approach was used to determine the left atrial volume index (LAVI), which was then adjusted for body surface area (BSA). To calculate the left ventricular mass index (LVMI), the Devereux formula was utilized, which was then adjusted by BSA. The study was authorized by the local ethics committee, and it was carried out in accordance with the Helsinki Declaration (IRB number: 22/261—from Hamidiye School of Medicine Scientific Ethical Committee). This study was described according to the report guideline.
2. Laboratory analysis
After at least 8 hours of fasting, blood specimen collection was performed in the early morning. The FBG and lipid profile, including serum TG, were measured using conventional biochemical techniques. If the TG level was ≤400 mg/dL, the Friedewald equation was used to calculate low-density lipoprotein cholesterol. The TyG index was calculated as: TyG Index=Ln [Fasting TG (mg/dL)×FBG (mg/dL)/2].
3. Outcomes
The mean follow-up duration of the investigation was 44.3±3.9 months. MACEs were defined as cardiovascular mortality, non-fatal myocardial infarction (MI), and undergoing percutaneous coronary intervention (PCI) or aortocoronary bypass grafting (ACBG) in long-term follow-up. Outpatient visits, phone interviews, and national and hospital medical databases were used to gather information on long-term MACEs.
4. Statistical analysis
SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used for all statistical tests. To illustrate categorical data, frequencies and percentages were employed. To compare categorical data between groups, the χ2 test was performed. All continuous variables had non-normal distributions. As a consequence, the medians (interquartile ranges) for these variables were presented. To assess the normality of variable distributions, the Kolmogorov-Smirnov test was applied. To analyze all variables with skewed distributions, the Kruskal-Wallis test was performed. To identify variables independently related to long-term MACEs, univariable and multivariable Cox regression models were used (using the enter method). Parameters having a p-value of 0.05 in the univariable analysis were integrated into the multivariable Cox regression analysis. The cumulative survival of patients based on the TyG index was shown using the Kaplan-Meier and log-rank tests. The optimum cut-off value of the TyG index for long-term MACEs in all patients and patients without diabetes was determined using a nonparametric receiver operating characteristic (ROC) curve analysis. All data were presented in the form of hazard ratios (HRs) and 95% confidence intervals (CIs). As the level of statistical significance, a p-value of ≤0.05 was chosen.
RESULTS
483 patients with high cardiovascular risk were included in this analysis. Long-term MACEs were observed in 87 (18%) patients. The participants in this research were separated into patients without long-term MACEs (n=396) and those with long-term MACEs (n=87).
The baseline characteristics, echocardiographic data, and laboratory results for all patients are presented in Table 1. The patients with long-term MACEs were older and had a higher male-to-female ratio, SBP, and DBP. Diabetes mellitus (DM), HT, and hyperlipidemia were more prevalent in patients with long-term MACEs. In terms of echocardiographic data, the LVEF, LAVI, and LVMI were similar in both groups. In regard to laboratory measurements, the patients with long-term MACEs had significantly higher levels of FBG, creatinine, TG, the TyG index, and HbA1c and significantly lower high-density lipoprotein cholesterol (HDL-C) levels. In total, 66 patients developed non-fatal MI and 83 patients underwent PCI and/or ACBG during long-term follow-up. The overall all-cause mortality was 3.7% (n=18).
Table 1. Baseline characteristics, laboratory parameters, and echocardiographic findings of all patients.
| Characteristics | Patients without incident MACEs (n=396) | Patients with incident MACEs (n=87) | p-value | |
|---|---|---|---|---|
| Age (yr) | 58.0 (53.0–63.0) | 65.0 (61.0–68.0) | <0.001 | |
| Sex, male | 171 (43.2) | 64 (73.6) | <0.001 | |
| BMI (kg/m2) | 28.9 (25.8–32.6) | 28.4 (25.4–31.4) | 0.370 | |
| SBP (mmHg) | 142 (132–151) | 152 (143–159) | <0.001 | |
| DBP (mmHg) | 82 (72–90) | 86 (75–97) | 0.007 | |
| Heart rate (beat per min) | 76 (69–82) | 76 (69–83) | 0.874 | |
| Risk factors | ||||
| HT | 270 (68.2) | 75 (86.2) | 0.001 | |
| Diabetes mellitus | 164 (41.4) | 63 (72.4) | <0.001 | |
| Hyperlipidemia | 76 (19.2) | 26 (29.9) | 0.027 | |
| Smoking | 172 (43.4) | 41 (47.1) | 0.530 | |
| Echocardiographic data | ||||
| LVEF | 61 (60–63) | 61 (60–62) | 0.605 | |
| LAVI (mL/m2) | 29.0 (24.2–34.0) | 29.6 (24.4–35.5) | 0.886 | |
| LVMI (g/m2) | 85.5 (72.8–94.9) | 89.3 (81.6–97.6) | 0.063 | |
| Laboratory analysis | ||||
| FBG (mg/d) | 106 (97–126) | 132 (104–165) | <0.001 | |
| Creatinine (mg/dL) | 0.81 (0.74–0.91) | 0.86 (0.77–1.00) | 0.012 | |
| BUN (mg/dL) | 14 (13–18) | 15 (12–20) | 0.777 | |
| AST (U/L) | 20 (17–24) | 19 (16–23) | 0.219 | |
| ALT (U/L) | 21 (16–28) | 20 (15–28) | 0.840 | |
| LDL-C (mg/dL) | 134 (114–163) | 142 (122–161) | 0.598 | |
| HDL-C (mg/dL) | 43 (38–51) | 41 (35–48) | 0.013 | |
| TG (mg/dL) | 146 (106–202) | 189 (147–289) | <0.001 | |
| TyG index | 8.2 (5.6–11.7) | 12.6 (8.5–21.3) | <0.001 | |
| Hs-CRP (mg/dL) | 0.00 (0.00–0.30) | 0.00 (0.00–0.30) | 0.645 | |
| Hs-troponin (ng/mL) | 0.02 (0.01–0.03) | 0.02 (0.01–0.03) | 0.251 | |
| HbA1c (%) | 6.0 (5.7–6.5) | 6.5 (6.0–7.1) | <0.001 | |
| TSH (mIU/L) | 1.5 (1.0–2.2) | 1.3 (0.9–2.0) | 0.161 | |
| T4 (ng/dL) | 1.0 (0.9–1.0) | 1.0 (0.9–1.1) | 0.639 | |
| Long-term MACEs | ||||
| Non-fatal MI | 0 (0.0) | 66 (75.9) | ||
| PCI-ACBG | 0 (0.0) | 83 (95.4) | ||
| Cardiovascular mortality | 0 (0.0) | 18 (20.7) | ||
| Follow-up (mon) | 45 (41–48) | 43 (41–48) | ||
Continuous variables are presented as median (interquartile range), nominal variables are presented as frequency (%).
MACE, major adverse cardiovascular event; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HT, hypertension; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; LAVI, left atrial volume index; LVMI, left ventricular mass index; FBG, fasting blood glucose; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; TyG, triglyceride-glucose; hs-CRP, high-sensitive C-reactive protein; hs-troponin, high-sensitivity troponin; TSH, thyroid-stimulating hormone; MI, myocardial infarction; PCI, percutaneous coronary intervention; ACBG, aortocoronary bypass grafting.
The findings of univariable and multivariable Cox proportional regression analysis for independent predictors of long-term MACEs can be seen in Table 2. Age, male sex, SBP, DBP, HT, DM, TG, FBG, the TyG index, creatinine, HDL-C, and HbA1c each predicted the occurrence of long-term MACEs using univariable Cox proportional regression analysis. After including all of the aforementioned variables in a multivariable Cox proportional regression analysis, age, male sex, DM, and the TyG index (HR, 1.003; 95% CI, 1.001–1.006; p=0.011) were shown to be independently related to long-term MACEs.
Table 2. Univariable and multivariable model for long-term major adverse cardiovascular events.
| Characteristics | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 1.125 (1.089–1.163) | <0.001 | 1.153 (1.114–1.194) | <0.001 |
| Male sex | 2.990 (1.856–4.817) | <0.001 | 3.848 (2.324–6.372) | <0.001 |
| SBP | 1.031 (1.018–1.045) | <0.001 | 1.030 (1.011–1.048) | 0.001 |
| DBP | 1.019 (1.005–1.034) | 0.008 | - | - |
| HT | 2.422 (1.317–4.456) | 0.004 | - | - |
| DM | 2.870 (1.793–4.592) | 0.001 | 2.862 (1.688–4.852) | <0.001 |
| TG* | 1.004 (1.003–1.006) | <0.001 | - | - |
| FBG* | 1.007 (1.004–1.010) | <0.001 | - | - |
| TyG index | 1.004 (1.002–1.015) | <0.001 | 1.003 (1.001–1.006) | 0.011 |
| Creatinine | 3.744 (1.143–12.258) | 0.029 | - | - |
| HDL-C | 0.980 (0.959–1.002) | 0.072 | - | - |
| HbA1c | 1.199 (1.064–1.352) | 0.003 | - | - |
All clinically relevant parameters were included in the model.
SBP, systolic blood pressure; DBP, diastolic blood pressure; HT, hypertension; DM, diabetes mellitus; TG, triglyceride; TyG, triglyceride-glucose; HDL-C, high-density lipoprotein cholesterol; HbA1c, hemoglobin A1c; HR, odds ratio; CI, confidence interval.
*These parameters were not included in the multivariable model as they are components of the TyG index.
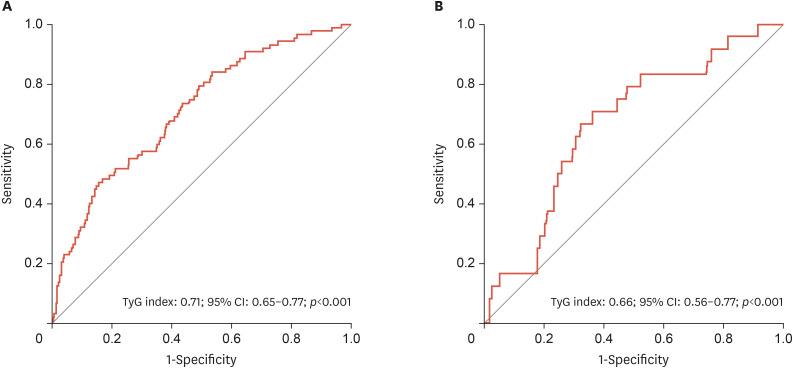
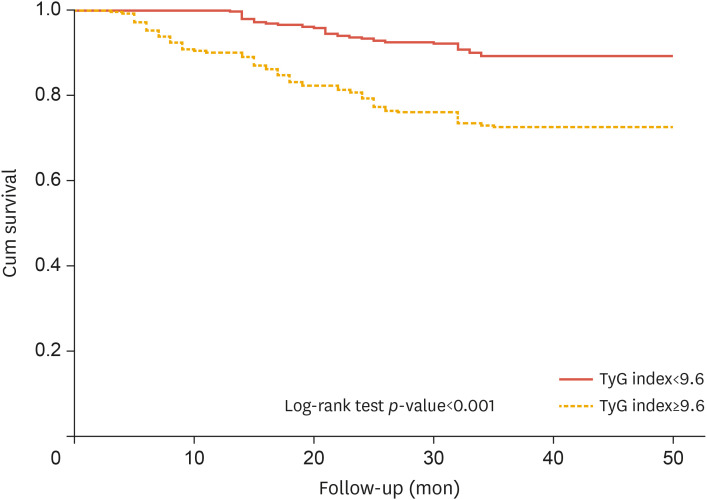
The ROC curve revealed that the optimum value of the TyG index to predict long-term MACEs in the overall study cohort was >9.68, with 65% sensitivity and 63% specificity (area under the curve [AUC], 0.71; 95% CI, 0.65–0.77; p<0.001) (Fig. 1A). Meanwhile, the optimum value of TyG index to predict long-term MACEs in patients without diabetes was >8.28, with 66% sensitivity and 67% specificity (AUC, 0.66; 95% CI, 0.56–0.77; p<0.001) (Fig. 1B). According to the Kaplan-Meier survival curves, individuals with high cardiovascular risk who had a TyG index of more than 9.68 had a lower probability of surviving during long-term follow-up (log-rank test: χ2=24.07) (Fig. 2).
Fig. 1. (A) A ROC curve analysis of the TyG index for long-term MACEs for patients with high cardiovascular risk. (B) A ROC curve analysis of the TyG index in long-term MACEs for patients without diabetes who had high cardiovascular risk.
ROC, receiver operating characteristic; TyG, triglyceride-glucose; MACE, major adverse cardiovascular event; CI, confidence interval.
Fig. 2. Kaplan-Meier survival analysis of long-term MACEs according to cut-off values determined through receiver operating characteristic curve analysis.
TyG, triglyceride-glucose; MACE, major adverse cardiovascular event.
DISCUSSION
Age, male sex, DM, and the TyG index were demonstrated to be independent predictors of long-term MACE occurrence in patients with high cardiovascular risk. This investigation is the first study evaluating the predictive value of the TyG index in terms of long-term MACEs in patients with high cardiovascular risk.
Age, male sex, and DM are well-known risk factors for cardiovascular disease.11 The TyG index has been shown to have associations with poor outcomes in several cardiovascular diseases, including in patients with stable coronary artery disease, less developed collateral networks in the coronaries, and chronic total occlusions.12,13 However, the precise pathophysiology underlying the relationship between the TyG index and cardiovascular disease remains obscure. The TyG index calculation includes fasting TG and FBG levels, which are globally accepted as risk factors for atherosclerosis. IR has also been linked to coronary artery disease in recent decades.14 Interestingly, Guerrero-Romero et al.15 showed that the TyG index served as a marker of IR, which may explain the close relationship between the TyG index and coronary artery disease IR, which is significantly correlated with the TyG index, leads to endothelial dysfunction, coagulation disorders, myocardial cell death, smooth muscle cell dysfunction, cardiac fibrosis, and ventricular stiffness.16,17 However, other investigations have also failed to show a relationship between the TyG index and adverse cardiovascular events.18,19 Undiagnosed disorders in glucose and TG metabolism may be the underlying reason for those non-significant findings. Differences in treatment modalities between patients with diabetes and dyslipidemia may also have caused the lack of a relationship between the TyG index and adverse cardiovascular events in these studies. Despite inconsistent results among investigations of the TyG index, most studies have reported a clear relationship between the TyG index and cardiovascular diseases, such as acute coronary syndrome, in-stent restenosis, arterial stiffness, coronary artery calcification, and heart failure.7,20,21,22,23 However, there is an evidence gap regarding the relationship between the TyG index and long-term MACEs in patients with high cardiovascular risk. Our study presents important follow-up data, including both patients with and without diabetes who had high cardiovascular risk, and our findings emphasize the substantial role of the TyG index in primary prevention. The TyG index can be suggested as a surrogate marker in patients with high cardiovascular risk for long-term MACE prediction. Thus, patients with a higher TyG index may require aggressive treatment strategies concomitant with healthy lifestyle habits in order to prevent MACEs. The TyG index, which is easily calculable in all patients, may play a role in directing primary prevention strategies in patients with high cardiovascular risk.
Our study has several limitations. First, there might have been an elevated risk for numerous confounding factors due to the retrospective study design. However, all consecutive patients with high cardiovascular risk were included and patients’ follow-up was applied in compliance with a standard protocol including outpatient visits and phone calls. Second, the mortality reasons were not elaborated upon since we did not know the causes of death for most patients in the study population. Therefore, all-cause mortality was analyzed as an outcome.
In conclusion, the TyG index was demonstrated to be an independent predictor of long-term MACE occurrence in patients with high cardiovascular risk who had not been previously diagnosed with ASCVD.
Footnotes
Funding: None.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Hayıroğlu Mİ, Ayhan G.
- Data curation: Çiçek V, Palice A.
- Formal analysis: Hayıroğlu Mİ.
- Investigation: Hayıroğlu Mİ, Çınar T.
- Methodology: Hayıroğlu Mİ, Çınar T.
- Resources: Çiçek V.
- Writing - original draft: Hayıroğlu Mİ, Çınar T.
- Writing - review & editing: Hayıroğlu Mİ, Tekkeşin Aİ.
References
- 1.Barquera S, Pedroza-Tobías A, Medina C, Hernández-Barrera L, Bibbins-Domingo K, Lozano R, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46:328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy KJ, Singh M, Bangit JR, Batsell RR. The role of insulin resistance in the pathogenesis of atherosclerotic cardiovascular disease: an updated review. J Cardiovasc Med (Hagerstown) 2010;11:633–647. doi: 10.2459/JCM.0b013e328333645a. [DOI] [PubMed] [Google Scholar]
- 4.Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PLoS One. 2019;14:e0212963. doi: 10.1371/journal.pone.0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Son DH, Lee HS, Lee YJ, Lee JH, Han JH. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32:596–604. doi: 10.1016/j.numecd.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Sun H, Zhang W, Xi Y, Shi X, Yang Y, et al. Elevated triglyceride-glucose index predicts risk of incident ischaemic stroke: the Rural Chinese Cohort Study. Diabetes Metab. 2021;47:101246. doi: 10.1016/j.diabet.2021.101246. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Liu K, Chen M, Liu Y, Gao A, Hu C, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20:137. doi: 10.1186/s12933-021-01332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akbar MR, Pranata R, Wibowo A, Irvan, Sihite TA, Martha JW. The association between triglyceride-glucose index and major adverse cardiovascular events in patients with acute coronary syndrome – dose-response meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31:3024–3030. doi: 10.1016/j.numecd.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 10.Otterstad JE. Measuring left ventricular volume and ejection fraction with the biplane Simpson’s method. Heart. 2002;88:559–560. doi: 10.1136/heart.88.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Smedt D, De Bacquer D, De Sutter J, Dallongeville J, Gevaert S, De Backer G, et al. The gender gap in risk factor control: effects of age and education on the control of cardiovascular risk factors in male and female coronary patients. The EUROASPIRE IV study by the European Society of Cardiology. Int J Cardiol. 2016;209:284–290. doi: 10.1016/j.ijcard.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Jin JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10:6137–6146. doi: 10.21037/jtd.2018.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao A, Liu J, Hu C, Liu Y, Zhu Y, Han H, et al. Association between the triglyceride glucose index and coronary collateralization in coronary artery disease patients with chronic total occlusion lesions. Lipids Health Dis. 2021;20:140. doi: 10.1186/s12944-021-01574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rewers M, Zaccaro D, D’Agostino R, Haffner S, Saad MF, Selby JV, et al. Insulin sensitivity, insulinemia, and coronary artery disease: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27:781–787. doi: 10.2337/diacare.27.3.781. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 16.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21:68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, et al. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res Clin Pract. 2007;77(Suppl 1):S161–S164. doi: 10.1016/j.diabres.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 18.Cho YR, Ann SH, Won KB, Park GM, Kim YG, Yang DH, et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci Rep. 2019;9:6129. doi: 10.1038/s41598-019-42700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46:189–197. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19:80. doi: 10.1186/s12933-020-01054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17:41. doi: 10.1186/s12933-018-0692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16:108. doi: 10.1186/s12933-017-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo W, Zhao L, Mo F, Peng C, Li L, Xu Y, et al. The prognostic value of the triglyceride glucose index in patients with chronic heart failure and type 2 diabetes: a retrospective cohort study. Diabetes Res Clin Pract. 2021;177:108786. doi: 10.1016/j.diabres.2021.108786. [DOI] [PubMed] [Google Scholar]