Abstract
There are unmet needs for pharmacologic agents beyond current medications, such as statins, to effectively lower low-density lipoprotein cholesterol levels to target goals, especially in patients with very high or extremely high risk. Pharmacological targeting of mRNA represents an emerging, innovative approach with the potential to expand upon current therapies. In RNA-targeted therapeutics, a novel approach is the use of chemically modified oligonucleotides to inhibit the production of target proteins at their sites of gene coding. There are two main classes of RNA-targeted therapeutics: single-stranded antisense oligonucleotides (ASOs) and double-stranded small inhibiting RNAs. ASOs are synthetic molecules with a length of 15–30 nucleotides that are designed specifically to bind to a target mRNA in a sequence-specific manner. Using these agents to inhibit the translation of key regulatory proteins, such as apolipoprotein CIII, apolipoprotein(a), and angiopoietin-like protein 3, has demonstrated treatment efficacy for dyslipidemia. Many cardiovascular outcome trials with ASOs are ongoing. As clinicians, we must carefully monitor the long-term safety and efficacy of this new modality through large clinical trials in the future.
Keywords: Antisense oligonucleotides, Dyslipidemia, Apolipoprotein C-III, Apolipoprotein(a), Angiopoietin-like protein 3
INTRODUCTION
Low-density lipoprotein (LDL) is a crucial risk factor for the initiation and progression of atherosclerotic cardiovascular disease (ASCVD).1,2 Clinical trials have consistently shown that reductions in low-density lipoprotein cholesterol (LDL-C) levels by statins reduce morbidity and mortality in patients with ASCVD,3,4 and there is no argument with major guidelines that recommend statins as first-line therapy to lower LDL-C levels.5,6 However, in a subgroup of very high-risk patients, statin monotherapy or a combination therapy of statins with other lipid-lowering agents does not effectively lower LDL-C to reduce major adverse cardiovascular events. These include patients with familial hypercholesterolemia, those who are refractory to existing lipid-lowering agents, and those who are intolerant to statins.7 Clearly, there is a need for pharmacological agents to lower LDL-C levels to target goals in very high-risk patients that cannot be met by current therapeutics.
Traditional small-molecule lipid-lowering agents such as statins and fibrates target the catalytic or regulatory domain of proteins. For example, statins competitively inhibit 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase,8 while fibrates activate peroxisome proliferator-activated receptor α (PPARα).9 However, small-molecule agents cannot effectively control proteins without a well-defined catalytic or regulatory domain, such as apolipoprotein CIII (apoCIII), apolipoprotein(a) (apo[a]), and angiopoietin-like protein 3 (ANGPTL3). These proteins can be targeted with monoclonal antibodies, but large amounts of antibodies would be required to completely inactivate these proteins, and such high plasma concentrations would generate adverse levels of immune complexes. These factors also impact safety and cost issues and have limited the use of monoclonal antibody drugs other than proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors in this field.10
RNA-targeted therapeutics represent an innovative approach to LDL-C control through the use of chemically modified oligonucleotides to inhibit the production of target proteins at their sites of gene expression. There are two main classes of RNA-targeted therapeutics: single-stranded antisense oligonucleotides (ASOs) and double-stranded small inhibiting RNA.11 These therapies block important target proteins in lipoprotein metabolism.
In this review, we focus on ASO technology and ASO agents targeting apoCIII, apo(a), and ANGPTL3 that have emerged as new therapeutic modalities in lipid-lowering therapy. Regarding ASO agents, the rationale for target identification, pharmacology, and clinical trial results are discussed.
ANTISENSE OLIGONUCLEOTIDE TECHNOLOGY
ASOs are single-stranded, synthetic molecules with a length of 15–30 nucleotides, designed to bind specifically to a target mRNA in a sequence-specific manner.12 ASOs are chemically modified by processes such as phosphorothioate backbone modifications, where phosphorothioate is used as a substitute for the phosphodiester linkages between nucleotide bases. For ASO drugs, this modification confers increased resistance to nucleases and increases binding activity to plasma proteins. As a result, it enhances plasma half-life and facilitates drug delivery to target tissues.13
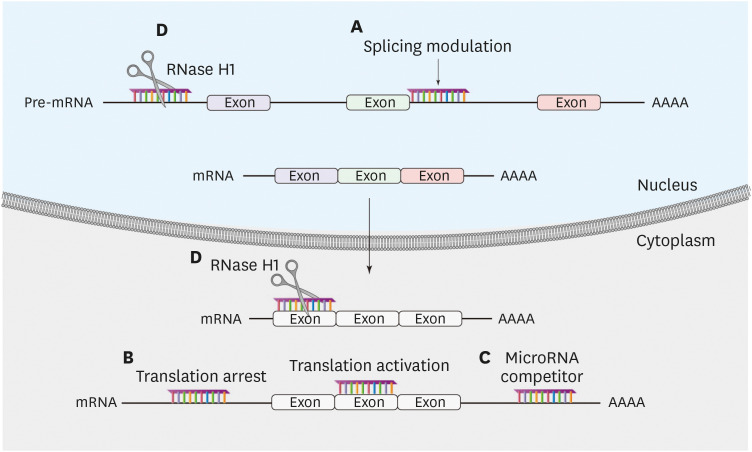
ASOs can modulate gene expression by two different mechanisms (Fig. 1).11 First, ASOs bind to and occupy the target mRNA without triggering RNA degradation (occupancy-only mediated). This mechanism results in changes in RNA processing, inhibition or enhancement of translation, and inhibition of interactions of the target mRNA with key proteins. Second, ASOs induce targeted mRNA degradation (enzymatic RNA degradation) through the cleavage of target mRNAs by RNase H1. ASOs contain chemically modified RNA nucleotides flanking a central region composed of 8–10 DNA nucleotides. The RNA nucleotides enhance affinity to complementary sequences, while the DNA nucleotides serve as a substrate for RNase H1. The degradation process of mRNA by RNase H1 is specific for RNA in an RNA-DNA duplex and takes place in both the cytoplasm and nucleus.14
Fig. 1. Modulation of gene expression by ASOs. ASOs can modulate gene expression by two different mechanisms. First, ASOs bind and occupy the target mRNA without triggering RNA degradation (occupancy-only mediated): (A) splicing modulation by base pairing with sequence elements in pre-mRNA to inhibit or enhance the utilization of splicing sites; (B) translation modulation by base pairing with mRNA, either to inhibit or activate translation through binding to inhibitory elements; (C) microRNA modulation either by base pairing with microRNA to inhibit the function of the microRNA or by base pairing with microRNA-binding sites of a particular mRNA to eliminate the effect of a particular microRNA. Second, ASOs induce target mRNA degradation (enzymatic RNA degradation): (D) DNA-like ASOs that trigger complementary RNA cleavage by RNase H1.
ASO, antisense oligonucleotide.
APOLIPOPROTEIN CIII
1. Rationale for target identification
Plasma triglycerides are a combination of triglyceride-rich lipoproteins and their remnant lipoprotein particles. Although triglycerides themselves are not considered a crucial risk factor for ASCVD, elevated levels of plasma triglyceride-rich lipoproteins and their remnants have been causally linked to ASCVD and explain important concepts of remnant CVD risks.15,16 Elevated triglyceride levels result in the elevation of very low-density lipoprotein (VLDL) and chylomicron remnants, activating cholesteryl ester transfer protein, and increased levels of small, dense LDL-C. These remnant particles and small, dense LDL-C contribute to cholesterol deposition and the growth of atheromatous plaques.17
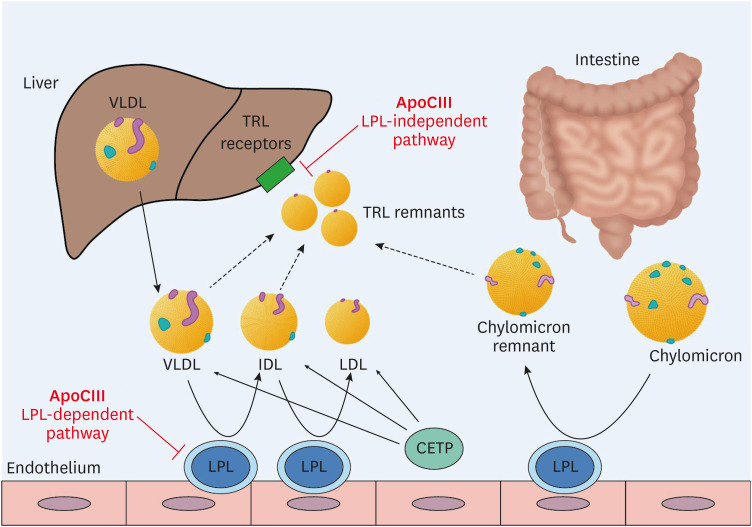
ApoCIII is a key regulator of triglyceride-rich lipoprotein metabolism (Fig. 2). It is a potent inhibitor of lipoprotein lipase (LPL), the enzyme responsible for the lipolysis of triglycerides in VLDL and chylomicron particles. In addition, by LPL-independent pathways, ApoCIII inhibits the hepatic clearance of VLDL and chylomicron remnants.18 High plasma apoCIII levels are associated with an increased risk of coronary heart disease (CHD).19 , 20 In addition, loss-of-function mutations in the APOC3 gene are associated with 40% lower triglyceride levels and a 40% lower risk of CHD than observed in individuals without those mutations.21,22 The cardiovascular benefits of serum triglyceride reduction are not consistent.23 However, considering the contribution of triglyceride-rich lipoproteins to atherosclerosis and the association of loss-of-function mutations in the APOC3 gene with a lower risk of CHD, apoCIII appears to be a promising target in the prevention of ASCVD.
Fig. 2. The metabolism of TRLs and the role of apoCIII. Triglyceride-rich VLDL is released from the liver and converted to IDL by lipoprotein lipase (LPL). Chylomicrons are formed in the intestine and transformed into chylomicron remnants by LPL. ApoCIII regulates TRL metabolism by inhibiting the activity of LPL (LPL-dependent pathway) and by interfering with hepatic clearance of TRL remnants (LPL-independent pathway).
TRL, triglyceride-rich lipoprotein; apoCIII, apolipoprotein CIII; VLDL, very low-density lipoprotein; IDL, intermediate-density lipoprotein; LPL, lipoprotein lipase; CETP, cholesteryl ester transfer protein.
2. Pharmacology
The first drug specifically targeting apoCIII mRNA was volanesorsen (previously identified as ISIS 304801 or ISIS-APOCIIIRx). Volanesorsen is a 2′-methoxyethyl-modified ASO with phosphorothioate substitutions administered subcutaneously once a week.24,25 Subsequently, IONIS-APOCIII-LRx (previously identified as AKCEA-APOCIII-LRx or ISIS 678354) was developed. This agent has the same nucleotide sequence as unconjugated volanesorsen, but additionally contains a triantennary N-acetyl galactosamine (GalNAc) complex. It has been tested with subcutaneous injections weekly or every 4 weeks.26
3. Clinical trial results
Volanesorsen has been tested in patients with elevated triglyceride levels and in patients with familial chylomicronemia syndrome (FCS), an autosomal recessive disease of chylomicron metabolism associated with severe hypertriglyceridemia and recurrent pancreatitis due to a deficiency in lipoprotein lipase function.
In a phase 2 trial (NCT01529424), treatment with volanesorsen resulted in a significant reduction in triglyceride levels in patients with hypertriglyceridemia.27 At the highest dose of 300 mg weekly, apoCIII and triglyceride levels were reduced by 80% and 70%, respectively, when volanesorsen was administered as monotherapy, and by 71% and 64%, respectively, when it was added to fibrate as a combination therapy.
In three patients with FCS, volanesorsen reduced triglyceride levels by up to 86% through an LPL-independent pathway.28 The APPROACH trial (NCT02211209) was a 52-week phase 3 trial of 66 FCS patients with fasting triglyceride levels ≥750 mg/dL.25 After treatment with 300 mg of volanesorsen weekly, apoCIII levels were reduced by 84% at 3 months, and triglyceride levels were reduced by 77%. Seventy-seven percent of patients in the volanesorsen group achieved triglyceride levels of < 750 mg/dL. Sixty percent and 45% of patients in the volanesorsen group exhibited injection-site reactions and thrombocytopenia with platelet levels of <100,000/μL, respectively, suggesting that we must monitor platelet levels closely in future clinical settings.
COMPASS (NCT02300233) was a 26-week phase 3 trial of 113 patients with fasting triglyceride levels ≥500 mg/dL.29 After treatment with 300 mg of volanesorsen weekly, triglyceride levels were reduced by 71% at 3 months, representing an absolute reduction of 869 mg/dL. In the volanesorsen group, 24% of patients exhibited injection-site reactions, one patient exhibited thrombocytopenia with platelet levels <50,000/μL, and one patient exhibited serum sickness.
In May 2019, volanesorsen was approved in the European Union for the treatment of adult patients with FCS.30 A phase 2/3 trial of volanesorsen in patients with familial partial lipodystrophy is underway (NCT02527343).
Meanwhile, in a phase 2 trial of patients with hypertriglyceridemia and established ASCVD or high cardiovascular risk (NCT03385239), treatment with IONIS-APOCIII-LRx for 6 months resulted in triglyceride reductions of 23% with 10 mg every 4 weeks, 56% with 15 mg every 2 weeks, 60% with 10 mg every 4 weeks, and 60% with 50 mg every 4 weeks, compared with an increase of 6% in the placebo group.31 A phase 3 trial of IONIS-APOCIII-LRx in patients with FCS is underway (NCT04568434). We can expect more clinical outcomes from apoCIII target treatment in the future, especially if it can significantly reduce the hard outcomes of ASCVD in addition to its dramatic triglyceride-lowering effects.
APOLIPOPROTEIN(a)
1. Rationale for target identification
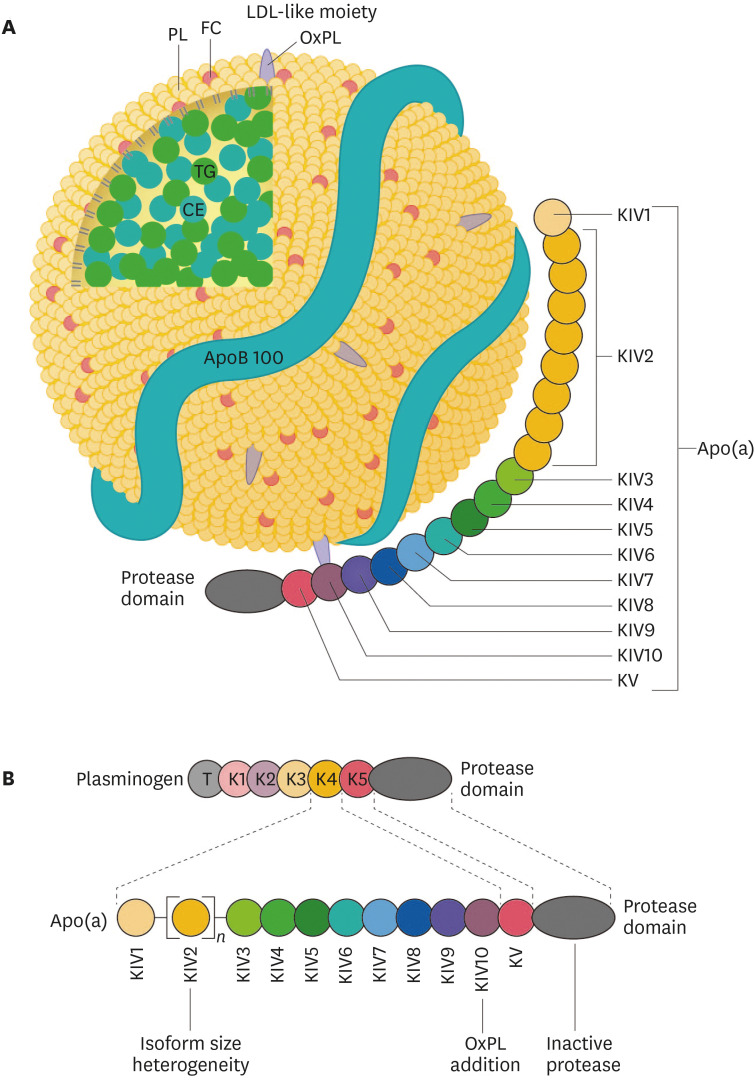
Lipoprotein(a) (Lp[a]) consists of an LDL-like moiety covalently linked to apo(a). The LDL-like moiety contains a single molecule of apolipoprotein B100, an outer phospholipid-risk shell and unesterified free cholesterol, and a lipid core consisting of cholesteryl esters and triglycerides (Fig. 3). Apo(a) shows a high degree of homology with plasminogen. Whereas plasminogen consists of a tail domain, 5 kringle domains, and a protease domain, apo(a) consists of 10 different domains homologous to a plasminogen kringle 4 (named KIV in apo[a]), followed by a kringle 5-like domain, and a protease-like domain. Apo(a) consists of 10 different types of KIVs, of which KIV type 2 (KIV2) is present in different copy numbers in the various apo(a) isoforms, whereas only a single copy of the other KIV domains is present in all apo(a) isoforms.32
Fig. 3. Structure of Lp(a). (A) Lp(a) consists of a LDL-like moiety covalently linked by a single disulfide bond to the glycoprotein apo(a). The LDL-like moiety contains a single molecule of apoB100, an outer shell of PLs and unesterified FC, and a neutral lipid core consisting of CEs (primarily) and TGs. OxPLs are predominantly covalently bonded to apo(a), but are also found covalently linked to apoB100 and free in the lipid moiety. (B) Apo(a) consists of 10 different domains homologous to plasminogen K4 (named KIV in apo[a]), a KV, and an inactive, protease-like domain.
Lp(a), lipoprotein(a); LDL, low-density lipoprotein; apo(a), apolipoprotein(a); apoB100, apolipoprotein B100; PL, phospholipid; FC, free cholesterol; CE, cholesteryl ester; TG, triglyceride; OxPL, oxidized phospholipid; K4, kringle 4; KV, kringle 5-like domain; T, tail domain.
Lp(a) concentrations are well known to have strong associations with coronary heart disease, stroke, and aortic valve stenosis.33,34 The exact mechanisms by which Lp(a) accelerates these disorders have not been fully elucidated, but the oxidized phospholipids present on apo(a) might play an important role by promoting endothelial dysfunction, lipid deposition, inflammation, and osteogenic differentiation.32 A case-control study showed that two LPA gene variants were strongly associated with both increased Lp(a) levels and an increased risk of coronary disease.35 In addition, the number of KIV2 repeats in apo(a), which correlates inversely with levels of Lp(a), exhibited a negative association with the risk of myocardial infarction.36 A Mendelian randomization analysis showed that a reduction in Lp(a) by 101.5 mg/dL had an equivalent association with CHD risk to a 38.7 mg/dL reduction in LDL-C levels.37
No therapeutic agents have been approved for lowering Lp(a) specifically. Studies of agents approved for lowering Lp(a) nonspecifically, including niacin, PCSK9 inhibitors, mipomersen, and estrogen, demonstrated no direct cardiovascular benefits on Lp(a) levels. However, the patients in these studies were not recruited based on elevated Lp(a) levels, and because of the left-skewed distribution of Lp(a), it was not possible to estimate the importance of an absolute reduction of Lp(a) for ASCVD events precisely from these results alone.38
2. Pharmacology
The first drug specifically targeting apo(a) mRNA was IONIS-APO(a)Rx (previously labeled ISIS-APO[a]Rx). IONIS-APO(a)Rx is 2′-O-methoxyethyl-modified ASO with phosphorothioate substitutions that is administered subcutaneously.39 Pelacarsen (previously denoted AKCEA-APO[a]LRx or IONIS-APO[a]LRx or TQJ230) is a modified form of IONIS-APO(a)Rx that is conjugated with a triantennary GalNAc complex, and it showed more than 30 times higher potency than the parent ASO.40
3. Clinical trial results
In a phase 1 trial, six doses of IONIS-APO(a)Rx resulted in significant reductions of Lp(a) levels in a dose-dependent manner: 39% in the 100-mg group; 59% in the 200-mg group, and; 77% in the 300-mg group.39 In a phase 2 trial, treatment with IONIS-APO(a)Rx produced an Lp(a) level reduction of 67% in cohort A and 72% in cohort B.40
In a phase 1 trial, pelacarsen resulted in an Lp(a) level reduction of up to 92%.40 In a phase 2 trial, which included patients with established ASCVD and Lp(a) levels of >60 mg/dL, pelacarsen was administered in ascending doses at intervals of 1 to 4 weeks. After 6 months of treatment, Lp(a) was successfully reduced by 35% at a dose of 20 mg every 4 weeks, 56% at 40 mg every 4 weeks, 58% at 20 mg every 2 weeks, 72% at 60 mg every 4 weeks, and 80% at 20 mg every week, as compared with 6% with placebo. There were no significant differences in adverse events regarding platelet counts, liver and renal toxicity, and influenza-like symptoms. As the most common adverse event, 27% of patients in the pelacarsen group exhibited injection-site reactions.41
A phase 3 trial of pelacarsen in patients with established ASCVD and Lp(a) levels of >70 mg/dL is currently ongoing (NCT04023552).
ANGIOPOIETIN-LIKE PROTEIN 3
1. Rationale for target identification
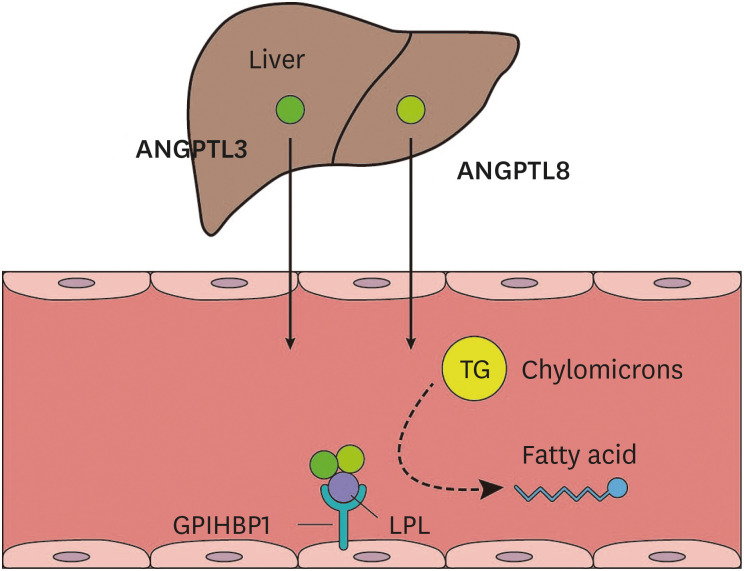
ANGPTL3, which is produced in the liver and secreted into the circulation, inhibits lipoprotein lipase and endothelial lipase, thereby influencing triglyceride and high-density lipoprotein cholesterol (HDL-C) levels (Fig. 4).42,43 The mechanism whereby ANGPTL3 regulates LDL-C levels is not clear, but endothelial lipase-dependent VLDL clearance may be involved.44 Human studies have shown that loss-of-function mutations in the ANGPTL3 gene are associated with low levels of triglycerides, LDL-C, and HDL-C.45,46 An earlier study showed that participants heterozygous for ANGPTL3 loss-of-function variants exhibited approximately 50% lower ANGPTL3 levels than those without these variants, and had a 39% lower risk of coronary artery disease.47 Another study showed that participants heterozygous for ANGPTL3 loss-of-function variants demonstrated a 17% reduction in triglyceride levels, a 12% reduction in LDL-C levels, and a 34% reduction in risk of coronary artery disease.48 Treatment with evinacumab, a monoclonal antibody against ANGPTL3, resulted in a decrease in atherosclerotic lesion area and necrotic content in an atherosclerosis-prone mouse model, and a decrease in triglyceride levels by up to 76% and LDL-C levels of up to 23% in humans.47
Fig. 4. TG metabolism and the role of ANGPTL3. Dietary fat is transported through the blood as part of chylomicrons. TGs in the chylomicrons are hydrolyzed by LPL. The GPIHBP1 transports LPL from the cell surface to the capillary endothelium. ANGPTL3, produced in the liver and secreted into the circulation, inhibits LPL in peripheral tissues. The function of ANGPTL3 as an LPL inhibitor is dependent on ANGPTL8, which is also produced in the liver and forms a complex with ANGPTL3.
TG, triglyceride; ANGPTL3, angiopoietin-like protein 3; ANGPTL8, angiopoietin-like protein 8; LPL, lipoprotein lipase; GPIHBP1, glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1.
2. Pharmacology
Vupanorsen (previously denoted as AKCEA-ANGPTL3-LRx or IONIS-ANGPTL3-LRx or ISIS 703802) is a GalNAc-conjugated ASO. Its 20 nucleotides are linked by 13 phosphorothioate bonds and 6 phosphodiester bonds. It is administered subcutaneously.49
3. Clinical trial results
In a phase 1 trial, participants with triglyceride levels > 90 mg/dL were treated with a single dose (20, 40, or 80 mg) or multiple doses (10, 20, 40, or 60 mg per week for 6 weeks) of vupanorsen.49 After 6 weeks of treatment, participants in the multiple-dose groups demonstrated a reduction in ANGPTL3 levels (47% to 85%), triglycerides (33% to 63%), LDL-C (1% to 33%), VLDL cholesterol (28% to 60%), non-HDL-C (10% to 37%), apolipoprotein B (3% to 26%), and apoCIII (19% to 59%). There were no serious adverse events. In a phase 2 trial, participants with triglyceride levels >150 mg/dL, type 2 diabetes, and hepatic steatosis were treated with vupanorsen (40 or 80 mg every 4 weeks, or 20 mg every week) subcutaneously.50 After 6 months of treatment, significant reductions in triglycerides of 36%, 53%, and 47%, and in ANGPTL3 of 41%, 59%, and 56% were observed in the groups that received 40 mg every 4 weeks, 80 mg every 4 weeks, and 20 mg every week, respectively. Treatment with 80 mg of vupanorsen every 4 weeks reduced apoCIII levels by 58%, remnant cholesterol by 38%, total cholesterol by 19%, non-HDL-C by 18%, HDL-C by 24%, and apolipoprotein B by 9%. The most common adverse events were injection-site pruritus (14%) and injection-site erythema (12%). No patient exhibited a platelet level <100,000/mm3.
CONCLUSION
A summary of ASOs targeting apoCIII, apo(a), and ANGPTL3 is provided in Table 1. The use of antisense technology has made it possible to inhibit the unique protein targets of dyslipidemia with high specificity and high potency. Although this technology has led to major advances in lipid-lowering therapy, delivery to target tissues other than the liver, biological barriers to permeability, the formation of anti-drug antibodies, and oral availability remain major challenges. Despite these challenges, the application of ASOs to targets of dyslipidemia has paved new paths for the treatment of dyslipidemia and prevention of ASCVD. We look forward to the results of ongoing cardiovascular outcome trials using ASO drugs.
Table 1. ASOs targeting apoCIII, apo(a), and ANGPTL3.
| Target | ApoCIII | Apo(a) | ANGPTL3 |
|---|---|---|---|
| Rationale for target identification | • Observational evidence | • Observational and genetic evidence | • Loss-of-function mutations in ANGPTL3 gene |
| • Loss-of-function mutations in APOC3 gene | • Mendelian randomization studies | ||
| Pharmacology | • Volanesorsen (ISIS 304801, ISIS-APOCIIIRx): weekly subcutaneous injection | • Pelacarsen (AKCEA-APO[a]LRx, IONIS-APO[a]LRx, TQJ230): GalNAc-conjugated ASO, subcutaneous injection, administration every 1 to 4 weeks | • Vupanorsen (AKCEA-ANGPTL3-LRx, IONIS-ANGPTL3-LRx, ISIS 703802): GalNAc-conjugated ASO, subcutaneous injection, administration every 1 to 4 weeks |
| • IONIS-APOCIII-LRx (AKCEA-APOCIII-LRx, ISIS 678354): GalNAc-conjugated volanesorsen, subcutaneous injection, administration every 1 to 4 weeks | |||
| Clinical trial results | • Volanesorsen: significant reductions in apoCIII and triglycerides (>70%) in hypertriglyceridemia and FCS | • Phase 1 and 2 trials: reduction of lipoprotein(a) by up to 80% | • Phase 1 trial of vupanorsen with reductions in triglyceride (47% to 85%) and LDL-C (1% to 33%) levels |
| • Volanesorsen: injection site reactions and thrombocytopenia in FCS | • Phase 2 study of vupanorsen in patients with hypertriglyceridemia, type 2 diabetes, and nonalcoholic fatty liver disease: reductions in triglyceride levels (36% to 47%) | ||
| • Volanesorsen: European Union approval in adult patients with FCS (2019) | |||
| • IONIS-APOCIII-LRx: significant reductions in apoCIII, triglycerides, and apoB in mild hypertriglyceridemia | |||
| <Ongoing> | <Ongoing> | ||
| • Phase 2/3 trial of volanesorsen in patients with hypertriglyceridemia and familial partial lipodystrophy (NCT02527343) | • A phase 3 trial of pelacarsen in patients with established ASCVD and Lp(a) levels of >70 mg/dL (NCT04023552) | ||
| • Phase 3 trial of IONIS-APOCIII-LRx in patients with FCS (NCT04568434) |
ASO, antisense oligonucleotide; apoCIII, apolipoprotein CIII; apo(a), apolipoprotein(a); ANGPTL3, angiopoietin-like protein 3; GalNAc, N-acetyl galactosamine; FCS, familial chylomicronemia syndrome; apoB, apolipoprotein B; LDL-C, low-density lipoprotein cholesterol; ASCVD, atherosclerotic cardiovascular disease; Lp(a), lipoprotein(a).
Footnotes
Funding: None.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Kim K, Choi SH.
- Supervision: Kim K, Choi SH.
- Visualization: Kim K, Choi SH.
- Writing - original draft: Kim K, Choi SH.
- Writing - review & editing: Kim K, Choi SH.
References
- 1.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn CH, Choi SH. New drugs for treating dyslipidemia: beyond statins. Diabetes Metab J. 2015;39:87–94. doi: 10.4093/dmj.2015.39.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 4.Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415. doi: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Kim K, Choi SH. In: Stroke revisited: dyslipidemia in stroke. Lee SH, Kang MK, editors. Singapore: Springer Singapore; 2021. Safety considerations of pharmacological treatment; pp. 203–219. [Google Scholar]
- 8.Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 10.Tsimikas S. RNA-targeted therapeutics for lipid disorders. Curr Opin Lipidol. 2018;29:459–466. doi: 10.1097/MOL.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 11.Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics. Cell Metab. 2018;27:714–739. doi: 10.1016/j.cmet.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Levin AA. Treating disease at the RNA level with oligonucleotides. N Engl J Med. 2019;380:57–70. doi: 10.1056/NEJMra1705346. [DOI] [PubMed] [Google Scholar]
- 13.Crooke ST, Vickers TA, Liang XH. Phosphorothioate modified oligonucleotide-protein interactions. Nucleic Acids Res. 2020;48:5235–5253. doi: 10.1093/nar/gkaa299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018;46:1584–1600. doi: 10.1093/nar/gkx1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 16.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg HN, Packard CJ, Chapman MJ, Borén J, Aguilar-Salinas CA, Averna M, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42:4791–4806. doi: 10.1093/eurheartj/ehab551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borén J, Packard CJ, Taskinen MR. The roles of apoC-III on the metabolism of triglyceride-rich lipoproteins in humans. Front Endocrinol (Lausanne) 2020;11:474. doi: 10.3389/fendo.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacks FM, Alaupovic P, Moye LA, Cole TG, Sussex B, Stampfer MJ, et al. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 2000;102:1886–1892. doi: 10.1161/01.cir.102.16.1886. [DOI] [PubMed] [Google Scholar]
- 20.van Capelleveen JC, Bernelot Moens SJ, Yang X, Kastelein JJ, Wareham NJ, Zwinderman AH, et al. Apolipoprotein C-III levels and incident coronary artery disease risk: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2017;37:1206–1212. doi: 10.1161/ATVBAHA.117.309007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 22.TG and HDL Working Group of the Exome Sequencing Project; National Heart, Lung, and Blood Institute. Crosby J, Peloso GM, Auer PL, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marston NA, Giugliano RP, Im K, Silverman MG, O’Donoghue ML, Wiviott SD, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140:1308–1317. doi: 10.1161/CIRCULATIONAHA.119.041998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham MJ, Lee RG, Bell TA, 3rd, Fu W, Mullick AE, Alexander VJ, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112:1479–1490. doi: 10.1161/CIRCRESAHA.111.300367. [DOI] [PubMed] [Google Scholar]
- 25.Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019;381:531–542. doi: 10.1056/NEJMoa1715944. [DOI] [PubMed] [Google Scholar]
- 26.Alexander VJ, Xia S, Hurh E, Hughes SG, O’Dea L, Geary RS, et al. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur Heart J. 2019;40:2785–2796. doi: 10.1093/eurheartj/ehz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373:438–447. doi: 10.1056/NEJMoa1400283. [DOI] [PubMed] [Google Scholar]
- 28.Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371:2200–2206. doi: 10.1056/NEJMoa1400284. [DOI] [PubMed] [Google Scholar]
- 29.Gouni-Berthold I, Alexander VJ, Yang Q, Hurh E, Steinhagen-Thiessen E, Moriarty PM, et al. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9:264–275. doi: 10.1016/S2213-8587(21)00046-2. [DOI] [PubMed] [Google Scholar]
- 30.Paik J, Duggan S. Volanesorsen: first global approval. Drugs. 2019;79:1349–1354. doi: 10.1007/s40265-019-01168-z. [DOI] [PubMed] [Google Scholar]
- 31.Tardif JC, Karwatowska-Prokopczuk E, Amour ES, Ballantyne CM, Shapiro MD, Moriarty PM, et al. Apolipoprotein C-III reduction in subjects with moderate hypertriglyceridaemia and at high cardiovascular risk. Eur Heart J. 2022;43:1401–1412. doi: 10.1093/eurheartj/ehab820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol. 2019;16:305–318. doi: 10.1038/s41569-018-0153-2. [DOI] [PubMed] [Google Scholar]
- 33.Emerging Risk Factors Collaboration. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 35.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 36.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 37.Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a mendelian randomization analysis. JAMA Cardiol. 2018;3:619–627. doi: 10.1001/jamacardio.2018.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 39.Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 40.Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 41.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 42.Shimizugawa T, Ono M, Shimamura M, Yoshida K, Ando Y, Koishi R, et al. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J Biol Chem. 2002;277:33742–33748. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- 43.Shimamura M, Matsuda M, Yasumo H, Okazaki M, Fujimoto K, Kono K, et al. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler Thromb Vasc Biol. 2007;27:366–372. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]
- 44.Adam RC, Mintah IJ, Alexa-Braun CA, Shihanian LM, Lee JS, Banerjee P, et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J Lipid Res. 2020;61:1271–1286. doi: 10.1194/jlr.RA120000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest. 2009;119:70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377:211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, et al. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol. 2017;69:2054–2063. doi: 10.1016/j.jacc.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med. 2017;377:222–232. doi: 10.1056/NEJMoa1701329. [DOI] [PubMed] [Google Scholar]
- 50.Gaudet D, Karwatowska-Prokopczuk E, Baum SJ, Hurh E, Kingsbury J, Bartlett VJ, et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J. 2020;41:3936–3945. doi: 10.1093/eurheartj/ehaa689. [DOI] [PMC free article] [PubMed] [Google Scholar]