Abstract
Context
Studies on cardiometabolic health in transgender and gender-diverse youth (TGDY) are limited to small cohorts.
Objective
This work aimed to determine the odds of cardiometabolic-related diagnoses in TGDY compared to matched controls in a cross-sectional analysis, using a large, multisite database (PEDSnet).
Methods
Electronic health record data (2009-2019) were used to determine odds of cardiometabolic-related outcomes based on diagnosis, anthropometric, and laboratory data using logistic regression among TGDY youth vs controls. The association of gender-affirming hormone therapy (GAHT) with these outcomes was examined separately among TGDY. TGDY (n = 4172) were extracted from 6 PEDSnet sites and propensity-score matched on 8 variables to controls (n = 16 648). Main outcomes measures included odds of having cardiometabolic-related diagnoses among TGDY compared to matched controls, and among TGDY prescribed GAHT compared to those not prescribed GAHT.
Results
In adjusted analyses, TGDY had higher odds of overweight/obesity (1.2; 95% CI, 1.1-1.3) than controls. TGDY with a testosterone prescription alone or in combination with a gonadotropin-releasing hormone agonist (GnRHa) had higher odds of dyslipidemia (1.7; 95% CI, 1.3-2.3 and 3.7; 95% CI, 2.1-6.7, respectively) and liver dysfunction (1.5; 95% CI, 1.1-1.9 and 2.5; 95% CI, 1.4-4.3) than TGDY not prescribed GAHT. TGDY with a testosterone prescription alone had higher odds of overweight/obesity (1.8; 95% CI, 1.5-2.1) and hypertension (1.6 95% CI, 1.2-2.2) than those not prescribed testosterone. Estradiol and GnRHa alone were not associated with greater odds of cardiometabolic-related diagnoses.
Conclusion
TGDY have increased odds of overweight/obesity compared to matched controls. Screening and tailored weight management, sensitive to the needs of TGDY, are needed.
Keywords: gender dysphoria, pediatric, cardiometabolic, cholesterol, body mass index, hormone therapy
Transgender individuals are individuals whose gender identity differs from their sex designated at birth, and 1.8% of adolescents in the United States identify as transgender (1). Transgender individuals seeking care may be diagnosed with gender dysphoria (2), which is characterized as the psychological distress that results from an incongruence between one’s sex assigned at birth and one’s gender identity, as defined by the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (3). Guidelines from the Endocrine Society and World Professional Association for Transgender Health support the use of gender-affirming hormone therapy (GAHT) for eligible adolescents and adults (2).
Studies in adults in the United States and Europe suggest that GAHT may have an adverse effect on cardiometabolic health (4, 5). In Europe, transgender women on estradiol (E2) therapy have a higher risk of stroke and venous thromboembolism than both cisgender reference women and men (whose gender identity is congruent with sex designated at birth) (4). In 2 large cohorts, transgender women have increased risk of myocardial infarction than cisgender women (but not cisgender men) (4, 6, 7). These studies are limited by self-report survey design with no information about GAHT use (6, 7) and inclusion of ethinyl estradiol, which is not currently recommended as GAHT because of adverse effects on cardiometabolic health (8). In the Kaiser STRONG cohort that uses electronic health record (EHR) data (9), both prevalent and incident type 2 diabetes were more common in adult transgender women than cisgender women, but not compared to cisgender men (5). In contrast, a recent large study from the Amsterdam Cohort did not show any difference in the incidence of type 2 diabetes in transgender men or women as compared to those with the same birth-assigned sex (10). The effect of testosterone on cardiometabolic health is less clear (4, 7, 11-13). Whereas one study showed transgender men on testosterone therapy have a higher risk of myocardial infarction than cisgender women (but not cisgender men) (4), others have shown increased risk compared to cisgender women and men (7). There is no clear association between testosterone GAHT and stroke, venous thromboembolism, or type 2 diabetes (4, 5, 10).
There are several gaps in the literature, which has maintained a narrow view on evaluation of GAHT and cardiometabolic health without assessing other factors. Transgender and gender-diverse people have health disparities in cardiovascular morbidity and mortality that are likely driven, at least in part, by psychosocial stressors across the lifespan, including stigma, harassment, violence, and discrimination including in the health care setting, which likely contributes to a lower likelihood of receiving preventive care services (14, 15). Furthermore, outcomes available on cardiometabolic health in transgender and gender diverse youth (TGDY) are primarily from small, single-center analyses, some without a comparison group (16-19), with most studies focused on adults (5–7, 9).
This multicenter, retrospective, cross-sectional study aimed to better define cardiometabolic-related diagnoses in TGDY in the PEDSnet cohort. We aimed to 1) evaluate the risk of diagnoses related to cardiometabolic health among TGDY compared to matched controls, and 2) evaluate the potential association of various GAHTs on cardiometabolic-related diagnoses among TGDY.
Materials and Methods
Patients
This study was conducted using PEDSnet, A Pediatric Learning Health System network, and includes data from the following PEDSnet institutions: Children’s Hospital Colorado, Children’s Hospital of Philadelphia, Nemours Children’s Health, Nationwide Children’s Hospital, St. Louis Children’s Hospital, and Seattle Children’s Hospital. PEDSnet is a Partner Network Clinical Data Research Network in PCORnet, the National Patient Centered Clinical Research Network, an initiative funded by the Patient Centered Outcomes Research Institute.
Clinical data are available from the EHR of these health systems from 2009 onward for patients with an in-person encounter with a provider. TGDY are defined in this study as having a diagnosis of gender dysphoria or related diagnosis (by PEDSnet concept ID, as previously described [20], which include codes extracted from the EHR problem list or diagnosis code from any encounter). The data for all TGDY (age >2 years at last visit) and at least one outpatient visit from 2009 to 2019 were extracted from the PEDSnet database in November 2019. We chose one outpatient visit as a criterion for cases and controls to not oversample those seen in the health systems for urgent/emergent care only. A random sample of 197 039 patients with at least one outpatient visit in the same time period who did not have a diagnosis of gender dysphoria were used as a pool of controls (controls did not include individuals with a difference of sex development or sex chromosome aneuploidy, as these were other case populations of interest described in separate publications). To ensure these controls were representative of the general PEDSnet population, we evaluated the prevalence of well-characterized pediatric diagnoses (asthma, type 1 diabetes, and acute lymphoid leukemia) and the prevalence in the control pool was similar to PEDSnet as a whole.
Outcomes
Cardiometabolic-related outcomes investigated in this study included overweight/obesity, dyslipidemia, liver dysfunction, hypertension, dysglycemia, and polycystic ovary syndrome (PCOS). These outcomes were captured using SNOMED concept codes and were defined as having either a diagnosis (billing code, problem list) or at least 2 abnormal measurements (anthropometric or laboratory value) recorded in the EHR (Table 1). The following percentages reflect diagnoses for the entire case/control population obtained by billing codes/problem lists (only), 2 abnormal measurements (only), or both, respectively: overweight/obesity: 1.2%, 20.9%, 12.0%; dyslipidemia: 2.9%, 1.7%, 1.1%; liver dysfunction: 0.2%, 8.1%, 0.2%; dysglycemia: 0.5%, 1.3%, 0.4%; hypertension: 1.7%, 5.2%, 0.9% (PCOS was defined by diagnostic codes alone). For continuous variables, normal and abnormal values were defined using academic society guidelines related to each outcome and the Centers for Disease Control definition of overweight and obesity in youth and adolescents (see Table 1) (21-23). We used dichotomous classifications for disease presence vs absence for the primary outcomes of interest as the clinical applicability is established; however, analysis of continuous data resulted in similar conclusions (Table 2). In the multivariable models, we chose to keep overweight/obesity as dichotomous rather than continuous as the effect of body mass index (BMI) on the health outcomes of interest is nonlinear. Medications of interest were defined using anatomical therapeutic chemical (ATC) or RxNorm codes, structured vocabularies to capture and group similar and synonymous medications into categorical classes: GnRHa (L02AE), E2 (G03C, not including combined oral contraceptives, G03A), testosterone (G03B), combined oral contraceptive pills (COCPs) (G03AA, G03AB), progestins norethindrone and medroxyprogesterone (7514, 6691), spironolactone (9997), and antipsychotics (N05A).
Table 1.
Criteria and codes used for defining binary outcomes
| Outcome | Diagnoses, vital signs, or laboratory value criteria | SNOMED-CT or LOINC codes |
|---|---|---|
| Overweight/Obesity | Increased BMI | 4849901 |
| Obesity | 414916001; 414915002 | |
| Overweight | 238131007 | |
| BMI > 85% if age < 18 yb (23) | 39156-5 | |
| BMI > 25 if age > 18 yb | 39156-5 | |
| Dyslipidemia (24) | Dyslipidemia | 370992007 |
| Hyperlipidemia | 55822004 | |
| HDL < 45 mg/dL if age < 18 yb | 2085-9 or 9833-5 | |
| HDL < 40 mg/dL if age > 18 yb | 2085-9 or 9833-5 | |
| Triglycerides > 100 mg/dL if age < 10 yb | 2571-8 | |
| Triglycerides > 130 mg/dL if age 10-19 yb | 2571-8 | |
| Triglycerides > 150 mg/dL if age > 19 yb | 2571-8 | |
| LDL > 100 mg/dL if age < 18 yb | 18262-6, 47213-4, 2089-1, 13457-7 | |
| LDL > 130 mg/dL if age > 18 yb | 18262-6, 47213-4, 2089-1, 13457-7 | |
| Total cholesterol > 200 mg/dLb | 2093-3 | |
| Dysglycemiaa | Impaired glucose tolerance | 9414007 |
| Impaired fasting glycemia | 390951007 | |
| Diabetes mellitus type 2 | 44054006 | |
| Chronic hyperglycemia | 170765005 | |
| Dysglycemia | 426255005 | |
| Nondiabetic hyperglycemia | 700449008 | |
| Metabolic stress hyperglycemia | 237624007 | |
| Poor glycemic control | 237622006 | |
| Hyperglycemia due to diabetes mellitus | 822995009 | |
| Hemoglobin A1c > 5.7%b | 4548-4 or 17856-6 | |
| Hypertension | Benign hypertension | 10725009 |
| Essential hypertension | 59621000 | |
| Diastolic hypertension | 48145000 | |
| Prehypertension | 702817009 | |
| Finding of increased blood pressure | 24184005 | |
| BP 90% if age < 13 yb (21) | 8460-8, 8454-1, 8459-0, 8455-8, 8461-6 | |
| SBP > 120 or DPB > 80 if age > 13 yb | or 8453-3 | |
| Liver dysfunction | ALT level abnormal | 166646003 |
| Nonalcoholic fatty liver | 197315008 | |
| Nonalcoholic steatohepatitis | 442685003 | |
| Increased AST level | 160931000119108 | |
| AST level abnormal | 166668008 | |
| Elevated liver enzymes level | 707724006 (omit 707734002) | |
| AST or ALT > 97.5% if age < 17 yb (25) | 1920-8 or 1742-6 | |
| AST > 35 if age > 17 yb (22) | 1920-8 | |
| ALT > 33 if age > 17 yb (22) | 1742-6 | |
| Polycystic ovary syndrome | N/A | 237055002 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; N/A, not applicable; SBP, systolic blood pressure.
a Excluded individuals with a diagnosis of type 1 diabetes mellitus.
b Required 2 or more separate events meeting these criteria to minimize abnormalities secondary to acute illness or spurious results.
Table 2.
Values for outcomes and missing data for the entire transgender and gender-diverse youth cohort and controls
| TGDY cases n = 4172 | Controls n = 16 453 | ||||
|---|---|---|---|---|---|
| No. (%) | Median (25th-75th percentile) | No. (%) | Median (25th-75th percentile) | P | |
| Systolic BP, mm Hg | 1701 (40.8) | 116 (108-124) | 6026 (36.6) | 114 (106-121) | < .0001 |
| Diastolic BP, mm Hg | 1701 (40.8) | 67 (62-73) | 6026 (36.6) | 66 (60-72) | < .0001 |
| BMI | 3829 (91.8) | 23 (20-28) | 14 633 (88.9) | 22 (19-26) | < .0001 |
| BMI, % | 3829 (91.8) | 77 (41-95) | 14 633 (88.9) | 69 (39-90) | < .0001 |
| ALT, IU/L | 1627 (39.0) | 24 (17-31) | 3940 (23.9) | 24 (16-32) | .70 |
| AST, IU/L | 1449 (34.7) | 25 (20-32) | 3798 (23.1) | 27 (21-36) | < .0001 |
| Total cholesterol, mg/dL | 1633 (39.1) | 156 (138-178) | 1907 (11.6) | 156 (137-180) | .95 |
| HDL (mg/dL) | 1618 (38.8) | 46 (39-55) | 1837 (11.1) | 47 (39-56) | .13 |
| LDL, mg/dL | 1142 (27.4) | 87 (70-106) | 1266 (7.7) | 86 (70-104) | .42 |
| Triglycerides, mg/dL | 1633 (39.1) | 93 (67-135) | 2021 (12.3) | 91 (66-132) | .44 |
| HbA1c, % | 532 (12.8) | 5.3 (5.1, 5.5) | 959 (5.8) | 5.4 (5.2-6.7) | < .0001 |
No. represents the total number of people with a test and the percentage of cases and controls with a test performed. P values are from a Wilcoxon rank sum test and show statistically significant differences in the values between cases and controls.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TGDY, transgender and gender-diverse youth.
Statistical Analysis
We identified 4172 TGDY for analysis. Propensity scores were used to match controls to cases (approximately 4:1; 44 cases were matched 3:1 because of limited availability of controls). A total of 16 648 controls were included in the analysis. A priori covariates used for matching include: year of birth, age at last visit, sex listed in the chart (EHR sex), race, ethnicity, insurance status (public vs private vs none), duration in the PEDSnet database (time between first and last encounter), and site (20). Cases and controls were matched on the predicted probability of having the diagnosis (gender dysphoria) using a greedy match algorithm and a caliper of width of 0.1. The balance of covariates between the cases and control groups (ie, the similarity of the covariate distributions) was evaluated as a reduction in standardized mean difference, using a decision criterion of less than 0.20 to indicate that a covariate was balanced, as previously demonstrated (26).
Demographic and other descriptive characteristics were compared using either a chi-square test of proportions or a Wilcoxon rank sum test. Our primary outcomes examined the differences in odds of having a diagnosis related to cardiometabolic health between those with (cases) and without (controls) a diagnosis of gender dysphoria, regardless of use of GAHT. These were examined using logistic regression with generalized estimating equations to account for potential correlation among the matched cases and controls. An interaction term was used in the model for EHR sex to evaluate whether the association between outcomes and case/control status differed by sex. In the majority of cases, EHR sex was consistent with the patient’s presumed sex assigned at birth based on medications prescribed or a diagnosis of PCOS. Those TGDY with a male EHR sex had the following prescriptions: progestin (n = 4, 0.28%), COCPs (n = 12, 0.85%), intrauterine device (n = 6, 0.43%), and etonogestrel implant (n = 0, 0%), and diagnosis of PCOS (n = 1, 0.02%). Multivariable regression analyses were adjusted for overweight/obesity, diagnosis of depression, and an antipsychotic medication prescription (20, 27-29). A total of 44% of TGDY and 9.6% of controls had depression, and 13.0% of TGDY and 4.5% of controls were on antipsychotic medication.
We included only TGDY in analyses that examined the association between GAHT and cardiometabolic-related diagnoses. TGDY with prescriptions for the following GAHTs: GnRHa monotherapy, testosterone monotherapy, E2 monotherapy, testosterone and GnRHa, and E2 and GnRHa were compared to TGDY who were never prescribed any GAHT. The E2 group did not include COCPs, as these were included in a separate analysis. We were not able to determine if cardiometabolic-related diagnoses occurred before or after receiving a prescription for GAHT given limitations of the data set. Analyses were adjusted for EHR sex, age at last visit, duration in PEDSnet, overweight/obesity, depression, and antipsychotic prescription. Overweight/obesity was nearly universal among individuals diagnosed with PCOS; therefore, this term was not included in that model. Additionally, we examined common medications used for menstrual suppression among TGDY with a female EHR sex (norethindrone, medroxyprogesterone, COCPs) given their association with the outcomes evaluated (9, 30, 31). We also examined the antiandrogen, spironolactone. All analyses were performed using SAS v9.4.
We used a more conservative P value of less than .01 to account for multiple comparisons. This was derived from a Bonferroni correction adjusting for 6 outcomes (.008) and rounded to the nearest hundredths place. Data with n less than 11 in any cell were not reported as per PEDSnet policy.
Results
Demographic characteristics for cases and controls are shown in Table 3.
Table 3.
Demographics for cases and controlsa
| TGDY cases n = 4172 (%) | Controls n = 16 648 (%) | P | |
|---|---|---|---|
| Sex listed in chart | .48 | ||
| Female | 2766 (66.3) | 11 130 (66.9) | |
| Male | 1407 (33.7) | 5518 (33.1) | |
| Race | .97 | ||
| White | 3027 (72.5) | 12 065 (72.5) | |
| Unknown | 401 (9.6) | 1570 (9.4) | |
| Other | 390 (9.3) | 1610 (9.7) | |
| Black | 257 (6.2) | 1016 (6.1) | |
| Asian | 98 (2.3) | 387 (2.3) | |
| Ethnicity | .74 | ||
| Non-Hispanic | 3538 (84.8) | 14 121 (84.8) | |
| Hispanic | 354 (8.5) | 1452 (8.7) | |
| Unknown | 281 (6.7) | 1075 (6.5) | |
| Insurance | .06 | ||
| Private | 2530 (60.6) | 10 127 (60.8) | |
| Public | 1287 (30.8) | 5105 (30.7) | |
| Other | 253 (6.1) | 898 (5.4) | |
| Unknown | 103 (2.5) | 518 (3.1) | |
| Site | .73 | ||
| 1 | 604 (14.5) | 2427 (14.6) | |
| 2 | 1041 (24.9) | 4341 (26.1) | |
| 3 | 1005 (24.1) | 3877 (23.3) | |
| 4 | 296 (7.1) | 1153 (6.9) | |
| 5 | 937 (22.5) | 3717 (22.3) | |
| 6 | 290 (6.9) | 1133 (6.8) | |
| Duration in PEDSnet, y | 5.7 (1.7-11.1) | 6.1 (1.6-11.1) | .48 |
| Age at first visit, y | 10.0 (4.4-14.6) | 9.8 (4.1-14.3) | .08 |
| Age at last visit, y | 16.7 (14.6-18.3) | 16.6 (14.3-18.4) | .64 |
| Total No. of outpatient visits | 10 (4-26) | 8 (3-22) | < .01 |
P values from a chi-square test of proportions for No. (%) data and Wilcoxon rank sum test for continuous data.
Abbreviation: TGDY, transgender and gender-diverse youth.
a Data are shown as No. (%) or median (25th-75th percentile).
Odds of Cardiometabolic Diagnoses Among Transgender and Gender-Diverse Youth vs Controls
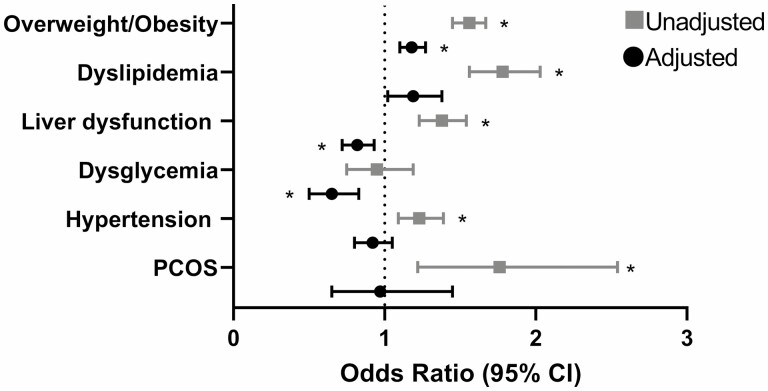
Prevalence of cardiometabolic diagnoses among TGDY and controls is in Table 4. TGDY had higher unadjusted odds of having the following cardiometabolic-related diagnoses compared to controls (Fig. 1): overweight/obesity (P < .0001), dyslipidemia (P < .0001), liver dysfunction (P < .0001), and hypertension (P < .001). Among those with a female EHR sex, TGDY had higher odds of PCOS (P < .01) compared to controls. Overall, there was no difference in the unadjusted odds of dysglycemia (see Fig. 1). In the adjusted models, TGDY had higher odds of overweight/obesity compared to controls (P < .0001, adjusted for depression and antipsychotic prescription) and lower odds of dysglycemia (P < .001) and liver dysfunction (P = .003) compared to controls (adjusted for overweight/obesity, depression, and antipsychotic prescription).
Table 4.
Prevalence of cardiometabolic-related diagnoses
| TGDY vs controls | TGDY prescribed GAHT compared to TGDY not prescribed GAHT | |||||||
|---|---|---|---|---|---|---|---|---|
| TGDY cases No. (%) | Control No. (%) | No GnRHa No. (%) | GnRHa No. (%) | No T No. (%) | T No. (%) | No E2 No. (%) | E2 No. (%) | |
| Overweight/obese | 1764 (42.3) | 5263 (32.0) | 1661 (42.5) | 103 (38.6) | 1273 (39.4) | 491 (52.3) | 1558 (42.1) | 206 (43.5) |
| Dyslipidemia | 351 (8.4) | 806 (4.9) | 340 (8.7) | 11 (4.1) | 218 (6.7) | 133 (14.2) | 298 (8.1) | 53 (11.2) |
| Liver dysfunction | 460 (11.0) | 1360 (8.3) | 434 (11.1) | 26 (9.7) | 326 (10.1) | 134 (14.3) | 389 (10.5) | 71 (15.0) |
| Dysglycemia | 90 (2.2) | 375 (2.3) | 85 (2.2) | 5 (1.9) | 72 (2.2) | 18 (1.9) | 77 (2.1) | 13 (2.7) |
| Hypertension | 373 (8.9) | 1214 (7.4) | 351 (9.0) | 22 (8.2) | 268 (8.3) | 105 (11.2) | 304 (8.2) | 69 (14.6) |
| PCOS | 42 (1.5) | 96 (0.9) | 42 (1.1) | 0 (0) | 33 (1.0) | 9 (1.0) | 39 (1.1) | 3 (0.6) |
Abbreviations: E2, estradiol; GAHT, gender-affirming hormone therapy; GnRHa: gonadotropin-releasing hormone agonist; PCOS, polycystic ovary syndrome; T, testosterone; TGDY, transgender and gender-diverse youth.
Figure 1.
Odds of cardiometabolic-related diagnoses among TGDY vs controls. The forest plots show the odds ratios and 95% CIs of cardiometabolic-related diagnoses among TGDY compared to controls. Higher odds ratios (> 1) indicate that TGDY are more likely to have the listed diagnosis. Lower odds ratios (< 1) indicate that TGDY are less likely to have the listed diagnosis. Unadjusted analyses are shown in gray squares; adjusted analyses (for overweight/obesity, depression, and antipsychotic medication use) are shown in black circles. Overweight/obesity and PCOS not adjusted for overweight/obesity. *P < .01. PCOS, polycystic ovary syndrome; TGDY, transgender and gender-diverse youth.
When EHR sex was included as an interaction term, there were statistically significant interactions for overweight/obesity (P < .0001) and dysglycemia (P < .01). TGDY with a female EHR sex had higher odds of overweight/obesity than female controls (odds ratio 1.8; 95% CI, 1.7-2.0; P < .0001). TGDY with a male EHR sex had lower odds of dysglycemia than male controls (0.6; 95% CI, 0.4-0.9; P = .01).
Odds of Cardiometabolic Diagnoses Among Transgender and Gender-Diverse Youth on Gender-Affirming Hormone Therapy
Overall, 1412 (33.8%) of TGDY had a GAHT prescription listed in the chart: 267 (6.4%) had a prescription for GnRHa alone, 832 (19.9%) had a prescription for testosterone without a GnRHa, 349 (8.4%) for E2 without a GnRHa, 106 (2.5%) for testosterone with a GnRHa, and 125 (3.0%) for E2 with a GnRHa. The prevalence of cardiometabolic diagnoses among TGDY with a GAHT prescription compared to those without a GAHT prescription is shown in Table 4. Among the TGDY with female EHR sex, 112 (4.1%) had a prescription for a progestin (norethindrone, medroxyprogesterone) and 199 (7.2%) for COCP; among TGDY with a male EHR sex, 332 (23.6%) had a prescription for spironolactone.
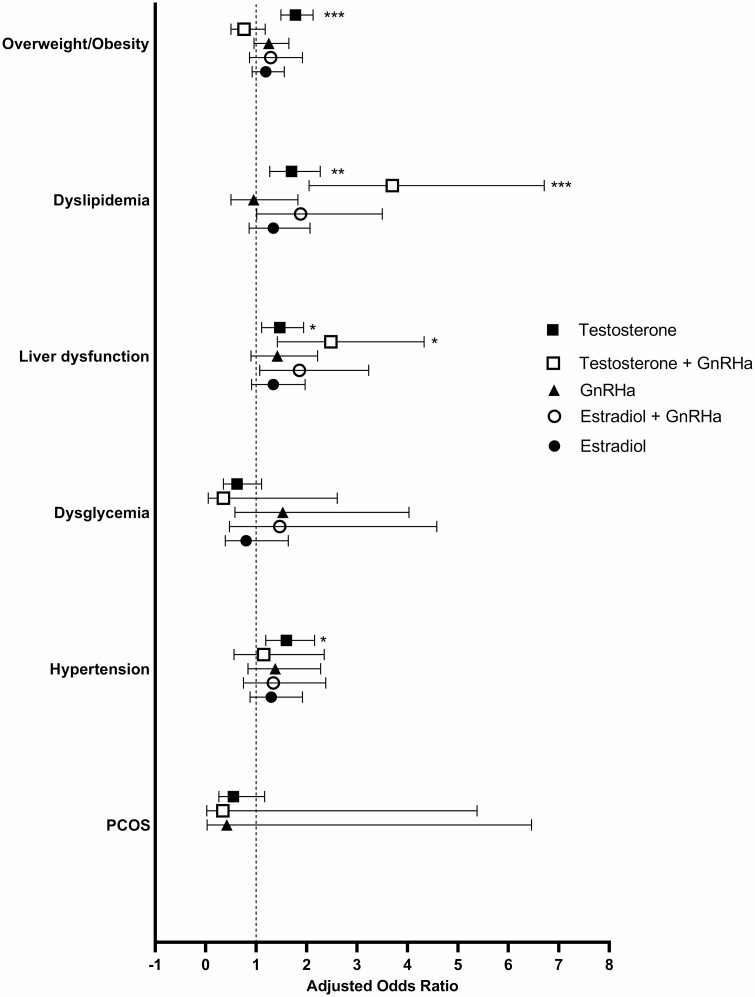
Among TGDY, those with a prescription for testosterone (without GnRHa) had higher odds of having overweight/obesity (1.8; 95% CI, 1.5-2.1; P < .0001), dyslipidemia (1.7; 95% CI, 1.3-2.3; P < .01), liver dysfunction (1.5; 95% CI, 1.1-1.9; P ≤ .01), and hypertension (1.6; 95% CI, 1.2-2.2; P < .01) compared to TGDY not on GAHT in adjusted models (Fig. 2, no differences in which outcomes were significant or directionality in unadjusted models). TGDY with a testosterone and GnRHa prescription had increased odds of dyslipidemia (3.7; 95% CI, 2.0-6.7; P < .0001) and liver dysfunction (2.5; 95% CI, 1.4-4.3; P < .01) compared to those TGDY who were never prescribed GAHT in adjusted models (see Fig. 2, no differences in which outcomes were significant or directionality in unadjusted models).
Figure 2.
Odds of cardiometabolic outcomes by prescription among transgender and gender-diverse youth. The forest plots show the adjusted odds ratios and 95% CIs of cardiometabolic health outcomes among TGDY prescribed these medications compared to those not prescribed these medications. Higher odds ratios (> 1) indicate that TGDY prescribed these medications are more likely to have the listed diagnosis compared to TGDY not prescribed these medications. Lower odds ratios (< 1) indicate that TGDY on these medications are less likely to have the listed diagnosis compared to TGDY not on these medications. *P < .01, **P < .001, ***P < .0001. TGDY, transgender and gender-diverse youth.
TGDY with a prescription for E2 had higher odds of dyslipidemia (1.9; 95% CI, 1.3-2.7; P = .001), liver dysfunction (1.6; 95% CI, 1.2-2.3; P < .01), and hypertension (2.3; 95% CI, 1.7-3.2; P < .0001) than TGDY without a prescription for E2 in unadjusted analyses (see Fig. 2), but in the adjusted analyses, these were no longer statistically significant. Individuals prescribed both E2 and GnRHa had higher odds of liver dysfunction (2.1; 95% CI, 1.3-3.4; P < .01) and hypertension (2.1; 95% CI, 1.2-3.6; P < .01) in unadjusted analyses (see Fig. 2), but in the adjusted analyses, these were no longer statistically significant.
There was no statistically significant difference in the odds of any cardiometabolic-related diagnosis in unadjusted or adjusted for GnRHa alone.
TGDY with a female EHR sex prescribed norethindrone or medroxyprogesterone had similar odds of each cardiometabolic outcome as those not prescribed these medications (data not shown). TGDY with a female EHR sex prescribed COCPs had higher odds of overweight/obesity (1.7; 95% CI, 1.2-2.2; P < .001) and dyslipidemia (2.1; 95% CI, 1.4-3.2; P < .001) compared to those not prescribed COCPs. When a spironolactone prescription was included in the model, the results did not change (data not shown).
The overall prevalence of cardiometabolic-related conditions among TGDY prescribed GAHT were relatively uncommon (≤ 15%) aside from overweight/obesity (see Table 4). The values for blood pressure, BMI, and laboratory tests for TGDY and matched controls are shown in Table 2. Although statistically different between TGDY and controls, there is not a clinically significant difference overall between groups for blood pressure, lipid measures, liver transaminases, or glycated hemoglobin A1c. TGDY were more likely to have laboratory tests performed than control youth (percentage with missing data in Table 2).
Discussion
This multicenter, cross-sectional analysis shows that TGDY have increased odds having a diagnosis of overweight/obesity, dyslipidemia, liver dysfunction, hypertension, and PCOS compared to controls in unadjusted analyses. However, after adjusting for important covariates, only the increased odds of overweight/obesity remained statistically significant. This attenuation speaks to the effect overweight/obesity, depression, and antipsychotic use may have on cardiometabolic health in this population.
Although we were unable to evaluate specifically for minority or psychosocial stress in this study, it has been well documented that chronic stress related to stigma and discrimination contribute to adverse health outcomes, including increased risk of cardiometabolic-related conditions in minoritized populations (30). Minority stress theory describes the health disparities that can be explained by stressors in a hostile culture and result in harassment, maltreatment, discrimination, and victimization (31). Stress and minority stress can result in biologic changes ranging from systemic disease to gene expression (30). People who experience chronic stress are at higher risk of metabolic syndrome (32). Epigenetic changes that occur in this setting can upregulate inflammatory processes that in turn can disrupt typical physiology related to cardiovascular, metabolic, and endocrine function (30). Thus, it is important to recognize the role minority stress is likely playing in the difference in outcomes seen for the entire TGDY cohort compared to matched controls, as all TGDY likely experience some degree of minority stress related to misgendering, negative self-belief, harassment, violence, bathroom use, ability to change gender marker on official documents, access and coverage for gender-affirming medical care, and the legality of such care (15, 33, 34). However, despite the development of a conceptual framework adapting the minority stress model for clinical work with gender-minority populations (34), and recommendations by the American Heart Association for research that incorporates minority stress theory, available data are limited (14). Finally, although the odds of these diagnoses were higher among TGDY compared to controls, the prevalence of these conditions overall was low (with the exception of overweight/obesity).
The increased risk for overweight/obesity among TGDY compared to matched controls as well as among those with a testosterone prescription should also be considered in light of legislation in half the states in the United States proposing banning participation on the sporting team that aligns with their gender identity for transgender minors (35). Other studies have shown that TGDY are less physically active compared to their cisgender counterparts (36, 37). A systematic review found that the primary barrier to participation in sports was “the lack of inclusive and comfortable environments” and that TGD people mostly had negative experiences in sports (38). TGD individuals on GAHT, which is related to higher body satisfaction, are more engaged in physical activity than people not on hormones (37), though many transgender males will avoid or limit physical activity and sports participation before chest-masculinizing surgery (39). While increased participation in sports and physical activity may improve physical and mental health (40, 41), bans on TGDY participation in sports will increase stigma and discrimination and worsen health and fitness for TGDY (42). Bans on sports participation may lead to a further worsening of overall risk of poor cardiometabolic health as TGDY age into adulthood (40).
However, sex steroids also play a role in cardiometabolic health. Our findings suggest TGDY who received prescriptions for testosterone were more likely to have diagnoses of overweight/obesity, dyslipidemia, liver dysfunction, and hypertension compared to those without these prescriptions. We were not able to evaluate whether these diagnoses preceded or followed the initial testosterone prescription and represent an association, not causality. It is also possible that those individuals who received a GAHT prescription may be experiencing worse gender dysphoria and worse minority stress compared to those who did not receive a prescription, which would effect outcomes.
Overweight/Obesity
We found that TGDY had higher odds of overweight/obesity compared to controls. Additionally, when overweight/obesity was included in the adjusted models, the odds of the cardiometabolic-related conditions evaluated here were attenuated. However, there was a statistically significant interaction by sex, with TGDY with a female EHR sex being at high risk for overweight/obesity compared to female controls. Furthermore, testosterone, but not other GAHTs, was associated with a diagnosis of overweight/obesity. Our group (16) and others (18, 43) have shown an increase in BMI after testosterone initiation in transgender adolescents, and an overall higher prevalence of overweight/obesity as defined by BMI in their cohort when compared to rates in adolescents nationally (16). This finding is also consistent with a meta-analysis showing an increase in BMI with testosterone use in transgender adults (44). However, BMI may be a poor metric in this population because testosterone use is associated with increased muscle mass, which results in weight gain (45). Although we have only BMI data and not body composition in PEDSnet, prior studies have examined body composition in transgender youth, showing that adolescents on testosterone or E2 therapy who have had body fat measured by dual-energy x-ray absorptiometry are intermediate between matched cisgender male and female controls (17). GnRHa therapy results in an increase in body fat and decrease in lean mass after initiation (5, 9), and cross-sectional analyses show that those on GnRHa therapy alone have a higher percentage body fat than BMI-matched cisgender controls (46). Those prescribed COCPs, which are used for menstrual suppression in TGDY with female EHR sex, had high odds of overweight obesity. As these adolescents are also prescribed testosterone, the use of COCPs could be further influencing the association between overweight/obesity and testosterone prescription. Screening for overweight/obesity and mental health concerns, as well as tailored weight management sensitive to the needs of this population, are needed and may be particularly important for TGDY who were assigned female at birth and those starting testosterone.
Dyslipidemia
Meta-analyses show an association between GAHT and dyslipidemia (12). Specifically, an increase in triglycerides and low-density lipoprotein cholesterol and a decrease in high-density lipoprotein cholesterol were seen with the initiation of testosterone, and an increase in triglycerides was seen with the initiation of E2 (2, 12). A number of studies published after this 2017 meta-analysis have consistently described an association of dyslipidemia with testosterone use in transgender adolescents, namely decreased high-density lipoprotein cholesterol (16, 17, 19, 43) and increased triglycerides (19). However, many studies do not demonstrate an association between E2 use and dyslipidemia (17, 19, 43). Our data similarly show higher odds of dyslipidemia in TGDY who are prescribed testosterone alone or in combination with GnRHa, and we do not show an increased odds of dyslipidemia with E2 alone, or E2 and GnRHa, when compared to TGDY not prescribed GAHT. Our results support the Endocrine Society guidelines, which recommend monitoring lipid profiles in adolescents during puberty induction with testosterone (2). Additionally, TGDY prescribed COCPs had higher odds of dyslipidemia than those not prescribed COCPs. As COCPs are used for menstrual suppression in those who may also be prescribed testosterone for GAHT, their use may be influencing the association between dyslipidemia and testosterone prescription. Available laboratory data from all TGDY and controls show no difference in measured cholesterol values (see Table 2), suggesting this is likely not a clinically significant outcome. Additionally, we show a significant attenuation of the odds of dyslipidemia in TGDY compared to controls in our adjusted model. It remains important to discuss modifiable risk factors such as overweight/obesity in this population to address this risk.
Liver Dysfunction
In our unadjusted model, TGDY had higher odds of liver dysfunction than controls, but after adjusting for overweight/obesity, depression, and antipsychotic prescription, the odds of liver dysfunction were lower in TGDY compared to matched controls, highlighting the importance of these other factors in risk. Additionally, we hypothesize that lower odds are due to routine testing in TGDY on GAHT leading to a higher number of tests, with fewer controls undergoing testing of transaminases. Controls who are tested most likely have a higher pretest probability of an abnormal value than TGDY undergoing routine surveillance. While there were statistically significant differences in laboratory data between TGDY and controls, these differences in transaminases are not clinically meaningful. We found an association between testosterone prescription and increased odds of liver dysfunction. While the Endocrine Society guidelines previously recommend monitoring transaminases for individuals on testosterone therapy (but not GnRHa or E2) (47), the most recent guidelines (2017) do not (2). One study by Jarin et al (43) showed no change in transaminases in TGDY after initiation of testosterone, and a statistically significant decrease in alanine aminotransferase in TGDY after initiation of E2 therapy.
Hypertension
TGDY had higher odds of having hypertension than controls in the unadjusted, but not adjusted, model. TGDY prescribed testosterone alone, but not other GAHT regimens, also had higher odds of hypertension in the unadjusted and adjusted models. Studies of hypertension in TGDY have had mixed results, ranging from a statistically, but likely not clinically significant, increase in blood pressure after testosterone initiation (19), to no change with either testosterone or E2 (43), and another showing TGDY on testosterone had lower blood pressure than BMI-matched controls (17). Monitoring blood pressure (as recommended by the Endocrine Society guidelines) before and after hormone initiation, as well as in youth generally, remains important until larger, prospective studies are performed.
Dysglycemia
We found that TGDY have lower odds of having dysglycemia than controls. This is likely due to the increased number of tests in the TGDY group, as some clinicians routinely evaluate dysglycemia in this group. We hypothesize that the controls who are being evaluated for dysglycemia have a higher pretest probability of having pathology than those TGDY who may be screened more routinely.
Polycystic Ovary Syndrome
We found that TGDY with a female EHR sex had higher odds of having a PCOS diagnosis than controls in the unadjusted but not adjusted models. Early studies suggested higher rates of PCOS may exist in transgender individuals who were assigned female at birth (48, 49, 50, 51), though a recently published review that used more uniform diagnostic criteria for PCOS shows similar rates of gender dysphoria in individuals with PCOS as compared to controls (52). Given that our study relied on diagnosis capture from available EHR data, we are unable to determine what criteria were used to diagnose PCOS in our cohort.
Strengths of this study include the size of our data set, use of a large, matched control group, multicenter data capture through PEDSnet, and the wide geographic distribution of participating sites. Additionally, we used objective data including blood pressure, laboratory values, height and weight, as well as medications prescribed in addition to diagnosis codes. There are also several limitations. We are not able to deduce relationships between the timing of the outcomes of interest related to timing of gender dysphoria diagnosis or initiation of GAHT. All results were evaluated cross-sectionally given the limitations of the data set. Additionally, we captured only diagnoses that were listed in the problem list or as a billing diagnosis, and medications that were listed or prescribed within each participating institution’s EHR. We did not evaluate any gender-affirming surgical procedures such as masculinizing chest surgery, which may have an effect on BMI and other cardiometabolic outcomes. While we were able to capture objective laboratory and anthropometric data, if patients had laboratory data collected at a non-PEDSnet site, those values would not be captured here.
It is important to highlight that TGDY in our study were more likely to have laboratory (but not anthropometric) measurements than controls, which could lead to more opportunities to receive a cardiometabolic-related diagnosis. Given that guideline-based practice recommends baseline laboratory evaluation before starting and while on GAHT (2), it is very likely that mild perturbations in values with little clinical significance would be captured at a higher rate in the TGDY when compared to controls who are not typically undergoing routine laboratory testing. However, control youth may have been more likely to have a particular laboratory test for a given clinical indication. For example, the interquartile range for glycated hemoglobin A1c for control youth is wider than that for TGDY and capture individuals with a diabetes-range A1c. This may represent bias in the control group, which could be a less metabolically healthy group than the general population. The propensity-score match on several variables and matching 4 controls to every TGDY case helped address any potential bias introduced. Although several results were statistically significant, for all laboratory data comparisons, differences were not clinically meaningful. Additionally, while there are higher odds of cardiometabolic-related diagnoses in TGDY prescribed GAHT, these outcomes remained uncommon among this population (< 15% for all outcomes other than overweight/obesity), which shows that while associations exist, these are largely not prevalent diagnoses. Finally, comparisons within the TGDY group were not matched comparisons, and there may be important differences between those who do vs do not receive a GAHT prescription.
In conclusion, TGDY are more likely to have a higher cardiometabolic risk profile when compared to matched controls without GD, particularly with regard to overweight/obesity among people with a female EHR sex. Importantly, our results differed significantly after adjusting for overweight/obesity, depression, and antipsychotic medication prescription, highlighting the importance of addressing these aspects of health in TGDY. This important finding shows that addressing modifiable health risk factors such as overweight/obesity through participation in activities through organized sports, as well as counseling on diet, could have a significant impact on the health of TGDY. The difference in absolute median values of cardiometabolic laboratory data between groups was statistically significant but not clinically meaningful. Overall, it is reassuring that neither statistically significant nor clinically meaningful differences were seen for youth who had received E2 or GnRHa. Future work should incorporate a minority stress framework into understanding these outcomes, and clinicians and researchers should continue to follow cardiometabolic outcomes and recommend interventions to improve cardiometabolic health. Finally, large, longitudinal studies are needed to further evaluate health risk profiles and protective factors for TGDY overall, and those initiating GAHT.
Acknowledgments
We would like to thank the PEDSnet Data Coordinating Center for their support in the data acquisition and all PEDSnet site contributors. We would also like to thank the team at the Adult and Child Center for Health Outcomes Research and Delivery Science (ACCORDS) including prior analysts Jacob Thomas, Bridget Mosley, and Angela Moss, who worked on data cleaning and the early analyses, and Elizabeth Juarez-Colunga, who helped develop the initial analysis plan.
Glossary
Abbreviations
- BMI
body mass index
- COCPs
combined oral contraceptive pills
- DBP
diastolic blood pressure
- E2
estradiol
- EHR
electronic health record
- GAHT
gender-affirming hormone therapy
- GnRHa
gonadotropin-releasing hormone agonist
- PCOS
polycystic ovary syndrome
- SBP
systolic blood pressure
- TGDY
transgender and gender-diverse youth
Contributor Information
Anna Valentine, University of Colorado Anschutz Medical Campus, Department of Pediatrics, Aurora, Colorado 80045, USA; Children’s Hospital Colorado, Division of Endocrinology, Aurora, Colorado 80045, USA.
Shanlee Davis, University of Colorado Anschutz Medical Campus, Department of Pediatrics, Aurora, Colorado 80045, USA; Children’s Hospital Colorado, Division of Endocrinology, Aurora, Colorado 80045, USA.
Anna Furniss, University of Colorado Adult & Child Consortium for Health Outcomes Research and Delivery Sciences (ACCORDS), Aurora, Colorado 80045, USA.
Nadia Dowshen, University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania 19104, USA.
Anne E Kazak, Thomas Jefferson University, Sidney Kimmel Medical College, Philadelphia, Pennsylvania 19107, USA.
Christopher Lewis, Washington University School of Medicine, St Louis, Missouri 63110, USA.
Danielle F Loeb, University of Colorado Anschutz Medical Campus, Department of Medicine, Aurora, Colorado 80045, USA.
Leena Nahata, Center for Biobehavioral Health, Abigail Wexner Research Institute, Columbus, Ohio 43215, USA; Division of Endocrinology, Nationwide Children’s Hospital, Columbus, Ohio 43205, USA.
Laura Pyle, University of Colorado Anschutz Medical Campus, Department of Pediatrics, Aurora, Colorado 80045, USA; University of Colorado School of Public Health, Department of Biostatistics and Informatics, Aurora, Colorado 80045, USA.
Lisa M Schilling, University of Colorado Anschutz Medical Campus, Department of Medicine, Aurora, Colorado 80045, USA; University of Colorado Data Science to Patient Value Initiative, Aurora, CO 80045, USA.
Gina M Sequeira, Seattle Children’s Research Institute, Seattle, Washington 98121, USA.
Natalie Nokoff, University of Colorado Anschutz Medical Campus, Department of Pediatrics, Aurora, Colorado 80045, USA; Children’s Hospital Colorado, Division of Endocrinology, Aurora, Colorado 80045, USA.
Financial Support
This work was supported in part by the National Institutes of Health/National Institute of Child Health and Human Development (National Institutes of Health (NIH)/National Institute of Child Health and Human Development (NICHD) Nos. K23HD092588 and R03HD102773 to S.D.), the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI No. K23HL151868 to N.N.), the Doris Duke Foundation (S.D. and N.N.), the National Institute of Diabetes and Digestive and Kidney Diseases (No. T325T32DK063687 to A.V.), the Pediatric Endocrine Society (A.V.), and the Society for Adolescent Health and Medicine (A.V.). Contents are the authors’ sole responsibility and do not necessarily represent views of the funders. The funders had no role in the design or conduct of the study.
Disclosures
N.N. previously consulted for Antares Pharma, Inc and Neurocrine Biosciences. L.S. previously contributed to a digital innovation project with Eli Lily, Inc. The other authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Johns MM, Lowry R, Andrzejewski J, et al. Transgender identity and experiences of violence victimization, substance use, suicide risk, and sexual risk behaviors among high school students—19 states and large urban school districts, 2017. Morb Mortal Wkly Rep. 2019;68(3):67-71. doi: 10.15585/mmwr.mm6803a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903. doi: 10.1210/jc.2017-01658 [DOI] [PubMed] [Google Scholar]
- 3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed.American Psychiatric Association; 2013:xliv, 947. [Google Scholar]
- 4. Nota NM, Wiepjes CM, de Blok CJM, Gooren LJG, Kreukels BPC, den Heijer M. Occurrence of acute cardiovascular events in transgender individuals receiving hormone therapy. Circulation. 2019;139(11):1461-1462. doi: 10.1161/CIRCULATIONAHA.118.038584 [DOI] [PubMed] [Google Scholar]
- 5. Islam N, Nash R, Zhang Q, et al. Is there a link between hormone use and diabetes incidence in transgender people? Data from the STRONG cohort. J Clin Endocrinol Metab. 2022;107(4):e1549-e1557. doi: 10.1210/clinem/dgab832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nokoff NJ, Scarbro S, Juarez-Colunga E, Moreau KL, Kempe A. Health and cardiometabolic disease in transgender adults in the United States: behavioral risk factor surveillance system 2015. J Endocr Soc. 2018;2(4):349-360. doi: 10.1210/js.2017-00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alzahrani T, Nguyen T, Ryan A, et al. Cardiovascular disease risk factors and myocardial infarction in the transgender population. Circ Cardiovasc Qual Outcomes. 2019;12(4):e005597. doi: 10.1161/circoutcomes.119.005597 [DOI] [PubMed] [Google Scholar]
- 8. Sitruk-Ware R, Nath A. Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Pract Res Clin Endocrinol Metab. 2013;27(1):13-24. doi: 10.1016/j.beem.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 9. Quinn VP, Nash R, Hunkeler E, et al. Cohort profile: study of Transition, Outcomes and Gender (STRONG) to assess health status of transgender people. BMJ Open. 2017;7(12):e018121. doi: 10.1136/bmjopen-2017-018121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Velzen D, Wiepjes C, Nota N, et al. Incident diabetes risk is not increased in transgender individuals using hormone therapy. J Clin Endocrinol Metab. 2022;107(5):e2000-e2007. doi: 10.1210/clinem/dgab934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dutra E, Lee J, Torbati T, Garcia M, Merz CNB, Shufelt C. Cardiovascular implications of gender-affirming hormone treatment in the transgender population. Maturitas. 2019;129:45-49. doi: 10.1016/j.maturitas.2019.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, et al. Sex steroids and cardiovascular outcomes in transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2017;102(11):3914-3923. doi: 10.1210/jc.2017-01643 [DOI] [PubMed] [Google Scholar]
- 13. Fernandez JD, Tannock LR. Metabolic effects of hormone therapy in transgender patients. Endocr Pract. 2016;22(4):383-388. doi: 10.4158/EP15950.OR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Streed CG Jr, Beach LB, Caceres BA, et al. Assessing and addressing cardiovascular health in people who are transgender and gender diverse: a scientific statement from the American heart association. Circulation. 2021;144(6):e136-e148. doi: 10.1161/CIR.0000000000001003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M.. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality; 2016. [Google Scholar]
- 16. Valentine A, Bonny A, Chelvakumar G, et al. Cardiometabolic parameters among transgender adolescent males on testosterone therapy and body mass index-matched cisgender females. Transgend Health. 2021;6(6):369-373. doi: 10.1089/trgh.2020.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nokoff NJ, Scarbro SL, Moreau KL, et al. Body composition and markers of cardiometabolic health in transgender youth compared with cisgender youth. J Clin Endocrinol Metab. 2020;105(3):e704-e714. doi: 10.1210/clinem/dgz029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sequeira GM, Kidd K, El Nokali NE, et al. Early effects of testosterone initiation on body mass index in transmasculine adolescents. J Adolesc Health. 2019;65(6):818-820. doi: 10.1016/j.jadohealth.2019.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olson-Kennedy J, Okonta V, Clark LF, Belzer M. Physiologic response to gender-affirming hormones among transgender youth. J Adolesc Health. 2018;62(4):397-401. doi: 10.1016/j.jadohealth.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nunes-Moreno M, Buchanan C, Cole FS, et al. Behavioral health diagnoses in youth with gender dysphoria compared with controls: a PEDSnet study. J Pediatr. 2021;241:147-153.e1. doi: 10.1016/j.jpeds.2021.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flynn JT, Kaelber DC, Baker-Smith CM, et al. Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. doi: 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 22. Kwo PY, Cohen SM, Lim JK. ACG Clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35. doi: 10.1038/ajg.2016.517 [DOI] [PubMed] [Google Scholar]
- 23. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth charts for the United States: methods and development. Vital Health Stat 11. 2002;( 246):1-190. [PubMed] [Google Scholar]
- 24. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bussler S, Vogel M, Pietzner D, et al. New pediatric percentiles of liver enzyme serum levels (alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase): effects of age, sex, body mass index, and pubertal stage. Hepatology. 2018;68(4):1319-1330. doi: 10.1002/hep.29542 [DOI] [PubMed] [Google Scholar]
- 26. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pramyothin P, Khaodhiar L. Metabolic syndrome with the atypical antipsychotics. Curr Opin Endocrinol Diabetes Obes. 2010;17(5):460-466. doi: 10.1097/MED.0b013e32833de61c [DOI] [PubMed] [Google Scholar]
- 28. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med. 2015;373(14):1307-1317. doi: 10.1056/NEJMoa1502821 [DOI] [PubMed] [Google Scholar]
- 29. Warrier V, Greenberg DM, Weir E, et al. Elevated rates of autism, other neurodevelopmental and psychiatric diagnoses, and autistic traits in transgender and gender-diverse individuals. Nat Commun. 2020;11(1):3959. doi: 10.1038/s41467-020-17794-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flentje A, Heck NC, Brennan JM, Meyer IH. The relationship between minority stress and biological outcomes: a systematic review. J Behav Med. 2020;43(5):673-694. doi: 10.1007/s10865-019-00120-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674-697. doi: 10.1037/0033-2909.129.5.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332(7540):521-525. doi: 10.1136/bmj.38693.435301.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lefevor GT, Boyd-Rogers CC, Sprague BM, Janis RA. Health disparities between genderqueer, transgender, and cisgender individuals: an extension of minority stress theory. J Couns Psychol. 2019;66(4):385-395. doi: 10.1037/cou0000339 [DOI] [PubMed] [Google Scholar]
- 34. Hendricks ML, Testa RJ. A conceptual framework for clinical work with transgender and gender nonconforming clients: an adaptation of the Minority Stress Model. Prof Psychol Res Pr. 2012;43(5):460-467. doi: 10.1037/a0029597 [DOI] [Google Scholar]
- 35. Equality Federation. Accessed January 14, 2022. https://www.equalityfederation.org/state-legislation
- 36. Muchicko MM, Lepp A, Barkley JE. Peer victimization, social support and leisure-time physical activity in transgender and cisgender individuals. Leisure/Loisir. 2014;38(3-4):295-308. doi: 10.1080/14927713.2015.1048088 [DOI] [Google Scholar]
- 37. Jones BA, Haycraft E, Bouman WP, Arcelus J. The levels and predictors of physical activity engagement within the treatment-seeking transgender population: a matched control study. J Phys Act Health. 2018;15(2):99-107. doi: 10.1123/jpah.2017-0298 [DOI] [PubMed] [Google Scholar]
- 38. Jones BA, Arcelus J, Bouman WP, Haycraft E. Sport and transgender people: a systematic review of the literature relating to sport participation and competitive sport policies. Sports Med. 2017;47(4):701-716. doi: 10.1007/s40279-016-0621-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mehringer JE, Harrison JB, Quain KM, Shea JA, Hawkins LA, Dowshen NL. Experience of chest dysphoria and masculinizing chest surgery in transmasculine youth. Pediatrics. 2021;147(3):e2020013300. doi: 10.1542/peds.2020-013300 [DOI] [PubMed] [Google Scholar]
- 40. Barrera E, Millington K, Kremen J. The medical implications of banning transgender youth from sport participation. JAMA Pediatr. 2022;176(3):223-224. doi: 10.1001/jamapediatrics.2021.4597 [DOI] [PubMed] [Google Scholar]
- 41. McMahon EM, Corcoran P, O’Regan G, et al. Physical activity in European adolescents and associations with anxiety, depression and well-being. Eur Child Adolesc Psychiatry. 2017;26(1):111-122. doi: 10.1007/s00787-016-0875-9 [DOI] [PubMed] [Google Scholar]
- 42. Hughes LD, Dowshen N, Kidd KM, Operario D, Renjilian C, Gamarel KE. Pediatric provider perspectives on laws and policies impacting sports participation for transgender youth. LGBT Health. 2022;9(4):247-253. doi: 10.1089/lgbt.2021.0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jarin J, Pine-Twaddell E, Trotman G, et al. Cross-sex hormones and metabolic parameters in adolescents with gender dysphoria. Pediatrics. 2017;139(5):e20163173. doi: 10.1542/peds.2016-3173 [DOI] [PubMed] [Google Scholar]
- 44. Velho I, Fighera TM, Ziegelmann PK, Spritzer PM. Effects of testosterone therapy on BMI, blood pressure, and laboratory profile of transgender men: a systematic review. Andrology. 2017;5(5):881-888. doi: 10.1111/andr.12382 [DOI] [PubMed] [Google Scholar]
- 45. Klaver M, Dekker MJHJ, de Mutsert R, Twisk JWR, den Heijer M. Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: a meta-analysis. Andrologia. 2017;49(5). doi: 10.1111/and.12660 [DOI] [PubMed] [Google Scholar]
- 46. Nokoff NJ, Scarbro SL, Moreau KL, et al. Body composition and markers of cardiometabolic health in transgender youth on gonadotropin-releasing hormone agonists. Transgend Health. 2021;6(2):111-119. doi: 10.1089/trgh.2020.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine Society. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(9):3132-3154. doi: 10.1210/jc.2009-0345 [DOI] [PubMed] [Google Scholar]
- 48. Baba T, Endo T, Honnma H, et al. Association between polycystic ovary syndrome and female-to-male transsexuality. Hum Reprod. 2007;22(4):1011-1016. doi: 10.1093/humrep/del474 [DOI] [PubMed] [Google Scholar]
- 49. Balen AH, Schachter ME, Montgomery D, Reid RW, Jacobs HS. Polycystic ovaries are a common finding in untreated female to male transsexuals. Clin Endocrinol (Oxf). 1993;38(3):325-329. doi: 10.1111/j.1365-2265.1993.tb01013.x [DOI] [PubMed] [Google Scholar]
- 50. Liu M, Murthi S, Poretsky L. Polycystic ovary syndrome and gender identity. Yale J Biol Med. 2020;93(4):529-537. [PMC free article] [PubMed] [Google Scholar]
- 51. Futterweit W, Weiss RA, Fagerstrom RM. Endocrine evaluation of forty female-to-male transsexuals: increased frequency of polycystic ovarian disease in female transsexualism. Arch Sex Behav. 1986;15(1):69-78. doi: 10.1007/BF01542305 [DOI] [PubMed] [Google Scholar]
- 52. Calvar C, Fernández M, Duran Y, Ballester GP, Landini S. Polycystic ovary syndrome (Pcos) in female-to-male (Ftm) transsexual persons. Presented at the 18th European Congress of Endocrinology; May 2016, Munich Germany. Endocrine Abstracts. 2016;41:EP682. doi:10.1530/endoabs.41.EP682 [Google Scholar]
- 53. Davis SM, Nokoff NJ, Furniss A, et al. Population-based assessment of cardiometabolic-related diagnoses in youth with Klinefelter syndrome: a PEDSnet study. J Clin Endocrinol Metab. 2022;107(5):e1850-e1859. doi: 10.1210/clinem/dgac056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.