Abstract
Context
Urinary bladder paraganglioma (UBPGL) is rare.
Objective
We aimed to characterize the presentation and outcomes of patients diagnosed with UBPGL.
Methods
We conducted a multicenter study of consecutive patients with pathologically confirmed UBPGL evaluated between 1971 and 2021. Outcomes included repeat bladder surgery, metastases, and disease-specific mortality.
Results
Patients (n=110 total; n=56 [51%] women) were diagnosed with UBPGL at a median age of 50 years (interquartile range [IQR], 36-61 years). Median tumor size was 2 cm (IQR, 1-4 cm). UBPGL was diagnosed prior to biopsy in only 37 (34%), and only 69 (63%) patients had evaluation for catecholamine excess. In addition to the initial bladder surgery, 26 (25%) required multiple therapies, including repeat surgery in 10 (9%). Synchronous metastases were present in 9 (8%) patients, and 24 (22%) other patients with UBPGL developed metachronous metastases at a median of 4 years (IQR, 2-10 years) after the initial diagnosis. Development of metachronous metastases was associated with younger age (hazard ratio [HR] 0.97; 95% CI, 0.94-0.99), UBPGL size (HR 1.69; 95% CI, 1.31-2.17), and a higher degree of catecholamine excess (HR 5.48; 95% CI, 1.40-21.39). Disease-specific mortality was higher in patients with synchronous metastases (HR 20.80; 95% CI, 1.30-332.91). Choice of initial surgery, genetic association, sex, or presence of muscular involvement on pathology were not associated with development of metastases or mortality.
Conclusions
Only a minority of patients were diagnosed before biopsy/surgery, reflecting need for better diagnostic strategies. All patients with UBPGL should have lifelong monitoring for development of recurrence and metastases.
Keywords: catecholamine, micturition, diagnosis, prognosis
Paragangliomas (PGLs) are rare tumors that originate from extra-adrenal neuroendocrine tissue, with one-third to one-half being located in the thoracoabdominal region (1, 2). Urinary bladder PGL (UBPGL), a PGL that arises from the paraganglia within the urinary bladder wall, is one of the rarest types of thoracoabdominal PGLs, representing around 0.7% of all PGLs and < 0.05% of all bladder tumors (2-4).
Approximately 50% of patients with thoracoabdominal PGLs have pathogenic variants in pheochromocytoma/paraganglioma (PPGL) susceptibility genes, with pathogenic variants in the succinate dehydrogenase (SDH) B and D subunits being the most common (1, 5). Patients with thoracoabdominal PGLs, and pathogenic SDHB variants in particular, demonstrate an increased risk of metastatic disease (1, 5). Presentation of PGLs depends on the presence of catecholamine excess, location, and size, with many patients diagnosed incidentally on imaging, and, occasionally, through case detection testing for a known genetic association (6).
Scarce data are available on the presentation and outcomes of patients with UBPGL. Based on the 2 systematic reviews that summarized case reports and small case series, patients with UBPGL may present with symptoms of catecholamine excess triggered by micturition in 30% to 53%, with hematuria in 35% to 47%, or are incidentally discovered on imaging in 3% to 10% (7, 8). The reported proportion of patients with UBPGL demonstrating catecholamine excess vary widely between 55% and 91% (7-9). Genetic association is inconsistently reported, present in 12% to 63% of patients with UBPGL, and 9% to 52% for SDHB in particular (9-11). The best therapeutic strategy in patients with UBPGL is also unclear. In a recent systematic review that included 27 patients from small case series (range, 2-4 patients) and 167 case reports, 40% of patients with UBPGL were treated with transurethral resection of bladder tumor (TURBT), 45% with partial cystectomy, 4% with radical cystectomy, and 11% of patients were treated with undefined surgical approach (8). Outcomes of patients with UBPGL are also incompletely characterized. Based on prior reports, 14% of surgically treated UBPGL recur; however, factors associated with recurrence have not been identified (7). Rates of synchronous and metachronous metastatic disease in patients with UBPGL, and overall and disease-specific survival rates are inconsistently reported or difficult to estimate due to very limited sample size in current reports.
To address the multiple gaps in the literature, we designed a large multicenter study with the objectives to characterize the presentation and outcomes of patients diagnosed with UBPGL and to determine the factors associated with recurrence, metastatic disease, and survival.
Methods
Study Design and Participants
We conducted a multicenter study in accordance with the local ethical/Institutional Review Board (IRB) committees. All patients provided written consent, or a consent waiver was used depending on the local IRB requirements. The study included 5 centers from United States, 4 centers from Scandinavia, and 1 center from Brazil. All participating centers contributed consecutive cohorts of patients with pathologically proven UBPGL diagnosed during a median inclusion period of 31 years (IQR, 8-50 years). Enrollment periods depended on the availability of medical records to assure consecutive enrollment (Supplemental Table 1 (12)).
We recorded data on demographic factors, mode of discovery, genetic profile, family history of PPGL, PPGL characteristics, presence of recurrence and metastases, biochemical profile, imaging data, therapeutic modalities, and histopathological data. Biochemical evaluations varied according to local practices and availability. When plasma and urine metanephrines/catecholamines concentrations were above the normal reference ranges, based on local reference ranges, patients were considered to demonstrate catecholamine excess. Tumor size was determined based on the largest diameter of the UBPGL on histopathology or imaging (in patients not treated by surgery). UBPGL was considered multinodular when multiple distinct bladder PGL lesions were present. In patients with multinodular UBPGL, tumor size was defined as the largest diameter among all the nodules.
We defined metastatic PPGL based on the criteria of World Health Organization (WHO) as presence of PPGL at non-chromaffin sites (e.g., bone, lymph node, and liver) (13). Metastasis was considered synchronous if diagnosed at or within 3 months of the primary diagnosis of UBPGL, and metachronous, if diagnosed at ≥ 3 months after initial diagnosis with primary UBPGL diagnosis. In contrast, other non-bladder PPGLs were defined as concurrent PPGL if diagnosed at or within 3 months of the primary diagnosis with UBPGL and as new PPGL if diagnosed at ≥ 3 months after initial diagnosis with primary UBPGL.
Outcomes included development of recurrent UBPGL, need for second bladder surgery, number of therapies for UBPGL, development of metachronous metastases, overall survival, and disease-specific survival. Disease-specific survival was defined as the time period between the initial diagnosis of UBPGL and the time when patients died due to PGL.
To investigate whether the time period of diagnosis was associated with the mode of UBPGL discovery and the degree of workup for catecholamines excess, we analyzed variables based on time of diagnosis using the following intervals: ≤ 2001, 2002-2011, and 2012-2021.
To investigate the incidence rate of UBPGL in a population, we accessed the Denmark nationwide population-based database of all pathological examinations conducted since 1996. We estimated the incidence rate of new patients with UBPGL per million person-years with 95% confidence intervals, assuming that new cases followed a Poisson distribution. First, we extracted data on all Danish residents with a diagnosis code for PPGL from 1997 to 2016 (average annual population 5.46 million, total 109.26 million years of follow-up). Secondly, we included all patients whose pathological examinations mentioned “bladder,” “urine,” or “vesical.” Finally, we manually reviewed pathological reports to confirm the diagnosis of UBPGL (Supplementary Figure 1 (12)).
Statistics
All continuous variables were summarized as medians and interquartile ranges (IQR), while categorical variables were presented as frequencies and percentages. Mann-Whitney U test (for continuous variables) and the Chi-square test (for categorical variables) were used for subgroup analyses (genetic association and catecholamines excess). Spearman test was applied for correlation analysis between continuous variables and ordered categories. Multiple pairwise analyses were applied to estimate the differences in the mode of UBPGLs discovery and the rate of catecholamine evaluation.
In patients initially operated by either TURBT or partial cystectomy, univariable Cox analyses (hazard ratio [HR] and 95% CI) were used to identify factors associated with metachronous UBPGL metastasis, repeat bladder surgery and disease-specific survival.
Epidemiological analyses were conducted in Stata Statistical Software, Release 16.1 (Stata Corp, College Station, TX). We estimated incidence rate of new patients with UBPGL per million person-years with 95% CIs, assuming new cases followed a Poisson distribution.
All other statistical analyses were conducted by R for macOS (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria), and P < 0.05 was defined as statistically significant.
Results
General Characteristics of Patients With UBPGL
We identified 110 patients (56, [51%] women) diagnosed with UBPGL at a median age of 50 years (IQR, 36-61 years). In 99 patients with available data, only 20 (20%) patients reported a family history of PPGLs. Of 61 patients who had had genetic testing, 34 (56%) were confirmed to have a pathogenic variant in a gene associated with PPGL (SDHB in 26, SDHC in 2, SDHA in 2, SDHAF2 in 1, SDHD in 1, and VHL in 2). At the time of UBPGL diagnosis, 21 (19%) patients had concurrent primary PPGLs at other sites.
Mode of Discovery
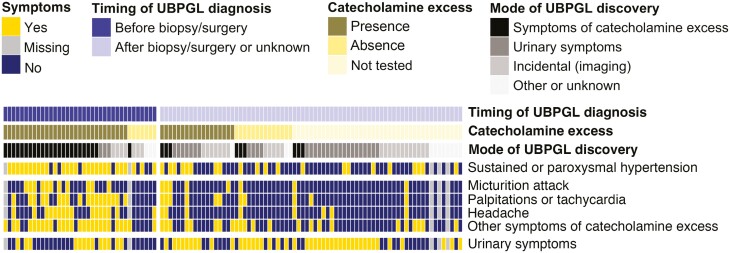
Patients were discovered with UBPGL incidentally on imaging (31 [28%]), due to symptoms of catecholamine excess (33 [30%]), during evaluation for urinary symptoms including hematuria, urination pain, and pelvic pain (32 [29%]), during evaluation for other reasons (10 [7%]), or unknown (4 [4%]) (Table 1, Fig. 1). Although a total of 70 (64%) of patients reported symptoms of catecholamine excess, only about one-third of patients (37 [34%]) were diagnosed with UBPGL before surgery/biopsy, most of whom presented with symptoms of catecholamine excess (34 [92%], induced by micturition in 11 [30%]) (Fig. 1). The majority of patients (71 [65%]) were diagnosed only based on histopathology, either after biopsy (n = 32) or after surgery (n = 39). In this subgroup of 71 patients, 43 (61%) presented with micturition-related symptoms, including hematuria (30 [42%]), micturition disturbance (19 [27%]), and pain on urination (8 [11%]) (Fig. 1). When asked in retrospect, 36 patients (51%) reported symptoms of catecholamine excess (triggered by micturition in 8 patients). In 9 of 39 patients who were diagnosed with UBPGL only postoperatively, workup for catecholamine excess was available prior to surgery. However, the diagnosis of UBPGL was dismissed due to absence of catecholamine excess (n = 5), borderline catecholamine excess (n = 3) combined with negative functional UBPGL imaging (n = 2), or failure to recognize UBPGL despite catecholamine excess in a patient with other PGLs (n = 1).
Table 1.
Characteristics of patients with urinary bladder paraganglioma
| Variable | Patients with UBPGLn = 110 |
|---|---|
| Women, n (%) | 56 (51%) |
| Age at diagnosis with UBPGL, years, median (IQR) | 50 (36-61) |
| Mode of discovery, n (%) | |
| Symptoms of catecholamine excess | 33 (30%) |
| Urinary symptoms | 32 (29%) |
| Incidental on imaging | 31 (28%) |
| Genetic case detection | 3 (3%) |
| Incidental on pathology | 3 (3%) |
| Mass effect symptoms | 1 (1%) |
| Other abnormalities | 3 (3%) |
| Unknown | 4 (4%) |
| Family history of PPGL, n (%) | |
| Yes | 20 (18%) |
| No | 79 (72%) |
| Unknown | 11 (10%) |
| Genetic association, n (%) | |
| SDHB | 26 (24%) |
| SDHA | 2 (2%) |
| SDHAF2 | 1 (1%) |
| SDHC | 2 (2%) |
| SDHD | 1 (1%) |
| VHL | 2 (2%) |
| Tested, negative | 27 (25%) |
| Not tested or unknown | 49 (45%) |
| UBPGL characteristics | |
| Multinodular, n (%) | 16 (15%) |
| Muscular invasion, n (%), available for n = 71 | 40 (56%) |
| UBPGL tumor size a , cm, median (IQR) | 2 (1-4) |
| Catecholamine excess, n (%) | |
| Present | 48 (44%) |
| Not present | 21 (19%) |
| Unknown | 41 (37%) |
| Degree of catecholamine excess, n (%) | |
| ≥10 times above the ULN | 14 (29%) |
| 4-10 times above the ULN | 13 (27%) |
| 2-4 times above the ULN | 8 (17%) |
| 1-2 times above the ULN | 10 (21%) |
| Unknown | 3 (6%) |
| Concomitant PPGL, n (%) | |
| Concurrent PPGL | 21 (19%) |
| New PPGL | 15 (14%) |
| Initial therapy for UBPGL, available for n = 108 | |
| Surgery, n (%) | 102 (94%) |
| Partial cystectomy | 61 (60%) |
| TURBT | 28 (27%) |
| Radical cystectomy | 5 (5%) |
| Other or unspecified surgery | 8 (8%) |
| Nonsurgical management, n (%) | 6 (6%) |
| Additional therapy for UBPGL, available for n = 106 | |
| Repeat bladder surgery, n (%) | 10 (9%) |
| Patients treated with more than 1 therapy, n (%) | 26 (25%) |
| Recurrent UBPGL, n (%), available for n = 104 | 13 (13%) |
| Interval to recurrence, years, median (IQR) | 3 (2-5) |
| Persistent UBPGL, n (%), available for n = 106 | 10 (9%) |
|
Metastatic PPGL, n (%), available for n = 108 Synchronous |
33 (30%) 9 (8%) |
| Metachronous | 24 (22%) |
| Time to metachronous metastasis, years, median (IQR) | 4 (2-10) |
| Progression of metastatic disease, n (%), available for n = 32 | |
| Indolenta | 13 (41%) |
| Progressive | 19 (59%) |
| Duration of follow-up after metastasis development, years, median (IQR) | 4 (1-7) |
| Follow-up data | |
| Duration of follow-up, years, median (IQR) | 4 (1-11) |
| Status at the end of follow-up, n (%) | |
| Alive | 89 (81%) |
| Deceased of PPGL | 11 (10%) |
| Deceased of other reasons | 10 (9%) |
Abbreviations: IQR, interquartile range; PPGL, pheochromocytoma/paraganglioma; SDH, Succinate dehydrogenase complex; TURBT, transurethral resection of bladder tumor; UBPGL, urinary bladder paraganglioma; ULN, upper limit of normal; VHL, Von Hippel-Lindau.
aIndolent disease was defined as metastatic disease that had stable tumor size and numbers of metastatic spread based on imaging study at last follow-up. Incidental on pathology referred to UBPGLs that were incidentally found on the pathology post resection of other pelvic tumors. Tumor size was determined based on the largest diameter of the UBPGL on histopathology or imaging (in patients not treated by surgery); in patients with multinodular UBPGL, tumor size was defined as the largest diameter among all the nodules.
Figure 1.
Mode of discovery of urinary bladder paraganglioma. Other symptoms of catecholamine excess included dizziness, tremor, syncope, flushing, paleness, sweating, anxiety, nausea, vomiting, abdominal pain, diarrhea, constipation, hypotension, chest pain, back pain, tinnitus, and weight loss. Urinary symptoms referred to hematuria, micturition disturbances, pain on urination, and pelvic pain. Other or unknown mode of discovery included evaluation for a known genetic association, evaluation for mass effect symptoms, incidentally after resection of other pelvic tumors, evaluation for other abnormalities (abnormal creatine or estimated glomerular filtration rate), and unknown. Types of biopsy included fine needle aspiration and diagnostic transurethral resection. Abbreviations: UBPGL, urinary bladder paraganglioma.
Biochemical Findings
A total of 69 (63%) patients had biochemical evaluation for catecholamine excess (Table 2). Catecholamine excess was diagnosed in 48 (44%) patients.
Table 2.
Characteristics of patients with UBPGL according to presence or absence of catecholamine excess
| Variable | Catecholamine excess presentn = 48 | Catecholamine excess absent n = 21 |
P value |
|---|---|---|---|
| Baseline data | |||
| Women, n (%) | 28 (58%) | 10 (48%) | 0.575 |
| Age at diagnosis with UBPGL, years, median (IQR) | 42 (31-55) | 58 (36-67) | 0.037 |
| Mode of discovery, n (%) | 0.015 | ||
| Symptoms of catecholamine excess | 26 (54%) | 4 (19%) | |
| Other | 22 (46%) | 17 (81%) | |
| SDHB genetic association, n (%) | 16 (48%) | 7 (58%) | 0.805 |
| UBPGL characteristics | |||
| Multinodular, n (%) | 12 (25%) | 0 (0%) | 0.014 |
| Muscular invasion, n (%), available for n = 43 | 16 (57%) | 8 (57%) | 1.000 |
| UBPGL tumor size a , cm, median (IQR) | 4 (2-6) | 1 (1-2) | <0.001 |
| History or active concomitant PPGL | |||
| PPGL past history, n (%) | 5 (10%) | 3 (14%) | 0.692 |
| Concurrent PPGL, n (%) | 17 (35%) | 2 (10%) | 0.055 |
| Synchronous metastatic PPGL, n (%) | 8 (17%) | 1 (5%) | 0.482 |
| Therapeutic data | |||
| Initial therapy for UBPGL | 0.939 | ||
| Surgery, n (%) | 45 (94%) | 20 (99%) | |
| Partial cystectomy | 30 (67%) | 15 (75%) | |
| TURBT | 7 (16%) | 3 (15%) | |
| Radical cystectomy | 3 (7%) | 0 (0%) | |
| Other or unspecified surgery | 5 (11%) | 2 (10%) | |
| Nonsurgical management, n (%) | 3 (6%) | 1(5%) | |
| Patients treated with more than 1 therapy a , n (%) | 16 (33%) | 2 (10%) | 1.000 |
| Outcome data of patients initially managed with TURBT or partial cystectomy, n = 55 | |||
| Repeat bladder surgery, n (%) | 3 (8%) | 2 (11%) | 1.000 |
| Recurrent UBPGL, n (%) | 3 (8%) | 1 (6%) | 1.000 |
| Interval to recurrence, years, median (IQR) | 4 (3-4) | 3 (3-3) | 1.000 |
| Persistent UBPGL, n (%) | 2 (6%) | 1 (6%) | 1.000 |
| Metachronous metastatic PPGL, n (%), available for n = 54 | 11 (31%) 3 (2-5) |
2 (11%) 1 (1-1) |
0.179 0.046 |
| Time to metastasis, years, median (IQR) Progression of synchronous and metachronous metastatic disease, n (%), available for n = 16 |
0.083 |
||
| Indolenta | 3 (23%) | 2 (100%) | |
| Progressive | 10 (77%) | 0 (0%) | |
| Duration of follow-up after metastasis development, years, median (IQR) |
4 (2-6) | 4 (4-5) | 0.931 |
| Follow-up data | |||
| Status at the end of follow-up | 0.543b | ||
| Alive, n (%) | 32 (86%) | 16 (89%) | |
| Deceased of PPGL, n (%) | 3 (8%) | 0 (0%) | |
| Deceased of other reasons, n (%) | 2 (5%) | 2 (11%) | |
| Duration of follow-up, years, median (IQR) | 5 (2-10) | 4 (2-7) | 0.511 |
Abbreviations: IQR, interquartile range; PPGL, pheochromocytoma/paraganglioma; SDH, Succinate dehydrogenase complex; TURBT, transurethral resection of bladder tumor; UBPGL, urinary bladder paraganglioma.
P values were estimated by Mann-Whitney U test for continuous variables and Chi-square test for categorical variables.
aAdditional therapy included repeat surgery, chemotherapy, nuclear medicine therapy, targeted therapy, and local therapy with radiation or ablation. Indolent disease was defined as metastatic disease that had stable tumor size and numbers of metastatic spread based on imaging study at last follow-up. Tumor size was determined based on the largest diameter of the UBPGL on histopathology or imaging (in patients not treated by surgery); in patients with multinodular UBPGL, tumor size was defined as the largest diameter among all the nodules.
b P value was according to patients who died of PPGL vs patients who were alive or deceased for other reasons.
Patients with catecholamine excess were more likely to present with a larger UBPGL size and to have multinodular UBPGLs (Table 2). Higher degree of catecholamine excess was associated larger tumor size (r = 0.6; P < 0.001).
Imaging Workup
At the time of initial presentation, median size of UBPGLs was 2 cm (IQR, 1-4), and 16 (15%) patients had multinodular UBPGLs. Imaging modalities that initially localized UBPGL included ultrasonography (20 [18%]), computed tomography (CT) (64 [58%]), magnetic resonance imaging (MRI) (44 [40%]), metaiodobenzylguanidine (MIBG) scintigraphy (24 [22%]), Ga-68 [DOTA-Tyr3]-octreotate (DOTATATE)-positron emission tomography (PET) scan (14 [13%]), and F-18 fluorodeoxyglucose (FDG)-PET scan (11, 13%) (Supplemental Table 2 (12)). Almost half of the patients (53 [48%]) had more than one imaging study prior to considering biopsy, surgery, or other management. MIBG scintigraphy had the highest false negative rate (10/24 [42%]), followed by Ga-68 DOTATATE-PET/CT (5/14 [36%]), ultrasonography (5/20 [25%]), FDG-PET/CT (2/11 [18%]), CT (9/64 [14%]), and MRI (4/44 [9%]). Our results demonstrated that false negative results were not associated with catecholamine excess or presence of SDHB pathogenic variants (Supplementary Table 2 (12)).
Management
Following the diagnoses of UBPGL, 103 (94%) patients were treated with surgery, 4 (4%) were treated with other therapies, 1 (1%) patient was managed conservatively, while no information on therapy was available in 2 patients (Table 1). Overall, 26 (25%) out of 106 patients with available data were treated with multiple therapies that included repeat bladder surgery in 10 (38%), chemotherapy in 14 (54%), nuclear medicine therapy (MIBG therapy and peptide receptor radionuclide therapy) in 7 (27%), targeted molecular therapy (tyrosine kinase inhibitors, e.g., sunitinib) in 5 (19%), and local therapy with radiation or ablation in 4 (15%).
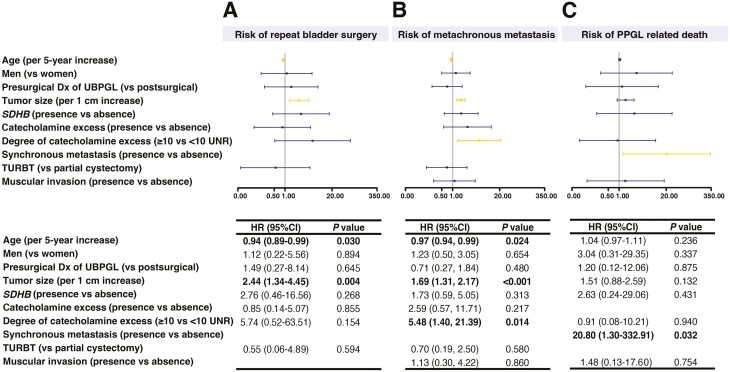
Out of the 102 patients who were initially treated with surgery, the surgical approaches included partial cystectomy (61 [60%]), TURBT (28 [27%]), radical cystectomy (5 [5%]), and other or unspecified surgery (8 [8%]). Among 89 patients who were initially treated with the two most common surgical approaches, partial cystectomy or TURBT (Table 3), 16 (18%) patients required multiple therapies, of whom 6 (7%) patients required repeat bladder surgery due to recurrent UBPGL (n = 3) or persistent UBPGL due to incomplete resection (n = 3) (Table 3). Patients with suspected UBPGL prior to surgery were more likely to be treated with partial cystectomy (46 [75%] vs TURBT 7 [25%]). The group of patients treated with partial cystectomy vs TURBT were also more likely to present with symptoms of and to be tested for catecholamine excess (Table 3). In comparison to patients treated with TURBT, those treated with partial cystectomy were younger (Table 3, Supplemental Table 3 (12)). Repeat bladder surgery was performed in 6 patients (5 patients initially treated with partial cystectomy and 1 patient initially treated with TURBT), as shown in Table 3 and Supplemental Table 3 (12)). Univariable Cox analysis showed that younger age (per 5 years increase in age, HR 0.94; 95% CI, 0.89-0.99) and a larger tumor size (per 1 cm increase in UBPGL size, HR 2.44; 95% CI, 1.34-4.45) were associated with repeat bladder surgery (Fig. 2A). In contrast, sex, type of initial surgery, presence of synchronous metastases or concurrent PPGL, SDHB pathogenic variant, and catecholamine excess were not associated with the risk of repeat bladder surgery (Fig. 2A).
Table 3.
Characteristics and outcomes of patients with urinary bladder paraganglioma treated with partial cystectomy and transurethral resection of bladder tumor
| Variable | TURBTn = 28 | Partial cystectomy n = 61 |
P value |
|---|---|---|---|
| Age at diagnosis with UBPGL, years, median (IQR) | 61 (52-66) | 47 (34-57) | 0.001 |
| Timeline of UBPGL discovery in relation to surgery | <0.001 | ||
| Noninvasive diagnosis or biopsy, before surgery | 7 (25%) | 46 (75%) | |
| Based on histopathology, after surgery | 21 (75%) | 15 (25%) | |
| Mode of discovery and diagnosis, n (%) | 0.002 | ||
| Symptoms of catecholamine excess | 1 (4%) | 23 (38%) | |
| Other symptoms | 27 (96%) | 36 (62%) | |
| SDHB genetic association, n (%), available for n = 52 | 2 (15%) | 19 (49%) | 0.073 |
| UBPGL characteristics | |||
| Multinodular, n (%) | 4 (14%) | 5 (8%) | 0.454 |
| Muscular invasion, n (%), available for n = 62 | 12 (55%) | 24 (60%) | 0.883 |
| UBPGL tumor size a , cm, median (IQR) | 2 (1-3) | 2 (2-4) | 0.112 |
| Catecholamine excess | 0.001 | ||
| Not tested, n (%) | 18 (67%) | 14 (23%) | |
| Present, n (%) | 6 (22%) | 31 (52%) | |
| Absent, n (%) | 3 (11%) | 15 (25%) | |
| History or active concomitant PPGL | |||
| PPGL past history, n (%) | 1 (4%) | 6 (10%) | 0.426 |
| Concurrent PPGL, n (%) | 1 (4%) | 10 (16%) | 0.162 |
| Synchronous metastatic PPGL, n (%) | 0 (0%) | 3 (5%) | 0.549 |
| Therapeutic data, available for n = 87 | |||
| Patients treated with more than 1 therapy a , n (%) | 2 (7%) | 14 (24%) | 0.117 |
| Outcome data | |||
| Repeat bladder surgery, n (%) | 1 (4%) | 5 (8%) | 0.659 |
| Recurrent UBPGL, n (%), available for n = 87 | 3 (11%) | 5 (9%) | 0.801 |
| Interval to recurrence, years, median (IQR) | 2 (2-4) | 4 (3-6) | 0.208 |
| Persistent UBPGL, n (%) | 0 (0%) | 3 (5%) | 0.548 |
| Metachronous metastatic PPGL, n (%), available for n = 88 | 3 (11%) | 16 (27%) | 0.157 |
| Time to metastasis, years, median (IQR) | 3 (2-6) | 4 (2-8) | 0.955 |
|
Progression of synchronous and metachronous metastatic
disease, n (%), available for n = 21 |
0.553 | ||
| Indolenta | 2 (67%) | 7 (39%) | |
| Progressive | 1 (33%) | 11 (61%) | |
| Duration of follow-up after metastasis development, years, median (IQR) |
4 (2-8) | 4 (1-6) | 0.960 |
| Follow-up data | |||
| Status at the end of follow-up | 0.304b | ||
| Alive, n (%) | 25 (89%) | 55 (87%) | |
| Deceased of PPGL, n (%) | 0 (0%) | 4 (7%) | |
| Deceased of other reasons, n (%) | 3 (11%) | 4 (7%) | |
| Duration of follow-up, years, median (IQR) | 4 (1-6) | 5 (3-11) | 0.052 |
P values were estimated by Mann-Whitney U test for continuous variables and Chi-square test for categorical variables.
Abbreviations: IQR, interquartile range; PPGL, pheochromocytoma/paraganglioma; SDH, Succinate dehydrogenase complex; TURBT, transurethral resection of bladder tumor; UBPGL, urinary bladder paraganglioma.
aAdditional therapy included repeat surgery, chemotherapy, nuclear medicine therapy, targeted therapy, and local therapy with radiation or ablation. Indolent disease was defined as metastatic disease that had stable tumor size and numbers of metastatic spread based on imaging study at last follow-up. Tumor size was determined based on the largest diameter of the UBPGL on histopathology or imaging (in patients not treated by surgery); in patients with multinodular UBPGL, tumor size was defined as the largest diameter among all the nodules.
b P value was according to patients who died of PPGL vs patients who were alive or deceased for other reasons.
Figure 2.
Outcomes of patients with urinary bladder paraganglioma. A. Factors associated with repeat bladder surgery. B. Factors associated with metachronous metastasis. C. Determinants of disease-specific survival. Abbreviations: Dx, diagnosis; HR, hazard ratio; PPGL, pheochromocytoma and paraganglioma; SDHB, Succinate dehydrogenase complex iron sulfur subunit B; TURBT, transurethral resection of bladder tumor; UBPGL urinary bladder paraganglioma; UNR, upper limit of normal reference range. Hazard ratios and P values were based on univariable Cox analysis. Tumor size was determined based on the largest diameter of the UBPGL on histopathology or imaging (in patients not treated by surgery). UBPGL was considered multinodular when multiple distinct bladder PGL lesions were present. In patients with multinodular UBPGL, tumor size was defined as the largest diameter among all the nodules.
Metastatic Disease
At the time of diagnosis with UBPGL, 9 (8%) patients presented with synchronous metastases (Table 1). Patients with synchronous metastatic disease were more likely to be younger (median age of 43 years [IQR, 21-48] vs 57 years [IQR, 43-64]; P = 0.006), to have concurrent PPGL (9 [100%] vs 12 [12%]; P < 0.001), and to present with catecholamine excess (8 [89%] vs 24 [31%]; P < 0.001) and a larger UBPGL (median size of 4 cm [IQR, 3-6] vs 2 cm [IQR, 1-3]; P = 0.001), when compared with patients without metastases. Presentation with synchronous metastatic disease was not associated with sex or SDHB pathogenic variants (data not shown).
In addition to patients presenting with synchronous metastases, 24 (22%) other patients with UBPGL developed metachronous metastases at a median of 4 years (IQR, 2-10 years) after initial diagnosis with UBPGL (Table 1). By the end of available follow-up, 33 (30%) patients had metastatic PGL (Table 4). The most common metastatic site was bone (22 [67%]), followed by lymph node (19 [58%]), lung (8 [24%]), and liver (3 [9%]). Thirteen (39%) patients had metastases at multiple sites. Patients with metastatic disease were younger, had larger UBPGL size, and were more likely to demonstrate a higher degree of catecholamine excess, when compared to patients with nonmetastatic UBPGL (Table 4). SDHB pathogenic variant, however, was not associated with metastatic disease (Table 4).
Table 4.
Characteristics and outcomes of patients with benign and malignant urinary bladder paraganglioma
| Variable | Nonmetastatic n = 73 | Metastatica n = 33 |
P value |
|---|---|---|---|
| Baseline data | |||
| Women, n (%) | 40 (55%) | 16 (48%) | 0.695 |
| Age at diagnosis with UBPGL, years, median (IQR) | 57 (42-64) | 38 (21-47) | <0.001 |
| Mode of discovery, n (%), available for n = 104 | 0.001 | ||
| Symptoms of catecholamine excess | 15 (21%) | 18 (56%) | |
| Other | 57 (79%) | 14 (44%) | |
| SDHB genetic association, n (%) | 15 (35%) | 12 (52%) | 0.302 |
| UBPGL characteristics | |||
| Multinodular, n (%) | 10 (14%) | 5 (15%) | 1.000 |
| Muscular invasion, n (%), available for n = 69 | 31 (56%) | 7 (50%) | 0.899 |
| UBPGL tumor size a , cm, median (IQR) | 2 (1-3) | 4 (3-6) | <0.001 |
| Catecholamine excess, available for n = 71 | 0.009 | ||
| Present, n (%) | 23 (56%) | 24 (89%) | |
| Absent, n (%) | 18 (44%) | 3 (11%) | |
| Degree of catecholamine excess, n (%), available or n = 44 | < 0.001 | ||
| ≥10 times above the ULN | 1 (5%) | 12 (55%) | |
| < 10 times the ULN | 20 (95%) | 10 (45%) | |
| History or active concomitant PPGL | |||
| PPGL past history, n (%) | 5 (7%) | 4 (12%) | 0.455 |
| Concurrent PPGL, n (%) | 6 (8%) | 14 (42%) | <0.001 |
| Therapeutic data | |||
| Initial therapy for UBPGL | 0.005 | ||
| Surgery, n (%) | 71 (97%) | 30 (91%) | |
| Partial cystectomy | 41 (58%) | 19 (63%) | |
| TURBT | 25 (35%) | 3 (10%) | |
| Radical cystectomy | 2 (3%) | 3 (10%) | |
| Other or unspecified surgery | 3 (4%) | 5 (17%) | |
| Nonsurgical management, n (%) | 2 (3%) | 3 (9%) | |
| Patients treated with more than 1 therapy a , n (%) | 4 (6%) | 21 (66%) | <0.001 |
| Outcome data of patients initially managed with TURBT or partial cystectomy, n = 88 | |||
| Repeat bladder surgery, available for n = 86 | 1 (2%) | 5 (24%) | 0.003 |
| Recurrent UBPGL, n (%), available for n = 86 | 3 (5%) | 5 (24%) | 0.020 |
| Interval to recurrence, years, median (IQR) | 4 (4-4) | 3 (2-5) | 0.693 |
| Persistent UBPGL, n (%), available for n = 86 | 0 (0%) | 3 (14%) | 0.013 |
| Follow-up data | |||
| Status at the end of follow-up | 0.005b | ||
| Alive, n (%) | 60 (91%) | 18 (82%) | |
| Deceased of PPGL, n (%) | 0 (0%) | 4 (18%) | |
| Deceased of other reasons, n (%) | 6 (9%) | 0 (0%) | |
| Duration of follow-up, years, median (IQR) | 4 (1-7) | 8 (4-15) | 0.012 |
P values were estimated by Mann-Whitney U test for continuous variables and Chi-square test for categorical variables.
Abbreviations: IQR, interquartile range; PPGL, pheochromocytoma/paraganglioma; SDH, Succinate dehydrogenase complex; TURBT, transurethral resection of bladder tumor; UBPGL, urinary bladder paraganglioma; ULN, upper limit of normal.
aAdditional therapy included repeat surgery, chemotherapy, nuclear medicine therapy, targeted therapy, and local therapy with radiation or ablation. Indolent disease was defined as metastatic disease that had stable tumor size and numbers of metastatic spread based on imaging study at last follow-up. Metastatic urinary bladder paraganglioma included all the synchronously and metachronously metastatic urinary bladder paraganglioma. Tumor size was determined based on the largest diameter of the UBPGL on histopathology or imaging (in patients not treated by surgery); in patients with multinodular UBPGL, tumor size was defined as the largest diameter among all the nodules.
b P value was according to patients who died of PPGL vs patients who were alive or deceased for other reasons.
Univariable Cox analysis demonstrated that younger patients (HR 0.97; 95% CI, 0.94-0.99 per each 5-year increase in age), and those with larger UBPGL (per 1 cm increase in size, HR 1.69; 95% CI, 1.31-2.17), were at a higher risk of developing metastatic disease. In addition, development of metastatic disease was associated with the degree of catecholamine excess (HR 5.48; 95% CI, 1.40-21.39) if catecholamine/metanephrine concentrations were ≥ 10 times upper limit of normal. However, the choice of initial surgery (TURBT vs partial cystectomy), SDHB pathogenic variant association, sex, and presence of muscular involvement on pathology were not associated with development of metastases (Fig. 2B).
Survival
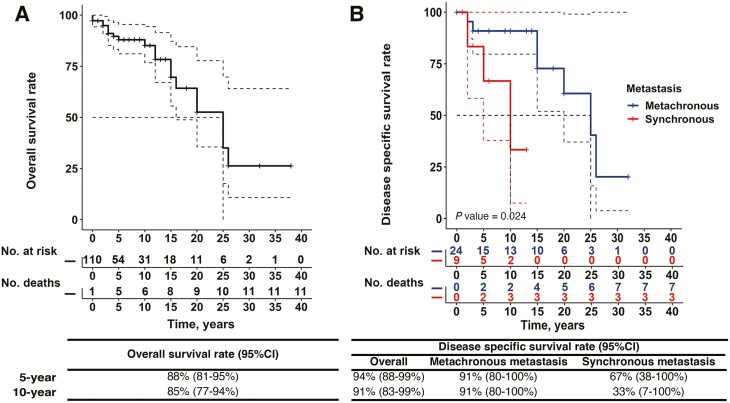
During a median follow-up of 4 years (IQR, 1-11 years), 21 (19%) patients died. Metastatic PPGL was the cause of death in 10 (9%) patients. No deaths occurred in the perioperative period due to catecholamine excess in patients with an unrecognized UBPGL. Univariable Cox analysis demonstrated that disease-specific mortality was higher in patients with synchronous metastatic disease (HR 20.80; 95% CI, 1.30-332.91 per year) when compared with patients without synchronous metastatic disease (Fig. 2C). The 5-year and 10-year overall survival of patients with UBPGL were 88% (95% CI, 81-95%) and 85% (95% CI, 77-94%), respectively (Fig. 3A). The 5- year and 10-year disease-specific survival of patients with UBPGL were 94% (95% CI, 88-99%) and 91% (95% CI, 83-99%) respectively; however, these were much lower in patients presenting with synchronous metastases vs those with metachronous metastases (Fig. 3B).
Figure 3.
Prognosis of patients with urinary bladder paraganglioma. A. The overall survival of patients with UBPGL. B. The disease-specific survival of patients with synchronous metastasis (red) and with metachronous metastasis (blue). P value was estimated by Kaplan-Meier curve and was based on patients with synchronous metastasis and with metachronous metastasis.
Epidemiology and the Impact of Year of Diagnosis on Presentation and Outcomes
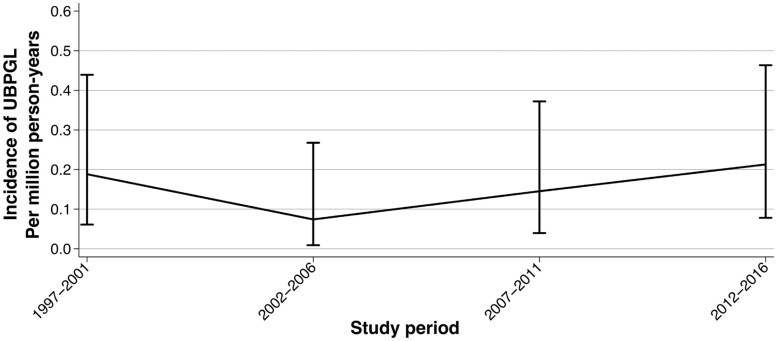
In the population-based study in Denmark, 1102 individuals were registered with a pathological diagnosis of PPGL between 1997 and 2016, of whom 17 patients (1.5%) were diagnosed with UBPGL (Supplementary Figure 1 (12)). This corresponded to an average incidence rate of 0.16 new cases (95% CI, 0.09-0.25) per million person-years. Incidence did not change over time (Fig. 4).
Figure 4.
Incidence rate of urinary bladder paraganglioma in Denmark 1997-2016. Abbreviation: UBPGL, urinary bladder paraganglioma.
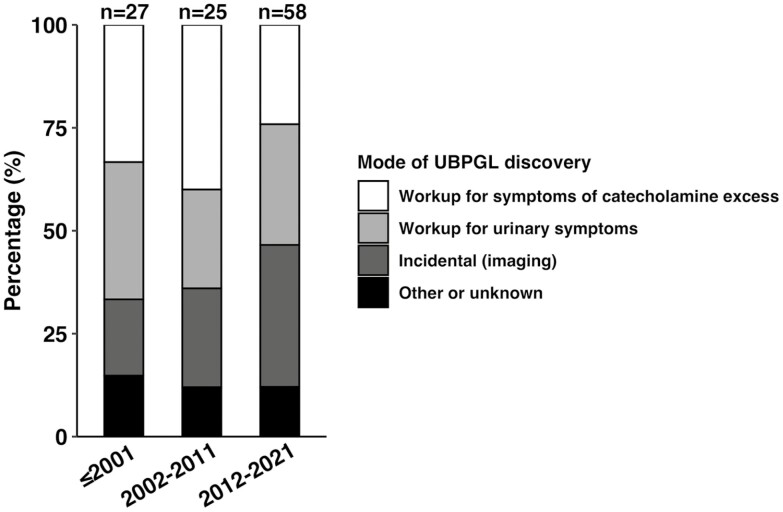
In the multicenter study, 27 (25%) of the 110 patients were diagnosed before 2001, 25 (23%) patients were diagnosed during 2002-2011, and 58 (53%) during 2012-2021. The mode of UBPGL discovery and the proportion of patients undergoing assessment for catecholamine excess were not significantly different among study periods (≤2001, 2002-2011, and 2012-2021) (Fig. 5).
Figure 5.
Mode of discovery of urinary bladder paraganglioma in different study period. There were no significant differences in the mode of UBPGL discovery in 3 study periods. 9 (33%), 10 (40%), and 14 (24%) of UBPGL were discovered by workup for symptoms of catecholamine excess in ≤ 2001, 2002-2011, and 2012-2021, P = 0.323; 9 (33%), 6 (24%), and 17 (29%) of UBPGL were detected during workup for urinary symptoms, P = 0.761; 5 (19%), 6 (24%), and 20 (34%) of UBPGL were found incidentally on imaging, P = 0.276; 4 (15%), 3 (12%) and 7 (12%) of UBPGL were discovered by other mode or with unknown discovery mode, P = 0.933. The rate of catecholamine workup (16 [59%], 19 [76%] and 37 [64%] P = 0.418) were also not significantly different among these 3 study periods. Abbreviations: UBPGL, urinary bladder paraganglioma. P value was calculated based on multiple pairwise analysis.
Discussion
In this large multicenter study, we characterized the presentation, management, and outcomes of patients with UBPGL. We found that only a third of patients were diagnosed with UBPGL prior to surgery/biopsy, and only 63% underwent workup for catecholamine excess, with the majority of patients having catecholamine-secreting tumors. Younger age and larger UBPGL size were associated with the need for repeat bladder surgery. Similarly, younger age and larger tumor size, but also a higher degree of catecholamine excess were associated with development of metachronous metastatic disease. Synchronous metastatic disease was associated with PPGL-related mortality.
We found that the majority of patients were diagnosed with UBPGL only after biopsy or surgery. This is likely explained by the high proportion of patients in whom the urinary bladder mass was discovered incidentally on imaging or during workup for urinary symptoms. Notably, half of the patients diagnosed based on histopathology did report symptoms of catecholamine excess that were either misinterpreted or not elicited during initial assessment. Consistent with our findings, several other studies showed that most patients with UBPGL present with symptoms of catecholamine excess, uniquely induced by micturition in some patients (7, 9). However, as reported in one systematic review, more than half of patients with UBPGL were diagnosed only after surgery (8). The inadequate evaluation for catecholamine excess in patients with UBPGL indicates suboptimal recognition of this disease and suggests the need for collaborative endocrinology-urology effort to improve current diagnostic strategies in these patients. Interventions for such collaborative effort should be further tested, and could potentially include multispecialty tumor board reviews, interdisciplinary educational sessions, or computerized algorithms and reminders for catecholamine excess workup in any patient scheduled for a bladder tumor biopsy or surgery.
Overall, we found that catecholamine excess was documented in 70% of patients with available preoperative workup. The proportion of patients with UBPGL associated with catecholamine excess in our study was higher than previously reported (7, 8). Notably, evaluation for catecholamine excess was performed only in 63% of patients in our study, and in 54% to 73% of patients in other studies (7, 8), suggesting that PGL was not included in the differential diagnosis at the time of presentation. Making an appropriate diagnosis of catecholamine-secreting PPGL prior to biopsy or surgery is crucial to avoid life-threatening perioperative complications (14, 15).
In our study, we found no significant increase in incidence of UBPGL over the years. We found that at the time of diagnosis the median age was 50 years, and there was no sex predominance. The median age of diagnosis in our study was higher than previously reported, possibly due to selection bias in the systematic review that included case reports (7). We also found that the median size of UBPGLs in our study was significantly lower at the time of initial diagnosis (2 cm as opposed to 2.7-3.7 cm), reflecting publication bias toward more severe cases in previous studies (8).
The majority of patients with UBPGL were treated with either partial cystectomy or TURBT. Partial cystectomy was more commonly chosen when the diagnosis of UBPGL was made preoperatively, in younger patients, and in patients with documented catecholamine excess. In contrast, tumor size and presence of synchronous metastases were not associated with the choice of initial procedure. Notably, 9% of patients needed a second bladder surgery, more common in younger patients with larger UBPGL, but not affected by the type of initial surgery (partial cystectomy vs TURBT). The impact of preoperative diagnosis on the choice of surgery was previously reported (8); however, no other studies investigated the impact of type of surgery on the need for repeat surgery. We were not able to fully explore the reasons for the higher risk of repeat bladder surgery in younger patients in our study. Potential explanations include a higher likelihood of a more aggressive disease in younger patients who also presented with a larger UBPGL size.
We found that 30% of patients in our study developed metastatic disease: synchronous in 8% of patients and metachronous in 22%. Younger patients with larger UBPGL and higher degree of catecholamine excess were more likely to develop metachronous metastases during follow-up. We found that only synchronous metastatic disease was associated with PPGL-related mortality. Previous smaller studies reported wide ranges of metastatic disease in patients with UBPGL, from 7% to 48% and an overall good prognosis (4, 7, 9). Direct comparisons are difficult due to scarce data on survival in other studies, heterogeneous durations of follow-up, and lack of differentiation between the survival of patients with synchronous vs metachronous metastases. Our group has previously reported that timing of metastatic development after the initial diagnosis plays a role in overall prognosis of patients with metastatic PPGL (16), consistent with findings in the current study. As it is difficult to accurately predict development of metastases, every patient with UBPGL needs to have lifelong follow-up.
We found that 18% of patients presenting with UBPGL had family history of PPGL, and the majority of patients undergoing germline genetic testing had pathogenic SDHx variants, most commonly in SDHB. Previous studies reported that the presence of SDHB variants was a risk factor of metastasis and poor prognosis (17, 18). In a large study of patients with metastatic PGL in all locations, SDHB was not associated with aggressive disease (16). In our study, we found a higher prevalence of SDHB pathogenic variant in younger patients but did not find an association of SDHB pathogenic variants with development of metastases or mortality. Another smaller study of 27 patients with UBPGL, of whom 52% demonstrated SDHB pathogenic variants, also showed no relationship between SDHB pathogenic variants and metastatic disease (9).
Strengths of our study included a relatively large sample size that allowed us to perform subgroup analyses related to the presence and degree of catecholamine excess and the type of initial surgery, as well as to identify factors associated with development of metachronous metastases and PPGL mortality. We have also had access to the population-based data that allowed us to determine incidence of UBPGL over the period. Limitations of our study included selection, information bias, and limited duration of follow-up. Not all patients had a standardized evaluation. The approach to the workup for catecholamine excess varied and was missing in a large proportion of patients in whom the diagnosis of UBPGL was made postoperatively. Given the timeline of our study, and difficulties with reimbursement for genetic testing in certain countries, not all patients had genetic testing, and the approach to testing differed. The imaging approach also varied depending on the institutional preferences and availability. We used the largest size of tumor rather than the volume of tumor in our analyses, which was likely a less accurate measure of tumor bulk. Considering the small numbers of events, multivariable analysis was not possible. Certain variables included in the univariable analysis (tumor size and the degree of catecholamine excess) were correlated, which should be accounted for in any future and larger studies if multivariable analysis is considered. Finally, our study did not include any patients from Asian countries, though several smaller studies that originated from Asia reported similar results. Considering the rarity of UBPGL, a prospective study is unlikely to be feasible.
In conclusion, we characterized the clinical presentation and outcomes of patients with UBPGL. UBPGL was diagnosed noninvasively in only 34% of patients, and evaluation for catecholamine excess was performed in only 63% prior to surgery, suggesting that PGL was not in the initial differential diagnosis. We showed that younger patients with larger UBPGL and a higher degree of catecholamine excess were at higher risk for repeat bladder surgery and the development of metachronous metastases. Overall disease-specific survival was excellent except in patients presenting with synchronous metastatic disease. Age, UBPGL size, initial surgery type, and presence of SDHB pathogenic variants were not associated with PPGL mortality.
Glossary
Abbreviations
- CT
computed tomography
- HR
hazard ratio
- IQR
interquartile range
- MIBG
metaiodobenzylguanidine
- PGL
paraganglioma
- PPGL
pheochromocytoma/paraganglioma
- TURBT
transurethral resection of bladder tumor
- UBPGL
urinary bladder paraganglioma
Contributor Information
Kai Yu, Adrenal Center, Division of Endocrinology and Metabolism, West China Hospital, Sichuan University, Chengdu, Sichuan, 610041, China; Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN, 55905, USA.
Andreas Ladefoged Ebbehøj, Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, 8200, Denmark; Department of Clinical Medicine, Aarhus University, Aarhus, 8200, Denmark.
Hiba Obeid, Department of Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan, Ann Arbor, MI, 48105, USA.
Anand Vaidya, Center for Adrenal Disorders, Brigham and Women’s Hospital, Harvard Medical School, MA, 02115, USA.
Tobias Else, Department of Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan, Ann Arbor, MI, 48105, USA.
Heather Wachtel, Department of Surgery, Division of Endocrine and Oncologic Surgery, University of Pennsylvania, PA, 19104, USA.
Ailsa Maria Main, Department of Endocrinology and Metabolism, Copenhagen University Hospital, Copenhagen, 2100, Denmark; Faculty of Health and Medical Sciences, Copenhagen University, Copenhagen, 2100, Denmark.
Esben Søndergaard, Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, 8200, Denmark; Department of Clinical Medicine, Aarhus University, Aarhus, 8200, Denmark.
Louise Lehmann Christensen, Department of Endocrinology, Odense University Hospital, Odense, 5000, Denmark.
Christofer Juhlin, Department of Oncology-Pathology, Karolinska Institute, Solna, 17164, Sweden; Department of Pathology and Cancer Diagnostics, Karolinska University Hospital, Solna, 17176, Sweden.
Jan Calissendorff, Department of Molecular Medicine and Surgery, Karolinska Institute, Stockholm, 17176, Sweden; Department of Endocrinology, Karolinska University Hospital, Stockholm, 17176, Sweden.
Debbie L Cohen, Department of Medicine, Renal Division, University of Pennsylvania, PA, 19104, USA.
Bonita Bennett, Department of Medicine, Renal Division, University of Pennsylvania, PA, 19104, USA.
Marianne Skovsager Andersen, Department of Endocrinology, Odense University Hospital, Odense, 5000, Denmark.
Catharina Larsson, Department of Oncology-Pathology, Karolinska Institute, Solna, 17164, Sweden.
Madson Q Almeida, Unidade de Adrenal, Laboratório de Hormônios e Genética Molecular LIM/42, Serviço de Endocrinologia e Metabologia, Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, São Paulo, 05403-900, Brasil; Servico de Endocrinologia, Instituto do Câncer do Estado de São Paulo (ICESP), Faculdade de Medicina da Universidade de São Paulo, São Paulo, 05403-900, Brasil.
Lauren Fishbein, Division of Endocrinology, University of Colorado, Denver, CO, 80045, USA.
Stephen A Boorjian, Department of Urology, Mayo Clinic, Rochester, MN 55905, USA.
William F Young, Jr, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN, 55905, USA.
Irina Bancos, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN, 55905, USA.
Funding
This research was partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the U.S. National Institutes of Health (NIH) under award K23DK121888 (to I.B.) and by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award KL2 TR001879 (to H.W.). The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health.
Disclosures
A.V. declares consulting fees from Mineralys, Corcept, and HRA Pharma, unrelated to the current work. I.B. declares consulting fees from Recordati, Corcept, Spruce, Sparrow, Adrenas, Progenics, and HRA Pharma unrelated to this work. L.F. declares consulting fees from Lantheus/Progenics unrelated to this work. T.E. served on advisory boards for Lantheus, HRA Pharma, Corcept, and Merck and received funding for institutionally contracted studies from Strongbridge, Merck, and Corcept. Other authors declare no competing financial interests.
Data Availability
Some datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Curras-Freixes M, Inglada-Perez L, Mancikova V, et al. Recommendations for somatic and germline genetic testing of single pheochromocytoma and paraganglioma based on findings from a series of 329 patients. J Med Genet. 2015;52(10):647-656. [DOI] [PubMed] [Google Scholar]
- 2. Erickson D, Kudva YC, Ebersold MJ, et al. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab. 2001;86(11):5210-5216. [DOI] [PubMed] [Google Scholar]
- 3. Niu Q, Lu Y, Xu S, et al. Clinicopathological characteristics and survival outcomes of bladder neuroendocrine carcinomas: a population-based study. Cancer Manag Res. 2018;10:4479-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Purnell S, Sidana A, Maruf M, Grant C, Agarwal PK. Genitourinary paraganglioma: demographic, pathologic, and clinical characteristics in the Surveillance, Epidemiology, and End Results database (2000-2012). Urol Oncol. 2017;35(7):457.e9-457.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schulte KM, Talat N, Galata G, et al. Genetics and the clinical approach to paragangliomas. Horm Metab Res. 2014;46(13):964-973. [DOI] [PubMed] [Google Scholar]
- 6. Gruber LM, Hartman RP, Thompson GB, et al. Pheochromocytoma Characteristics and Behavior Differ Depending on Method of Discovery. J Clin Endocrinol Metab. 2019;104(5):1386-1393. [DOI] [PubMed] [Google Scholar]
- 7. Beilan JA, Lawton A, Hajdenberg J, Rosser CJ. Pheochromocytoma of the urinary bladder: a systematic review of the contemporary literature. BMC Urol. 2013;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li M, Xu X, Bechmann N, et al. Differences in clinical presentation and management between pre- and postsurgical diagnoses of urinary bladder paraganglioma: is there clinical relevance? A systematic review. World J Urol. 2022;40(2):385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martucci VL, Lorenzo ZG, Weintraub M. Association of urinary bladder paragangliomas with germline mutations in the SDHB and VHL genes. Urol Oncol. 2015;33(4):167.e13-–167.e20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park S, Kang SY, Kwon GY, et al. Clinicopathologic Characteristics and Mutational Status of Succinate Dehydrogenase Genes in Paraganglioma of the Urinary Bladder: A Multi-Institutional Korean Study. Arch Pathol Lab Med. 2017;141(5):671-677. [DOI] [PubMed] [Google Scholar]
- 11. Mason EF, Sadow PM, Wagner AJ, et al. Identification of succinate dehydrogenase-deficient bladder paragangliomas. Am J Surg Pathol. 2013;37(10):1612-1618. [DOI] [PubMed] [Google Scholar]
- 12. Yu K, Ebbehoj A, Obeid H, et al. Supplementary data: presentation, management, and outcomes of urinary bladder paraganglioma: results from a multi-center study. Uploaded July 8, 2022. Figshare. doi: 10.6084/m9.figshare.19351967.v2. [DOI]
- 13. Lam AK. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr Pathol. 2017;28(3):213-227. [DOI] [PubMed] [Google Scholar]
- 14. McCorkell SJ, Niles NL. Fine-needle aspiration of catecholamine-producing adrenal masses: a possibly fatal mistake. AJR Am J Roentgenol. 1985;145(1):113-114. [DOI] [PubMed] [Google Scholar]
- 15. Quayle FJ, Spitler JA, Pierce RA, Lairmore TC, Moley JF, Brunt LM. Needle biopsy of incidentally discovered adrenal masses is rarely informative and potentially hazardous. Surgery. 2007;142(4):497-502; discussion 502; discussion 502-494. [DOI] [PubMed] [Google Scholar]
- 16. Hamidi O, Young WF Jr., Iniguez-Ariza NM, et al. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J Clin Endocrinol Metab. 2017;102(9):3296-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822-3828. [DOI] [PubMed] [Google Scholar]
- 18. Assadipour Y, Sadowski SM, Alimchandani M, et al. SDHB mutation status and tumor size but not tumor grade are important predictors of clinical outcome in pheochromocytoma and abdominal paraganglioma. Surgery. 2017;161(1):230-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.