Abstract
Seed size is determined by the coordinated growth of the embryo, endosperm, and integument. Growth of the integument is initiated by signal molecules released from the developing endosperm or embryo. Although recent studies have identified many components that regulate seed size by controlling integument growth, the upstream signals and the signal transduction pathway that activate these components after double fertilization are unclear. Here, we report that the receptor-like kinase ERECTA (ER) controls seed size by regulating outer integument cell proliferation in Arabidopsis thaliana. Seeds from er mutants were smaller, while those from ER-overexpressing plants were larger, than those of control plants. Different from its role in regulating the development of other organs, ER regulates seed size via a novel mechanism that is independent of its intracellular domain. Our genetic and biochemical data show that a MITOGEN-ACTIVATED PROTEIN KINASE (MAPK) signaling pathway comprising MAPK-KINASE 4/5, MAPK 3/6 (MPK3/6), DA1, and UBIQUITIN SPECIFIC PROTEASE 15 (UBP15) functions downstream of ER and modulates seed size. MPK3/6 phosphorylation inactivates and destabilizes DA1 to increase the abundance of UBP15, promoting outer integument cell proliferation and increasing seed size. Our study illustrates a nearly completed ER-mediated signaling pathway that regulates seed size and will help uncover the mechanism that coordinates embryo, endosperm, and integument growth after double fertilization.
ERECTA regulate seed size by regulating the proliferation of outer integument cells via an MKK4/5-MPK3/6-DA1-UBP15 signaling pathway in Arabidopsis, and is independent of its intracellular domain.
IN A NUTSHELL.
Background: Seed development begins with double fertilization. Double fertilization releases a yet-to-be identified signal that triggers cell proliferation followed by cell expansion of outer integument cells, which later develop into the seed coat. This rapidly increases the volume of developing seeds, making room for the developing embryo. Many genes that regulate seed size by controlling the development of outer integument cells have been identified. Among these genes, peptidase DA1 cleaves UBIQUITIN SPECIFIC PROTEASE 15 (UBP15), reducing UBP15 protein abundance and inhibiting the proliferation of outer integument cells. However, the upstream signaling pathway that activates DA1-UBP15 remains unclear.
Question: In an attempt to identify more seed size-controlling genes, we established that the receptor-like kinase ERECTA (ER) plays an important role in regulating seed size in Arabidopsis. We were very interested to know how double fertilization activates ER and what the downstream signaling pathway is that mediates the regulation of seed size by ER.
Findings: We found that ER promotes outer integument cell proliferation by regulating the DA1-UBP15 module via the MKK4/5-MPK3/6 pathway in Arabidopsis. ER-activated MPK3/6 interacts directly with and phosphorylates DA1, which destabilizes and inactivates DA1 protein, causing the accumulation of UBP15 protein to promote the proliferation of outer integument cells. Our study reveals a nearly completed ER-MKK4/5-MPK3/6-DA1-UBP15 signaling pathway that promotes the proliferation of outer integument cells in developing seeds. We also showed that in this signaling pathway, ER does not need its cytoplasmic domains to regulate outer integument cell proliferation, indicating ER might function as a co-receptor.
Next steps: Identifying the ligand and the ER interacting receptor (possibly another receptor-like kinase) that bind ER and regulate outer integument cell proliferation is challenging. However, doing so would help elucidate the mechanism by which double fertilization initiates outer integument cell proliferation to coordinate embryo and endosperm development during seed formation.
Introduction
Being sessile, plants have evolved various adaptive traits to allow them to thrive and seed size is one such important trait. Larger seeds provide more nutrients for young seedlings to grow and deal with a harsh environment, while smaller seeds can be dispersed more favorably. Harvested seeds (grains) are the most important staple food for humans. Therefore, uncovering the mechanisms that govern seed development could contribute to future agricultural productivity.
Seed development begins with double fertilization. Subsequently, the embryo and endosperm start to develop. At the same time, it is believed that a signal molecule is released from the developing embryo or endosperm to initiate the division and/or expansion of outer integument cells in Arabidopsis thaliana, which increases the volume of the developing seed to make room for the future enlarged embryo (Roszak and Köhler, 2011; Figueiredo and Köhler, 2014). Therefore, seed size is regulated coordinately by the growth of the embryo, endosperm, and maternal outer integument tissues (Sundaresan, 2005; Nowack et al., 2010; Li et al., 2019). In recent decades, hundreds of genes have been reported to be involved in regulating seed development and seed size (Chaudhury et al., 2001; Berger et al., 2006; Lau et al., 2012; Li et al., 2019). However, the nature of this embryo- or endosperm-originated signal is unknown and the signal transduction pathway by which the signal molecule regulates outer integument cell division and expansion is unclear (Wang et al., 2021a, 2021b).
In general, extracellular signals must first be perceived by various receptors before they can stimulate cellular responses. Therefore, identifying the receptors that function during specific growth and developmental stages, and resolving the mechanisms by which these receptors transduce the perceived signals, will help reveal how cell fate is determined in multicellular organisms. Among the receptors involved in cell–cell communication in plants, the receptor-like kinase (RLK) family is the largest. It has >600 members in Arabidopsis and >1,000 members in rice (Oryza sativa), which corresponds to >2% of each genome (Shiu and Bleecker, 2003; Dardick et al., 2007). These RLKs play essential roles in regulating many aspects of plant growth and development (De Smet et al., 2009; Jamieson et al., 2018; Escocard de Azevedo Manhães et al., 2021).
ERECTA (ER) is a leucine-rich repeat (LRR)-RLK. At the plasma membrane, ER interacts with its co-receptor TOO MANY MOUTHS (TMM) and/or members of the SOMATIC EMBRYOGENESIS RECEPTOR KINASE family of RLKs to regulate stomata development and plant immune responses (Lee et al., 2012; Meng et al., 2015; Jordá et al., 2016). Additionally, ER family RLKs play essential roles in regulating shoot apical meristem development (Chen et al., 2013), cell and organ elongation (Torii et al., 1996; Uchida et al., 2012), outer integument formation (Pillitteri et al., 2007), stamen development (Hord et al., 2008), and leaf primordium initiation (Chen et al., 2013). As a typical RLK, ER functions were found to depend on its intracellular kinase activity to transduce perceived external signals to downstream targets. The mutation of invariable amino acids in the kinase domain inactivated ER, producing a phenotype that was nearly identical to that of the loss-of-function mutant er105 (Lease et al., 2001). Downstream of ER, MITOGEN-ACTIVATED PROTEIN KINASE (MAPK) signaling is involved in transducing the ER signal (Bergmann et al., 2004; Meng et al., 2012) but the targets of ER-activated MAPKs are unclear.
In this study, we established that ER plays an important role in regulating seed size by promoting the proliferation of outer integument cells. Interestingly, we discovered that ER may function as a co-receptor in regulating seed size because deletion of the intracellular domain of ER did not affect its role in outer integument cell proliferation in vivo. Further investigation of the ER signaling mechanism revealed that a MAPK-KINASE 4/5 (MKK4/5)-MAPK 3/6 (MPK3/6)-DA1-UBIQUITIN SPECIFIC PROTEASE 15 (UBP15) signaling pathway functions downstream of ER to regulate seed size. Together, our findings identify a signal transduction pathway that acts maternally to regulate seed size. The discovery that ER may function as both a receptor and co-receptor to regulate different aspects of plant growth and development provides insight into the organ/tissue-specific regulatory mechanisms of RLKs.
Results
ER regulates seed size in Arabidopsis
We noticed that the average seed area and seed weight of the Landsberg (Lan) erecta (Ler) ecotype Arabidopsis plants are consistently smaller and lighter, respectively, than those of the Columbia (Col-0) ecotype. Given that ER plays an essential role in regulating organ size (Shpak et al., 2004; Ferjani et al., 2007; Tisne et al., 2008; van Zanten et al., 2009) and a loss-of-function mutant of an ER homolog in rice produced smaller grains (Guo et al., 2020), we hypothesized that a loss of function of ER is responsible for the smaller seed size observed in Ler ecotype plants. To test this hypothesis, we cloned the ER genomic sequence (gERCol) from Col-0 plants, which included the 2,037-bp promoter, 5′-untranslated region, and 26 introns but lacked the stop codon and 3′-untranslated region. gERCol encodes an identical ER protein to that encoded by Lan ecotype plants, which are considered as the wild-type (WT) for Ler (Rédei, 1962). When expressed in Ler plants, the gERCol increased the seed size in Ler plants to a level similar to that in Lan ecotype plants (Supplemental Figure S1).
We also expressed ERpro:ER-4myc in the ER loss-of-function mutant er105 (Col-0 background) and T3 homozygous transgenic seeds were harvested for comparison. The er105 seeds were significantly smaller and lighter. Depending on the expression level, the ERpro:ER-4myc transgene could either restore the er105 seeds to a WT size or to a size that was larger than that seen in the WT control (Figure 1 and Supplemental Figure S2). However, plants with a higher expression level of ER tend to have a higher frequency of ER co-suppression in their offspring. To ensure the reliability and reproducibility of our work, transgenic plants with a moderately increased expression level of ER were used for the genetic crosses, because the ER co-suppression rate from the offspring of these plants is low. Together, these results suggest that ER plays an important role in regulating seed size in Arabidopsis.
Figure 1.
ER regulates seed size in Arabidopsis. A, Dry seeds from WT (Col-0), er105 mutant, and ERpro:ER-4myc expressing er105 mutation (gER/er105). Scale bar, 0.5 mm. Lines #13, #18, and #29 are three independent transgenic lines. B, Immunoblot showing ER-4Myc levels in the transgenic plants using anti-Myc antibodies. C, Quantitation of the area and weight of the dry seeds shown in (A). Error bars indicate the mean ± sd (left panel, n > 30). Statistical differences are indicated by different lowercase letters (one-way ANOVA, P < 0.05).
ER regulates seed size independently of its intracellular domain
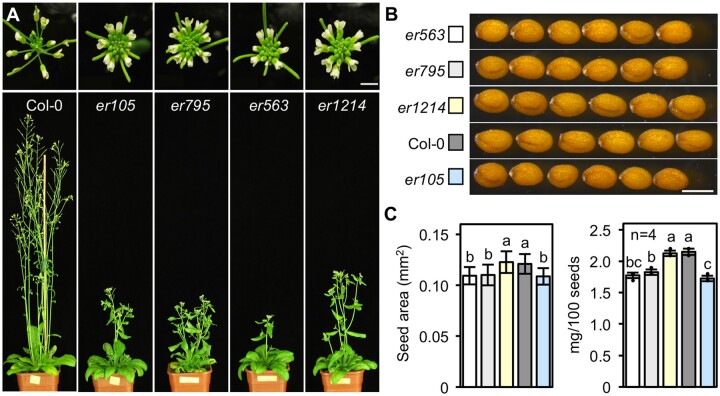
In a screen of EMS-mutagenized Col-0 seeds, three additional er mutants were isolated and named: er563, er795, and er1214. The inflorescence stem height and architecture, silique shape, and pedicel length of these er mutants were similar to those observed in er105 (Figure 2A and Supplemental Figure S3). These mutants were backcrossed with Col-0 plants for two generations to clean up the background and the seed size of the mutants was analyzed. As predicted, the seed size and seed weight of er563 and er795 were significantly smaller and lighter than those of the WT control. Surprisingly, the seeds of er1214 were almost the same size and weight as those of the Col-0 control (Figure 2, B and C), suggesting that er1214 carries a novel mutation, which could separate the regulatory function of ER in seed size determination from its other regulatory roles (e.g. inflorescence and silique shape establishment and pedicel elongation).
Figure 2.
er1214 is a novel er mutant that has normal seed size. A, Six-week-old WT (Col-0) and the newly isolated er mutant plants. The upper panel shows the compact inflorescences of the er mutants. Scale bar, 5 mm. B, Dry seeds from the plants shown in (A). Scale bar, 0.5 mm. C, Quantification of the area and weight of the dry seeds shown in (B) (graphic fills match the colors defined in Panel B). Error bars indicate the mean ± sd (left panel, n > 30). Statistical differences are indicated by different lowercase letters (one-way ANOVA, P < 0.05).
Sequencing results showed that nucleotides +1,006 and +1,007 of ER (starting from the ATG and including an intron) in the er563 mutant were missing, causing a frameshift and generating a premature stop codon that is 12 nucleotides after the mutation site. In er795, a C-to-T transversion at position +849 was found that resulted in a premature stop codon at amino acid 100. In comparison, er1214 carried a G-to-A transversion at position +4,210, which prevented excision of an intron and generated a premature stop codon that is 45 nucleotides after the mutation site. The deduced ER mutant proteins in er563 and er795 contained only 124 and 99 amino acids, respectively, and thus were considered non-functional. In contrast, er1214 encoded a truncated ER protein with 632 amino acids, which contains the intact extracellular domain and the transmembrane domain but lacks the cytoplasmic domains (Supplemental Figure S4).
The above results suggested that the kinase domain is required for ER to regulate inflorescence stem growth and architecture, silique formation, and pedicel elongation, but not for seed size determination in Arabidopsis. To confirm this hypothesis, a fragment of the gERCol encoding a truncated protein containing the complete extracellular and transmembrane domains, but with a short cytoplasmic tail containing 16 amino acids, was cloned, fused with a C-terminal GFP tag (ERpro:ERΔk-GFP), and transformed into er105 mutant plants. Similar to the er1214 mutant, the ERpro:ERΔk-GFP/er105 transgenic plants had short and compact inflorescences, short pedicels, and blunt-ended short siliques (Supplemental Figure S5). Depending on the accumulated ERΔk-GFP protein level, ERpro:ERΔk-GFP transgenic plant seeds were similar or larger in size than those observed in the WT (Figure 3 and Supplemental Figure S6). In contrast, expression of ERpro:ER-GFP in er105 mutant plants restored the er105-related phenotypes to a WT appearance (Figure 3, A and B and Supplemental Figure S5). Taken together, these results confirm that, different from its mechanism in regulating inflorescence morphology, silique shape, and pedicel elongation, ER regulates seed size via a mechanism that is independent of its cytoplasmic domain.
Figure 3.
The intracellular domain is not required for ER to regulate seed size. A, Inflorescence apices and whole plants from 7-week-old WT (Col-0), er105, er1214, and er105 lines expressing ERpro:ER-GFP (gER/er105) or ERpro:ERΔK-GFP (gERΔK/er105). Scale bar, 5 mm in upper panel and 2 cm in lower panel. B, Immunoblot showing ER-GFP and ERΔK-GFP levels in the plants shown in (A). C and D, Fully elongated siliques with pedicels (C) and dry seeds (D) from the plants shown in (A). Scale bar, 0.5 mm. E, Quantification of the area of the dry seeds shown in (D) (graphic fills match the colors defined in Panel D). Error bars indicate the mean ± sd (n > 30). Statistical differences are indicated by different letters (one-way ANOVA, P < 0.05).
ER acts maternally to regulate seed size
Seed size is mainly determined by the coordinated growth of the maternal integument, embryo, and endosperm. To determine whether ER regulates seed size in a maternal or zygotic manner, we performed a reciprocal cross between Col-0 and er105 plants. Seeds harvested from er105 plants pollinated with WT pollen were similar in size to self-pollinated er105 seeds. Similarly, the size of seeds from Col-0 plants pollinated with er105 mutant pollen was comparable to that of seeds from self-pollinated Col-0 plants (Figure 4, A and B). We also examined self-pollinated F2 seeds from our Col-0/er105 and er105/Col-0 crosses and found that the seed size was consistently similar to that in the WT control (Supplemental Figure S7). These results suggest that ER regulates seed size maternally.
Figure 4.
ER acts maternally to regulate seed size. A, Fully matured F1 seeds from Col-0 × Col-0, Col-0 × er105, er105 × Col-0, and er105 × er105 crosses. Scale bar, 0.5 mm. B, Quantitation of the relative area and the weight of the F1 dry seeds harvested from the crosses shown in (A) (graphic fills match the colors defined in Panel A). Error bars indicate the mean ± sd (left panel, n > 30). C, The perimeter and number of cells in the outer integument of seeds from Col-0, er105, er105 plants expressing ERpro:ER-4myc (gER-4myc) or ERpro:ERΔK-4myc (gERΔK-4myc) at 0 and 8 DAP. Error bars indicate the mean ± sd (n > 30). D, Confocal microscopic examination of the tissue-specific localization pattern of ER-YFP in seeds at 2 DAP. Scale bar, 20 μm. Statistically significant differences in (B) and (C) are indicated by different lowercase letters (one-way ANOVA, P < 0.05).
ER regulates outer integument cell proliferation
The maternal integument develops into the seed coat in mature seeds. During development, outer integument cells first undergo division to increase the overall cell number and then undergo cell expansion to increase the cell volume (Le et al., 2010). We found that under our growth conditions, the outer integument cell numbers started to increase dramatically at 1 day after pollination (DAP), and plateaued at 2.5 DAP (Supplemental Figure S8). The seed size continued to increase sharply after 2.5 DAP, possibly due to cell expansion. At 8 DAP, both the seed size and outer integument cell number reached a level that was very close to that in seeds at 14 DAP (Supplemental Figure S8), when the seeds were fully developed and had started to dehydrate (Le et al., 2010).
ER was previously reported to regulate organ shape and organ growth by regulating cell proliferation (Shpak et al., 2003, 2004). Thus, to investigate whether ER regulates seed size maternally by a similar mechanism, we manually pollinated the pistils of er105, ERpro:ER-4myc/er105 and ERpro:ERΔk-4myc/er105 plants using their own pollen and examined the outer integument cell number and seed size at 8 DAP. As shown in Figure 4C, significant decreases in outer integument cell number and seed size were observed in the er105 seeds at 8 DAP. These phenotypes were specifically caused by the er mutation, as the expression of ERpro:ER-4myc and ERpro:ERΔk-4myc could fully restore the er105 seed size and number of outer integument cells to WT levels (Figure 4C).
Using ERpro:ER-YFP/er105 transgenic plants, we examined the tissue and cellular localization of ER-YFP in seeds at 2 DAP and found that ER-YFP was distributed throughout the seeds and was clearly localized at the periphery of the outer integument cells (Figure 4D). Together with the phenotypes of the er105 mutant and the complemented transgenic seeds, our results strongly suggest that ER plays an important role in regulating seed size by controlling outer integument cell proliferation, but in an intracellular domain-independent manner.
ER, ER-LIKE (ERL) 1, and ERL2 function redundantly in seed size regulation
Besides ER, two ER homologs, ERL1 and ERL2, are encoded in the Arabidopsis genome. These three genes have been shown to function redundantly in regulating multiple aspects of plant development (Shpak et al., 2004, 2005; Pillitteri et al., 2007; Hord et al., 2008). To investigate whether ERL1 and ERL2 also function in parallel with ER to regulate seed size in Arabidopsis, matured dry seeds harvested from erl2-1, erl1-2, er105 erl2-1, er105 erl1-2, and erl1-2 erl2-1 mutants were compared and the seed size was quantified. We established that erl1-2 and erl2-1 seeds were smaller than Col-0 seeds, but larger than er105 seeds. In addition, seeds from the er105 erl1-2 and er105 erl2-1 double mutants were significantly smaller than those of the er105 mutant, but the erl1-2 erl2-1 seeds were similar in size as those of the erl1-2 or erl2-1 single mutants (Supplemental Figure S9, A and B). Quantification of the numbers of outer integument cells and seed perimeters in 0 and 8 DAP mutant seeds also showed similar patterns as observed in the dried seed size (Supplemental Figure S9C). These results suggest that ER, ERL1, and ERL2 function redundantly, but ER is more important in regulating outer integument cell proliferation and seed size in Arabidopsis.
The observation that ERL1 and ERL2 also participate in regulating seed size raised a concern: is it possible that the WT -like seed size observed in er1214 is due to gene redundancy? To rule out this possibility, we first examined the expression level of ER, ERL1, and ERL2 in 0–2 DAP siliques, rosette leaves, and inflorescences of er105 and er1214 mutant. Surprisingly, ER expression was significantly decreased in the er1214 mutant, possibly due to a feedback regulation of ER by its signaling pathway. In comparison, ERL1 and ERL2 expression was slightly increased in the young siliques of the er1214 mutant, but the expression of these genes in rosette leaves and inflorescences was similar in Col-0 and er1214 plants (Supplemental Figure S10). These results suggest that feedback upregulation of ER, ERL1, and ERL2 does not underlie the WT-like size of er1214 seeds.
We also generated er1214 erl1-2 and er1214 erl2-1 double mutants by genetic crossing and examined the seed size of these plants. If the seed size phenotype of er1214 is due to gene redundancy, we should observe similar seed size-related phenotypes between er1214 erl1-2 and er105 erl1-2, or between er1214 erl2-1 and er105 erl2-1 plants. Quantification showed that the outer integument cell numbers in 0 and 8 DAP seeds of er1214 erl1-2 or er1214 erl2-1 mutants were significantly increased when compared with those in er105 erl1-2 or er105 erl2-1 mutants, but at similar levels as in the erl1-2 or erl2-1 mutant, respectively. Consistent with this result, the size of matured dry seeds (Supplemental Figure S9B) and seed perimeter in 0 and 8 DAP seeds (Supplemental Figure S9C) of er1214 erl1-2 or er1214 erl2-1 mutants were larger than those of er105 erl1-2 or er105 erl2-1 mutants, but similar to those of the erl1-2 or erl2-1 mutant. Together, these results provide additional evidence that ER regulates seed size in an intracellular domains-independent manner.
ER functions upstream of DA1 to regulate outer integument cell proliferation
A pathway involving DA1 and UBP15 plays a central role in regulating seed size by stimulating outer integument cell proliferation (Li and Li, 2015). In this pathway, the peptidase DA1 is a negative regulator, while UBP15 is a positive regulator (Li et al., 2008; Du et al., 2014). Upon ubiquitination by the E3 ligase DA2 and EOD1, the peptidase activity of DA1 is activated. DA1 then interacts physically with and cleaves UBP15, reducing its protein abundance (Dong et al., 2017). Increased cellular levels of UBP15 caused by overexpressing or reducing DA1 expression could increase both the size of seeds and the growth of other organs, such as petals, by promoting cell proliferation (Xia et al., 2013; Du et al., 2014; Dong et al., 2017).
We thus next asked whether ER regulates the proliferation of outer integument cells via the DA1-UBP15 pathway. To answer this question, we generated da1-1 er105 double mutant, da1-1 er105 erl2-1 triple mutant, ER-overexpressing (ER-OX) ubp15-1 mutant (ERpro:ER-4myc/ubp15, because it has extra copies of the ER gene in the genome, Supplemental Figure S11), UBP15-overexpressing (UBP15-OX) er105 and er105 erl2-1 mutants (UBP15pro:UBP15-GFP/er105 and UBP15pro:UBP15-GFP/er105 erl2-1, respectively), and DA1-overexpressing er105 erl1-2 mutant (DA1pro:DA1-YFP/er105 erl1-2) plants by genetic crossing. The size and outer integument cell numbers in seeds at 8 DAP from these mutants were analyzed. The size and outer integument cell numbers in 0 and 8 DAP seeds were increased in ER-OX, loss-of-function mutant da1-1, or UBP15-OX plants, but decreased in er105, er105 erl2-1, and ubp15-1 mutants, when compared with those observed in Col-0 seeds, respectively (Figure 5, A–E). Furthermore, da1-1 mutation and UBP15-OX restored the reduced size and outer integument cell number phenotypes of the er105 and er105 erl2-1 mutant seeds to levels similar to those in the da1-1 or UBP15-OX control (Figure 5, A–D and Supplemental Figure S12). In addition, the ubp15-1 mutation restored the increased seed size and outer integument cell number phenotypes of the ER-OX seeds to levels seen in the ubp15-1 mutant (Figure 5E). Similar results for flower size were also observed in these plants (Supplemental Figure S13).
Figure 5.
ER family members act upstream of DA1 to regulate outer integument cell proliferation. A–E, Quantification of the number of cells and perimeter of the outer integument in seeds at 0 or 8 DAP. The plants used in the genetic crosses were: er105 (er), er105 erl2-1 (er erl2), da1-1, UBP15pro:UBP15-GFP (gUBP15), ERpro:ER-4myc (gER-4myc), and ubp15-1 (ubp15). Error bars indicate the mean ± sd (n > 14). Statistically significant differences are indicated by different lowercase letters (one-way ANOVA, P < 0.05). F, Immunoblots showing the abundance of DA1-YFP and UBP15-GFP in 2 DAP seeds from DA1pro:DA1-YFP (gDA1-YFP)- or gUBP15-GFP-expressing WT (Col-0), er105 erl1-2 (er erl1), or er105 erl2-1 (er erl2) mutant plants, respectively, using anti-GFP antibody. G, Quantitation of the relative DA1-YFP or UBP15-GFP immunoblot signals in (F) using the anti-tubulin signal as an equal loading control. Error bars indicate the mean ± sd. Statistically significant differences are indicated by *(P < 0.05) or **(P < 0.01), two-tailed Student’s t test.
Immunoblotting using anti-GFP antibody showed that the abundance of DA1-YFP was increased in seeds of the er105 erl1-2 mutant at 2 DAP, while the abundance of UBP15-GFP was decreased in seeds of the er105 and er 105 erl2-1 mutants at 2 DAP in comparison with the WT control (Figure 5, F and G and Supplemental Figure S14). Taken together, these genetic and biochemical data suggest that ER functions upstream of the DA1-UBP15 pathway to regulate seed and flower size.
ER regulates outer integument cell proliferation via the MKK4/5-MPK3/6-DA1-UBP15 pathway
MKK4/5-MPK3/6 signaling functions downstream of ER and regulates many aspects of plant growth and development (Wang et al., 2007; Hord et al., 2008; Lee et al., 2012; Meng et al., 2012). Thus, we questioned whether a similar mechanism is involved in the regulation of seed size by ER. To answer this question, we first examined the seed size and outer integument cell number at 8 DAP in seeds from mpk3, mpk6, and mkk4 mkk5 mutant plants, as well as from plants expressing a constitutively active form of MKK4 (MKK4DD) under the control of the ER promoter (ERpro:FLAG-MKK4DD). The seeds of the mpk3 and mpk6 mutants were similar in size to WT seeds, whereas the outer integument cell number in the seeds of both mutants was significantly reduced compared with that in the Col-0 control. In comparison, the seed size and outer integument cell number were reduced in mkk4 mkk5 mutant plants but increased in ERpro:FLAG-MKK4DD-expressing plants (Supplemental Figure S15).
Next, we generated ERpro:ER-GFP/mpk3, ERpro:FLAG-MKK4DD/er105 erl1-2, da1-1 mpk3, ERpro:FLAG-MKK4DD/ubp15-1, UBP15pro:UBP15-GFP/mpk3, and UBP15pro:UBP15-GFP/mkk4 mkk5 mutants by genetic crosses. Microscopy examination showed that the seed size and outer integument cell numbers in 8 DAP seeds of ERpro:ER-GFP/mpk3 and ERpro:FLAG-MKK4DD/er105 erl1-2 plants were similar to those observed in mpk3 and ERpro:FLAG-MKK4DD plants, respectively, suggesting MKK4 and MPK3 function downstream of ER in regulating seed size (Figure 6, A and B). In comparison, seed size and outer integument cell numbers in 8 DAP seeds of mpk3 da1-1, ERpro:FLAG-MKK4DD/ubp15-1, UBP15pro:UBP15-GFP/mpk3, or UBP15pro:UBP15-GFP/mkk4 mkk5 were similar to those in seeds from da1-1, ubp15-1, or UBP15pro:UBP15-GFP plants, respectively, indicating that the DA1-UBP15 pathway functions downstream of MKK4 and MPK3 in the seed size determination process (Figure 6, C–F).
Figure 6.
The MKK4/5-MPK3/6-DA1-UBP15 signaling pathway functions downstream of ER to regulate outer integument cell proliferation. A–F, Quantification of the number of cells and the perimeter of the outer integument in seeds at 0 and 8 DAP. The mutant or transgenic plants used in the genetic crosses were: er105 erl1-2 (er erl1), ubp15-1 (ubp15), mkk4 mkk5 (mkk4/5), mpk3, da1-1, ERpro:ER-GFP (gER-GFP), ERpro:FLAG-MKK4DD (4DD), and UBP15pro:UBP15-GFP (gUBP15). Error bars indicate the mean ± sd (n > 15). Statistically significant differences are indicated by different lowercase letters (one-way ANOVA, P < 0.05).
Immunoblotting using anti-GFP antibody and seeds at 0–2 DAP revealed that, consistent with its larger seed size phenotype, the ERpro:FLAG-MKK4DD transgenic plants accumulated less DA1-GFP, but more UBP15-GFP (Figure 7, A–D) in 0–2 DAP seeds. As UBP15 is a positive regulator of seed size and is a direct substrate of DA1, these genetic and biochemical data strongly suggest that ER regulates outer integument cell proliferation via the MKK4/5-MPK3/6-DA1-UBP15 pathway during seed development.
Figure 7.
The ER-MKK4 pathway regulates DA1 and UBP15 protein abundance and MPK6 phosphorylation in developing seeds. Immunoblots showing the levels of DA1-YFP (A) and UBP15-GFP (C) in plants expressing DA1pro:DA1-YFP (gDA1-YFP) or UBP15pro:UBP15-GFP (gUBP15-GFP) alone or with ERpro:FLAG-MKK4DD, using anti-GFP antibody and total proteins extracted from seeds at 0–2 DAP. B and D, Quantification of the relative DA1-YFP or UBP15-GFP protein abundance shown in A or C. E, 0–2 DAP siliques harvested from ERpro:FLAG-MKK4DD expressing Col-0 and er105 erl1-2 (er erl1-2) plants were used for immunoblot using anti-FLAG antibody. Anti-tubulin immunoblot was used as an equal loading control. F, Quantification of FLAG-MKK4DD protein intensity in immunoblots showed in (E). G, Immunoblot examination of phosphorylated MPK6 and MPK3 in 0–2 DAP seeds harvested from Col-0, er105, and er1214 plants. Anti-phospho-p44/42 MAPK (ERK1/2) antibody was used to detect phosphorylated MPK6 and MPK3; anti-MPK6, anti-MPK3, and anti-tubulin antibodies were used for equal loading control purpose. H, Quantification of phosphorylated MPK6 in immunoblots shown in (G). Values are mean ± sd. Statistically significant differences are indicated by *P < 0.05, **P < 0.01 (two-tailed Student’s t test), or ns, not significant.
MPK6 phosphorylates and inactivates DA1
To uncover the molecular mechanism by which MKK4/5-MPK3/6 mediates the ER-DA1-UBP15 pathway regulated seed size formation, we first assessed whether protein abundance and/or kinase activity of MKK4/5 or MPK3/6 is regulated by ER. As shown in Figure 7, E and F, the abundance of MKK4DD is at a similar level in 0–2 DAP siliques of Col-0 and er105 erl1-2 mutant plants. While in 0–2 DAP seeds from Col-0, er105, and er1214 plants, immunoblotting using anti-MPK3, anti-MPK6, and anti-pERK1/2 (recognizes phosphorylated and activated MPK3/6 proteins) antibodies showed that MPK6 activation is significantly reduced in the er105 mutant, but at a similar level in Col-0 and er1214 plants (Figure 7, G and H).
Next, we tested if MPK3 and MPK6 interact directly with DA1. As shown in Figure 8A, MPK3 and MPK6 interacted directly with DA1 in yeast two-hybrid (Y2H) assays. The interaction was further confirmed in firefly luciferase complementation assays using Nicotiana benthamiana epidermal cells that co-expressing DA1-nLuc and cLuc-MPK3 or cLuc-MPK6 (Figure 8B), and in vivo by a co-immunoprecipitation assay using the inflorescences of DA1pro:DA1-YFP transgenic Arabidopsis plants (Figure 8C).
Figure 8.
MPK3 and MPK6 interact directly with DA1. A, Y2H assays of the interactions among MPK3, MPK6, and DA1. The positive interactions were evaluated by yeast cells grown on SD media that lacking Leu, Trp, and His (− LTH) in the presence of 10 mM 3-AT. B, Firefly luciferase complementation assays show that DA1 interacts directly with MPK3 and MPK6. The leaves of four-week-old N. benthamiana plants were co-infiltrated with A. tumefaciens (strain GV3101) cells containing the indicated vector pairs. Images were collected 36 h after infiltration. Scale bar, 1 cm. C, Co-immunoprecipitation of DA1 with MPK3 or MPK6 in Arabidopsis. Total protein was extracted from the inflorescences of Col-0 and DA1pro:DA1-YFP (gDA1-YFP) transgenic plants. DA1-YFP protein was immunoprecipitated using GFP-Trap beads and the immunoblots were probed with antibodies against GFP, MPK3, or MPK6.
Capillary isoelectric focusing (cIEF) analysis showed that in the presence of ATP the isoelectric point of recombinant GST-DA1 decreased when it was mixed with recombinant MKK4DD-activated His-MPK6 (Supplemental Figure S16), suggesting that DA1 can be phosphorylated by MPK6. In a mass-spectrometric analysis of in vitro MPK6 phosphorylated DA1, two MPK6 phosphorylation sites (Ser119 [unambiguous] and Ser498 [ambiguous]) were identified on DA1 (Supplemental Figure S17). These two serine residues are adjacent to a proline residue, which fits the description of the minimal SP/TP consensus MPK phosphorylation dipeptide motif (Pitzschke, 2015). Besides, Ser119 and Ser498, analysis of the DA1 protein sequence showed that Thr268 is also next to a proline and could be another MPK phosphorylation site on DA1.
To investigate whether MPK6 phosphorylation modulates DA1 function, we replaced Ser119, Thr268, and Ser498 with non-phosphorylatable Ala residues (DA13A) or with Glu residues (DA13E) to mimic the constitutively phosphorylated form of DA1 by site-directed mutagenesis. An in vitro phosphorylation assay showed that MPK3 and MPK6 were no longer able to phosphorylate DA13A protein, indicating that Ser119, Thr268, and Ser498 are major MPK3/6 phosphorylation sites on DA1 protein (Supplemental Figure S18). We then expressed constructs encoding mutated forms of DA1 tagged with YFP at the C-terminus in da1-1 mutant, after which the abilities of these DA1 proteins to recover the large seed phenotype of da1-1 were compared. The expression of both WT DA1pro:DA1WT-YFP and DA1pro:DA13A-YFP recovered the increased size and outer integument cell number phenotype of da1-1 seeds to a WT level, while the expression of DA1pro:DA13E-YFP only slightly decreased the size and outer integument cell number of da1-1 seeds, albeit DA13E-YFP abundance is higher than DA13A-YFP and at a similar level as DA1WT-YFP (Figure 9, A and B and Supplemental Figure S19). Immunoblotting showed that, in comparison with the reduced abundance of DA1WT-YFP protein in ERpro:FLAG-MKK4DD expressing plants (Figure 7A), the abundance of DA13A-YFP protein was at a similar level between WT background and transgenic plants that express ERpro:FLAG-MKK4DD (Figure 9, C and D), suggesting that phosphorylation of DA1 by MPK3/6 affects the stability of DA1 in developing seeds.
Figure 9.
MPK6 phosphorylation inactivates and destabilizes DA1. A and B, Quantification of seed area or the outer integument cell numbers in dried (A) or 14 DAP (B) seeds from WT plants (Col-0), da1-1 mutant plants, and da1-1 mutant plants expressing DA1pro:DA1WT-YFP (gDA1WT-YFP), gDA13A-YFP, or gDA13E-YFP. # indicates different transgenic lines. Error bars indicate the mean ± sd (n > 30). Statistically significant differences are indicated by different lowercase letters (one-way ANOVA, P < 0.05). C, 0–2 DAP siliques harvested from transgenic plants expressing gDA13A-YFP, alone or with ERpro:FLAG-MKK4DD, were used for immunoblots. D, Quantification of the relative DA13A-YFP protein abundance shown in (C) using anti-tubulin immunoblots as an equal loading control (two-tailed Student’s t test).
Discussion
After double fertilization, the seed development begins. Outer integument cells proliferate and then the cells expand (Haughn and Chaudhury, 2005; Ingouff et al., 2006). As shown in Supplemental Figure S8, seed size increased rapidly from 1 to 6 DAP. In comparison, the embryo and endosperm increased in size relatively slowly at this stage (Orozco-Arroyo et al., 2015). Therefore, the quick increase in seed size before 6 DAP was predominantly controlled by the fast growth of the outer integument layers, which provides the space for future embryo and endosperm development. In recent years, a number of genes that regulate seed size by controlling the proliferation of outer integument cells have been identified (Li and Li, 2016; Li et al., 2019); however, the signal molecule that initiates the division of outer integument cells after double fertilization, and the signal transduction pathway induced by this molecule, is unclear.
This study offers several lines of evidence suggesting that ER is an RLK that regulates seed size maternally by promoting outer integument cell proliferation. First, the seeds from Ler ecotype plants and multiple ER loss-of-function mutants were smaller than that in Col-0 WT plant, while those from transgenic plants overexpressing ER were larger. These results suggest that ER plays a major role in regulating seed size. Second, reciprocal crosses showed that ER regulates seed size in a maternal manner. Third, at 8 DAP, when the cell size and outer integument cell number almost reached their maximum levels (Supplemental Figure S8), the outer integument cell numbers and seed sizes were reduced in er, er erl1, and er erl2 mutant plants, but increased in ER-OX transgenic plants. These results are in agreement with those of previous reports, which showed that ER regulates organ architecture and size via the control of cell proliferation (Shpak et al., 2003, 2004). Fourth, confocal laser microscopy observations of ERpro:ER-YFP transgenic plants showed that ER is expressed in outer integument cells. Together with a recent study, which showed that a loss-of-function mutant of an ER homolog in rice had a reduced grain length but an increased grain width (Guo et al., 2020), these data suggest that ER family RLKs play a general role in regulating seed morphology in both dicots and monocots.
As a typical RLK, ER has an extracellular domain that contains 20 LRR domains, a single-pass transmembrane domain, and a cytoplasmic domain with Ser/Thr kinase activity (Lease et al., 2001). Although kinase activity was believed to be critical for ER to regulate various growth and developmental processes (Lease et al., 2001; Meng et al., 2015), evidence also showed that a kinase-dead form of ER (ERK565E) could partially rescue stem and pedicel elongation in er erl1 erl2 mutant plants, suggesting that ER regulates different developmental processes through both kinase activity-dependent and -independent mechanisms (Kosentka et al., 2017). It is likely that the intracellular domain of ERK565E could activate downstream signaling components via a protein–protein interaction. In this study, we found that the novel ER mutant er1214 exhibited typical er null mutant-like phenotypes (e.g. semi-dwarfism, compact inflorescences, short pedicels, and blunt-end siliques), but the seed size was similar to that in Col-0 control plants. Given that er1214 does not have an intracellular domain, these results suggest that ER regulates seed size via a novel mechanism that is independent of its cytosolic domain.
In Arabidopsis, there is a group of receptor-like proteins (RLPs) at the plasma membrane, which includes TMM, CLAVATA2 (CLV2), and CHITIN ELICITOR BINDING PROTEIN (CEBiP). These RLPs typically contain an extracellular domain, a transmembrane domain, and a short cytoplasmic tail that lacks an obvious signaling domain (Wang et al., 2008). These RLPs may interact with RLKs and transduce extracellular signals to downstream effector proteins. For example, upon ligand perception, TMM could form a heterodimer with ER (Lee et al., 2012); CLV2 could form a heterodimer with CORYNE (CRN), a kinase that contains a transmembrane domain and a very small extracellular domain (Bleckmann et al., 2010); and CEBiP could form multiple oligomers with itself and another RLK, CHITIN ELICITOR RECEPTOR KINASE 1 (Shimizu et al., 2010) to regulate stomata patterning, shoot and root meristem development, and plant immune responses (Kaku et al., 2006; Müller et al., 2008; Shimizu et al., 2010; Somssich et al., 2016). Although ER contains a fully functional kinase domain in its intracellular domain, the cytoplasmic domain-independent function of er1214 suggests that ER serves as a co-receptor in developing outer integument cells, and might form a heterodimer with another RLK- or CRN-like protein kinase to transduce perceived signals and initiate outer integument cell proliferation.
Besides ER, a few RLKs have been reported to regulate seed size. LecRK-VIII.2 positively regulates seed size by promoting both cell expansion and proliferation in the seed coat (Xiao et al., 2021), whereas FERONIA and BRASSINOSTEROID (BR) INSENSITIVE 1 were reported to regulate seed size by inhibiting and promoting cell expansion in outer integument cells, respectively (Jiang et al., 2013; Yu et al., 2014). XIAO is an LRR-RLK encoded in the rice genome. In the xiao mutant, both cell division and seed size are greatly reduced. It was reported that XIAO regulates plant growth by regulating BR signaling, but it remains to be determined whether XIAO regulates seed size by regulating cell division, cell expansion, or both (Jiang et al., 2011). Similarly, rice CRINKLY4 regulates grain size by coordinately regulating epidermal cell size and cell numbers (Chun et al., 2020). It remains to be determined if these RLKs mediated cellular signaling would interact with each other sequentially or synergistically to regulate outer integument cell proliferation and expansion in developing seeds.
Among the genetic networks that regulate seed size maternally, the DA1-UBP15 pathway controls seed size by regulating the proliferation of outer integument cells in Arabidopsis and spikelet hull cells in rice (Du et al., 2014; Li and Li, 2015). In Arabidopsis, the E3 ligase DA2 ubiquitinates and activates the peptidase DA1, which promotes the cleavage of UBP15 by DA1 and inhibits the proliferation of outer integument cells (Dong et al., 2017). There are four DA1 homologs encoded in the rice genome. Interestingly, overexpression of HOMOLOG OF DA1 ON RICE CHROMOSOME 3 (HDR3) has been shown to increase grain size, indicating that HDR3 is a positive regulator of rice grain size (Gao et al., 2021). Meanwhile, it was reported that GRAIN WIDTH ON CHROMOSOME 2 (a DA2 homolog in rice) is a negative regulator and OsUBP15 is a positive regulator of grain size (Shi et al., 2019; Hao et al., 2021), and that OsDA1 (another DA1 homolog in rice) can interact directly with ubiquitin and OsUBP15 (Shi et al., 2019). It remains to be determined whether the DA1-UBP15 pathway functions in a similar way in Arabidopsis and rice plants. Nevertheless, despite the important role of the DA1-UBP15 pathway in regulating seed size, the upstream signal that activates this pathway remains unclear.
Downstream of ER, the MKK4/5-MPK3/6 signaling pathway was shown to mediate many ER-regulated growth and developmental processes, including stomata formation, embryo development, inflorescence architecture, silique length, morphology determination, and pedicel elongation (Wang et al., 2007, 2021a, 2021b; Meng et al., 2012; Zhang et al., 2017). The MAPK pathway was also shown to play an important role in regulating grain size by regulating the proliferation of lemma cells in rice (Xu et al., 2018a, 2018b). In Arabidopsis, it was shown that mkk4/5 double mutant seeds had the same width as but were shorter than WT seeds, while seeds from mpk6 plants were the same length and width as WT seeds (Zhang et al., 2017). However, another study showed that mpk6 mutant seeds were larger than WT seeds (Xiao et al., 2021). In agreement with the findings of Zhang et al. (2017), at 8 DAP, seeds from mpk3 or mpk6 mutant plants were similar in size to WT seeds, but the cell numbers in the outer integument of both mutant seed types were significantly reduced compared with WT seeds. In comparison, both the seed size and outer integument cell number were reduced in mkk4/5 double mutant seeds but increased in seeds expressing ERpro:FLAG-MKK4DD. These results provide additional evidence supporting the idea that the MKK4/5-MPK3/6 pathway plays a major role in regulating the proliferation of outer integument cells. The reason why the mpk3 and mpk6 seeds were similar in size to WT seeds at 8 DAP could be that MPK3/6 also play a negative role in regulating the expansion of outer integument cells.
Our genetic evidence shows that the MKK4/5-MPK3/6 pathway functions upstream of DA1-UBP15 and downstream of ER in regulating integument cell proliferation. Additionally, we found that MPK3/6 could interact directly with and phosphorylate DA1. Substitution of the MPK3/6 phosphorylation sites on DA1 by the amino acid Ala stabilizes DA13A protein in ERpro:FLAG-MKK4DD plants, while the ability to rescue the large seed phenotype of the da1-1 mutant is greatly reduced if the MPK3/6 phosphorylation sites on DA1 were substituted by Glu (DA13E). Taken together, these results suggest that the phosphorylation of DA1 by MPK3/6 might destabilize and inactivate DA1 protein, cause the accumulation of UBP15, and promote the proliferation of outer integument cells. Therefore, our results link previously unrelated signaling modules and reveal a nearly completed signaling pathway that transduces signals from ER to MKK4/5, MPK3/6, DA1, and UBP15, and which promotes the proliferation of outer integument cells in developing seeds after double fertilization. However, several questions remain unanswered. For example, ER may use either cytoplasmic domain-dependent or -independent mechanisms to regulate various developmental processes, but both mechanisms appear to depend on MKK4/5-MPK3/6 to transduce the upstream signal. It would be interesting to establish how different RLK complexes activate the same signaling module and regulate different cellular responses. Future identification of the ligand and receptor that can bind with ER and regulate integument cell proliferation will help reveal the mechanism that coordinates integument cell growth with embryo and endosperm development during seed formation.
Materials and methods
Plant materials and growth conditions
The Arabidopsis mutant and transgenic plants used in this study, including er105, erl1-2, erl2-1, mpk3, mpk6, mkk4 mkk5, ubp15-1 (SALK_018601), da1-1, UBP15pro:UBP15-GFP, and ERpro:FLAG-MKK4DD, are in the Col-0 background and have been reported previously (Torii et al., 1996; Shpak et al., 2004; Li et al., 2008, 2014; Meng et al., 2012; Du et al., 2014; Zhang et al., 2017). Mutants expressing ERpro:ER-4myc, ERpro:ER-GFP, UBP15pro:UBP15-GFP, DA1pro:DA1-YFP, DA1pro:DA13A-YFP, and ERpro:FLAG-MKK4DD were generated by genetic crossing. F3 segregated homozygous progenies were used for genetic and immunoblot analysis. The plants were grown at 22°C in a growth room equipped with T5 4000 K fluorescent tubes (Philips) under long-day conditions (16 h of light at 100 μmol/m2s, followed by 8 h of darkness). Double or triple mutants generated by genetic crossing and F3 segregated homozygous plants were used for phenotypical or biochemical analysis.
Constructs and plant transformation
The ER genomic sequence (gER), which includes the 2,037-bp promoter, 5′-untranslated region, and all introns but lacks the stop codon and 3’-untranslated region; the intracellular domain deletion form of the gER (gERΔK); and the DA1 genomic sequence (gDA1), which includes the 2,099-bp promoter, 5′-untranslated region, and all introns but lacks the stop codon and 3′-untranslated region, were amplified from Col-0 plants using the primers shown in Supplemental Table S1. The PCR products were purified, cloned into pCR8/GW/TOPO (Invitrogen, Waltham, MA, USA), and then subcloned into Gateway compatible binary vectors to generate C-terminal GFP, YFP, or 4myc fusion tags using LR Clonase (Invitrogen). The destination vectors were introduced into Agrobacterium tumefaciens strain GV3101 and transformed into Col-0 or er105 mutant plants by the floral dip method (Clough and Bent, 1998). All the transgenic lines used in this study were homozygous T3 lines.
Morphological analysis and microscopy
Dry seeds were observed under a Leica M205 FA microscope and photographed with a Leica DFC310 FX digital imaging system (Leica Microsystems, Wetzlar, Germany). The produced digital images were used for area measurement with ImageJ software. To determine the outer integument cell number and perimeter of developing seeds at different DAP, developing seeds were dissected out and mounted in clear solution (chloral hydrate:water:glycerol, 8:3:1 [w:v:v]). The seeds were then cleared for 30 min and observed in differential interference contrast mode under a ZEISS imager A1 microscope equipped with a ZEISS AxioCam MRc5 CCD camera (Carl Zeiss AG, Oberkochen, Germany). Outer integument cell numbers were directly counted under the microscope. Digital images were taken and used for seed perimeter measurement with ImageJ software. Alternatively, unfertilized ovules were dissected out, mounted in water, and observed directly under a confocal laser-scanning microscope (FV3000; Olympus, Shinjuku, Tokyo, Japan).
Protein–protein interaction assays
The Y2H Gold-Gal4 system (Clontech, Mountain View, CA, USA) was used for our Y2H assays. The full-length coding sequences of DA1, MPK3, and MPK6 were inserted into pGBKT7 and pGADT7 to prepare the bait and prey constructs, respectively, which were transformed into yeast strain Y2H Gold and screened for interactions on drop-out medium with 10 mM 3′AT.
For the firefly luciferase complementation assays, the full-length coding sequences of DA1, MPK3, and MPK6 were cloned into pCAMBIA-cLuc or pCAMBIA-nLuc binary vectors to generate N-terminus (nLuc) or C-terminus (cLuc) fused and truncated luciferase tags on the target proteins. Firefly luciferase complementation assays were performed according to Zhang et al. (2016).
For the co-immunoprecipitation assay, the leaves of DA1pro:DA1-YFP and Col-0 plants were harvested. Total protein was extracted using NEBT buffer (25 mM HEPES, pH 7.5, 50 mM KCl, 10 mM MgCl2, 1 mM EDTA, 1 mM DTT, 1% Triton X-100, and protease inhibitor cocktail). After centrifugation at 4°C at 20,000 × g for 10 min, the supernatant was incubated with GFP-Trap Magnetic Agarose Beads (Chromotek, Planegg-Martinsried, Germany, Lot 81210001A) at 4°C for 30 min with constant rotation. The beads were then washed three times with wash buffer (25 mM HEPES, pH 7.5, 50 mM KCl, and 0.1% Triton X-100) and eluted with 2 × SDS loading buffer at 95°C for 10 min. The immunopurified proteins were subjected to immunoblot analysis using anti-GFP (Roche, Basel, Switzerland, Cat. No. 11814460001, 0.2 μg/mL), anti-AtMPK3 (Sigma, Ronkonkoma, NY, USA, Product No. M8318, 0.5 μg/mL), or anti-AtMPK6 antibodies (Sigma, Product No. A7104, 0.5 μg/mL).
Protein purification and in vitro phosphorylation assays
The coding sequences of MPK6, MPK3, MKK4DD (T224D/S230D), and DA1 were cloned into pET28b, pMAL-c2x, or pGEX4T-1 expression vectors and introduced into the Rosetta-gami 2 Escherichia coli host strain to produce His-, MBP-, and GST-tagged recombinant proteins, respectively. To generate MKK4-activated MPK3 or MPK6, the vectors that produce MBP-tagged MKK4DD and 6His-tagged MPK3 or MPK6 proteins were co-transformed into the E. coli host strain; only 6His-tagged MPK3 or MPK6 proteins were purified for in vitro phosphorylation assays. The E. coli-expressed recombinant proteins were induced and purified by standard protocols with glutathione Sepharose 4B Beads (GE Healthcare, Chicago, IL, USA), amylose agarose beads (New England Biolabs, Ipswich, MA, USA), and Ni-NTA resin (Qiagen, Hilden, Germany), respectively.
An in vitro kinase assay was performed in 25 μL of kinase reaction buffer (20 mM Tris–HCl, pH 7.5, 50 mM KCl, 10 mM MgCl2, and 200 μM ATP) with 5 μg GST-DA1 and 500 ng MKK4-activated His-MPK6. The mixture was incubated at 30°C for 3 h. The reaction was stopped by adding 6× SDS sample buffer and heating at 70°C for 15 min. Proteins were separated by SDS–PAGE and in-gel digested for identification by mass spectrometry of the phosphorylation sites.
cIEF-immunoassay
cIEF-immunoassays were carried out on a NanoPro100 instrument (ProteinSimple, San Jose, CA, USA). After the in vitro phosphorylation of GST-DA1 by His-MPK6 at 30°C for 3 h, an aliquot of the reaction solution (set to 0.1 mg/mL of the final protein concentration) was mixed with Premix G2 pH 3–10 Separation and pI Standard Ladder by thorough vortexing. The mixed sample, 1:50 diluted anti-GST antibody (Abmart, #M20007), 1:100 diluted horseradish peroxidase-conjugated secondary antibody (ProteinSimple, Lot #86143), and 1:1 mixed Luminol-Peroxide solutions were loaded into a NanoPro plate as instructed by the manufacturer. Isoelectric focusing was carried out at 21,000 μW for 40 min. Focused proteins were immunoblotted within the capillaries after UV illumination for 100 s at factory settings. The incubation time for the primary and secondary antibodies was 4 and 1 h, respectively. Prior to each antibody incubation, the capillaries were washed twice for 5 min. Chemiluminescence detection was carried out for 30–960 s. Data were analyzed with Compass software (ProteinSimple).
Immunoblots
Fresh harvested tissue was ground to fine powder in the presence of liquid nitrogen. SDS sample buffer (0.125 M Tris–HCl, pH 6.8, 4% SDS, 20% glycerol, 2% β-mercaptoethanol, bromophenol blue) was added at a ratio of 20 µL/10 mg tissue powder with 1× protease inhibitor cocktail (G-Biosciences, St. Louis, MO, USA). After heating at 95°C for 5 min, the mixture was centrifuged at 4°C, 12,000 g for 10 min, and the supernatant was saved for SDS–PAGE and immunoblotting using anti-FLAG (Abmart, Shanghai, China, #M20008), anti-myc (MBL International, Woburn, MA, USA, M192-3), anti-GFP (Roche, Cat. No. 11814460001), anti-AtMPK3/anti-AtMPK6 (Sigma, M8318/A7104), and Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (Cell Signaling Technology, Danvers, MA, USA, #9101) antibodies. Immunoblots using anti-HSP90 (Agrisera, Vännäs, SWEDEN, Lot 1010) or anti-Tubulin (Sigma, T5168) antibodies were used as equal loading control.
Quantitative real-time PCR
Fresh plant tissue was harvested and ground to fine powder in the presence of liquid nitrogen. Total RNA was extracted using RNA-easy Isolation Reagent (Vazyme, Nanjing, China) following the protocol provided by the manufacturer. cDNA was reverse transcribed using HiScript III RT SuperMix (Vazyme). Quantitative real-time PCR was performed using ChamQ Universal SYBR qPCR Master Mix (Vazyme) and a CFX96 Thermal Cycler (Bio-Rad, Hercules, CA, USA). Relative expression level of target genes was determined by the comparative threshold cycle method and was normalized to that of PP2A. The sequences of the gene-specific primers used are listed in Supplemental Table S1.
Experimental replicates and statistical analysis
All data presented in this article have been confirmed independently by at least three biological replicates. The term “biological replicates” means that plants were grown at different times. Tissues or developing seeds harvested from different plants grown at the same time were pooled for immunoblot or microscopy observation, and were considered as one biological replicate. Because no treatment was used, a two-tailed Student’s t test and one-way ANOVA test were performed to evaluate the differences between different data sets using Microsoft Excel software. Statistically significant differences are indicated by different lowercase letter (P < 0.05, ANOVA test), one asterisk (P < 0.05, Student’s t test) or two asterisks (P < 0.01, Student’s t test). Summaries of statistical analyses are provided in Supplemental Data Set S1.
Accession numbers
Sequence data from this article can be found in TAIR under the following accession numbers: ERECTA (AT2G26330), ERL1 (AT5G62230), ERL2 (AT5G07180), DA1 (AT1G19270), UBP15 (AT1G17110), MPK3 (AT3G45640), MPK6 (AT2G43790), MKK4 (AT1G51660), and MKK5 (AT3G21220).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression of ER rescued the small seed phenotype of Ler.
Supplemental Figure S2. ER overexpression increases seed size in Arabidopsis.
Supplemental Figure S3. Silique phenotype of isolated er mutants.
Supplemental Figure S4. Schematic diagram showing the protein structure of ER and er proteins.
Supplemental Figure S5. The intracellular domain is required for ER to regulate silique morphology.
Supplemental Figure S6. An increase in the abundance of ERΔk-GFP protein in Col-0 plants increases seed size.
Supplemental Figure S7. Seed size of Col-0, er105 homozygous and heterozygous plants.
Supplemental Figure S8. The size and outer integument cell numbers of developing seeds in the WT.
Supplemental Figure S9. ER, ERL1, and ERL2 function redundantly in regulating outer integument cell proliferation.
Supplemental Figure S10. Relative expression of ER, ERL1, and ERL2 in er105 and er1214 mutants.
Supplemental Figure S11. The expression level of ER transcript and ER-4myc protein in ERpro:ER-4myc expressing plants.
Supplemental Figure S12. DA1 functions downstream of ER in regulating seed size.
Supplemental Figure S13. Flower size is regulated by the ER-DA1-UBP15 pathway.
Supplemental Figure S14. UBP15 protein accumulates less in the er105 mutant.
Supplemental Figure S15. The MKK4/5-MPK3/6 pathway regulates outer integument cell proliferation.
Supplemental Figure S16. MPK6 phosphorylates DA1 in vitro.
Supplemental Figure S17. The in vitro MPK6 phosphorylated DA1 peptides identified by mass spectrometry.
Supplemental Figure S18. Ser119, Thr268, and Ser498 are major MPK3/6 phosphorylation sites on DA1.
Supplemental Figure S19. MPK3/6 phosphorylation sites modulate the ability of DA1 to regulate seed size.
Supplemental Table S1. Primers used in this study.
Supplemental Data Set S1. Statistical table.
Supplementary Material
Acknowledgments
We thank Dr. Zuhua He (Institute of Plant Physiology and Ecology, CAS) for providing the er105 mutant and ERECTA overexpressing transgenic seeds; Dr. Juan Xu (Zhejiang University) for providing the mkk4 mkk5 double mutant and ERpro:FLAG-MKK4DD/Col-0 transgenic seeds; Dr. Xiaoping Gou (Lanzhou University) for providing the erl1-2 and erl2-1 mutants; and Dr. Ying Sun (Hebei Normal University, China) for providing MPK3 and MPK6 pENTRTM/D-TOPO clones and mpk3, mpk6 seeds.
Funding
This study was supported by grants from the National Natural Science Foundation of China (32170323, 2014CB943404), the Department of Education of Hebei Province, China (LJRC025), and the Postgraduate Innovation Fund of Hebei Province (CXZZBS2018099).
Conflict of interest statement. None declared.
Contributor Information
Xuedan Wu, Key Laboratory of Molecular and Cellular Biology of Ministry of Education, Hebei Collaboration Innovation Center for Cell Signaling, Hebei Key Laboratory of Molecular and Cellular Biology, College of Life Sciences, Hebei Normal University, Shijiazhuang 050024, China.
Xingbo Cai, Key Laboratory of Molecular and Cellular Biology of Ministry of Education, Hebei Collaboration Innovation Center for Cell Signaling, Hebei Key Laboratory of Molecular and Cellular Biology, College of Life Sciences, Hebei Normal University, Shijiazhuang 050024, China.
Baowen Zhang, Key Laboratory of Molecular and Cellular Biology of Ministry of Education, Hebei Collaboration Innovation Center for Cell Signaling, Hebei Key Laboratory of Molecular and Cellular Biology, College of Life Sciences, Hebei Normal University, Shijiazhuang 050024, China.
Shuting Wu, Key Laboratory of Molecular and Cellular Biology of Ministry of Education, Hebei Collaboration Innovation Center for Cell Signaling, Hebei Key Laboratory of Molecular and Cellular Biology, College of Life Sciences, Hebei Normal University, Shijiazhuang 050024, China.
Ruiju Wang, Key Laboratory of Molecular and Cellular Biology of Ministry of Education, Hebei Collaboration Innovation Center for Cell Signaling, Hebei Key Laboratory of Molecular and Cellular Biology, College of Life Sciences, Hebei Normal University, Shijiazhuang 050024, China.
Na Li, State Key Laboratory of Plant Cell and Chromosome Engineering, CAS Centre for Excellence in Molecular Plant, Institute of Genetics and Development Biology, Chinese Academy of Sciences, Beijing 100101, China.
Yunhai Li, State Key Laboratory of Plant Cell and Chromosome Engineering, CAS Centre for Excellence in Molecular Plant, Institute of Genetics and Development Biology, Chinese Academy of Sciences, Beijing 100101, China.
Yu Sun, Key Laboratory of Molecular and Cellular Biology of Ministry of Education, Hebei Collaboration Innovation Center for Cell Signaling, Hebei Key Laboratory of Molecular and Cellular Biology, College of Life Sciences, Hebei Normal University, Shijiazhuang 050024, China.
Wenqiang Tang, Key Laboratory of Molecular and Cellular Biology of Ministry of Education, Hebei Collaboration Innovation Center for Cell Signaling, Hebei Key Laboratory of Molecular and Cellular Biology, College of Life Sciences, Hebei Normal University, Shijiazhuang 050024, China.
W.T. and Y.S. designed the research. X.W., X.C., and S.W. performed most of the research. B.Z. did the mass spectrometry analysis. R.W. screened the er mutant. N.L. and Y.L. helped prepare the genetic materials and expression vectors. X.W., X.C., Y.S., and W.T. wrote the article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Wenqiang Tang (tangwq@mail.hebtu.edu.cn).
References
- Berger F, Grini PE, Schnittger A (2006) Endosperm: an integrator of seed growth and development. Curr Opin Plant Biol 9: 664–670 [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR (2004) Stomatal development and pattern controlled by a MAPKKKinase. Science 304: 1494–1497 [DOI] [PubMed] [Google Scholar]
- Bleckmann A, Weidtkamp-Peters S, Seidel CAM, Simon R (2010) Stem cell signaling in Arabidopsis requires CRN to localize CLV2 to the plasma membrane. Plant Physiol 152: 166–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury AM, Koltunow A, Payne T, Luo M, Tucker MR, Dennis ES, Peacock WJ (2001) Control of early seed development. Annu Rev Cell Dev Biol 17: 677–699 [DOI] [PubMed] [Google Scholar]
- Chen MK, Wilson RL, Palme K, Ditengou FA, Shpak ED (2013) ERECTA family genes regulate auxin transport in the shoot apical meristem and forming leaf primordial. Plant Physiol 162: 1978–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun Y, Fang J, Zafar SA, Shang J, Zhao J, Yuan S, Li X (2020) MINI SEED2 (MIS2) encodes a receptor-like kinase that controls grain size and shape in rice. Rice 13: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dardick C, Chen J, Richter T, Ouyang S, Ronald P (2007) The rice kinase database. A phylogenomic database for the rice kinome. Plant Physiol 143: 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Voss U, Jürgens G, Beeckman T (2009) Receptor-like kinases shape the plant. Nat Cell Biol 11: 1166–1173 [DOI] [PubMed] [Google Scholar]
- Dong H, Dumenil J, Lu FH, Na L, Vanhaeren H, Naumann C, Klecker M, Prior R, Smith C, McKenzie N, et al. (2017) Ubiquitylation activates a peptidase that promotes cleavage and destabilization of its activating E3 ligases and diverse growth regulatory proteins to limit cell proliferation in Arabidopsis. Genes Dev 31: 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Li N, Chen L, Xu Y, Li Y, Zhang Y, Li C, Li Y (2014) The ubiquitin receptor DA1 regulates seed and organ size by modulating the stability of the ubiquitin specific protease UBP15/SOD2 in Arabidopsis. Plant Cell 26: 665–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escocard de Azevedo Manhães AM, Ortiz-Morea FA, He P, Shan L (2021) Plant plasma membrane-resident receptors: surveillance for infections and coordination for growth and development. J Integr Plant Biol 63: 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferjani A, Horiguchi G, Yano S, Tsukaya H (2007) Analysis of leaf development in fugu mutants of Arabidopsis reveals three compensation modes that modulate cell expansion in determinate organs. Plant Physiol 144: 988–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo DD, Köhler C (2014) Signaling events regulating seed coat development. Biochem Soc Trans 42: 358–363 [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhang N, Wang WQ, Shen SY, Bai C, Song XJ (2021) The ubiquitin-interacting motif-type ubiquitin receptor HDR3 interacts with and stabilizes the histone acetyltransferase GW6a to control the grain size in rice. Plant Cell 33: 3331–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Lu ZQ, Shan JX, Ye WW, Dong NQ, Lin HX (2020) ERECTA1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to control spikelet number by regulating cytokinin metabolism in rice. Plant Cell 32: 2763–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Wang D, Wu Y, Huang K, Duan P, Li N, Xu R, Zeng D, Dong G, Zhang B, et al. (2021) The GW2-WG1-OsbZIP47 pathway controls grain size and weight in rice. Mol Plant 14: 1266–1280 [DOI] [PubMed] [Google Scholar]
- Haughn G, Chaudhury A (2005) Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci 10: 472–477 [DOI] [PubMed] [Google Scholar]
- Hord CL, Sun YJ, Pillitteri LJ, Torii KU, Wang H, Zhang S, Ma H (2008) Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases. Mol Plant 1: 645–658 [DOI] [PubMed] [Google Scholar]
- Ingouff M, Jullien PE, Berger F (2006) The female gametophyte and the endosperm control cell proliferation and differentiation of the seed coat in Arabidopsis. Plant Cell 18: 3491–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson PA, Shan L, He P (2018) Plant cell surface molecular cypher: receptor-like proteins and their roles in immunity and development. Plant Sci 274: 242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Bao L, Jeong SY, Kim SK, Xu C, Li X, Zhang Q (2011) XIAO is involved in the control of organ size by contributing to the regulation of signaling and homeostasis of brassinosteroids and cell cycling in rice. Plant J 70: 398–408 [DOI] [PubMed] [Google Scholar]
- Jiang W, Huang H, Hu Y, Zhu S, Wang Z, Lin W (2013) Brassinosteroid regulates seed size and shape in Arabidopsis. Plant Physiol 162: 1965–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordá L, Sopeña-Torres S, Escudero V, Nuñez-corcuera B, Delgado-Cerezo M, Torii KU, Molina A (2016) ERECTA and BAK1 receptor like kinases interact to regulate immune responses in Arabidopsis. Front Plant Sci 7: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, Minami E, Shibuya N (2006) Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci USA 103: 11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosentka PZ, Zhang L, Simon YA, Satpathy B, Maradiaga R, Mitoubsi O, Shpak ED (2017) Identification of critical functional residues of receptor-like kinase ERECTA. J Exp Bot 68: 1507–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S, Slane D, Herud O, Kong J, Jürgens G (2012) Early embryogenesis in flowering plants: setting up the basic body pattern. Annu Rev Plant Biol 63: 483–506 [DOI] [PubMed] [Google Scholar]
- Le BH, Cheng C, Bui AQ, Wagmaister JA, Henry KF, Pelletier J, Kwong L, Belmonte M, Kirkbride R, Horvath S, et al. (2010) Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc Natl Acad Sci USA 107: 8063–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lease KA, Lau NY, Schuster RA, Torii KU, Walker JC (2001) Receptor Serine/threonine protein kinases in signaling: analysis of the erecta receptor-like kinase of Arabidopsis thaliana. New Phytol 151: 133–143 [DOI] [PubMed] [Google Scholar]
- Lee JS, Kuroha T, Hnilova M, Khatayevich D, Kanaoka MM, McAbee JM, Sarikaya M, Tamerler C, Torii KU (2012) Direct interaction of ligand receptor pairs specifying stomatal patterning. Genes Dev 26: 126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Wang G, Zhao JL, Zhang LQ, Ai LF, Han YF, Sun DY, Zhang SW, Sun Y (2014) The receptor-like kinase SIT1 mediates salt sensitivity by activating MAPK3/6 and regulating ethylene homeostasis in rice. Plant Cell 26: 2538–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Li Y (2015) Maternal control of seed size in plants. J Exp Bot 66: 1087–1097 [DOI] [PubMed] [Google Scholar]
- Li N, Li Y (2016) Signaling pathways of seed size control in plants. Curr Opin Plant Biol 33: 23–32 [DOI] [PubMed] [Google Scholar]
- Li N, Xu R, Li Y (2019) Molecular networks of seed size control in plants. Annu Rev Plant Biol 70: 435–463 [DOI] [PubMed] [Google Scholar]
- Li Y, Zheng L, Corke F, Smith C, Bevan MW (2008) Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev 22: 1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Chen X, Mang H, Liu C, Yu X, Gao X, Torri KU, Shan L (2015) Differential function of Arabidopsis SERK family receptor-like kinases in stomatal patterning. Curr Biol 25: 2361–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Wang H, He Y, Liu Y, Walker JC, Torii KU, Zhang S (2012) A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates Arabidopsis inflorescence architecture by promoting localized cell proliferation. Plant Cell 24: 4948–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Bleckmann A, Simon R (2008) The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 20: 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowack MK, Ungru A, Bjerkan KN, Grini PE, Schnittger A (2010) Reproductive cross-talk: seed development in flowering plants. Biochem Soc Trans 38: 604–612 [DOI] [PubMed] [Google Scholar]
- Orozco-Arroyo G, Paolo D, Ezquer I, Colombo L (2015) Networks controlling seed size in Arabidopsis. Plant Reprod 28: 17–32 [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Bemis SM, Shpak ED, Torii KU (2007) Haploinsufficiency after successive loss of signaling reveals a role for ERECTA-family genes in Arabidopsis ovule development. Development 134: 3099–3109 [DOI] [PubMed] [Google Scholar]
- Pitzschke A (2015) Modes of MAPK substrate recognition and control. Trends Plant Sci 20: 49–55 [DOI] [PubMed] [Google Scholar]
- Rédei GP (1962) Supervital mutants of Arabidopsis. Genetics 47: 443–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak P, Köhler C (2011) Polycomb group proteins are required to couple seed coat initiation to fertilization. Proc Natl Acad Sci USA 108: 20826–20831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Nakano T, Takamizawa D, Desaki Y, Ishii-Minami N, Nishizawa Y, Minami E, Okada K, Yamane H, Kaku H, et al. (2010) Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J 64: 204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB (2003) Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol 132: 530–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Ren Y, Liu L, Wang F, Zhang H, Tian P, Pan T, Wang Y, Jing R, Liu T, et al. (2019) Ubiquitin specific protease 15 has an important role in regulating grain width and size in rice. Plant Physiol 180: 381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, Berthiaume CT, Hill EJ, Torii KU (2004) Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131: 1491–1501 [DOI] [PubMed] [Google Scholar]
- Shpak ED, Lakeman MB, Torii KU (2003) Dominant negative receptor uncovers redundancy in the Arabidopsis ERECTA leucine-rich repeat receptor like kinase signaling pathway that regulates organ shape. Plant Cell 15: 1095–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU (2005) Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309: 290–293 [DOI] [PubMed] [Google Scholar]
- Somssich M, Bleckmann A, Simon R (2016) Shared and distinct functions of the pseudokinase CORYNE (CRN) in shoot and root stem cell maintenance of Arabidopsis. J Exp Bot 16: 4901–4915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V. (2005) Control of seed size in plants. Proc Natl Acad Sci USA 102: 17887–17888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisne S, Reymond M, Vile D, Fabre J, Dauzat M, Koornneef M, Granier C (2008) Combined genetic and modeling approaches reveal that epidermal cell area and number in leaves are controlled by leaf and plant developmental processes in Arabidopsis. Plant Physiol 148: 1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y (1996) The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8: 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Shimada M, Tasaka M (2012) Modulation of the balance between stem cell proliferation and consumption by ERECTA family genes. Plant Signal Behav 7: 1506–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zanten M, Snoek LB, Proveniers MC, Peeters AJ (2009) The many functions of ERECTA. Trends Plant Sci 14: 214–218 [DOI] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S (2007) Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ellendorff U, Kemp B, Mansfield JW, Forsyth A, Mitchell K, Bastas K, Liu CM, Woods-Tör E, Zipfel C, et al. (2008) A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol 147: 503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Chen H, Ortega-perez M, Miao Y, Ma Y, Henschen A, Lohmann JU, Laubinger S, Bayer M (2021a) Independent Parental contributions initiate zygote polarization in Arabidopsis thaliana. Curr Biol 31: 1–7 [DOI] [PubMed] [Google Scholar]
- Wang W, Xiong H, Sun K, Zhang B, Sun MX (2021b) New insights into cell-cell communications during seed development in flowering plants. J Integr Plant Biol 64: 215–229 [DOI] [PubMed] [Google Scholar]
- Xia T, Li N, Dumenil J, Li J, Kamenski A, Bevan MW, Gao F, Li Y (2013) The ubiquitin receptor DA1 interacts with the E3 ubiquitin ligase DA2 to regulate seed and organ size in Arabidopsis. Plant Cell 25: 3347–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Hu S, Zou X, Cai R, Liao R, Lin X, Yao R, Guo X (2021) Lectin receptor-like kinase LecRK-VIII.2 is a missing link in MAPK signaling mediated yield control. Plant Physiol 187: 303–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Yu H, Wang J, Duan P, Zhang B, Li J, Li Y, Xu J, Lyu J, Li N, et al. (2018a) A mitogen-activated protein kinase phosphatase influences grain size and weight in rice. Plant J 95: 937–946 [DOI] [PubMed] [Google Scholar]
- Xu R, Duan P, Yu H, Zhou Z, Zhang B, Wang R, Li J, Zhang G, Zhuang S, Lyu J, et al. (2018b) Control of grain size and weight by the OsMKKK10–OsMKK4–OsMAPK6 signaling pathway in rice. Mol Plant 11: 860–873 [DOI] [PubMed] [Google Scholar]
- Yu F, Li J, Huang Y, Liu L, Li D, Chen L, Luan S (2014) FERONIA receptor kinase controls seed size in Arabidopsis thaliana. Mol Plant 7: 920–922 [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang X, Zhao Z, Wang R, Huang X, Zhu Y, Yuan L, Wang Y, Xu X, Burlingame AL, et al. (2016) OsBRI1 activates BR signaling by preventing binding between the TPR and kinase domains of OsBSK3 via phosphorylation. Plant Physiol 170: 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wu H, Su J, Wang H, Zhu Q, Liu Y, Xu J, Lukowitz W, Zhang S (2017) Maternal control of embryogenesis by MPK6 and its upstream MKK4/MKK5 in Arabidopsis. Plant J 92: 1005–1019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.