Abstract
Context
Autosomal recessive hypophosphatemic rickets (ARHR) are rare, heritable renal phosphate-wasting disorders that arise from overexpression of the bone-derived phosphaturic hormone fibroblast growth factor 23 (FGF23) leading to impaired bone mineralization (rickets and osteomalacia). Inactivating mutations of Dentin matrix protein 1 (DMP1) give rise to ARHR type 1 (ARHR1). Short stature, prominent bowing of the legs, fractures/pseudofractures, and severe enthesopathy are prominent in this patient population. Traditionally, treatment consists of oral phosphate replacement and the addition of calcitriol but this approach is limited by modest efficacy and potential renal and gastrointestinal side effects.
Objective
The advent of burosumab (Crysvita), a fully humanized monoclonal antibody to FGF23 for the treatment of X-linked hypophosphatemia and tumor-induced osteomalacia, offers a unique opportunity to evaluate its safety and efficacy in patients with ARHR1.
Results
Monthly administration of burosumab to 2 brothers afflicted with the disorder resulted in normalization of serum phosphate, healing of pseudofracture, diminished fatigue, less bone pain, and reduced incapacity arising from the extensive enthesopathy and soft tissue fibrosis/calcification that characterizes this disorder. No adverse effects were reported following burosumab administration.
Conclusion
The present report highlights the beneficial biochemical and clinical outcomes associated with the use of burosumab in patients with ARHR1.
Keywords: FGF23, hypophosphatemic rickets, osteomalacia, pseudofractures, burosumab
Autosomal recessive hypophosphatemic rickets (ARHR) are inherited disorders of renal phosphate wasting, the commonality being increased serum levels of the bone-derived phosphaturic hormone fibroblast growth factor (FGF23) (1). ARHR type 1 (ADHR1) is a rare condition caused by inactivating mutations in the gene encoding dentin matrix acidic phosphoprotein 1 (DMP1), a noncollagenous phosphoprotein (2). Loss of DMP1 leads to increased production and secretion of FGF23 by skeletal cells (osteoblasts and osteocytes) (2, 3). In the kidney, its major target organ, FGF23 interacts with FGF receptor 1 (FGFR1) and the coreceptor α-klotho to inhibit tubular phosphate reabsorption and 1,25-dihydroxyvitamin D [1,25(OH)2D] synthesis (reviewed in [4]).
DMP1 is a member of the small integrin binding ligand N-linked glycoprotein (SIBLING) family, a group of noncollagenous extracellular matrix proteins involved in bone mineralization (5). Loss of DMP1 gives rise to increased serum levels of FGF23 by mechanisms yet to be determined. As is the case with other FGF23-related forms of hypophosphatemic rickets, the pathophysiology of ARHR1 is complex, involving a number of factors such as hypophosphatemia, low serum calcitriol concentrations, and direct effects of FGF23 that contribute to the various manifestations and the associated long-term morbidities of the disease (reviewed in [6]).
ARHR1 manifests during childhood with bone pain, skeletal deformities, and other typical clinical features of rickets/osteomalacia. During adulthood, clinical findings may include bone pain, fatigue, muscle weakness, repeated bone fractures and pseudofractures, and extensive enthesopathy (calcification of tendons, ligaments, and joint capsules) limiting mobility and functionality (2). Serum concentrations of 1,25(OH)2D are inappropriately normal or low for the prevailing hypophosphatemia, circulating levels of 25-hydroxyvitamin D (25[OH]D), calcium, and parathyroid hormone (PTH) are normal, and alkaline phosphatase (ALP) activity is increased, while tubular reabsorption of phosphate is diminished.
Historically, conventional management of renal phosphate-wasting disorders has been based on clinical experience gained from treating patients with X-linked hypophosphatemia (XLH), the most common genetic form of renal phosphate-wasting disorders. In this condition, mutations in the phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX), a protein encoded by the PHEX gene, result in increased circulating levels of FGF23, renal phosphate wasting, and impaired skeletal mineralization. Over the years, treatment has been limited to oral phosphate and active vitamin D analogs dosed several times daily in an attempt to improve skeletal mineralization by increasing serum phosphate (PO4). However, growth remains suboptimal in many patients (7), while nephrocalcinosis and secondary hyperparathyroidism have become recognized complications of therapy. Moreover, treatment with phosphate and 1,25(OH)2D is associated with further increases in circulating FGF23, thereby diminishing the therapeutic efficacy while increasing the potential for complications (8).
Burosumab (Crysvita) is a fully humanized immunoglobulin G1 monoclonal antibody to FGF23 indicated for the treatment of XLH in adults (9, 10) and pediatric patients aged 6 months and older (11-13), and more recently for patients with acquired tumor-induced osteomalacia, a rare condition characterized by the development of mesenchymal tumors that manifest paraneoplastic secretion of FGF23 (14). Burosumab binds the amino-terminal domain of FGF23 that interacts with the FGF-binding portion of the combination FGFR1/klotho receptor, preventing FGF23 from binding to and signaling from its receptor. Burosumab restores tubular reabsorption of phosphate from the kidney and increases the production of 1,25(OH)2D, which in turn enhances intestinal absorption of phosphate, thereby improving serum PO4 levels and bone mineralization. Off-label use of burosumab for the treatment of fibrous dysplasia in a child has also been reported (15).
Since high circulating FGF23 levels is the common unifying manifestation of all of these disorders, we postulated that treatment with burosumab should be a safe and effective therapeutic approach for patients with ARHR1.
Materials and Methods
Two brothers with ARHR born to consanguineous parents have been described (16). Homozygous mutations (Met1Val) in the gene encoding DMP1 were identified as the cause of the autosomal recessive form of hypophosphatemia, type 1, in this family. The Met1Val mutant, due to loss of the signal peptide, does not enter the trans-Golgi network and secretory pathway but rather localizes to the cytoplasm (17).
The Research and Ethics Review Office–CIUSSS West-Central Montreal approved the protocol and written informed consent was obtained from the patients to have their medical and radiographic data, and images used for publication. Clinical data were collected from medical records and the 2 affected adult siblings were assessed clinically, biochemically, and radiographically over several years.
Patient 1
Patient 1 is a 50-year-old man diagnosed with hypophosphatemia early in childhood and treated periodically with oral phosphate and active vitamin D analogs. Several osteotomies were performed in childhood and early adolescence in an attempt to correct the lower-limb deformities, mainly unsuccessful. Presently, he suffers from debilitating cervical and back pain resulting in a limited range of rotational and bending motion.
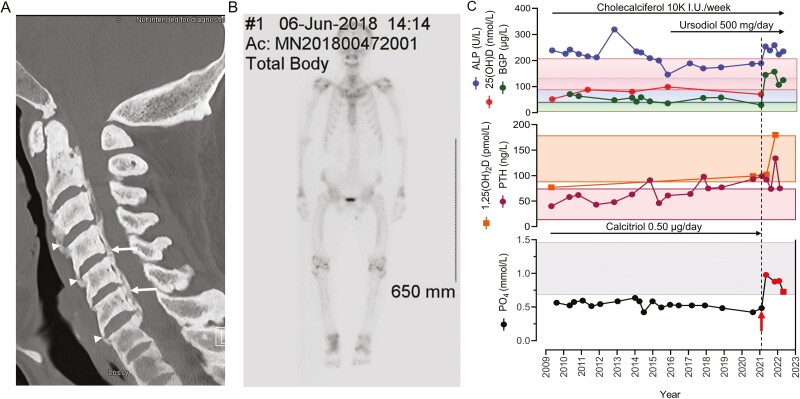
Comprehensive imaging studies disclosed extensive spinal ankylosis with calcification of the cervical, thoracic, and lumbar spinal ligaments and an extensive number of syndesmophytes leading to near-complete spine immobilization (Fig. 1A). As well, there was ossification of the posterior longitudinal ligament (see Fig.1A) resulting in canal stenosis throughout the cervical spine, becoming moderate to severe at C3 to C4 (16).
Figure 1.
A, Computed tomography scan imaging of the cervical spine at presentation, showing fusion of the vertebral bodies by syndesmophytes (arrowheads) and ossification of the posterior longitudinal ligament (arrows) leading to central canal stenosis. B, Whole-body Tc-99m-MDP bone scan demonstrating bone tracer uptake at the ankles, knees, hips, wrists, and shoulders, consistent with arthropathy. C, Longitudinal view of serum levels for alkaline phosphatase (ALP) activity, bone Gla-protein (BGP), 25-hydroxyvitamin D (25[OH]D) and 1,25-dihydroxyvitamin D (1,25[OH]2D), parathyroid hormone (PTH), and phosphate (PO4) before and following initiation of burosumab treatment (red arrow)/dashed line. Red circles indicate blood samples drawn 2 weeks following burosumab administration; red square indicates blood sample drawn just prior to the next burosumab dose. Normal ranges are indicated by the correspondingly colored rectangles. The treatment periods with ursodiol, cholecalciferol, and calcitriol are illustrated by black arrows.
A whole-body technetium-99m-methylene diphosphonate (Tc-99m-MDP) skeletal scintigraphy ordered by another service to exclude Paget disease demonstrated extensive arthropathy involving the ankles, knees, hips, wrists, and shoulders (Fig. 1B), but no pagetic bone lesions were identified.
At presentation, circulating levels of intact FGF23 were elevated (110 pg/mL, normal < 71 pg/mL) (16). Following the confirmatory results of the molecular diagnosis for ARHR1, conventional treatment with 1,25(OH)2D (0.5 μg daily) was instituted, and cholecalciferol (10 000 IU every week) was added, as serum levels were shown to be insufficient (52 nmol/L or 20.8 ng/mL). Daily calcium dietary intake was calculated between 810 and 840 mg per day and no supplementation was added. Serum 25(OH)D levels remained in the sufficient range (> 75 nmol/L), 1,25(OH)2D levels rose slightly and continued within the low-normal range, while serum PO4 levels did not change notably and remained low (Fig. 1C). No noteworthy changes in the levels of serum calcium were noted (not shown), as serum PTH levels remained mainly within normal range (see Fig. 1C). Bone Gla-protein (osteocalcin; herein BGP), a serum marker of bone formation (18), was modestly increased and remained so throughout the follow-up period.
On initial assessment, in the absence of ethanol abuse, serum liver enzyme levels (transaminases and γ-glutamyl transpeptidase [GGT]) and increased ALP activity were noted, and a hepatology consult was requested. Marked steatosis and epithelioid noncaseating coalescing granulomas that were negative for fungal stains were described on a liver needle biopsy. There were no ductal lesions, iron overload, fibrosis, or cholestasis. A magnetic resonance cholangiopancreatography study confirmed the absence of extrahepatic or intrahepatic biliary dilation or strictures that would suggest primary sclerosing cholangitis. Further extensive investigations failed to identify the cause of the liver granulomas and the patient was started empirically on ursodiol (500 mg/day). With time, serum liver enzyme levels normalized, although high serum ALP activity persisted.
In May 2021, insurance coverage for burosumab was obtained and treatment (80 mg subcutaneously every 4 weeks, 1 mg/kg; weight 80 kg; body mass index 31.2) was initiated 1 week after 1,25(OH)2D administration was discontinued. Following burosumab administration, serum PO4 levels normalized at the 2-week point post administration and remained within the normal range after 4 weeks, just before the subsequent injection, while those of BGP increased compared to pretreatment values (144.7 vs 29.5 µg/L) and have remained elevated 12 months into the treatment (see Fig. 1C). Similarly, noteworthy increases in 1,25(OH)2D levels (99 vs 180 pmol/L) were noted, while PTH levels following a minor initial drop, rose consequent to the rising serum PO4 (19) and decreasing FGF23 activity (20), and eventually normalized as levels of 1,25(OH)2D begun to rise. Serum ALP activity remained variable, initially rising and then falling, but not normalizing. A renal ultrasonographic study obtained 6 months into therapy disclosed no nephrolithiasis or nephrocalcinosis.
Burosumab has been very well tolerated and no adverse effects have been observed 1 year into treatment. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire section designed for the assessment of spine pain and stiffness and patient-reported health-related quality of life outcomes was used to obtain baseline evaluation and yearly follow-up assessment. On completion of the first year of treatment, total WOMAC scores decreased from 64 out of 96 to 53 out of 96 (for spine pain from 14/20 to 10/20, spine stiffness from 6/8 to 5/8, and difficulty performing daily physical activities from 44/68 to 38/68).
Patient 2
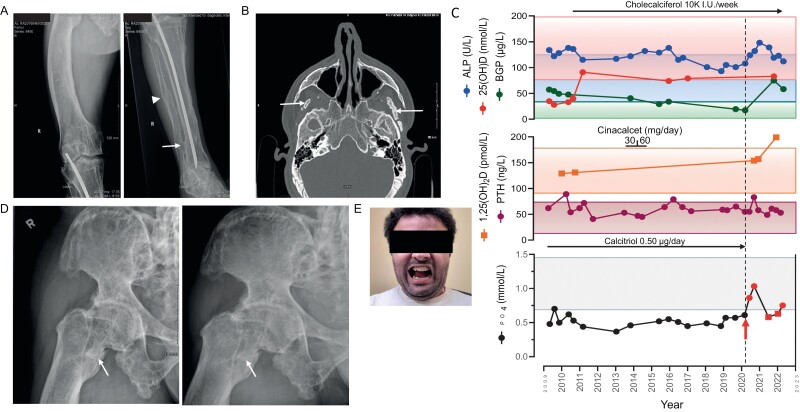
The 42-year-old-brother was similarly diagnosed early in childhood with renal phosphate wasting and hypophosphatemia and was treated periodically with oral phosphate and active vitamin D analogs. Several surgical attempts in childhood and early adolescence undertaken to correct the lower-limb deformities were mainly unsuccessful, leaving him with severe bowing of the right femur (Fig. 2A). At presentation, serum intact FGF23 levels were elevated (87 pg/mL) (16). His main complaint was his inability to open his mouth wide enough to eat (less than 1-fingerbreadth) because of severe enthesopathy affecting the bilateral mandibular coronoid processes and calcifications/ossifications within the pterygoid musculature and inferior aspect of the bilateral temporalis muscles (Fig. 2B) (16).
Figure 2.
A, Radiograph demonstrating the marked bowing of the right femur (left panel). Intramedullary rod in the tibia. Sequela of a previous fracture that has healed (arrowhead), with distal tibia-fibula ankyloses at the level of the distal interosseous membrane (arrow) (right panel). B, Computed tomography scan imaging of the facial bones shows the ossification within the inferior aspect of the temporalis muscles bilaterally (arrows). C, Longitudinal view of serum levels for alkaline phosphatase (ALP) activity, bone Gla-protein (BGP), 25-hydroxyvitamin D (25[OH]D) and 1,25-dihydroxyvitamin D (1,25[OH]2D), parathyroid hormone (PTH), and phosphate (PO4) before and following initiation of burosumab treatment (red arrow)/dashed line. Red circles indicate blood samples drawn 2 weeks following burosumab administration; red squares indicate blood samples drawn just prior to the next burosumab dose. Normal ranges are indicated by the correspondingly colored rectangles. The treatment periods with cinacalcet, cholecalciferol, and calcitriol are shown. D, Imaging of the right femur demonstrates a horizontal line with sclerotic edges at the level of the lesser trochanter, indicative of a pseudofracture (arrow, left panel). The same femur, 5 months into treatment with burosumab, with only a very subtle fracture line still visualized, consistent with ongoing healing (arrow, right panel). E, Following the second surgery, illustrating the maximal mouth opening achieved (2 fingerbreadths as compared to less than 1 fingerbreadth before surgery).
Between July 2013 and October 2014, in addition to 1,25(OH)2D, cholecalciferol, and a dietary calcium intake of nearly 900 mg day that were instituted at diagnosis, the calcimimetic cinacalcet was added as adjuvant treatment (Fig. 2C). Initially given at a concentration of 30 mg/day for 7 months, the dose was raised subsequently to 60 mg/day for the following 8 months in an attempt to increase serum PO4 by decreasing serum PTH (21). Although PTH levels did decrease and serum PO4 levels trended higher, the changes were judged to be of minor clinical significance. Higher doses of cinacalcet were not tolerated and treatment with cinacalcet was terminated.
Raised serum liver enzyme levels in the absence of ethanol abuse were also noted in this patient but they were rather modest in comparison to his brother. Ultrasonographic assessment of the liver disclosed fatty infiltration, and in his case, no further investigation was instituted.
In September 2019, he experienced progressive, debilitating right hip pain, particularly when walking. A computed tomography scan of the right hip and pelvis revealed marked bone demineralization, and a horizontal line with sclerotic edges at the level of the lesser trochanter, consistent with a pseudofracture (Fig. 2D, left panel). Owing to unforeseen delays and the beginning of the SARS-CoV-2 pandemic, evaluation by the orthopedic service was delayed.
In the meantime, in May 2020, treatment with burosumab was initiated at a dose of 1 mg/kg (total 70 mg subcutaneously every 4 weeks; weight 69.5 kg; body mass index 29.5). Following the institution of treatment, serum PO4 levels normalized at the 2-week point post administration but decreased to below normal range at 4 weeks, just before the subsequent injection. BGP levels rose to more than 4 times the pretreatment value (75.4 vs 17.4 µg/L), 1,25(OH)2D increased (199 vs 154 pmol/L), PTH levels remained notably stable after an initial rise, and ALP activity increased initially with a subsequent decline (see Fig.2C).
In October 2020, more than 1 year after the diagnosis of the pseudofracture, patient 2 was assessed by the orthopedic service. At the time, 5 months into the treatment with burosumab, the hip pain had diminished significantly and a repeat radiographic examination revealed near-complete healing of the pseudofracture (see Fig. 2D, right panel). A renal ultrasound obtained following 18 months of treatment with burosumab disclosed no nephrolithiasis or nephrocalcinosis.
In November 2020, the patient underwent corrective surgery to release the fibrotic muscles impairing mastication and insertion of bilateral mandibular condyle prosthesis. A previous attempt, while initially successful in achieving a modest amelioration, failed completely within 2 years following the procedure, as his condition rapidly reverted in severity. Now 19 months following the second surgery, the opening of his mouth is maintained at 2 fingerbreadths, with no indication of deterioration (Fig. 2E).
Burosumab administration over the past 24 months has been well tolerated and no adverse effects of therapy have been observed. He describes diminished spine pain going up and down stairs and experiences less fatigue when performing light domestic duties (total WOMAC scores decreased from 66/96 to 45/96; for spine pain from 12/20 to10/20, for spine stiffness from 6/8 to 5/8, and difficulty in performing daily physical activities from 48/68 to 30/68). He is also pleased with the persistent improvement in his capacity to open his mouth sufficiently to eat, an outcome that failed early on following the initial surgical procedure. However, he is still bothered substantially by his right leg deformity when he ambulates for long periods, and corrective surgery is contemplated.
Results
DMP1 is a major extracellular matrix protein that plays a key role in osteogenesis. While highly expressed in osteocytes (22), it remains unknown how its loss affects so profoundly osteocytic and osteoblastic FGF23 expression and secretion. The inability of osteocytes to mature when Dmp1 is removed (2), and the inappropriately reduced levels of serum PO4 and 1,25(OH)2D that ensue in the context of high circulating FGF23 concentrations, negatively influence the process of skeletal mineralization and ultimately affect the skeletal and extraskeletal manifestations of the disease.
Chronic hypophosphatemia contributes to the burden of disease by potentiating diffuse muscle pain and early fatigue. Lower rates of muscle adenosine 5′-triphosphate (ATP) synthesis due to hypophosphatemia have been reported (23) that likely contribute to the impediment in mobility and functionality reported by our patients. Accordingly, correction of hypophosphatemia improves muscle ATP synthesis and is likely to affect positively on this symptomatology. Indeed, both patients after receiving burosumab achieved fasting serum PO4 concentrations well within the normal range at the midpoint between monthly dosing (2 weeks). This may explain in part the modest amelioration reported by our patients as muscle pain lessened, early fatigue diminished, and physical functionality improved.
PO4 levels in patient 2 showed fluctuations over time as they fell slightly below the lower limit of the normal range when measured at the end point of the dosing interval, just before administration of the ensuing dose. Similar fluctuations were reported in XLH patients (9), although the mean serum PO4 concentration was within the normal range at the end of the monthly dosing intervals. Neither of our patients had a serum PO4 level above the upper limit of the normal range at any time. No clinically significant changes occurred in serum calcium concentration or plasma PTH levels.
As in XLH, serum ALP activity increased initially in both our patients following burosumab administration (9, 11), but subsequently declined. In the absence of liver disease, bone-specific ALP activity comprises only approximately 50% of circulating ALP activity in adults (24). Therefore, bone-specific ALP has been recommended for the monitoring of patients with XLH (25) and likely patients with other forms of renal phosphate-wasting disorders. However, while bone-specific ALP is available, rarely is it used in the general clinical setting, as is the case with our patients. For patient 1, ALP activity remained well above normal levels, likely reflecting the hepatic abnormalities reported and his relatively poor compliance with ursodiol treatment.
Adults with ARHR1 may have a considerably more severe skeletal phenotype than other inherited forms of FGF23-related hypophosphatemic rickets (16, 26). Characterized by poor bone quality, osteomalacia predisposes patients to spontaneous fractures, pseudofractures, delayed fracture healing, and bone pain. Osteomalacia-related fractures are atraumatic lucencies extending across both bone cortices, whereas pseudofractures are described as atraumatic lucencies extending across one cortex. Predominantly in the femurs, tibia/fibula, and metatarsals, assessment of active fracture/pseudofracture sites in XLH patients treated with burosumab demonstrated a higher rate of complete healing in the burosumab group compared to placebo at week 24 (9). This time frame corresponds closely to the period that our patient 2 had been receiving burosumab treatment for the near-complete healing of the lesser tuberosity pseudofracture to take place.
Recent histomorphometric studies of paired transiliac bone biopsies obtained from adult XLH patients treated with burosumab demonstrated significant improvement in osteoid volume/bone volume ratio, osteoid thickness, osteoid surface/bone surface ratio, and mineralization lag time at week 48 compared to baseline (27). Although bone biopsies have not been performed on our patients, similar improvements in bone mineralization and remodeling are expected and may explain the spontaneous fracture healing and amelioration of skeletal pain. Lending support to this contention is the rapid and pronounced rise in serum osteocalcin levels that followed burosumab administration, reflecting improvement in the underlying osteomalacia and restoration of bone remodeling following the normalization of serum PO4 and increase in 1,25(OH)2D.
The enthesopathy and the extraskeletal tissue fibrosis/calcification observed in our 2 patients are extensive. Calcification of the paraspinal ligaments leads to near-complete immobilization of the spine, at times requiring laminectomy (28). Ossification of the posterior longitudinal ligament results in spinal cord compression associated with myelopathy or severe stenosis requiring surgical decompression (29). In its primary or idiopathic form, ossification of the posterior longitudinal ligament is a multifactorial disease influenced by environmental and genetic factors (ectonucleotide pyrophosphatase/phosphodiesterase 1 [ENPP1], bone morphogenetic protein 2 [BMP2], transforming growth factor B1 [TGFB1] [reviewed in (30)], and circulating periostin levels [31]). In its secondary or syndromic form, it is associated with monogenic diseases like hypophosphatemic rickets/osteomalacia (XLH and ARHR). Just as in patient 1, it most frequently involves the cervical spine, followed by the thoracic spine causing narrowing of the spinal canal and neurological impairment (32). One of the important future tasks is to evaluate whether chronic treatment with burosumab will halt the development or progression of the debilitating complications of extraskeletal soft-tissue calcification and enthesopathy that profoundly affect the quality of life of these patients.
While the underlying mechanisms for these tissue alterations remain unknown, it was suggested that neither osteoarthritis nor enthesopathy-related pain would be expected to improve with burosumab treatment (9). Nevertheless, their progression may be halted, as off-target proinflammatory and profibrotic effects of FGF23 have been described (33-36). It is conceivable that similar off-target FGF23 actions by activation of other FGF receptors, particularly FGFR4, could contribute to these pathophysiological processes (37, 38). Hence, activation of fibrocartilage cell hypertrophy in the enthesis contributing to the enthesopathy (39), and of myofibroblasts promoting fibrosis and calcification could be ascribed to lifelong exposure to elevated serum levels of bone-derived FGF23. Limiting the exposure time to high circulating FGF23 may therefore improve clinical outcomes, as observed in patient 2 following the second surgical procedure, with persistence in the surgical outcome not observed following the initial surgical attempt, which was not followed by burosumab administration. Nevertheless, for now, the question as to whether burosumab treatment is contributing to this persistence by preventing further fibrosis and calcification of the muscles remains to be determined. It is conceivable however that long-term treatment with burosumab may halt disease progression. Further in vitro and in vivo studies are needed to lend support to this supposition.
In patient 1, granulomas were identified on the liver biopsy specimen but the underlying etiology remains uncertain. Hepatic granulomas are localized, well-circumscribed collections of chronic inflammatory cells, usually identified on biopsy specimens. They are associated with infectious (Mycobacterium tuberculosis, fungal diseases, Q fever, and HIV or parasitic infections), noninfectious (sarcoidosis, autoimmune, with primary biliary cholangitis being the most common, drug-induced, and malignancies), as well as idiopathic etiologies (40). Elevations in serum ALP activity, transaminases, and GGT are the most common abnormalities but are nonspecific and rarely helpful in diagnosis. In our patient, treatment with ursodiol was instituted empirically and monitoring was undertaken with regular clinical, biochemical, and FibroScan evaluations. Interestingly, while hepatocytes lack α-klotho, they express high levels of FGFR4. In the absence of its classic coreceptor α-klotho, FGF23 is reported to increase hepatic and serum levels of inflammatory cytokines in an FGFR4-dependent manner (41). By directly activating FGFR4 and calcineurin/NFAT signaling in hepatocytes, FGF23 actions lead to increased expression and secretion of inflammatory cytokines. This direct, proinflammatory effect on hepatocytes by high serum FGF23 may be contributing to the localized collections of inflammatory cells that comprise the hepatic granulomas in our patient. It is conceivable, therefore that long-term treatment with burosumab may alleviate this complication, although the concept remains purely speculative at this point.
Discussion
The work presented here provides, to the best of our knowledge, the first report on the efficacy and safety of burosumab in patients with ARHR1. The biochemical and clinical responses in our 2 affected individuals highlight the potential benefits of adding burosumab to the treatment protocol of this adult patient population. Whether the same benefits will be observed in children with this disorder remains to be determined. Yet, the effectiveness of burosumab in a real-world pediatric clinical setting could conceivably halt disease progression if treatment is started early in life, so as to prevent the long-term morbidities associated with the disorder.
In conclusion, while a larger, perhaps multicenter study is needed to fully assess the role of burosumab in this patient population, the rarity of this disorder will undoubtedly pose a major obstacle to such an endeavor; for now, preliminary studies such as ours will serve to guide the treatment approach to improve the quality of life of these patients by preventing the long-term complications arising from excess circulating FGF23.
Acknowledgments
We thank our 2 patients for their commitment and contribution to the very first steps taken toward the eventual cure of this rare bone disorder. Artwork for figures was realized by A. A. Karaplis.
Glossary
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- ALP
alkaline phosphatase
- ARHR
autosomal recessive hypophosphatemic rickets
- ATP
adenosine 5′-triphosphate
- BGP
bone Gla-protein
- DMP1
dentin matrix acidic phosphoprotein 1
- FGF23
fibroblast growth factor 23
- GGT
γ-glutamyl transpeptidase
- PO4
phosphate
- PTH
parathyroid hormone
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
- XLH
X-linked hypophosphatemia
Contributor Information
Xiuying Bai, Lady Davis Institute for Medical Research, CIUSSS de Centre-Ouest-de-l’île-de-Montréal, Jewish General Hospital, McGill University, Montréal, Quebec, H3T 1E2, Canada.
Mark Levental, Department of Radiology, CIUSSS de Centre-Ouest-de-l’île-de-Montréal, Jewish General Hospital, McGill University, Montréal, Quebec, H3T 1E2, Canada.
Andrew C Karaplis, Lady Davis Institute for Medical Research, CIUSSS de Centre-Ouest-de-l’île-de-Montréal, Jewish General Hospital, McGill University, Montréal, Quebec, H3T 1E2, Canada; Department of Medicine, Division of Endocrinology, CIUSSS de Centre-Ouest-de-l’île-de-Montréal, Jewish General Hospital, McGill University, Montréal, Quebec, H3T 1E2, Canada.
Financial Support
This work was supported by the Canadian Institutes of Health Research (CIHR) Project (grant No. 156304).
Author Contributions
Xiuying Bai: funding acquisition, investigation, data curation, writing-review, and editing. Mark Levental: data curation, writing-review, and editing. Andrew C. Karaplis: conceptualization, funding acquisition, resources, project administration, supervision, writing of the original draft, writing-review, and editing. All three authors read and approved the submitted manuscript.
Disclosures
A.C.K. has received an honorarium from Ultragenyx Canada for participating on the Ultragenyx 2021 CRYSVITA XLH Advisory Board. X.B. and M.L. have nothing to disclose.
Data Availability
All data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Alizadeh Naderi AS, Reilly RF. Hereditary disorders of renal phosphate wasting. Nat Rev Nephrol. 2010;6(11):657-665. doi: 10.1038/nrneph.2010.121 [DOI] [PubMed] [Google Scholar]
- 2. Feng JQ, Ward LM, Liu S, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310-1315. doi: 10.1038/ng1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ichikawa S, Gerard-O’Riley RL, Acton D, et al. A mutation in the Dmp1 gene alters phosphate responsiveness in mice. Endocrinology. 2017;158(3):470-476. doi: 10.1210/en.2016-1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richter B, Faul C. FGF23 actions on target tissues—with and without klotho. Front Endocrinol (Lausanne). 2018;9:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44(Suppl 1):33-40. [PubMed] [Google Scholar]
- 6. Beck-Nielsen SS, Mughal Z, Haffner D, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14(1):58. doi: 10.1186/s13023-019-1014-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M. Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med. 1991;325(26):1843-1848. doi: 10.1056/NEJM199112263252604 [DOI] [PubMed] [Google Scholar]
- 8. Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab. 2010;95(4):1846-1850. doi: 10.1210/jc.2009-1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Insogna KL, Briot K, Imel EA, et al. AXLES 1 Investigators. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res. 2018;33(8):1383-1393. doi: 10.1002/jbmr.3475 [DOI] [PubMed] [Google Scholar]
- 10. Portale AA, Carpenter TO, Brandi ML, et al. Continued beneficial effects of burosumab in adults with X-Linked hypophosphatemia: results from a 24-week treatment continuation period after a 24-week double-blind placebo-controlled period. Calcif Tissue Int. 2019;105(3):271-284.doi: 10.1007/s00223-019-00568-3 [DOI] [PubMed] [Google Scholar]
- 11. Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378(21):1987-1998. doi: 10.1056/NEJMoa1714641 [DOI] [PubMed] [Google Scholar]
- 12. Whyte MP, Carpenter TO, Gottesman GS, et al. Efficacy and safety of burosumab in children aged 1-4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7(3):189-199. doi: 10.1016/S2213-8587(18)30338-3 [DOI] [PubMed] [Google Scholar]
- 13. Imel EA, Glorieux FH, Whyte MP, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393(10189):2416-2427. doi: 10.1016/S0140-6736(19)30654-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jan de Beur SM, Miller PD, Weber TJ, et al. Burosumab for the treatment of tumor-induced osteomalacia. J Bone Miner Res. 2021;36(4):627-635. doi: 10.1002/jbmr.4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gladding A, Szymczuk V, Auble BA, Boyce AM. Burosumab treatment for fibrous dysplasia. Bone. 2021;150:116004. doi: 10.1016/j.bone.2021.116004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karaplis AC, Bai X, Falet JP, Macica CM. Mineralizing enthesopathy is a common feature of renal phosphate-wasting disorders attributed to FGF23 and is exacerbated by standard therapy in hyp mice. Endocrinology. 2012;153(12):5906-5917. doi: 10.1210/en.2012-1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farrow EG, Davis SI, Ward LM, et al. Molecular analysis of DMP1 mutants causing autosomal recessive hypophosphatemic rickets. Bone. 2009;44(2):287-294. doi: 10.1016/j.bone.2008.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown JP, Delmas PD, Malaval L, Edouard C, Chapuy MC, Meunier PJ. Serum bone Gla-protein: a specific marker for bone formation in postmenopausal osteoporosis. Lancet. 1984;1(8386):1091-1093. doi: 10.1016/s0140-6736(84)92506-6 [DOI] [PubMed] [Google Scholar]
- 19. Centeno PP, Herberger A, Mun HC, et al. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat Commun. 2019;10(1):4693. doi: 10.1038/s41467-019-12399-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silver J, Naveh-Many T. FGF23 and the parathyroid glands. Pediatr Nephrol. 2010;25(11):2241-2245. doi: 10.1007/s00467-010-1565-3 [DOI] [PubMed] [Google Scholar]
- 21. Alon US, Levy-Olomucki R, Moore WV, Stubbs J, Liu S, Quarles LD. Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol. 2008;3(3):658-664. doi: 10.2215/CJN.04981107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toyosawa S, Shintani S, Fujiwara T, et al. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16(11):2017-2026. doi: 10.1359/jbmr.2001.16.11.2017 [DOI] [PubMed] [Google Scholar]
- 23. Pesta DH, Tsirigotis DN, Befroy DE, et al. Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J. 2016;30(10):3378-3387. doi: 10.1096/fj.201600473R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosalki SB, Foo AY, Burlina A, et al. Multicenter evaluation of Iso-ALP test kit for measurement of bone alkaline phosphatase activity in serum and plasma. Clin Chem. 1993;39(4):648-652. [PubMed] [Google Scholar]
- 25. Haffner D, Emma F, Eastwood DM, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol. 2019;15(7):435-455. doi: 10.1038/s41581-019-0152-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mäkitie O, Pereira RC, Kaitila I, et al. Long-term clinical outcome and carrier phenotype in autosomal recessive hypophosphatemia caused by a novel DMP1 mutation. J Bone Miner Res. 2010;25(10):2165-2174. doi: 10.1002/jbmr.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Insogna KL, Rauch F, Kamenický P, et al. Burosumab improved histomorphometric measures of osteomalacia in adults with X-linked hypophosphatemia: a phase 3, single-arm, international trial. J Bone Miner Res. 2019;34(12):2183-2191. doi: 10.1002/jbmr.3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riccio AR, Entezami P, Giuffrida A, Dowling J, Forrest G, German JW. Minimally invasive surgical management of thoracic ossification of the ligamentum flavum associated with X-linked hypophosphatemia. World Neurosurg. 2016;94:580.e5-580.e10. doi: 10.1016/j.wneu.2016.07.076 [DOI] [PubMed] [Google Scholar]
- 29. An HS, Al-Shihabi L, Kurd M. Surgical treatment for ossification of the posterior longitudinal ligament in the cervical spine. J Am Acad Orthop Surg. 2014;22(7):420-429. doi: 10.5435/JAAOS-22-07-420 [DOI] [PubMed] [Google Scholar]
- 30. Ikegawa S. Genetics of ossification of the posterior longitudinal ligament of the spine: a mini review. J Bone Metab 2014;21(2):127-132. doi: 10.11005/jbm.2014.21.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawaguchi Y, Kitajima I, Yasuda T, et al. Serum periostin level reflects progression of ossification of the posterior longitudinal ligament. JB JS Open Access. 2022;7(1):1-8. doi: 10.2106/JBJS.OA.21.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inamasu J, Guiot BH, Sachs DC. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006;58(6):1027-1039. doi: 10.1227/01.NEU.0000215867.87770.73 [DOI] [PubMed] [Google Scholar]
- 33. Hao H, Li X, Li Q, et al. FGF23 promotes myocardial fibrosis in mice through activation of β-catenin. Oncotarget. 2016;7(40):64649-64664. doi: 10.18632/oncotarget.11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith ER, Holt SG, Hewitson TD. FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-β autoinduction. Int J Biochem Cell Biol. 2017;92:63-78. doi: 10.1016/j.biocel.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 35. Böckmann I, Lischka J, Richter B, et al. FGF23-mediated activation of local RAAS promotes cardiac hypertrophy and fibrosis. Int J Mol Sci. 2019;20(18):1-16. doi: 10.3390/ijms20184634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kotyla PJ, Kruszec-Zytniewska A, Owczarek AJ, Olszanecka-Glinianowicz M, Chudek J. Fibroblast growth factor 23 to alpha-klotho index correlates with systemic sclerosis activity: a proposal for novel disease activity marker. J Clin Med. 2018;7(12):1-9. doi: 10.3390/jcm7120558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liang G, Katz LD, Insogna KL, Carpenter TO, Macica CM. Survey of the enthesopathy of X-linked hypophosphatemia and its characterization in Hyp mice. Calcif Tissue Int. 2009;85(3):235-246. doi: 10.1007/s00223-009-9270-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grabner A, Amaral AP, Schramm K, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22(6):1020-1032. doi: 10.1016/j.cmet.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwartz AG, Long F, Thomopoulos S. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development. 2015;142(1):196-206. doi: 10.1242/dev.112714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Culver EL, Watkins J, Westbrook RH. Granulomas of the liver. Clin Liver Dis (Hoboken). 2016;7(4):92-96. doi: 10.1002/cld.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh S, Grabner A, Yanucil C, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016;90(5):985-996. doi: 10.1016/j.kint.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.