Abstract
Objective
Gestational diabetes mellitus (GDM) is becoming a public health concern in low/middle-income countries, and is known to cause severe morbidity and mortality for mothers and newborns. However, evidence reported for the incidence and risk factors of GDM is scant in Ethiopia. We aimed to assess the incidence of, and risk factors for, GDM in Goba town, Southeast Ethiopia.
Design
Prospective cohort study.
Setting
Goba town, Southeast Ethiopia.
Participants
Four hundred eighty pregnant women on antenatal care follow-up from 30 April to 30 September 2021.
Primary and secondary outcomes
Incidence and risk factors of GDM using fasting capillary blood glucose. Log-binomial model was used to identify the risk factors of GDM. Adjusted relative risk (aRR), along with 95% CIs, were calculated to estimate the strength of associations.
Results
The cumulative incidence rate of GDM in this study was 15.7% (95% CI: 12.3% to 19.2%). Being unemployed (aRR=2.73; 95% CI: 1.36 to 5.47), having a family history of diabetes mellitus (DM) (3.01; 2.09 to 4.35), low physical activity (2.43; 1.11 to 5.32), inadequate dietary diversity (1.48; 1.29 to 1.92), anaemia (2.51; 1.32 to 3.54) and antenatal depression (4.95; 3.35 to 7.31) were significantly associated with GDM.
Conclusion
The cumulative incidence of GDM was relatively high among the study participants. Having antenatal depression symptoms, low physical activity, inadequate dietary diversity, being unemployed, anaemia and a family history of DM were significant risk factors for GDM.
Keywords: Diabetes in pregnancy, Antenatal, Diabetes & endocrinology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The prospective design is a strength of the study.
Fasting capillary blood glucose was used to diagnose gestational diabetes mellitus, which is less sensitive than venous blood glucose.
The oral glucose tolerance test was not used due to resource limitations.
Pre-pregnancy anthropometric measurements and body mass index were not determined among pregnant women, which might be part of the predictor variables.
Introduction
Gestational diabetes mellitus (GDM) is a glucose intolerance detected during pregnancy for the first time.1 It is becoming a public health concern in low/middle-income countries, and known to cause severe morbidity and mortality for mothers and newborns.2 3 Pregnancy itself induces changes in maternal glucose metabolism and insulin sensitivity, thereby increasing the demand for insulin production.4 The common period for the diagnosis of GDM is between 24 to 28 weeks of gestation.5 However, hyperglycaemia during early pregnancy was identified as a risk factor for developing GDM.6 Therefore, determining blood glucose levels as early as possible is important to decrease adverse pregnancy outcomes.7 8
Diabetes mellitus (DM) and other non-communicable diseases are becoming more prevalent in developing countries, including Ethiopia.9 Globally, diabetes prevalence is increasing rapidly, estimated 381 million in 2013 to 422 million people living with DM in 2015. According to the International Diabetes Federation (IDF), by 2035, the global burden of DM is projected to reach 592 million, or 1 in 10 will have DM.10 The IDF estimates that 16.2% of live births to women had some form of hyperglycaemia in pregnancy.11 In sub-Saharan Africa (SSA), the burden of GDM was found to be 14.28%.12 In Ethiopia, women are at greater risk of GDM despite having a lower mean body mass index (BMI).13 In a study conducted in Gondar town, the cumulative incidence rate was 12.8%.14 Other studies conducted in Wolita and Hadiya zones, the southern part of Ethiopia, reported the cumulative incidence rates of GDM to be 4.2%15 and 26.2%,16 respectively.
GDM is associated with a greater risk of neonatal macrosomia,17 18 shoulder dystocia, neonatal trauma, respiratory distress and increased admission to neonatal intensive care units.19 Women with hyperglycaemia detected during early pregnancy are at greater risk of adverse pregnancy outcomes,6 with an incidence rate of 30.3%.20 These include high blood pressure and birth difficulties, with the baby more prone to fractures and nerve damage.11 GDM also results in permanent type 2 DM (T2DM) in women, with an incidence rate ranging from 2.6% to 70%.21 22
A higher prevalence of GDM was observed in mothers with a family history of T2DM.18 23 24 Further, a study on Russian women also identified that a genetic variant in MTNR1B is associated with an increased risk of GDM.25 Previous stillbirth, high mid-upper arm circumference, anaemia,26 27 advanced maternal age,28 low physical activity and a sedentary lifestyle have also been shown to increase the risk of GDM.24 29 A higher BMI, abdominal circumference and fasting glycaemia in the first trimester of pregnancy revealed a 13-fold increased risk of GDM.30 The proportion of GDM increases with the number of pregnancies.31
In most cases, adverse pregnancy outcomes among women with GDM are preventable by optimising glycaemic control. Early screening and treatment of mothers with GDM can minimise the complications for both mothers and their babies.19 Once diagnosed with GDM, a woman has a substantial chance of developing T2DM following delivery, with some studies reporting a 5-year cumulative incidence rate of over 50%.32
Despite all the above facts, there are only a few studies on the incidence and associated factors of GDM in SSA,33 including in Ethiopia, particularly in the study setting. Therefore, we aimed to assess the incidence and risk factors of GDM in Goba town, Southeast Ethiopia.
Methods
Study design and setting
A facility-based prospective follow-up study was conducted among pregnant women in health centres of Goba town from 30 April to 30 September 2021. The pregnant women were followed from 20 weeks of gestation to 32 weeks of gestation. Goba is one of the administrative towns in the Bale Zone, located 445 km from Addis Ababa city. According to the 2019 fiscal year, the total population of Goba town was 51 562, and the estimated number of pregnant women was 1789.34 The town has two health centres and one referral hospital. The health centres in Goba town serve more than three-fourths of pregnant women for antenatal care (ANC) follow-up.
Source population
All pregnant women who started ANC were followed up at Harawa Sinja Health Center and Oda Baha Health Center, Goba town, Southeast Ethiopia.
Study population
Pregnant women with a gestational age of 20 weeks receiving ANC at Harawa Sinja Health Center and Oda Baha Health Center, Goba town, Southeast Ethiopia.
Inclusion criteria
Pregnant women who are in their 20 weeks of gestation, have singleton pregnancy, permanent residents of the study area, and without any known pre-existing or overt DM.
Exclusion criteria
Pregnant women who took medications that could affect glucose metabolisms, such as steroids, beta-adrenergic agonists and antipsychotic medications, and who have an acute febrile illness were excluded.
Sample size determination and sampling techniques
The sample size was determined using the following parameters: 95% CI, 5% margin error, 80% power and the prevalence of GDM from a previous study conducted in the Hadiya zone (26%).16 For incidence rate, based on the single-population formula, the sample size was determined to be 295. For risk factors (stillbirth,14 abortion15 and family history of DM27) of GDM, the double-population formula using Epi Info V.7 software was used to determine the sample size, and considering 15% loss to follow-up, 480 samples were included in this study (table 1). After the sample size was computed for both objectives, the largest sample size was taken.
Table 1.
Sample size calculation for risk factors of GDM in Goba town, Southeast Ethiopia, 2021
| Exposure variables | Proportion in non-exposed | Proportion in the exposed | Power of the study | Crude OR | Sample size | Total sample size after considering 15% loss to follow-up |
| History of stillbirth14 | 5.17% | 14% | 80 | 2.97 | 417 | 480 |
| History of abortion15 | 16.3% | 45.5% | 80 | 4.2 | 101 | 116 |
| History of GDM27 | 1.8% | 12% | 80 | 7.4 | 236 | 271 |
GDM, gestational diabetes mellitus.
All pregnant women with a gestational age of 20 weeks were included in this study until the required sample size has reached.
All pregnant women on ANC follow-up at two health centres in Goba town who fulfilled our inclusion criteria were included in the study.
Variables of the study
Dependent variable
GDM: defined as fasting capillary blood glucose between 92 and 125 mg/dL.1
Exposure variables
Sociodemographic variables such as pregnant women’s age, occupation, religion, ethnicity and educational status; behavioural variables (caffeine, alcohol, dietary diversity and smoking); reproductive-related factors; a medical history of GDM, pregnancy-induced hypertension, stillbirth, intrauterine fetal death or spontaneous abortion; health-related chronic disease; and family history of T2DM and GDM were considered in this study.
Data collection procedures
An interviewer-administered, structured questionnaire was prepared in English and translated into the local languages ‘Amharic’ and ‘Afan Oromo’. The questionnaire was back-translated into English to assure consistency. The questionnaire was checked by language experts (MA holders in language). A midwife with a Bachelor of Science degree was involved in the data collection activity. Three days of training was provided to data collectors to familiarise them with the study objectives, data collection methods, ethical issues and the questionnaire.
Both primary and secondary data (chart review) were collected. The baseline maternal and sociodemographic characteristics, behavioural, dietary diversity and antenatal depression status were collected using face-to-face interviews. Dietary diversity was assessed using a 24-hour food recall method by the Food and Nutrition Technical Assistance 2016 version of the Women’s Minimum Dietary Diversity measurement tool. The 4 or less Minimum Dietary Diversity Score was categorised as inadequate dietary diversity.35
The Edinburgh Postnatal Depression Scale screening tool was used to assess antenatal depression in the past week.36 The short form of the International Physical Activity Questionnaire (IPAQ) was employed to assess the physical activities in the last 7 days. Then, using metabolic equivalents-minutes per week of the IPAQ scoring protocol, pregnant women were categorised into high, moderate and low levels of physical activity.37
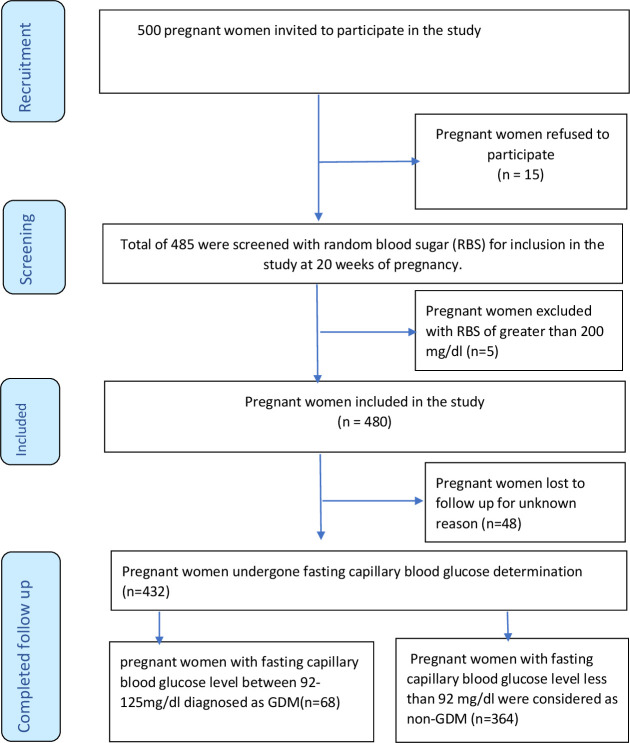
Fasting capillary blood glucose was performed for all pregnant women by capillary blood glucose, using a standard plasma-calibrated glucometer (Hemo Cue Glucose B-201+; Sweden). Even though the sensitivity of capillary blood glucose is lower than venous blood glucose, the international consensus is that it is acceptable in resource-poor settings for GDM diagnosis.5 Initially, a capillary blood glucose test (random blood glucose) was performed for all pregnant women at 20 weeks of gestation to rule out the presence of pre-existing or overt DM. Then, screening for GDM using fasting capillary blood glucose was performed at 24–28 weeks of gestational age (figure 1). A similar measurement was repeated at 32 weeks of gestation to identify the late occurrence of GDM. Participants diagnosed with GDM were referred immediately (linked) to healthcare providers who are experts in managing GDM. Follow-ups were assured through the public health facilities in close collaboration with experts and data collectors.
Figure 1.
Flow diagram of outcome ascertainment for pregnant women on ANC from 30 April to 30 September 2021. ANC, antenatal care; GDM, gestational diabetes mellitus.
Outcome ascertainment
In this study, initially, pregnant women were invited to participate, then screened for pre-existing DM using random capillary blood glucose. Pregnant women identified to have random capillary blood glucose greater than 200 mg/dL were excluded from the study. Finally, the included pregnant women underwent fasting capillary blood glucose measurement. Pregnant women with fasting capillary blood glucose between 92 and 125 mg/dL were diagnosed as GDM, and pregnant women with fasting capillary blood glucose levels less than 92 mg/dL were declared non-GDM.
Data quality control
The data quality was assured by applying a properly designed and pretested questionnaire. The tool was pretested on 5% of the sample size at the Baha Biftu Health Center 1 week before the actual data collection to establish its ability to elicit relevant information. In addition, the researchers ensured proper categorisation and coding of the questions. The investigators and a supervisor conducted regular supervision and follow-up. In addition, a regular check-up for completeness and consistency of the data was undertaken daily. Incomplete questionnaires were completed during the second appointment. The manufacturer’s instructions and standard operating procedures were strictly followed for all blood glucose measurements.
Data processing and analysis
The questionnaires were coded manually. The data were entered into EpiData V.3.1 and then exported from EpiData to Stata V.14 for analysis. Data were checked for missing values. Descriptive statistics were presented using frequencies, percentages, mean and SDs to describe study subjects. Multicollinearity was checked by looking at values of variance inflation factor (<7). Bivariate log-binomial regression analysis was employed to examine the relationship between the outcome and independent variables. All the variables with p≤0.2 in the bivariate log-binomial regression analyses were entered into a multivariable log-binomial regression model. This step helps to identify important associated factors for the dependent variables after controlling possible confounding factors. The crude and adjusted relative risks (aRRs) were used to estimate the strength of the association between predictors and outcome variables. Variables with a p value of <0.05 were considered statistically significant with the outcome variable.
Patient and public involvement
Patients and the public were not involved in the planning, designing and interpreting of the analysed data.
Results
Sociodemographic and economic characteristics of pregnant women
In this study, 500 pregnant women were invited to participate. Of the invited, 485 agreed to participate and were screened for pre-existing DM using random capillary blood glucose. Five pregnant women were identified to have random capillary blood glucose greater than 200 mg/dL, therefore excluded from the study. Of the remaining 480 pregnant women, 48 were lost to follow-up. The remaining 432 pregnant women had undergone fasting capillary blood glucose measurements. Sixty-eight pregnant women were identified to have fasting capillary blood glucose levels between 92 and 125 mg/dL, while 364 pregnant women had capillary blood glucose levels less than 92 mg/dL (figure 1).
The study included a total of 432 pregnant women, making the response rate 90%. The mean age of the pregnant women was 26.58 (SD ±5.88) years. Most of the participants (97.8%) were married, 88.9% were from the Oromo ethnic group and nearly half (47.5%) were Muslim by religion. One hundred thirty-seven (31.7%) women had attended secondary school education, while about 80.5% of pregnant women were unemployed (table 2).
Table 2.
Sociodemographic and economic characteristics of pregnant women attending ANC follow-up at health centres of Goba town, Southeast Ethiopia, April–September 2021 (n=432)
| Variables | Non-GDM n (%) | GDM n (%) | P value |
| Age in years | |||
| <25 | 159 (43.7) | 23 (33.8) | 0.027 |
| 25–29 | 120 (33.0) | 21 (30.9) | |
| 30–34 | 52 (14.3) | 13 (19.1) | |
| >34 | 33 (9.1) | 11 (16.2) | |
| Religion | |||
| Orthodox | 157 (43.1) | 28 (41.1) | 0.733 |
| Muslim | 173 (47.5) | 32 (47.1) | |
| Protestant | 34 (9.3) | 8 (11.8) | |
| Ethnicity | |||
| Oromo | 293 (80.5) | 55 (80.9) | 0.990 |
| Amhara | 61 (16.8) | 11 (16.2) | |
| Others* | 10 (2.7) | 2 (2.9) | |
| Educational status | |||
| No formal education | 53 (14.6) | 11 (16.3) | 0.538 |
| Primary school | 117 (32.1) | 19 (27.9) | |
| Secondary school | 118 (32.4) | 19 (27.9) | |
| College and above | 76 (20.9) | 19 (27.9) | |
| Occupational status | |||
| Employed | 60 (16.5) | 24 (35.3) | 0.017 |
| Non-employed | 304 (83.5) | 44 (64.7) | |
*Others (Gurage, Wolita).
ANC, antenatal care; GDM, gestational diabetes mellitus.
Clinical characteristics of study participants
The mean systolic blood pressure was 105.9 (SD ±10.2) mm Hg, and diastolic blood pressure was 66.4 (SD ±7.6) mm Hg. The pregnant women’s mean haemoglobin and random blood glucose levels were 11.9 (SD ±1.1) and 108 (SD ±16.7), respectively. Nearly one-third (33.8%) of the women were primigravida, around 27 (6.3%) had a family history of DM and 50 (11.6%) pregnant women were identified to have anaemia. The history of abortion and stillbirth was reported among 3% and 3.8% of pregnant women, respectively (table 3).
Table 3.
Obstetric history of study participants attending ANC follow-up at health centres of Goba town, Southeast Ethiopia, April–September 2021 (n=432)
| Variables | Non-GDM (n=364) | GDM (n=68) | P value |
| Gravidity | |||
| One | 143 (39.3) | 23 (33.9) | 0.515 |
| Two | 99 (27.2) | 22 (32.4) | |
| Three | 68 (18.6) | 11 (16.2) | |
| Four and above | 54 (14.3) | 12 (17.6) | |
| History of abortion/intrauterine fetal death | |||
| Yes | 5 (2.3) | 3 (6.7) | 0.115 |
| No | 216 (97.7) | 42 (93.3) | |
| History of stillbirth | |||
| Yes | 8 (3.6) | 2 (4.4) | 0.791 |
| No | 213 (96.4) | 43 (95.6) | |
| Confirmed pregnancy-induced hypertension in a previous pregnancy | |||
| Yes | 12 (5.4) | 2 (4.4) | 0.787 |
| No | 209 (94.6) | 43 (95.6) | |
| Confirmed GDM in a previous pregnancy | |||
| Yes | 2 (0.9) | 1 (2.2) | 0.446 |
| No | 119 (91.1) | 44 (97.8) | |
| Family history of diabetes | |||
| Yes | 17 (4.7) | 10 (14.7) | 0.002 |
| No | 347 (95.3) | 58 (85.3) | |
| Haemoglobin status | |||
| <0.11 g/L | 35 (9.6) | 15 (22.1) | 0.003 |
| ≥0.11 g/L | 329 (90.4) | 53 (77.9) | |
ANC, antenatal care; GDM, gestational diabetes mellitus.
A slightly higher proportion of DM family history was revealed among women with GDM than those without GDM (14.7% vs 4.7%). When pregnant women were compared in terms of anaemia status, those with GDM had a higher proportion than those without GDM (22.1% vs 9.6%) (table 3).
Behavioural and lifestyle characteristics of pregnant mothers
Out of total participants, alcohol and coffee intake during pregnancy was reported by 17.8% and 90%, respectively. Nearly one-third of women who consumed coffee reported consuming two cups of coffee per day. Most pregnant women (45.8%) reported having low physical activity, while about 1 in 10 pregnant women reported having probable antenatal depression symptoms. An inadequate dietary diversity score was reported in 6.3% of pregnant women participating in this study (table 4).
Table 4.
Behavioural characteristics of study participants attending ANC follow-up at health centres of Goba town, Southeast Ethiopia 2021 (n=432)
| Variable | Non-GDM (n=364) | GDM (n=68) | P value |
| History of alcohol intake during this pregnancy | |||
| Yes | 65 (17.9) | 12 (17.6) | 0.967 |
| No | 299 (82.1) | 56 (82.4) | |
| Type of alcohol | |||
| Local | 49 (75.4) | 8 (66.7) | 0.527 |
| Beer | 16 (24.6) | 4 (33.3) | |
| History of coffee intake in this pregnancy | |||
| Yes | 326 (89.6) | 63 (92.4) | 0.435 |
| No | 38 (10.4) | 5 (7.6) | |
| Number of cups of coffee per day | |||
| One | 83 (25.5) | 10 (15.9) | 0.002 |
| Two | 110 (33.7) | 16 (25.4) | |
| Three | 79 (24.2) | 16 (25.4 | |
| Four and above | 54 (16.7) | 21 (33.3) | |
| Physical activity status during pregnancy | |||
| Low | 156 (49.7) | 42 (61.8) | 0.013 |
| Moderate | 144 (39.6) | 20 (29.4) | |
| High | 64 (17.6) | 6 (8.8) | |
| Antenatal depression status | |||
| Probable | 24 (6.6) | 20 (29.4) | 0 |
| Possible | 43 (11.8) | 13 (19.1) | |
| None | 297 (81.6) | 35 (51.5) | |
| Dietary diversity score | |||
| <5 (inadequate) | 18 (4.9) | 9 (13.2) | 0.01 |
| ≥5 (adequate) | 346 (95.1) | 59 (86.8) | |
ANC, antenatal care; GDM, gestational diabetes mellitus.
Low physical activity was reported to be higher among pregnant women with GDM than those without GDM (61.8% vs 49.7%). Pregnant women with GDM were shown to have a higher proportion of antenatal depression when compared with those without GDM (29.4% vs 6.6%). Similarly, inadequate dietary diversity was revealed to be higher among pregnant women with GDM when compared with those without GDM (4.9% vs 13.2%) (table 4).
Incidence of GDM
During the study period, 432 pregnant women were followed for 4781 weeks. A total of 68 pregnant women developed GDM. The mean time of diagnosis of GDM is 26.1 (95% CI: 25.65 to 26.51) weeks of pregnancy. The overall incidence rate of GDM was 14.22 per 1000 weeks of follow-ups, and the cumulative incidence rate was 15.7% (95% CI: 12.3% to 19.2%) over 5 months.
Predictors of GDM among pregnant women
After adjustment for maternal age, employment status, family history of diabetes, haemoglobin status, physical activity, antenatal depression and dietary diversity, the adjusted log-binomial regression model has indicated that being unemployed (aRR=2.73; 95% CI: 1.36 to 5.47), having a family history of diabetes (aRR=3.01; 95% CI: 2.09 to 4.35), low physical activity (aRR=2.43; 95% CI: 1.11 to 5.32), inadequate dietary diversity (aRR=1.48; 95% CI: 1.29 to 1.92), anaemia (aRR=2.51; 95% CI: 1.32 to 3.54) and antenatal depression (aRR=4.95; 95% CI: 3.35 to 7.31) were significantly associated with GDM (table 5).
Table 5.
Bivariate and multivariable log-binomial regression analyses and predictors of GDM among pregnant women attending antenatal care at health centres, Goba town, Southeast Ethiopia: April–September 2021 (n=432)
| Variables | Non-GDM (n=364) | GDM (n=68) | Crude relative risk (cRR) (95% CI) | Adjusted relative risk (aRR) (95% CI) |
| Age of women in years | ||||
| <25 | 159 | 23 | 1 | 1 |
| 25–29 | 120 | 21 | 1.18 (0.68 to 2.04) | 1.36 (0.80 to 2.33) |
| 30–34 | 52 | 13 | 1.58 (0.85 to 2.94) | 1.53 (0.84 to 2.77) |
| >34 | 33 | 11 | 1.98 (1.04 to 3.75)+ | 1.84 (0.96 to 3.50) |
| Occupational status | ||||
| Employed | 91 | 8 | 1 | 1 |
| Non-employed | 273 | 60 | 2.23 (1.10 to 4.5)++ | 2.73 (1.36 to 5.47)** |
| Family history of DM | ||||
| Yes | 17 | 10 | 2.59 (1.49 to 4.47)+ | 3.01 (2.09 to 4.35)** |
| No | 347 | 58 | 1 | 1 |
| Total | 364 | 68 | ||
| Haemoglobin status | ||||
| <11 mg/dL | 35 | 15 | 2.16 (1.32 to 3.54)+ | 2.51 (1.70 to 3.69)** |
| ≥11 mg/dL | 329 | 53 | 1 | 1 |
| Total | 364 | 68 | ||
| History of coffee intake in this pregnancy | ||||
| Yes | 326 | 63 | 1.39 (0.59 to 3.27) | |
| No | 38 | 5 | 1 | |
| Total | 364 | 68 | ||
| Number of cups of coffee per day | ||||
| One | 83 | 10 | 1 | |
| Two | 110 | 16 | 1.18 (0.56 to 2.48) | |
| Three | 79 | 16 | 1.57 (0.75 to 3.27) | |
| Four and above | 54 | 21 | 2.60 (1.30 to 5.19)+ | |
| Physical activity status during this pregnancy | ||||
| Low | 156 | 42 | 2.71 (1.26 to 5.82)+ | 2.43 (1.11 to 5.32)* |
| Moderate | 144 | 20 | 0.57 (0.35 to 0.94)+ | 1.98 (0.88 to 4.47) |
| High | 64 | 6 | 1 | 1 |
| Antenatal depression status | ||||
| Probable | 24 | 20 | 4.31 (2.75 to 6.77)++ | 4.95 (3.35 to 7.31)** |
| Possible | 43 | 13 | 2.20 (1.24 to 3.89)+ | 2.12 (1.21 to 3.71)* |
| Dietary diversity score | ||||
| <5 | 18 | 9 | 1.57 (1.09 to 2.62)+ | 1.48 (1.29 to 1.92)** |
| ≥5 | 346 | 59 | 1 | 1 |
aRR: **p<0.001, *p<0.05; cRR: ++p<0.001, +<0.05.
List of variables used to adjust this model: maternal age, employment status, family history of diabetes, haemoglobin status, physical activity, antenatal depression and dietary diversity.
GDM, gestational diabetes mellitus.
Discussion
Our study aimed to assess the incidence and risk factors of GDM in Goba town, Southeast Ethiopia. In this study, the cumulative incidence rate of GDM among pregnant women attending ANC in health centres of Goba town was 15.7%. Our finding was almost similar to studies conducted in Gondar town, Ethiopia (12.8%)14 and Qingdao, China (17%).38 The current finding was higher than the study conducted in Wolita zone, Ethiopia (4.2%),13 and the possible reason might be due to the difference in the sample size.
Nevertheless, it was lower than a study conducted in Tanzania in which the cumulative incidence rate was found to be 19.5%.39 The cumulative incidence rate of GDM in this study was lower than the findings from a Nigerian study which was reported at 21.2%.40 Another study conducted in Hadiya zone, Ethiopia revealed a higher incidence rate than our finding (26.2%).16 These figures show that the incidence of GDM is increasing as other chronic medical conditions, which have been increasing with lifestyle shifts such as consuming fast food and increasing sedentary lifestyles. The variation might be due to variation in sample size and other sociodemographic variables.
Unemployment was shown to have a significant statistical association with GDM. As revealed in this study, non-employed pregnant women had 2.73 times higher risk of developing GDM than employed pregnant women. The current finding disagreed with a study conducted in Gondar town.14 The variation might be because of the difference in sociodemographic characteristics. Further, among unemployed women, low physical activity was shown to have a difference in pregnant women without GDM and those with GDM (46% vs 67%), respectively. This difference is supported by the evidence that employed adults were more likely to be physically active than non-employed.41 42 Similarly, employed pregnant women were physically active compared with the non-employed group.43 Physical inactivity, in turn, increases the risk of developing GDM.44 In this study, pregnant women with low physical activity were 2.43 times more at risk of GDM than pregnant women with high physical activity. This finding is supported by a study conducted in Gondar town14 and Amhara region, Ethiopia.27 Another study from Tanzania has identified low physical activity as a risk factor for GDM.45
The risk of developing GDM was 2.6 times higher in pregnant women with a family history of diabetes than in their counterparts. This finding is in agreement with a prospective cohort study conducted in Florida which revealed that the risk of GDM among women with a family history of DM increased twofold.46 Similarly, a study in Poland (Poznan city) identified family history of diabetes as an independent risk factor for GDM.47 This association could be because GDM has a genetic component that may predispose individuals to glucose intolerance during pregnancy, and T2DM shares a common genetic background with GDM.48
Anaemia was also shown to have an association with the occurrence of GDM. Our finding indicated that pregnant women with anaemia were 1.9 times at risk of developing GDM compared with pregnant women without anaemia. The finding is supported by a study conducted in Tanzania that revealed pregnant women with anaemia were at increased risk of developing GDM.26 Although there is no evidence to suggest that managing anaemia with iron supplementation increases the risk of GDM, some evidence suggests that pregnant women who get iron supplements for anaemia have excess iron deposited during pregnancy and are at an increased risk of developing GDM.49 Further, increased ferritin, haemoglobin and dietary heme intake was associated with an increased risk of GDM.50
The probability of developing GDM was 3.1 times more in the pregnant women who reported antenatal depression symptoms than those with no depression symptoms. The present result supported a study conducted in Chicago which revealed that women with GDM were 3.79 times more likely to have a history of depression.51 Similarly, a cohort study conducted in Canada has reported a twofold increased risk of depression among women with GDM more than those without GDM.52 The possible explanation could be depression results in hypercortisolemia, increasing insulin resistance.53
In this study, pregnant women with inadequate dietary diversity were 1.5 times more at risk of developing GDM than those with adequate dietary diversity. The finding agreed with the study conducted in Gondar town, where pregnant women with inadequate dietary diversity were at risk of developing GDM.14 This observation is possible because inadequate dietary diversity will decrease the probability of getting a high-fibre diet that controls blood sugar levels.54 Further, inadequate dietary diversity decreases the chance of getting antioxidants in food consumed, which is important to prevent or delay B cell dysfunction in diabetes by protecting against glucose toxicity.55
Our study was reported with the following limitations. First, we used fasting capillary blood glucose to diagnose GDM due to a lack of resources for oral glucose tolerance tests, which might affect the strength of recommendations. However, various studies have reported fasting capillary glucose as the most sensitive and specific test56 57 and recommended to be conducted in resource-limited settings.56 Second, even though capillary blood glucose is recommended in resource-limited settings, it is less sensitive than venous blood glucose. Third, pre-pregnancy anthropometric measurement and BMI were not determined among pregnant women, which may be part of the determinant factors.
Conclusion
The cumulative incidence of GDM was relatively high in Goba town. Having antenatal depression symptoms, anaemia, a family history of diabetes, low physical activity, inadequate dietary diversity and being unemployed were identified as risk factors for GDM. Therefore, increasing community awareness of physical exercise, increasing recreational activities and diversifying food intake during pregnancy are important. The study’s findings would be an input for decision-makers to combat GDM in Ethiopia.
Supplementary Material
Acknowledgments
We would like to thank all the participants of this study and data collectors. We would also like to extend our sincere gratitude to Madda Walabu University for providing financial support.
Footnotes
Twitter: @atlaw_daniel, @yohannesefa, @Twitter account- @Ayele61796612, @alelign10
Contributors: DA has contributed substantially to the conception and design, acquisition of data, analysis and interpretation of data. DA has written the draft manuscript and provided final approval of the version to be published. BS, TA, WN, AT, TR, YT, AM, ZTE, DS, HG, KB, DZ, AT, FD, FN, GB, ZS, ZF, ZR and VKC have made substantial contributions to the design, acquisition of data, analysis and interpretation of data. All authors revised the article critically for important intellectual content and provided final approval of the version to be published. All authors read and approved the final manuscript. DA acting as guarantor.
Funding: The data collection was supported by Madda Walabu University (grant number: Pr/89/699).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
The research protocol was approved by the Ethical Review Committee of Madda Walabu University (reference no: RDD/0097/13). All methods were conducted following the relevant tenets of the Helsinki Declaration. An official letter was obtained from the Goba town health office. Then, the letters were given to the Harawa Sinja Health Center and the Oda Baha Health Center heads. Finally, written consent was obtained from each study participant after explaining the study’s risks and benefits. The privacy of the respondents was secured throughout the data collection process, and anonymity and confidentiality of the data were maintained.
References
- 1.WHO . Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy, 2013 [PubMed]
- 2.Domanski G, Lange AE, Ittermann T, et al. Evaluation of neonatal and maternal morbidity in mothers with gestational diabetes: a population-based study. BMC Pregnancy Childbirth 2018;18:367. 10.1186/s12884-018-2005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KW, Ching SM, Ramachandran V, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2018;18:1–20. 10.1186/s12884-018-2131-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macaulay S, Dunger DB, Norris SA. Gestational diabetes mellitus in Africa: a systematic review. PLoS One 2014;9:e97871–11. 10.1371/journal.pone.0097871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simeoni U, Sobngwi E. The International Federation of Gynecology a nd Obstetrics (F I GO) Initiative on gestational diabetes mellitus : A pragmatic guide for diagnosis, management, and care. Int J Gynecol Obstet J 2015;3:173–211. 10.1016/S0020-7292(15)30033-3 [DOI] [Google Scholar]
- 6.Popova P, Tkachuk A, Dronova A, et al. Fasting glycemia at the first prenatal visit and pregnancy outcomes in Russian women. Minerva Endocrinol 2016;41:477–85. [PubMed] [Google Scholar]
- 7.Popova P, Castorino K, Grineva EN, et al. Gestational diabetes mellitus diagnosis and treatment goals: measurement and measures. Minerva Endocrinol 2016. [Epub ahead of print: 29 Jan 2016]. [PubMed] [Google Scholar]
- 8.Quaresima P, Visconti F, Chiefari E. Appropriate Timing of Gestational Diabetes Mellitus Diagnosis in Medium- and Low-Risk Women : Effectiveness of the Italian NHS Recommendations in Preventing Fetal Macrosomia. J Diabetes Res Res 2020;2020. 10.1155/2020/5393952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habtewold TD, Tsega WD, Wale BY. Diabetes mellitus in outpatients in Debre Berhan referral Hospital, Ethiopia. J Diabetes Res 2016;2016:3571368 10.1155/2016/3571368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IDF Diabetes Atlas Committee . The global burden of diabetes, 2013
- 11.International Diabetes Federation . Idf diabetes atlas, 2019
- 12.Muche AA, Olayemi OO, Gete YK. Prevalence and determinants of gestational diabetes mellitus in Africa based on the updated international diagnostic criteria: a systematic review and meta-analysis. Arch Public Health 2019;77:1–20. 10.1186/s13690-019-0362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffe A, Giveon S, Rubin C, et al. Gestational diabetes risk in a multi-ethnic population. Acta Diabetol 2020;57:263–9. 10.1007/s00592-019-01404-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muche AA, Olayemi OO, Gete YK. Prevalence of gestational diabetes mellitus and associated factors among women attending antenatal care at Gondar town public health facilities, Northwest Ethiopia. BMC Pregnancy Childbirth 2019;19:1–13. 10.1186/s12884-019-2492-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolka E, Deressa W, Reja A. Prevalence of gestational diabetes mellitus and associated factors in southern Ethiopia. Asian J Med Sci 2018;10:86–91. 10.3126/ajms.v10i1.21331 [DOI] [Google Scholar]
- 16.Larebo YM, Ermolo NA. Prevalence and risk factors of gestational diabetes mellitus among women attending antenatal care in Hadiya zone public hospitals, southern nation Nationality people region. Biomed Res Int 2021;2021:1–10. 10.1155/2021/5564668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karaçam Z, Çelİk D. The prevalence and risk factors of gestational diabetes mellitus in turkey: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2021;34:1331–41. 10.1080/14767058.2019.1635109 [DOI] [PubMed] [Google Scholar]
- 18.Yaping X, Chunhong L, Huifen Z. Risk factors associated with gestational diabetes mellitus: a retrospective case-control study. Int J Diabetes Dev Ctries 2021. 10.1007/s13410-021-00947-3 [DOI] [Google Scholar]
- 19.Wakwoya EB, Amante TD, Tesema KF. Gestational Diabetes Mellitus Is a Risk for Macrosomia : Case- Control Study in Eastern Ethiopia.. Confirmatory Results 2018. 10.1101/492355 [DOI] [Google Scholar]
- 20.Asmamaw A, Olayemi OO, Kebede Y. Gestational diabetes mellitus increased the risk of adverse neonatal outcomes : A prospective cohort study in Northwest Ethiopia. Midwifery J 2020;87. 10.1016/j.midw.2020.102713 [DOI] [PubMed] [Google Scholar]
- 21.Chivese T, Norris SA, Levitt NS. Progression to type 2 diabetes mellitus and associated risk factors after hyperglycemia first detected in pregnancy: a cross-sectional study in Cape town, South Africa. PLoS Med 2019;16:e1002865–18. 10.1371/journal.pmed.1002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muche AA, Olayemi OO, Gete YK. Predictors of postpartum glucose intolerance in women with gestational diabetes mellitus: a prospective cohort study in Ethiopia based on the updated diagnostic criteria. BMJ Open 2020;10:e036882–8. 10.1136/bmjopen-2020-036882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhotra A, Stewart A. Gestational diabetes and the neonate: challenges and solutions. Res Reports Neonatol 2015;31. 10.2147/RRN.S30971 [DOI] [Google Scholar]
- 24.Larrabure-Torrealva GT, Martinez S, Luque-Fernandez MA, et al. Prevalence and risk factors of gestational diabetes mellitus: findings from a universal screening feasibility program in Lima, Peru. BMC Pregnancy Childbirth 2018;18:1–9. 10.1186/s12884-018-1904-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popova PV, Klyushina AA, Vasilyeva LB, et al. Association of common genetic risk variants with gestational diabetes mellitus and their role in GDM prediction. Front Endocrinol 2021;12:1–7. 10.3389/fendo.2021.628582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mwanri AW, Kinabo J, Ramaiya K, et al. Prevalence of gestational diabetes mellitus in urban and rural Tanzania. Diabetes Res Clin Pract 2014;103:71–8. 10.1016/j.diabres.2013.11.021 [DOI] [PubMed] [Google Scholar]
- 27.Feleke BE. Determinants of gestational diabetes mellitus, a case-control study. J Matern Neonatal Med 2017;7. 10.1080/14767058.2017.1347923 [DOI] [PubMed] [Google Scholar]
- 28.Teh WT, Teede HJ, Paul E, et al. Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines. Aust N Z J Obstet Gynaecol 2011;51:26–30. 10.1111/j.1479-828X.2011.01292.x [DOI] [PubMed] [Google Scholar]
- 29.Muche AA, Olayemi OO, Gete YK. Effects of gestational diabetes mellitus on risk of adverse maternal outcomes: a prospective cohort study in Northwest Ethiopia. BMC Pregnancy Childbirth 2020;20:1–13. 10.1186/s12884-020-2759-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popova PV, Grineva EN, Gerasimov AS, et al. The new combination of risk factors determining a high risk of gestational diabetes mellitus. Minerva Endocrinol 2015;40:239–47. [PubMed] [Google Scholar]
- 31.Wolka E, Deressa W, Reja A. Prevalence of gestational diabetes mellitus and associated factors in southern Ethiopia. ASIAN J Med Sci 2019;10. 10.3126/ajms.v10i1.21331 [DOI] [Google Scholar]
- 32.Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstet Gynecol Clin North Am 2007;34:173–99. 10.1016/j.ogc.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mwanri AW, Kinabo J, Ramaiya K, et al. Gestational diabetes mellitus in sub-Saharan Africa: systematic review and metaregression on prevalence and risk factors. Trop Med Int Health 2015;20:983–1002. 10.1111/tmi.12521 [DOI] [PubMed] [Google Scholar]
- 34.Agency Cstatistical. Federal Democratic Republic of Ethiopia central statistical agency population projection of Ethiopia for all regions at Wereda level from 2014 – 2017, 2014. [Google Scholar]
- 35.FANTA . Minimum Dietary Diversity for Women: A Guide for Measurement [Internet], 2016. Available: http://www.fao.org/3/a-i5486e.pdf
- 36.Matthey S, Barnett B, White T. The Edinburgh postnatal depression scale. Br J Psychiatry 2003;182:368. 10.1192/bjp.182.4.368 [DOI] [PubMed] [Google Scholar]
- 37.Edwards MK, Loprinzi PD. Affective responses to acute bouts of aerobic exercise, mindfulness meditation, and combinations of exercise and meditation: a randomized controlled intervention. Psychol Rep 2019;122:465–84. 10.1177/0033294118755099 [DOI] [PubMed] [Google Scholar]
- 38.Li G, Wei T, Ni W, et al. Incidence and risk factors of gestational diabetes mellitus: a prospective cohort study in Qingdao, China. Front Endocrinol 2020;11:1–9. 10.3389/fendo.2020.00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Njete HI, John B, Mlay P, et al. Prevalence, predictors and challenges of gestational diabetes mellitus screening among pregnant women in northern Tanzania. Trop Med Int Health 2018;23:236–42. 10.1111/tmi.13018 [DOI] [PubMed] [Google Scholar]
- 40.Abbey M, Kasso T. First trimester fasting blood glucose as a screening tool for diabetes mellitus in a teaching hospital setting in Nigeria. AJMAH 2018;10:1–9. 10.9734/AJMAH/2018/39385 [DOI] [Google Scholar]
- 41.Amenu K, Gelibo T, Getnet M. Magnitude and determinants of physical inactivity in Ethiopia : Evidence from 2015 Ethiopia National NCD Survey. Ethiop J Heal Dev 2017;31. [Google Scholar]
- 42.Mengesha MM, Roba HS, Ayele BH, et al. Level of physical activity among urban adults and the socio-demographic correlates: a population-based cross-sectional study using the global physical activity questionnaire. BMC Public Health 2019;19:1–11. 10.1186/s12889-019-7465-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hailemariam TT, Gebregiorgis YS, Gebremeskel BF, et al. Physical activity and associated factors among pregnant women in Ethiopia: facility-based cross-sectional study. BMC Pregnancy Childbirth 2020;20:1–11. 10.1186/s12884-020-2777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishra S, Kishore S. Effect of physical activity during pregnancy on gestational diabetes mellitus. Indian J Endocrinol Metab 2018;22:661–71. 10.4103/ijem.IJEM_618_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mdoe MB, Kibusi SM, Munyogwa MJ, et al. Prevalence and predictors of gestational diabetes mellitus among pregnant women attending antenatal clinic in Dodoma region, Tanzania: an analytical cross-sectional study. BMJ Nutr Prev Health 2021;4:69–79. 10.1136/bmjnph-2020-000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278:1078–83. 10.1001/jama.1997.03550130052036 [DOI] [PubMed] [Google Scholar]
- 47.Lewandowska M. Gestational diabetes mellitus (GDM) risk for declared family history of diabetes, in combination with BMI categories. Int J Environ Res Public Health 2021;18. doi: 10.3390/ijerph18136936. [Epub ahead of print: 28 06 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwak SH, Jang HC, Park KS. Finding genetic risk factors of gestational diabetes. Genomics Inform 2012;10:239. 10.5808/GI.2012.10.4.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C, Rawal S. Dietary iron intake, iron status, and gestational diabetes. Am J Clin Nutr 2017;106:1672S–80. 10.3945/ajcn.117.156034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kataria Y, Wu Y, Horskjær P, et al. Iron status and gestational diabetes—a meta-analysis. Nutrients 2018;10:621–15. 10.3390/nu10050621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byrn M, Penckofer S. The relationship between gestational diabetes and antenatal depression. J Obstet Gynecol Neonatal Nurs 2015;44:246–55. 10.1111/1552-6909.12554 [DOI] [PubMed] [Google Scholar]
- 52.Pace R, Rahme E, Da Costa D, et al. Association between gestational diabetes mellitus and depression in parents: a retrospective cohort study. Clin Epidemiol 2018;10:1827–38. 10.2147/CLEP.S184319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber B, Schweiger U, Deuschle M, et al. Major depression and impaired glucose tolerance. Exp Clin Endocrinol Diabetes 2000;108:187–90. 10.1055/s-2000-7742 [DOI] [PubMed] [Google Scholar]
- 54.Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med 2020;17:e1003053. 10.1371/journal.pmed.1003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaneto H, Kajimoto Y, Miyagawa J, et al. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes 1999;48:2398–406. 10.2337/diabetes.48.12.2398 [DOI] [PubMed] [Google Scholar]
- 56.Saeedi M, Hanson U, Simmons D, et al. Characteristics of different risk factors and fasting plasma glucose for identifying GDM when using IADPSG criteria: a cross-sectional study. BMC Pregnancy Childbirth 2018;18:1–6. 10.1186/s12884-018-1875-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reyes-Muñoz E, Sandoval-Osuna NL, Reyes-Mayoral C, et al. Sensitivity of fasting glucose for gestational diabetes mellitus screening in Mexican adolescents based on international association of diabetes and pregnancy study groups criteria: a diagnostic accuracy study based on retrospective data analysis. BMJ Open 2018;8:e021617–7. 10.1136/bmjopen-2018-021617 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.