Abstract
Objective
To investigate the impact of gestational hypertension and pre-eclampsia on preterm birth.
Design
The data were collected from the China–US Collaborative Project for Neural Tube Defect Prevention; this was a large population-based cohort study.
Setting and participants
We selected participants registered in two southern provinces, for whom we had exact information on gestational blood pressure and pregnancy outcomes, and who were not affected by chronic hypertension. In total, 200 103 participants were recruited from 1993 to 1995.
Outcome measures
Preterm birth was defined as a singleton pregnancy and birth before 37 gestational weeks.
Results
The incidences of gestational hypertension and pre-eclampsia were 5.47% and 5.44%, respectively, for women who gave birth at full term, and 5.63% and 7.33%, respectively, for those who gave birth preterm. After adjusting for potential confounders, the risk ratios (RRs) of preterm birth in women with gestational hypertension and pre-eclampsia were 1.04 (95% CI 0.98 to 1.11) and 1.39 (95% CI 1.25 to 1.55), respectively. The associations were stronger for early-onset (<28 weeks of gestation) gestational hypertension (adjusted RR=2.13, 95% CI 1.71 to 2.65) and pre-eclampsia (adjusted RR=8.47, 95% CI 5.59 to 12.80).
Conclusions
Pre-eclampsia was associated with a higher risk of preterm birth. The early-onset gestational hypertension and pre-eclampsia were associated with more severe risks than late-onset conditions.
Keywords: Hypertension, EPIDEMIOLOGY, Fetal medicine, Prenatal diagnosis, OBSTETRICS
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Strengths of the study are the large sample size, prospective study design and detailed subtypes of hypertensive disorders of pregnancy.
Limitation of this study is our inability to control for certain confounding factors including smoking and alcohol consumption.
Although this study reported the effect of hypertensive disorders of pregnancy and subtypes thereof on preterm birth, further researches are needed to explore the mechanisms’ results.
Introduction
Preterm birth, a common adverse pregnancy outcome, is one of the leading causes of child death globally, especially in developing countries.1 It is estimated that, globally, annual preterm live births number 14.84 million, thus 10.6% of all births. Asian countries account for 52.9% of global preterm births; the proportion in China (7.8%) is the second highest worldwide.2 Preterm birth greatly increases the risks of infant mortality and morbidity, and the risks of long-term effects including respiratory syndrome and infections, which bring heavy medical financial burdens on the families and countries.3 4 The mechanism of preterm birth is still uncertain, and some studies suggested that elevated blood pressure levels during pregnancy may play an important role in the development of preterm birth.5 6
Pregnancy-induced hypertension, including gestational hypertension and pre-eclampsia, complicates 6%–10% of pregnancies.7 Gestational hypertension and pre-eclampsia trigger new-onset hypertension after 20 weeks of gestation, with or without proteinuria.8 It remains uncertain whether gestational hypertension and pre-eclampsia are separate diseases sharing common medical manifestations or the same disorder differing only in terms of spectral position.9 Some studies have reported that pregnancy-induced hypertension, especially pre-eclampsia, may be a driver of preterm birth.10 However, researchers have observed that early-onset and late-onset pre-eclampsia may have different triggers,11 and should be viewed as distinct conditions. Women with early-onset pre-eclampsia (characterised by reduced placental blood flow) exhibit a higher level of vascular resistance than do those with late-onset pre-eclampsia (which differs in terms of both origin and the haemodynamics).12 13 The reported associations between gestational hypertension and preterm birth are not consistent.14 Buchbinder et al14 found that (compared with subjects with normotensive and mild pre-eclampsia) women with severe gestational hypertension exhibited a significantly higher rate of preterm delivery. However, another study found that gestational hypertension was not associated with preterm birth.15
Therefore, we performed a large prospective cohort study to investigate the impacts of gestational hypertension and pre-eclampsia on preterm birth in China. In addition, we also assessed whether the time of disease onset (early-onset or late-onset pregnancy-induced hypertension) affected the preterm birth rate.
Materials and methods
Background and original cohort
The methods have been described previously.16 17 Commencing in 1993, the Chinese Ministry of Health conducted a public health campaign to prevent neural tube defects in 21 counties of two southern provinces (Zhejiang and Jiangsu) and one northern province (Hebei). During this campaign, all women who planned to get married, or who became pregnant, were registered in a pregnancy-monitoring system. This served as the principal record of antenatal care and the prime source of demographic information. All women were advised to take 400 µg of folic acid daily, commencing at the time of registration and continuing until completion of the first trimester of pregnancy. Pills were distributed at the time of registration. At the end of each month, healthcare workers recorded the dates of all menstrual periods and how many pills remained in each bottle. All reproductive events that occurred after 20 complete gestational weeks (live births, stillbirths and pregnancy terminations) were recorded, as were all structural congenital anomalies (regardless of the gestational week). The original cohort included 247 831 women registered between October 1993 and September 1995 who delivered infants by 31 December 1996.18
Selection of study subjects
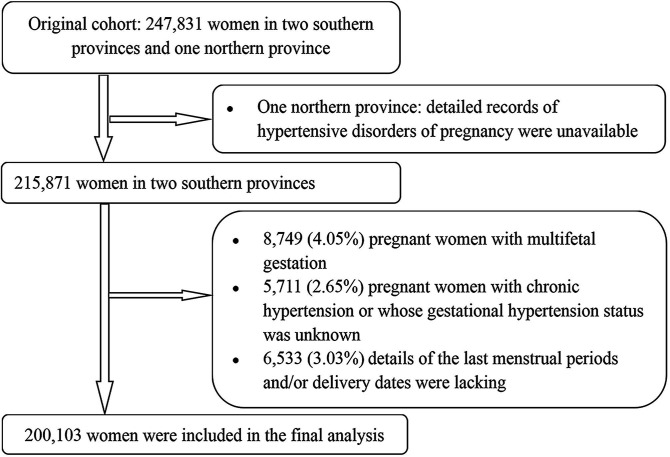
We selected participants registered in two southern provinces (Jiangsu and Zhejiang). Both provinces kept detailed records on pregnancy-induced hypertension. Of 215 871 women, we excluded 8749 (4.05%) with multifetal gestation, 5711 (2.65%) women with chronic hypertension or whose gestational hypertension diagnosis was unknown, and 6533 (3.03%) for whom details of the last menstrual periods and/or delivery dates were lacking. Ultimately, 200 103 women (92.70% of the target population) were included in the final analysis. Recruitment and derivation of the population used in the final analysis are shown in figure 1.
Figure 1.
Flow chart of participants.
Diagnosis of gestational hypertension and pre-eclampsia
An appropriate cuff bladder size was determined at each visit (based on arm circumference). Blood pressure was measured in the right arm using a mercury sphygmomanometer on at least two occasions separated by ≥6 hours. Gestational hypertension was defined as an absolute blood pressure ≥140/90 mm Hg after 20 weeks of gestation, or a blood pressure increment ≥30/15 mm Hg after 20 weeks of gestation compared with that of the first trimester.19 Pre-eclampsia (including eclampsia) was defined as a blood pressure of ≥140/90 mm Hg or a blood pressure increment 30/15 mm Hg after 20 weeks of gestation, with concurrent proteinuria (score 1+ as revealed by a dipstick test of a single random urine specimen collected after 20 weeks of gestation). Given the different heritabilities, clinical manifestations, and prognoses of early-onset and late-onset gestational hypertension and pre-eclampsia,18 20 we divided gestational hypertension and pre-eclampsia into two types: early-onset (<week 28 of gestation at diagnosis) and late-onset (at ≥week 28 of gestation at diagnosis).
Statistical analysis
We compared the mean age and body mass index (BMI), folic acid use, parity, ethnicity, educational level, and occupation between women with and without gestational hypertension. We used Student’s t-test to compare quantitative variables and the χ2 test to compare categorical variables. We calculated the incidences of preterm birth in women with and without gestational hypertension. Logistic regression models were used to estimate risk ratios (RRs) after adjusting for the principal underlying confounders including maternal age (continuous), BMI (continuous), educational level, occupation, parity, ethnicity and folic acid use. We compared the preterm birth incidences by the onset of hypertension and pre-eclampsia. The mean BMI was used if individual BMI data were lacking. All data were analysed using SPSS for Windows software (V.20.0). Statistical significance was defined as two-sided p<0.05.
Patient and public involvement
No patient was involved in the study design, in formulation of the study aims or the research questions, or in subject recruitment or study conduct. No patient was involved in the interpretation of results or manuscript preparation. We do not plan to disseminate the results to the study participants.
Results
Of the 200 103 women included in analysis, 19 115 (9.55%) evidenced gestational hypertension and 4912 (2.45%) pre-eclampsia. The baseline characteristics of the participants with gestational hypertension are shown in table 1. Nearly all were ethnically Han. Women with gestational hypertension were more likely to be older than others, to have a higher BMI, to be primiparous, to take folic acid and to be factory workers. The characteristics of women with and without pre-eclampsia are shown in online supplemental table 1. Women with pre-eclampsia were more likely to be older than others, to have a higher BMI, to be primiparous, to take folic acid and to have a lower education level.
Table 1.
Characteristics of women enrolled in the pregnancy-monitoring system by gestational hypertension status; China, 1993–1996
| Characteristics | Gestational hypertension group (n=19 115) | Non-gestational hypertension group (n=180 988) | P value | ||
| n* | % | n* | % | ||
| Age at pregnancy, (years, mean (SD)) |
25.04 (3.34) | 24.86 (3.21) | <0.001 | ||
| Body mass index at first visit (kg/m2, mean (SD)) | 20.77 (2.25) | 20.50 (2.09) | <0.001 | ||
| Primiparous | 16 277 | 85.15 | 149 967 | 82.86 | <0.001 |
| Han ethnicity | 18 977 | 99.28 | 179 670 | 99.27 | 0.923 |
| Folic acid use | 10 245 | 53.60 | 94 874 | 52.42 | 0.002 |
| Education level | 0.123 | ||||
| High school or higher | 2014 | 10.54 | 20 045 | 11.08 | |
| Junior high school | 11 380 | 59.53 | 107 253 | 59.26 | |
| Primary school or lower | 5672 | 29.67 | 53 175 | 29.38 | |
| Unknown | 49 | 0.26 | 515 | 0.28 | |
| Occupation | <0.001 | ||||
| Farmer | 10 930 | 57.18 | 106 992 | 59.12 | |
| Factory worker | 5409 | 28.30 | 49 257 | 27.22 | |
| Other† | 2754 | 14.41 | 24 480 | 13.53 | |
| Unknown | 22 | 0.12 | 259 | 0.14 | |
*Values for certain characteristics may be lower than the total numbers of subjects with or without gestational hypertension because some data were missing.
†Other includes businessman, teacher, day labourer and civil servant.
bmjopen-2021-058068supp001.pdf (50.8KB, pdf)
The overall incidence of preterm birth was 5.49%. The incidences for women with gestational hypertension and pre-eclampsia were 5.63% and 7.33%, respectively, and 5.47% and 5.44% for women with normal blood pressure. The RRs of preterm birth in women with gestational hypertension and pre-eclampsia were 1.03 (95% CI 0.97 to 1.10) and 1.38 (95% CI 1.23 to 1.53), respectively. Compared with the term birth group, the preterm birth group was more likely to be younger, of lower educational level and to not take folic acid (table 2). The RRs of preterm birth in women with gestational hypertension and pre-eclampsia were 1.04 (95% CI 0.98 to 1.11) and 1.39 (95% CI 1.25 to 1.55), respectively, after adjusting for the effects of major confounding factors (table 3). Sensitivity analysis was conducted after excluding newborns with major external birth defects (online supplemental table 2). The results were consistent with those described above. We also compared the preterm birth rates by early-onset or late-onset gestational hypertension. Early-onset hypertension was associated with an increased risk of preterm birth (adjusted RR=2.13, 95% CI 1.71 to 2.65), but the two types of pre-eclampsia exerted similar effects on preterm birth (early-onset: adjusted RR=8.47, 95% CI 5.59 to 12.80; late-onset: adjusted RR=1.30, 95% CI 1.16 to 1.46) (table 4).
Table 2.
Incidences and crude RRs of preterm birth by gestational hypertension and pre-eclampsia status, and other characteristics; China, 1993–1996
| Characteristic | No | Preterm birth | ||
| Incidence (%) | RR | 95% CI | ||
| Age (years) | ||||
| <20 | 896 | 10.6 | 1.92 | 1.55 to 2.38 |
| ≥20–<25 | 115 124 | 5.81 | 1 | — |
| ≥25–<30 | 59 960 | 5 | 0.85 | 0.82 to 0.89 |
| ≥30 | 24 123 | 4.9 | 0.84 | 0.78 to 0.89 |
| Body mass index (kg/m2) | ||||
| <18.5 | 28 576 | 5.54 | 1.01 | 0.96 to 1.07 |
| ≥18.5–<24 | 159 985 | 5.47 | 1 | — |
| ≥24–<28 | 10 610 | 5.45 | 1 | 0.91 to 1.09 |
| ≥28 | 932 | 6.33 | 1.17 | 0.90 to 1.52 |
| Education level | ||||
| High school or higher | 22 059 | 4.71 | 1 | — |
| Junior high school | 118 633 | 5.41 | 1.16 | 1.08 to 1.24 |
| Primary school or lower | 58 847 | 5.91 | 1.27 | 1.18 to 1.36 |
| Unknown | 564 | 5.85 | 1.26 | 0.88 to 1.80 |
| Occupation | ||||
| Farmer | 117 922 | 5.6 | 1 | — |
| Factory worker | 54 666 | 5.54 | 0.99 | 0.95 to 1.03 |
| Other* | 27 234 | 4.89 | 0.87 | 0.82 to 0.92 |
| Unknown | 281 | 2.49 | 0.43 | 0.20 to 0.91 |
| Parity | ||||
| Multiparous | 33 859 | 5.64 | 1 | — |
| Primiparous | 166 244 | 5.45 | 0.96 | 0.92 to 1.01 |
| Ethnicity | ||||
| Han | 198 647 | 5.48 | 1 | — |
| Other | 1456 | 6.46 | 1.19 | 0.97 to 1.47 |
| Folic acid use | ||||
| No | 94 984 | 5.9 | 1 | — |
| Yes | 105 119 | 5.1 | 0.86 | 0.82 to 0.89 |
| Gestational hypertension | ||||
| No | 180 988 | 5.47 | 1 | — |
| Yes | 19 115 | 5.63 | 1.03 | 0.97 to 1.10 |
| Pre-eclampsia | ||||
| No | 195 191 | 5.44 | 1 | — |
| Yes | 4912 | 7.33 | 1.38 | 1.23 to 1.53 |
*Other includes businessman, teacher, day labourer and civil servant.
RRs, risk ratios.
Table 3.
Associations between gestational hypertension and pre-eclampsia and preterm birth as revealed by multivariate logistic regression; China, 1993–1996
| Preterm birth | ||
| Risk factor | Adjusted RR | 95% CI |
| Age (continuous) | 1.04 | 1.03 to 1.05 |
| Factory worker | 1.00 | 0.96 to 1.05 |
| Other* | 0.93 | 0.87 to 1.00 |
| Unknown occupation | 0.40 | 0.19 to 0.87 |
| Junior high school | 1.10 | 1.02 to 1.18 |
| Primary school or lower | 1.22 | 1.13 to 1.32 |
| Unknown education | 1.38 | 0.95 to 1.99 |
| Folic acid use | 0.83 | 0.80 to 0.86 |
| Gestational hypertension | ||
| No | 1 | — |
| Yes | 1.04 | 0.98 to 1.11 |
| Pre-eclampsia | ||
| No | 1 | — |
| Yes | 1.39 | 1.25 to 1.55 |
*Other includes businessman, teacher, day labourer and civil servant.
RR, risk ratio.
Table 4.
Associations between early-onset and late-onset gestational hypertension and pre-eclampsia and the risk of preterm birth
| Onset period | Preterm birth | |||
| No | Incidence (%) | Crude RR (95% CI) | Adjusted RR (95% CI)* | |
| Gestational hypertension | ||||
| None† | 180 988 | 5.47 | 1 | 1 |
| Early-onset | 849 | 10.72 | 2.08 (1.67 to 2.58) | 2.13 (1.71 to 2.65) |
| Late-onset | 18 266 | 5.40 | 0.99 (0.92 to 1.06) | 1.00 (0.94 to 1.07) |
| Pre-eclampsia | ||||
| None† | 195 191 | 5.44 | 1 | 1 |
| Early-onset | 104 | 31.73 | 8.09 (5.35 to 12.23) | 8.47 (5.59 to 12.80) |
| Late-onset | 4808 | 6.80 | 1.27 (1.13 to 1.42) | 1.30 (1.16 to 1.46) |
*Adjusted for maternal age (continuous), BMI (continuous), education level, occupation, ethnicity, parity and folic acid use.
†Reference group.
BMI, body mass index; RR, risk ratio.
Discussion
Main findings of this study
This large prospective cohort study investigated whether gestational hypertension, pre-eclampsia and the onset time thereof were associated with the risk of preterm birth. We found that pre-eclampsia (particularly early-onset pre-eclampsia) was associated with an increased risk of preterm birth. Only early-onset gestational hypertension was significantly positively associated with preterm birth.
Our results are consistent with those of some previous studies.21–23 Bakker et al22 performed a prospective cohort study in the Netherlands to explore the association between blood pressure and preterm birth and reported an increased risk of preterm birth in a pre-eclampsia group compared with a non-hypertensive pregnant group (adjusted OR=5.89, 95% CI 2.63 to 13.14). However, no such association was observed in a gestational hypertension group, in agreement with our results. However, the cited work did not describe the onset of gestational hypertension or pre-eclampsia. A previous study suggested that onset time may affect the preterm birth rate.11 Another prospective cohort study showed that, compared with a normotensive group, women with pregnancy-induced hypertension were at an increased risk of preterm delivery (adjusted RR=5.1, 95% CI 3.4 to 7.8).23 Other studies reported similar results in different populations.24 25 However, a study in Zimbabwe found that the risk of preterm delivery did not vary between women with and without pregnancy-induced hypertension (p>0.05).26 The different findings may reflect differences in study design, population heterogeneity and the measurement methods.
Both the causes and outcomes of gestational hypertension and pre-eclampsia remain unclear. One study used survival curves to show that the risk factors imposed by both conditions were similar, but women with pre-eclampsia gave birth at a lower gestational age than did those with gestational hypertension.13 One retrospective case–control study showed that severe pre-eclampsia was more likely to be associated with preterm labour than gestational hypertension (OR=3.18, 95% CI 2.23 to 4.52).27 We also found that pre-eclampsia was associated with a higher risk of preterm birth than gestational hypertension. By contrast, one study subdivided the gestational hypertension and pre-eclampsia groups and found that severe gestational hypertension was associated with higher rates of adverse outcomes than was mild pre-eclampsia.14 These findings suggest that both high blood pressure and proteinuria may be strong indicators of preterm birth and should thus be carefully monitored.
The onset time of pregnancy-induced hypertension exerted different effects on preterm birth; early-onset subjects seemed to be at greater risk than late-onset cases.28 One study using population-based data found that an early-onset (<34 weeks) pre-eclampsia group exhibited a higher incidence of gestational age (34–36 weeks) than did a late-onset group (60.1% vs 23.4%).29 Another retrospective analysis also found that the rate of preterm birth was significantly higher in an early-onset than a late-onset group.30 One case series explored the maternal and neonatal outcomes of early-onset pre-eclampsia (before 26 weeks of gestation) and found high maternal complication rates and poor neonatal survival.31 All these findings are consistent with our results. One study used repeated antenatal blood pressure measurements to predict adverse outcomes and validated the results by performing a survey.32 The cited authors found that blood pressure data obtained after 28 weeks of gestation did not aid prediction of preterm birth. Some studies suggested that early-onset pregnancy-induced hypertension was associated with adverse neonatal outcomes, whereas late-onset hypertension exerted stronger effects on maternal disease.30 33 34 The earlier the complications, the longer the fetus was affected. Women with early-onset pregnancy-induced hypertension were more likely to give birth preterm.
Today, most preterm births in women with hypertensive disorders during pregnancy (especially pre-eclampsia) are medically induced. It is thus more meaningful to focus on gestational hypertension rather than pre-eclampsia. During the 1990s, many Chinese women did not seek maternal care, received little prenatal attention, and medical interventions seeking to prevent gestational hypertension and pre-eclampsia were delayed (or even absent) given a shortage of healthcare resources and poor insurance coverage. Especially in rural regions, Chinese women often lacked access to supplemental calcium, low-dose aspirin and magnesium sulfate, and were not educated in terms of lifestyle modification.35 36 In our cohort, the rates of caesarean section and induced delivery were 30.76% and 5.77%, respectively, in those with early-onset pre-eclampsia and 48.92% and 0.54%, respectively, in those with late-onset pre-eclampsia, thus much lower than today. In the 1990s in China, the medical philosophy was ‘let nature take its course’. Poorly controlled hypertension during pregnancy significantly increases maternal and fetal morbidity and mortality. The differences in the effects of gestational hypertension and pre-eclampsia on preterm birth that we found are those in (principally) women lacking active medical attention. This was thus an ‘epidemiological laboratory’ test; our pre-eclampsia data provide the theoretical baseline that will inform modern obstetric and paediatric guidelines.37
Possible mechanisms of this study
The pathophysiological mechanisms linking pregnancy-induced hypertension to preterm birth include inflammation, oxidative stress and endocrine disruption.38–40 Biomarkers of hypertensive disorders (including inflammatory interleukin-638 and reactive oxygen species39) play important roles in preterm birth.41 42 A recent study showed that hypertensive disorders may influence fetal growth by dysregulating the release of hormones including adipolines, triggering preterm birth.40 In particular, placental hypoperfusion induced by impaired development of placental blood vessels, and endothelial dysfunction, are the most common manifestations of high blood pressure and are also associated with reduced fetal growth.43 44
Strengths of this study
Our study had several strengths. First, this was a large, population-based, prospective cohort study; hypertensive data were collected during pregnancy (thus before birth). Selection and recall biases are minimal. The large sample size afforded excellent statistical power. Also, we considered the onset time of gestational hypertension and pre-eclampsia; this afforded more comprehensive insights. Finally, most participants were Han people living in similar regions, ensuring comparability.
Limitations of this study
Our work had certain limitations. Although we report the impacts of early-onset gestational hypertension and pre-eclampsia on preterm birth, we cannot discuss possible causal relationships; additional research is needed. Some confounding factors (eg, maternal smoking and alcohol consumption data) were unavailable. However, during the time of the study, few (especially rural, reproductive-age) Chinese women smoked or drank. A 1996 survey revealed that the smoking rate among Chinese women aged 20–29 years was less than 2%.45 Other maternal lifestyles such as physical activity, diet quality, and factors such as family income and gestational diabetes were also unavailable in this study. Further studies are needed to collect these pieces of information and test their possible modification effect. Another limitation is this study being population based. The original cohort included two southern provinces and one northern province; we excluded the latter because detailed clinical data on hypertension during pregnancy were unavailable.
We investigated the impacts of gestational hypertension and pre-eclampsia on preterm birth, and the effects of disease onset time. Women with pre-eclampsia were at a significantly higher risk of preterm birth. Early-onset hypertensive disorders of pregnancy affected the preterm birth rate more than did late-onset disorders.
Conclusion
In this large, prospective cohort study on Chinese women, early-onset gestational hypertension and pre-eclampsia both significantly increased the risk of preterm birth. Pre-eclampsia may be more detrimental than gestational hypertension. It is important to increase awareness of the health risks associated with hypertensive disorders of pregnancy, particularly the risk of preterm birth.
Supplementary Material
Acknowledgments
The authors thank all of the volunteers and staff involved in this research.
Footnotes
Contributors: HA, MJ and NL proposed the study concept and designed the analysis. HA performed the analyses and authored the first draft of the article. MJ, NL, LZ, ZL, HL and YZ provided advice on the first draft. RY and NL accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. All of the authors reviewed the manuscript and approved it before submission.
Funding: NL was supported by the National Natural Science Foundation of China (grant number: 81903327, 82173527), Beijing Natural Science Foundation (grant number: 7194285), Young Elite Scientist Sponsorship Program by CAST (YESS) (2018QNRC001), and National Clinical Research Center for Obstetrics and Gynecology (Peking University Third Hospital) (no. BYSYSZKF2021001). The original project was supported by a cooperative agreement between the US Centers for Disease Control and Prevention and Peking University (grant number: U01 DD000293).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Original data are available on request. Please contact the corresponding author for further information (linan01@pku.edu.cn).
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
The project was approved by the institutional review boards of the US Centers for Disease Control and Prevention and the Peking University Health Science Center. As most women in the (rural) study area were illiterate in the early 1990s, all women who took pills provided oral informed consent.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37–46. 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9. 10.1016/S0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- 4.Araújo BFde, Zatti H, Madi JM, et al. Analysis of neonatal morbidity and mortality in late-preterm newborn infants. J Pediatr 2012;88:259–66. 10.2223/jped.2196 [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Villar J, Sun W, et al. Blood pressure dynamics during pregnancy and spontaneous preterm birth. Am J Obstet Gynecol 2007;197:162.e1–162.e6. 10.1016/j.ajog.2007.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Li Z, Ye R, et al. Preconception blood pressure and risk of preterm birth: a large cohort study in China. J Hypertens 2016;34:2243–7. 10.1097/HJH.0000000000001069 [DOI] [PubMed] [Google Scholar]
- 7.Kintiraki E, Papakatsika S, Kotronis G, et al. Pregnancy-induced hypertension. Hormones 2015;14:211–23. 10.14310/horm.2002.1582 [DOI] [PubMed] [Google Scholar]
- 8.Villar J, Carroli G, Wojdyla D, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol 2006;194:921–31. 10.1016/j.ajog.2005.10.813 [DOI] [PubMed] [Google Scholar]
- 9.Li X, Tan H, Huang X, et al. Similarities and differences between the risk factors for gestational hypertension and preeclampsia: a population based cohort study in South China. Pregnancy Hypertens 2016;6:66–71. 10.1016/j.preghy.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 10.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009;33:130–7. 10.1053/j.semperi.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 11.Yung HW, Colleoni F, Dommett E, et al. Noncanonical mitochondrial unfolded protein response impairs placental oxidative phosphorylation in early-onset preeclampsia. Proc Natl Acad Sci U S A 2019;116:18109–18. 10.1073/pnas.1907548116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valensise H, Vasapollo B, Gagliardi G, et al. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension 2008;52:873–80. 10.1161/HYPERTENSIONAHA.108.117358 [DOI] [PubMed] [Google Scholar]
- 13.Shen M, Smith GN, Rodger M, et al. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PLoS One 2017;12:e0175914. 10.1371/journal.pone.0175914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchbinder A, Sibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol 2002;186:66–71. 10.1067/mob.2002.120080 [DOI] [PubMed] [Google Scholar]
- 15.Ray JG, Burrows RF, Burrows EA, et al. Mos HIP: McMaster outcome study of hypertension in pregnancy. Early Hum Dev 2001;64:129–43. 10.1016/S0378-3782(01)00181-5 [DOI] [PubMed] [Google Scholar]
- 16.Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. N Engl J Med Overseas Ed 1999;341:1485–90. 10.1056/NEJM199911113412001 [DOI] [PubMed] [Google Scholar]
- 17.Gindler J, Li Z, Berry RJ, et al. Folic acid supplements during pregnancy and risk of miscarriage. Lancet 2001;358:796–800. 10.1016/S0140-6736(01)05969-4 [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Ye R, Zhang L, et al. Folic acid supplementation during early pregnancy and the risk of gestational hypertension and preeclampsia. Hypertension 2013;61:873–9. 10.1161/HYPERTENSIONAHA.111.00230 [DOI] [PubMed] [Google Scholar]
- 19.Group CNGHW . National epidemiological investigation of gestational hypertension (in Chinese). Chin J Gynecol Obstet 1991;26:67–70 PMID:https://pubmed.ncbi.nlm.nih.gov/1860367/ [Google Scholar]
- 20.Hernández-Díaz S, Werler MM, Louik C, et al. Risk of gestational hypertension in relation to folic acid supplementation during pregnancy. Am J Epidemiol 2002;156:806–12. 10.1093/aje/kwf129 [DOI] [PubMed] [Google Scholar]
- 21.Fatemeh T, Marziyeh G, Nayereh G, et al. Maternal and perinatal outcome in nulliparious women complicated with pregnancy hypertension. J Pak Med Assoc 2010;60:707–10. [PubMed] [Google Scholar]
- 22.Bakker R, Steegers EAP, Hofman A, et al. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: the generation R study. Am J Epidemiol 2011;174:797–806. 10.1093/aje/kwr151 [DOI] [PubMed] [Google Scholar]
- 23.Berhe AK, Ilesanmi AO, Aimakhu CO, et al. Effect of pregnancy induced hypertension on adverse perinatal outcomes in Tigray regional state, ethiopia: a prospective cohort study. BMC Pregnancy Childbirth 2019;20:7. 10.1186/s12884-019-2708-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulualem G, Wondim A, Woretaw A. The effect of pregnancy induced hypertension and multiple pregnancies on preterm birth in Ethiopia: a systematic review and meta-analysis. BMC Res Notes 2019;12:91. 10.1186/s13104-019-4128-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Premkumar A, Baer RJ, Jelliffe-Pawlowski LL, et al. Hypertensive disorders of pregnancy and preterm birth rates among black women. Am J Perinatol 2019;36:148–54. 10.1055/s-0038-1660461 [DOI] [PubMed] [Google Scholar]
- 26.Muti M, Tshimanga M, Notion GT, et al. Prevalence of pregnancy induced hypertension and pregnancy outcomes among women seeking maternity services in Harare, Zimbabwe. BMC Cardiovasc Disord 2015;15:111. 10.1186/s12872-015-0110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C-M, Cheng P-J, Chang S-D. Maternal complications and perinatal outcomes associated with gestational hypertension and severe preeclampsia in Taiwanese women. J Formos Med Assoc 2008;107:129–38. 10.1016/S0929-6646(08)60126-6 [DOI] [PubMed] [Google Scholar]
- 28.Mol BWJ, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet 2016;387:999–1011. 10.1016/S0140-6736(15)00070-7 [DOI] [PubMed] [Google Scholar]
- 29.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 2013;209:544.e1–544.e12. 10.1016/j.ajog.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 30.Ni Y, Cheng W. Comparison of indications of pregnancy termination and prognosis of mothers and neonates in early- and late-onset preeclampsia. Hypertens Pregnancy 2016;35:315–22. 10.3109/10641955.2016.1143486 [DOI] [PubMed] [Google Scholar]
- 31.van Oostwaard MF, van Eerden L, de Laat MW, et al. Maternal and neonatal outcomes in women with severe early onset pre-eclampsia before 26 weeks of gestation, a case series. BJOG 2017;124:1440–7. 10.1111/1471-0528.14512 [DOI] [PubMed] [Google Scholar]
- 32.Macdonald-Wallis C, Silverwood RJ, de Stavola BL, et al. Antenatal blood pressure for prediction of pre-eclampsia, preterm birth, and small for gestational age babies: development and validation in two general population cohorts. BMJ 2015;351:h5948. 10.1136/bmj.h5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robillard P-Y, Dekker G, Chaouat G, et al. Historical evolution of ideas on eclampsia/preeclampsia: a proposed optimistic view of preeclampsia. J Reprod Immunol 2017;123:72–7. 10.1016/j.jri.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robillard P-Y, Dekker G, Chaouat G, et al. High incidence of early onset preeclampsia is probably the rule and not the exception worldwide. 20th anniversary of the reunion workshop. A summary. J Reprod Immunol 2019;133:30–6. 10.1016/j.jri.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 35.Gao Y, Barclay L. Availability and quality of emergency obstetric care in Shanxi province, China. Int J Gynaecol Obstet 2010;110:181–5. 10.1016/j.ijgo.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 36.Li F, Qin J, Zhang S, et al. Prevalence of hypertensive disorders in pregnancy in China: a systematic review and meta-analysis. Pregnancy Hypertens 2021;24:13–21. 10.1016/j.preghy.2021.02.001 [DOI] [PubMed] [Google Scholar]
- 37.Kucukgoz Gulec U, Ozgunen FT, Buyukkurt S, et al. Comparison of clinical and laboratory findings in early- and late-onset preeclampsia. J Matern Fetal Neonatal Med 2013;26:1228–33. 10.3109/14767058.2013.776533 [DOI] [PubMed] [Google Scholar]
- 38.Catov JM, Lewis CE, Lee M, et al. Preterm birth and future maternal blood pressure, inflammation, and intimal-medial thickness: the cardia study. Hypertension 2013;61:641–6. 10.1161/HYPERTENSIONAHA.111.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutherland MR, Bertagnolli M, Lukaszewski M-A, et al. Preterm birth and hypertension risk: the oxidative stress paradigm. Hypertension 2014;63:12–18. 10.1161/HYPERTENSIONAHA.113.01276 [DOI] [PubMed] [Google Scholar]
- 40.Magalhães ESdaS, Méio MDBB, Peixoto-Filho FM, et al. Pregnancy-induced hypertension, preterm birth, and cord blood adipokine levels. Eur J Pediatr 2020;179:1239–46. 10.1007/s00431-020-03586-8 [DOI] [PubMed] [Google Scholar]
- 41.Gilman-Sachs A, Dambaeva S, Salazar Garcia MD, et al. Inflammation induced preterm labor and birth. J Reprod Immunol 2018;129:53–8. 10.1016/j.jri.2018.06.029 [DOI] [PubMed] [Google Scholar]
- 42.Moore TA, Ahmad IM, Zimmerman MC. Oxidative stress and preterm birth: an integrative review. Biol Res Nurs 2018;20:497–512. 10.1177/1099800418791028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra VK, Hobel CJ, Sing CF. Placental blood flow and the risk of preterm delivery. Placenta 2009;30:619–24. 10.1016/j.placenta.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Everett TR, Lees CC. Beyond the placental bed: placental and systemic determinants of the uterine artery doppler waveform. Placenta 2012;33:893–901. 10.1016/j.placenta.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 45.Yang G, Fan L, Tan J, et al. Smoking in China: findings of the 1996 national prevalence survey. JAMA 1999;282:1247–53. 10.1001/jama.282.13.1247 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058068supp001.pdf (50.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Original data are available on request. Please contact the corresponding author for further information (linan01@pku.edu.cn).