Abstract
Context
Endothelial dysfunction may occur early in the development of cardiovascular and metabolic diseases; however, it remains often underestimated and studies rarely discriminate between diabetes types. We have examined endothelial function and its determinants during the early course of type 1 and type 2 diabetes.
Methods
Caucasian participants of the prospective German Diabetes Study (GDS) with known diabetes duration <1 year (n = 398) or without diabetes, but of similar age, body mass index (BMI) and sex distribution (n = 109), underwent measurements of flow-mediated dilation (FMD) and nitroglycerin-mediated dilatation (NMD). Whole-body insulin sensitivity (M-value) was assessed by hyperinsulinemic–euglycemic clamps and physical fitness (VO2max) by spiroergometry. A subset of individuals with type 1 or type 2 diabetes (n = 108) was re-evaluated after 5 years.
Results
At baseline, neither FMD nor NMD differed between people with diabetes and the matched glucose-tolerant groups. At the 5-year follow-up, decline in FMD (–13.9%, P = .013) of persons with type 2 diabetes was independent of age, sex, and BMI, but associated with baseline adipose tissue insulin resistance and indices of liver fibrosis. The M-value decreased in both type 1 and type 2 diabetes groups by 24% and 15% (both P < .001, respectively) over 5 years. Higher HbA1c, lower M-value, and lower VO2max at baseline was associated with lower FMD in both type 1 and type 2 diabetes.
Conclusion
Endothelial function decreases during the early course of type 2 diabetes. In addition to age and BMI, insulin sensitivity at diagnosis was the best predictor of progressive impairment in endothelial function in type 2 diabetes.
Keywords: endothelial function, flow-mediated dilatation, nitroglycerin-mediated dilatation, insulin resistance
Endothelial dysfunction occurs frequently in persons with diabetes mellitus and is associated with increased cardiovascular risk in longstanding diabetes (1). Thus, assessment of endothelial function has been employed aiming at better prediction of cardiovascular disease (2). Endothelial dysfunction is characterized by a reduced flow-mediated vasodilation due to decreased nitric oxide (NO) bioavailability (3). In people with diabetes, several mechanisms may explain endothelial dysfunction, such as impaired NO release, disturbed expression of receptors, and receptor-coupled mechanisms involved in vasorelaxation, reduced availability of the substrates for NO synthesis, progressive reduction of circulating progenitor cells, enhanced release of endothelium‐derived constricting factors, and decreased sensitivity of the vascular smooth muscle to NO signaling (4, 5). Particularly in type 2 diabetes (T2D), other abnormalities such as insulin resistance, ectopic lipid deposition as is the case in nonalcoholic fatty liver disease (NAFLD) and dysregulated fatty acid metabolism also contribute to elevated cardiovascular risk and may relate to endothelial dysfunction (6-8). The endothelial alterations may result from systemic release of free fatty acids or inflammatory cytokines from adipose tissue and liver (6, 8). Finally, chronic sedentary lifestyle and low physical fitness can accelerate endothelial dysfunction and cardiovascular risk (9).
Endothelial function can be measured by a noninvasive technique to estimate endothelial NO bioavailability during reactive hyperemia after blood flow restriction, and relies on its flow-mediated dilatation (FMD) of the brachial artery (1). Endothelium-independent nitroglycerin-mediated dilatation (NMD) allows one to evaluate smooth muscle sensitivity to NO as a vital mechanism involved in early atherosclerotic changes (10). However, there is a paucity of data regarding the dynamic changes in endothelial function assessed by FMD as well as NMD and the metabolic factors determining these changes in adults with recently diagnosed diabetes (11). Most studies were performed in patients with longstanding diabetes and did not directly compare type 1 diabetes (T1D) and T2D with matched glucose-tolerant control groups. However, it is clinically relevant to detect endothelial dysfunction as early as possible in order to identify those individuals with diabetes at the highest cardiovascular risk.
Thus, this study investigated the impact of changes in insulin sensitivity, ectopic lipid accumulation—specifically NAFLD—and physical fitness on endothelial function within the first year as well as 5 years after the diagnosis of T1D and T2D. We hypothesized that whole-body as well as adipose tissue insulin resistance and beta-cell dysfunction are the main drivers of the progression of endothelial dysfunction in recent-onset diabetes.
Materials and Methods
Volunteers
Caucasians with T1D (n = 179) or T2D (n = 218) and their respective age-, sex-, and body mass index (BMI)–matched glucose tolerant controls (CON1, n = 58; and CON2, n = 51) were recruited from the ongoing prospective observational German Diabetes Study (GDS) (Figure 1 (12)) (13). This analysis from a subgroup of the GDS, which was studied between 2009 and 2019, aimed at assessing endothelial dysfunction from FMD of the brachial artery as a surrogate of diabetes-related macrovascular risk. The GDS enrolls individuals with recently diagnosed diabetes (known disease duration: <12 months) and glucose-tolerant persons, aged 18-69 years, and with a planned follow-up of 20 years to monitor diabetes-related comorbidities and complications (13). Diagnosis of diabetes is based on the criteria of the American Diabetes Association (14). The glucose-tolerant control participants underwent a 75-g oral glucose tolerance test to exclude dysglycemia, had no first-degree relatives with known diabetes, and were examined only at baseline. Further exclusion criteria for all participants included diabetes due to other causes (genetic, pancreatic, etc.), pregnancy, acute or severe chronic cardiac, hepatic, renal, or psychiatric diseases, or immunosuppressive treatment. People with excessive alcohol consumption were excluded, as defined by >42 g alcohol on any single day and >88 g alcohol per week for women, and >65 g alcohol on any single day and >196 g alcohol per week for men (15). Persons with high-sensitivity C-reactive protein (hsCRP) values >1 mg/dL were excluded because acute inflammatory disease could not be ruled out, as were persons with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m², indicating relevant impairment of kidney function. Additionally, follow-up data after 5 years of disease duration from subgroups with T1D (n = 52) or T2D (n = 56) were included in this analysis.
Endothelial Function
FMD and NMD were measured using B mode ultrasonography on a Logiq S8 GE system (GE Healthcare, Munich, Germany) (16-18). Briefly, a blood pressure cuff was placed on the widest part of the proximal right forearm and inflated to 200 mmHg for 5 minutes to induce occlusion of the brachial artery; the subsequent release led to reactive hyperemia and NO bioavailability. The brachial artery was scanned on the anteromedial cubital region in longitudinal sections at baseline and after deflation. For NMD measurements patients received sublingually a single dose of 0.4 mg of glyceryl trinitrate spray (Nitrolingual akut® Spray, G. Pohl-Boskamp GmbH & Co. KG, Hohenlockstedt, Germany). Images of the brachial artery were obtained before and 3 minutes after administration. FMD and NMD were calculated as the quotient between the arterial baseline diameter and the diameter after cuff deflation and after medication administration, respectively, using Brachial-Analyzer software (Medical Imaging Applications, Coralville, Iowa, USA) (19). All data underwent intensive quality control check. Furthermore, 2 experienced operators performed the validation of FMD method in 29 consecutive participants of GDS using independent measurements of FMD. Inter-rater variability coefficients were computed and compared with current literature (16-18). The inter-rater agreement of the FMD measurements, rendering a coefficient of variation of 3% for the arterial diameter and 25% for FMD, is shown elsewhere (Figure 2 (12)).
Cardiovascular Parameters
Blood pressure was measured (Omron M400, Omron Healthcare GmbH, Mannheim, Germany) in the supine position following a 30-minute rest on both arms and both legs. The ankle–brachial index (ABI) was computed from the ratio of the systolic blood pressure measured at the ankle to that measured at the brachial artery (20). Heart rate was recorded together with an electrocardiogram in resting conditions.
Bioimpedance Analysis
Bioimpedance analysis was used for assessing fat mass and fat-free mass and calculating percent fat mass (BioElectrical Impedance Analyzer System, RJL Systems, Detroit, MI, USA) (13).
Modified Botnia Clamp Test
This test consists of an intravenous glucose tolerance test followed by a hyperinsulinemic–euglycemic clamp test with frequent measurements of glucose, C-peptide, and insulin (13). The intravenous glucose tolerance test was started by administrating an intravenous 30% glucose bolus of 1 mL/kg (body weight) followed by timed blood sampling for 60 minutes. An intravenous priming dose of human insulin (Insuman Rapid; Sanofi; Frankfurt, Germany) was then applied (10 mU × kg [body weight]–1× min–1 for 10 minutes) continued by constant infusion of 1.5 mU × kg (body weight)–1× min–1. Blood glucose concentration was maintained at 90 mg/dL by a variable 20% glucose infusion, and whole-body insulin sensitivity (M-value) was calculated from whole-body mean glucose infusion rates with glucose space correction during steady state (13). Fasting adipose tissue insulin sensitivity was assessed from the adipose tissue insulin resistance index (ADIPO-IR), which was calculated as fasting serum insulin × fasting plasma free fatty acid (FFA) (21).
Physical (Cardiorespiratory) Fitness
An incremental exhaustive exercise test on the cycle ergometer (Ergometrics 900; Ergoline, Bitz, Germany) was used to assess physical fitness (22). Heart rate, electrocardiogram, blood pressure, and respiratory gas exchange were continuously monitored. The maximal exhaustion was reached on average after 12 to 15 minutes of exercise with the load rate increasing by 16 W/min, and maximal oxygen consumption was calculated (VO2max).
Laboratory Analyses
Routine laboratory parameters were measured as described (13). The fatty liver index (FLI), as an estimate of liver steatosis, was computed from routine laboratory parameters using the equation (0.953 × log triglycerides + 0.139 × BMI + 0.718 × log γ-glutamyl transferase (GGT) + 0.053 × waist circumference – 15.745)/(1 + 0.953 × log triglycerides + 0.139 × BMI + 0.718 × log GGT + 0.053 × waist circumference – 15.745) × 100. The fibrosis index (FIB-4), as an estimate of liver fibrosis, was computed from routine laboratory parameters using the equation age × aspartate aminotransferase (AST)/platelet count × (alanine aminotransferase [ALT]) × ½) and used to describe NAFLD (23).
Statistics
Data are presented as mean (SD) for continuous variables and percentage for categorical variables. Skewed data were log-transformed before analysis. Matching was performed by propensity score for age, sex, and BMI. Additional analyses adjusted for age, sex, BMI, and baseline values were performed to exclude these variables as confounding factors where necessary. Associations between parameters have been evaluated using linear regression models and corresponding P values and were conducted separately for each diabetes group. Regression models were used to assess the best predictors of variable changes over time. P < .05 was considered to indicate statistically significant differences or correlations. Post hoc power calculation was based on the observed SD of 5.3 and 4.9 as estimated from the FMD mean values in the respective data sets, with sufficient statistical power for a medium effect size (Cohen’s d, mean difference divided by SD) of 0.5. Based on 1000 replicates each, the statistical power to detect such an effect was 0.92 and 0.90 in the T1D/CON1 and in the T2D/CON2 comparison, respectively. Statistical analyses were performed with SAS (version 9.4; SAS Institute, Cary, NC, USA).
Study Approval
The study was approved by the ethics committee of the Medical Faculty of the Heinrich-Heine University of Düsseldorf (reference number 4508), registered at Clinicaltrials.gov (identifier number: NCT01055093) and performed according to the Declaration of Helsinki as reported previously (13). All participants gave their written informed consent prior to study inclusion.
Results
Baseline
Clinical characteristics of the study population
Anthropometric and clinical data are shown in Table 1. People with T2D had higher BMI and waist-to-hip ratio than those with T1D (both P < .001), and higher waist-to-hip ratio than their respective controls (CON2; P < .001), independently of age and sex. The average self-reported daily alcohol intake was 16 g for men and 10 g for women. Fasting blood glucose, HbA1c, and hsCRP were higher in T1D than in T2D groups (P = .012, P = 0.001, P = .049), and, as expected, higher than in the respective control groups (T1D: P < .001, P < .001, P = 0.048; T2D: all P < .001). The majority of people with diabetes (76%) had good glycemic control at baseline (average HbA1c < 7% [53 mmol/mol]) according to current guidelines (24). Glucose-lowering medication is summarized elsewhere (Tables 2 and 3 (12)). For the most frequently used medication (metformin) additional analyses were performed to examine possible differences between treated and not-treated participants (Figure 3 (12)). There were no differences between participants on metformin and those on nonpharmacological treatment at baseline. The low number of participants in the other medication groups did not allow further statistical analyses (Tables 2 and 3 (12)).
Table 1.
Characteristics of the study population at baseline
| CON1 | T1D | CON2 | T2D | |
|---|---|---|---|---|
| N (m/f) | 58 (32/26) | 179 (103/76) | 51 (36/15) | 218 (152/66) |
| Known diabetes duration (months) | — | 6.1 ± 2.6 | — | 5.9 ± 3.1 |
| Age (years) | 37.0 ± 12.5 | 36.5 ± 11.1 | 50.8 ± 10.8 | 51.2 ± 10.3b |
| BMI (kg/m2) | 25.1 ± 3.9 | 24.9 ± 4.1 | 28.7 ± 3.7 | 30.1 ± 4.9b |
| WHR | 0.86 ± 0.08 | 0.88 ± 0.09 | 0.92 ± 0.07 | 0.96 ± 0.07a,b |
| Fat mass (%) | 20.1 ± 8.8 | 19.6 ± 8.6 | 28.1 ± 8.1 | 30.2 ± 10.3 |
| hsCRP (mg/dL) | 0.16 ± 0.32 | 0.22 ± 0.37a | 0.18 ± 0.27 | 0.30 ± 0.29a |
| Fasting blood glucose (mg/dL) | 88 ± 16 | 133 ± 41a | 89 ± 7 | 127 ± 32a,b |
| HbA1c (% (mmol/mol)) | 5.1 ± 0.2 | 6.6 ± 1.2a | 5.3 ± 0.3 | 6.4 ± 0.9a,b |
| eGFR (mL/min/1.73 m²) | 97.6 ± 13.5 | 101.8 ± 14.6 | 89.7 ± 11.9 | 90.0 ± 15.8 |
| Total cholesterol (mg/dL) | 186 ± 40 | 186 ± 42 | 205 ± 37 | 199 ± 44 |
| LDL-cholesterol (mg/dL) | 114 ± 38 | 111 ± 34 | 132 ± 34 | 128 ± 36 |
| HDL-cholesterol (mg/dL) | 65 ± 19 | 62 ± 18 | 59 ± 18 | 45 ± 13a,b |
| Triglycerides (mg/dL) | 99 ± 58 | 90 ± 62 | 142 ± 192 | 175 ± 197a |
| ALT (U/L) | 21.8 ± 9.5 | 23.7 ± 19.0 | 24.8 ± 10.4 | 33.7 ± 21.8a |
| AST (U/L) | 23.8 ± 6.6 | 21.7 ± 7.9a | 23.3 ± 6.6 | 25.7 ± 12.7 |
| GGT (U/L) | 20.8 ± 15.9 | 20.6 ± 16.8 | 28.6 ± 22.2 | 42.5 ± 65.72a,b |
| FFA (µmol/l) | 476 ± 190 | 656 ± 290a | 526 ± 190 | 638 ± 256a |
| ADIPO-IR (a.u.) | 3.1 ± 2.5 | 7.6 ± 8.6a | 4.5 ± 3.4 | 10.5 ± 7.7a,b |
Data are shown as absolute numbers or mean ± standard deviation, as applicable.
Abbreviations: ADIPO-IR, adipose tissue insulin resistance index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; a.u., arbitrary units; BMI, body mass index; CON1, age-, sex-, BMI-matched controls for the type 1 diabetes group; CON2, age-, sex-, BMI-matched controls for the type 2 diabetes group; eGFR, estimated glomerular filtration rate; FFA, free fatty acids; GGT, γ-glutamyl transferase; hsCRP, high-sensitivity C-reactive protein; HbA1c, glycated hemoglobin; T1D, type 1 diabetes; T2D, type 2 diabetes; WHR, waist-to-hip ratio.
a P ≤ .05 T1D or T2D vs CON1 or CON2, respectively.
b P ≤ .05 T1D vs T2D adjusted for age, sex, BMI.
Endothelial function and cardiovascular risk factors
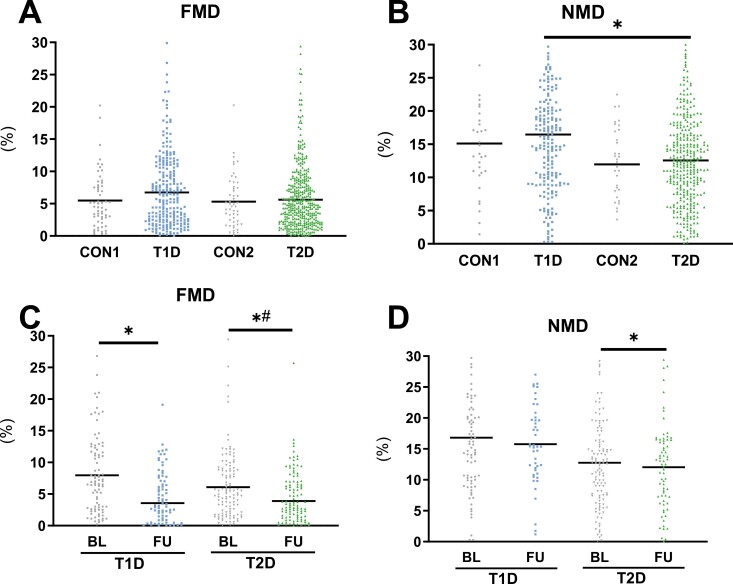
At baseline, people with T1D had higher NMD than those with T2D (P = .037). After adjustments for age, sex, and BMI, no difference was observed in endothelial function assessed by FMD and NMD across all groups (Fig. 1A and 1B; T1D vs CON1: FMD P = .101, NMD P = .257; T2D vs CON2: FMD P = .517, NMD P = .417; T1D vs T2D: FMD P = .261).
Figure 1.
Endothelial function of the study population, parameters of endothelial function in patients with newly diagnosed type 1 diabetes (T1D), type 2 diabetes (T2D), and matched glucose-tolerant controls (CON1 and CON2) showing flow-mediated dilation (FMD) (A, C) and nitroglycerin-mediated dilation (NMD) (B, D) at baseline (BL), and at the 5-year follow-up (FU) in a subgroup of patients with type 1 and type 2 diabetes. Scatter plot with mean. *P < .05. #P < .05 adjusted for age, sex, BMI, and baseline value.
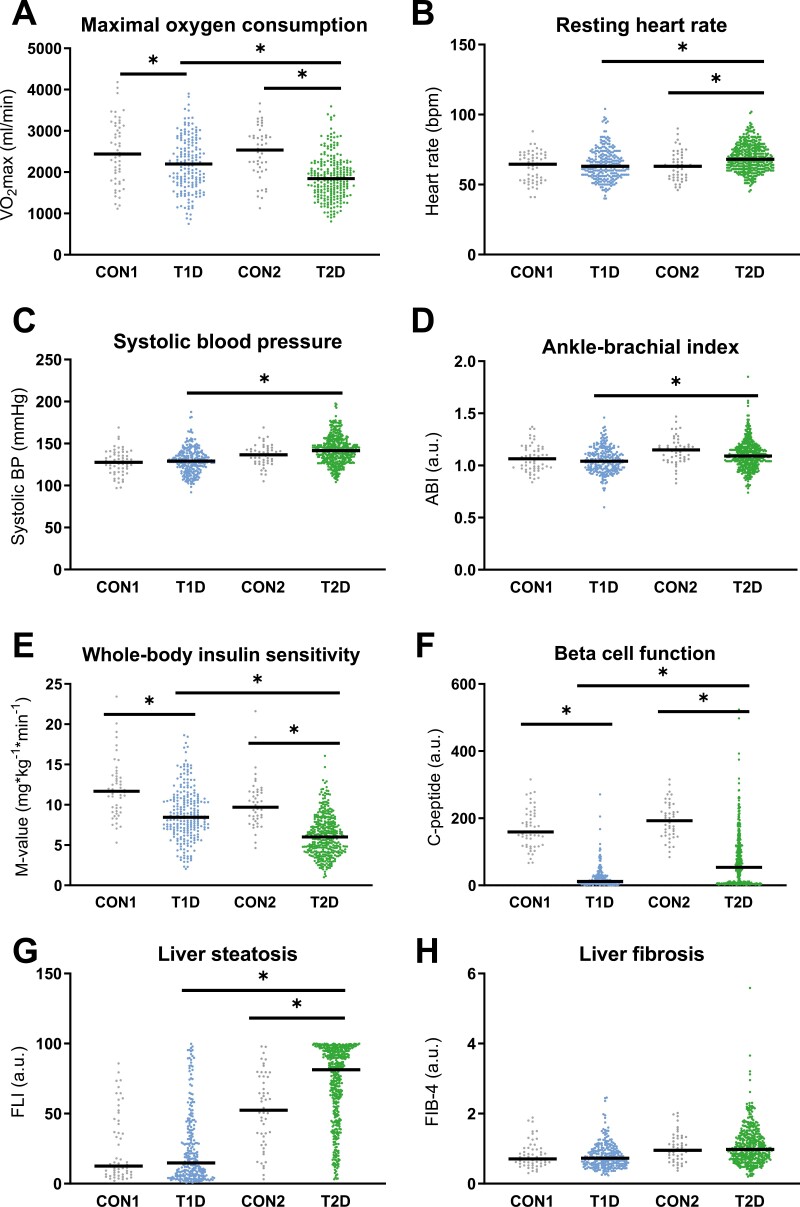
Both diabetes groups had lower VO2max than their respective controls (Fig. 2A; T1D vs CON1: P = .007; T2D vs CON2: P < .001), and participants with T2D also showed higher resting heart rate than the matched control (P = .001) and T1D groups (Fig. 2B; P = .003), and higher systolic blood pressure than the T1D groups (Fig. 2C; P = .017). However, there were no differences in diastolic blood pressure (T1D vs CON1: P = .764; T2D vs CON2: P = .728) or ABI (T1D vs CON1: P = .268; T2D vs CON2: P = .856) between participants with diabetes and control groups (Fig. 2D). The T2D group had lower high-density lipoprotein (HDL)-cholesterol than T1D (P < .001) and CON2 (P < .001), and higher triglycerides than CON2 (Table 1; P = .007). Of those with T1D, 176 (89.8%) had fasting triglyceride levels <150 mg/dL and 85 (43.8%) had low-density lipoprotein (LDL) levels <100 mg/dL, while of the patients with T2D only 157 (57.5%) and 55 (20.2%), respectively, achieved the recommended target values (25).
Figure 2.
Baseline characteristics of the study population, physical fitness (A), resting heart rate (B), systolic blood pressure (C), and ankle–brachial index (D), insulin sensitivity (E), beta-cell function (F), indices of liver steatosis (G) and liver fibrosis (H) in patients with newly diagnosed type 1 diabetes (T1D), type 2 diabetes (T2D) and matched glucose-tolerant controls (CON1 and CON2, respectively) at baseline. Scatter plot with mean. *P < .05.
Insulin sensitivity and liver function
Whole-body insulin sensitivity and beta-cell function were lower in T1D than in CON1 (12.0 ± 3.5 vs 9.0 ± 3.2 mg × kg–1 min–1, P < .001) and in T2D than in CON2 (10.2 ± 3.1 vs 6.7 ± 2.8 mg × kg–1× min–1, P < .001) (Fig. 2E and 2F). The adipose tissue insulin resistance index was higher in the T2D than in T1D group (P < .001), and in both groups it was higher than the respective controls. Both diabetes groups also had higher circulating FFA than the respective controls (Table 1; T1D vs CON1: ADIPO-IR P < .001, FFA P < .001; T2D vs CON2: ADIPO-IR P < .001, FFA P = .005).
Estimates of NAFLD were higher in T2D, as shown by increases in FLI compared with T1D (P < .001) and CON2 (Fig. 2G; P = .001) as well as in ALT and γGGT than CON2 (Table 1; P = .003 and P = .007).
5-Year Follow-Up
Clinical characteristics
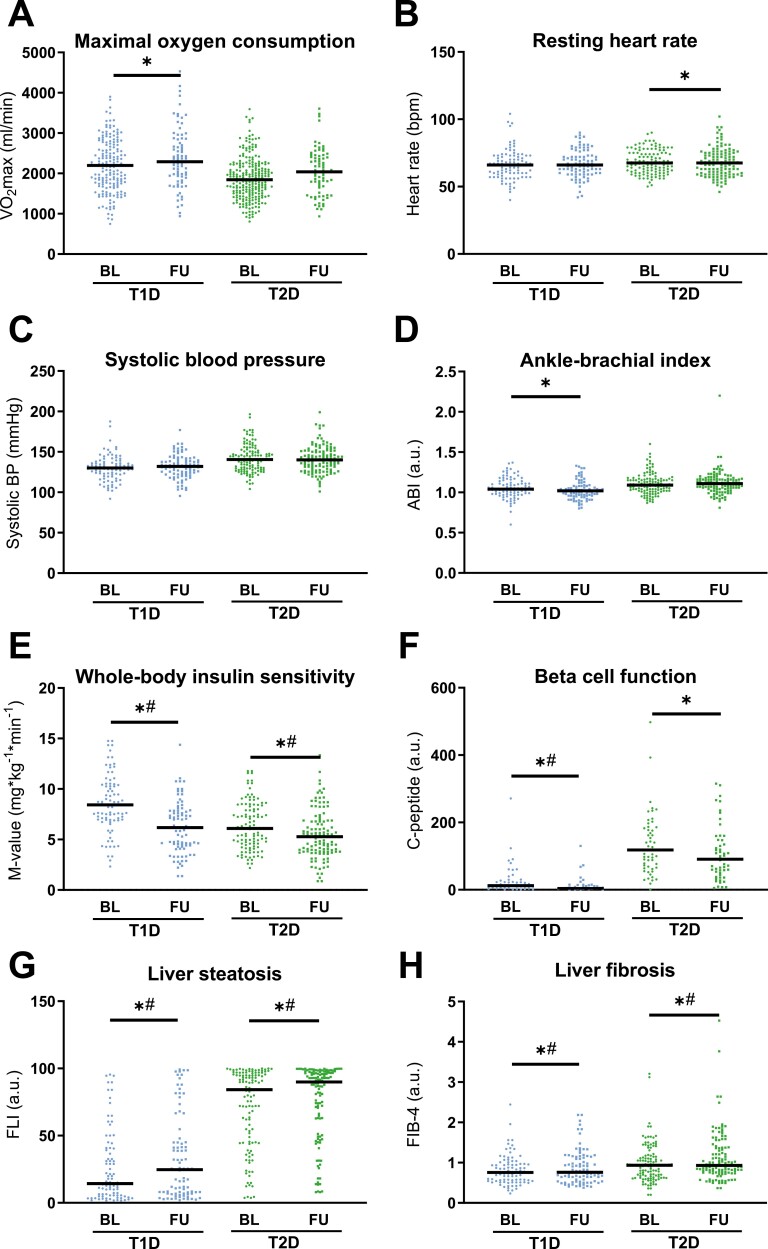
A subgroup of participants in both diabetes groups was evaluated at 5 years after diabetes diagnosis. BMI increased only in T1D (P = .003), whereas fat mass increased in both T1D (P = .001) and T2D (P =0.028) groups (Table 1 (12)). Glycemic control (fasting blood glucose, HbA1c) worsened independently of age, sex, and BMI in both groups (all P < .001).
Endothelial function and cardiovascular risk factors
FMD decreased in both T1D and T2D (Fig. 1C; P = .002 and P = .043), while NMD decreased only in T2D after 5 years (Fig. 1D; P = .025). Only in T2D did the decline in FMD remain after adjustment for age, sex, and BMI and the respective FMD value at baseline (–13.9%, P = .013; Fig. 1C and 1D). Interestingly, there were no changes from baseline to follow-up in VO2max (T1D P = .104, T2D P = .933), heart rate (T1D P = .635, T2D P = .870), systolic (T1D P = .227, T2D P = .169) or diastolic blood pressure (T1D P = .074, T2D P = .057), ABI (Fig. 3A-3D; T1D P = .330, T2D P = .964), or blood lipids (Table 1 (12); total cholesterol: T1D P = .352, T2D P = .502; LDL-cholesterol: T1D P = .668, T2D P = .282; HDL-cholesterol: T1D P = .747, T2D P = .274; triglycerides: T1D P = .429, T2D P = .276).
Figure 3.
Follow-up characteristics of the study population, Physical fitness (A), resting heart rate (B), systolic blood pressure (C), and ankle–brachial index (D), insulin sensitivity (E), beta-cell function (F), indices of liver steatosis (G), and liver fibrosis (H) in patients with type 1 diabetes (T1D), type 2 diabetes (T2D) at baseline (BL) as well as at the 5-year follow-up (FU). Scatter plot with mean. *P < .05. #P < .05 adjusted for age, sex, BMI, and baseline value.
Insulin sensitivity and liver function
Whole-body insulin sensitivity decreased by 24.3% and 14.9% in the T1D and T2D groups, respectively (both P < .001; Fig. 3E), whereas the decrease in beta-cell function remained significant after adjustment only in T1D (Fig. 3F; P < .001). Adipose tissue insulin sensitivity did not change within the groups at the 5-year follow-up (Table 1 (12); T1D P = .677, T2D P = .883). In both diabetes groups, NAFLD progressed, as demonstrated by increasing FLI (T1D P = .026, T2D P = .010) and FIB-4 (Fig. 3G and 3H; T1D P = .009, T2D P < .001). All these differences remained after adjustments for age, sex, and BMI. Further, people with T2D had higher GGT levels at 5 years (Table 2 (12); P = .047).
Associations Between Endothelial Function and Baseline Metabolic and Cardiovascular Parameters
Regression models revealed that higher HbA1c and lower M-value at baseline predicted FMD decline in both T1D and T2D (Figure 4A,B (12)). This finding was supported by an analysis comparing individuals with the highest and lowest insulin sensitivity (highest/lowest quartile) in each group (data not shown). Persons with T1D and high M-value at baseline developed no changes in FMD over 5 years, while FMD decreased in individuals with the lowest M-value at baseline (P = .002).
Moreover, higher VO2max at baseline was associated with a rise in FMD (P = .044) during 5 years in T2D, even after adjustment for age, sex, and BMI. On the other hand, elevated baseline FLI, triglycerides, and GGT predicted a greater increase in FMD in T2D (R2 = 0.36, P = .007, R2 = 0.48, P = .021, and R2 = 0.60, P = .031, respectively), while fat mass and hsCRP additionally predicted a decline of FMD in T1D (R2 = –0.16, P = .016 and R2 = –0.43, P = .012, respectively) after adjustments for age, sex, BMI, and baseline values.
In T1D, changes in FMD over 5 years positively correlated with changes in eGFR (P = .008, Figure 4C (12)), heart rate (P = .032), and VO2max (P = .038). People with T2D showed an inverse correlation between FMD and AST at baseline (P = .045). Additionally, baseline adipose tissue insulin resistance (P = .045) and FIB-4 (P = .029) were associated with decline in endothelial function in T2D. In T2D, there was a negative correlation between changes in FMD and ABI (P = .013, Figure 4D (12) ) as well as between changes in NMD and systolic blood pressure (P = .036).
Regression models revealed that age and eGFR best predict the changes in FMD within the T1D group (R2 = 0.76, all P < .05), while age, BMI, and M-value best predict the changes in FMD within the T2D group (R2 = 0.66, all P < .05) after adjustments for age, sex, BMI, and baseline values.
There were no significant differences for any correlation between parameters of endothelial dysfunction and beta-cell function in both diabetes groups.
Medications of persons with T1D and T2D are shown elsewhere (Table 3 (12)). The change in patterns compared with medications at baseline can be found elsewhere (Figures 5 and 6, respectively (12)).
Discussion
In this cohort of persons with recent-onset diabetes, there were no differences in FMD and NMD when compared with glucose-tolerant people after adjustments for age, sex, and BMI. However, persons with T2D develop an approximately 14% decline in endothelial function during the first 5 years of diabetes diagnosis, which is associated with estimates of adipose tissue insulin resistance and liver fibrosis at diagnosis. Further, this study identified glycemic control, whole-body insulin sensitivity, and physical fitness as key determinants of decreasing endothelial function in diabetes.
Previous studies examining endothelial function assessed by FMD in persons with diabetes revealed conflicting results. While recent meta-analyses showed impaired endothelial function in T1D (26) and T2D compared with healthy people (27), another recent population-based study suggested no association between diabetes and endothelial function (11). Previous studies reported reduced FMD only for overt T2D, but not for participants with impaired glucose tolerance (28) or at the time of diabetes onset (19). The present study adds to these data that endothelial dysfunction may not be necessarily present at diagnosis, but rapidly develop despite only minor changes in fat mass and glycemic control, and no changes in lipid profile in metabolically well-controlled patients with T2D during 5 years.
Chronic hyperglycemia impairs vascular function and could contribute to the increased cardiovascular risk in diabetes (29). However, it has been postulated that endothelial dysfunction may pre-date the onset of hyperglycemia in T2D, suggesting a role for insulin resistance, altered adipokine secretion patterns, or abnormal concentrations of metabolites other than glucose (30). Partly in line with this concept, the present study found that besides hyperglycemia, other metabolic alterations at baseline, such as clamp-derived whole-body (peripheral, mainly skeletal muscle) insulin resistance as well as fasting adipose tissue insulin resistance and physical fitness, are relevant predictors of impaired endothelial function in diabetes, independent of age, sex, and BMI. The present data suggest that peripheral insulin resistance rather than decline in beta-cell dysfunction seems to be the main driver of early endothelial dysfunction, particularly in T2D. Similarly, a previous analysis including participants of the GDS cohort demonstrated that both hyperglycemia and insulin resistance associate with modest alterations in cardiac autonomic function, specifically baroreflex dysfunction, in well-controlled recent-onset T2D individuals during the first 5 years (31). Mechanistically, these alterations could be linked to activation of the novel protein kinase C–diacylglycerol pathway (32), an established mechanism of insulin resistance in various tissues (7). Previous studies suggested that insulin may chronically modulate vascular tone and that activation of protein kinase C isoforms in the vascular tissues may be a primary event leading to endothelial dysfunction in insulin-resistant states (33). Insulin resistance and abnormal mitochondrial function represent frequently observed features of persons with T2D (7), but may also be a feature of T1D (34). From recent studies suggesting that mitochondrial dynamics are relevant to endothelial-cell function (35) and cardiovascular protection (36) it is tempting to speculate that interventions directed toward restoring mitochondrial function in humans with diabetes may be beneficial also for preventing and treating endothelial dysfunction. Although metformin treatment may affect mitochondrial function also in endothelium (37), only a few studies have analyzed its impact on endothelial function in persons with diabetes. One small-scale study found no effect of 3-day treatment with metformin on endothelial function in healthy humans (38), whereas another study reported improvements in endothelial function induced by 12-week metformin intake in persons with metabolic syndrome, but without diabetes (39).
Aside from endothelial dysfunction, NAFLD represents another risk factor for cardiovascular disease, likely via its relationship with insulin resistance, dyslipidemia, and T2D (40). Indeed, in T2D, parameters of liver function such as transaminases and surrogate markers for liver steatosis and fibrosis were associated with FMD, suggesting a link between hepatic alterations and cardiovascular risk, even at the early diabetes stages.
Pronounced dyslipidemia as a key atherogenic risk factor (41), along with reduced insulin sensitivity and hypertension, also contributes to elevated cardiovascular risk (42) and increases the hazard for cardiovascular events and cardiac vulnerability (43). Of note, in our cohort of recently diagnosed persons with T2D, only 19% were prescribed lipid-lowering medications, which may explain that about 80% and 43% did not meet guideline-recommended targets for LDL-cholesterol and triglycerides, respectively. This is in line with previous work showing that almost half of the study participants with T2D do not meet the recommended targets (44).
Other measures of cardiovascular protection include preserving kidney function as well as reduction of chronic inflammation and ectopic fat deposition, such as in the liver (45). These observations are strengthened by showing that eGFR is closely associated with endothelial dysfunction, specifically in those with T1D. However, novel glucose-lowering drugs with known cardiovascular and renal protective effects (46) were not prescribed frequently and early, as reflected by the low number of participants receiving sodium-glucose cotransporter-2 inhibitors or glucagon-like peptide 1 receptor agonists in the present cohort.
Recent studies suggested that NMD can be a more potent marker for predicting future cardiovascular events in persons at risk (10, 47). The alleged superiority of NMD compared with FMD in detecting early vascular changes might make it more suitable for monitoring disease progression and assessing cardiovascular risk in diabetes. In contrast, the present study showed a decrease in FMD in T2D, while NMD remained unchanged after adjustments for age, sex, and BMI and baseline NMD value. Interestingly, another study reported no difference in NMD between metabolically healthy and individuals at early stages of diabetes (28). This suggests that FMD could be more sensitive to subtle changes in endothelial function during the early course of the disease. However, endothelium-dependent vasodilatation in human forearm circulation is not fully dependent on NO, but also involves other mechanisms, including endothelium-dependent hyperpolarization (48). It is likely that endothelium-dependent hyperpolarization–dependent vasodilation also contributed to the observed differences between groups.
Some limitations of the current study need to be considered. By design, the GDS includes only Caucasian volunteers with well-controlled T1D and T2D, which does not allow one to generalize the results to all people with diabetes. On the other hand, the study benefits from gold-standard methodology and the recruitment of well-matched control groups. Further, the FMD technique is inherently susceptible to higher inter-rater variability, which we tried to overcome by assessment of the variation and allowing only trained and experienced operators to perform the measurements. The follow-up study represents a strength of the current study, but is limited in that no follow-up data are available for the control group. The loss to follow-up in the diabetes population mainly derives from the GDS being an ongoing study so that not all participants had reached the follow-up disease duration milestones at the time of analysis.
Conclusions
These results suggest that endothelial function decreases in T2D soon after diagnosis. Glycemic control, insulin sensitivity, along with physical fitness seem to be important determinants on the development of impaired endothelial function over the early course of the disease. Furthermore, the present data suggest that treatment concepts aiming at early amelioration of hyperglycemia and insulin resistance could contribute to prevention of progressive endothelial dysfunction in people with diabetes mellitus.
Acknowledgements
We would like to thank the staff of the German Diabetes Center for their excellent support. The GDS group consists of H. Al-Hasani, V. Burkart, A.E. Buyken, G. Geerling, C. Herder, A. Icks, K. Jandeleit-Dahm, J. Kotzka, O. Kuss, E. Lammert, W. Rathmann, V. Schrauwen-Hinderling, J. Szendroedi, S. Trenkamp, D. Ziegler, and M. Roden (speaker).
Glossary
Abbreviations
- ABI
ankle–brachial index
- ADIPO-IR
adipose-tissue insulin resistance index
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- eGFR
estimated glomerular filtration rate
- FFA
free fatty acids
- FIB-4
fibrosis index
- FLI
fatty liver index
- FMD
flow-mediated dilation
- GDS
German Diabetes Study
- GGT
γ-glutamyl transferase
- HDL
high-density lipoprotein
- hsCRP
high-sensitivity C-reactive protein
- LDL
low-density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- NMD
nitroglycerin-mediated dilatation
- NO
nitric oxide
- T1D
type 1 diabetes
- T2D
diabetes
- VO2max
maximal oxygen consumption
Contributor Information
Oana Patricia Zaharia, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany; German Center for Diabetes Research (DZD e. V.), Partner Düsseldorf, Neuherberg, Germany; Department of Endocrinology and Diabetology, Medical Faculty and University Hospital, Heinrich-Heine University, Düsseldorf, Germany.
Martin Schön, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany; German Center for Diabetes Research (DZD e. V.), Partner Düsseldorf, Neuherberg, Germany.
Luca Löffler, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany.
Klaus Strassburger, German Center for Diabetes Research (DZD e. V.), Partner Düsseldorf, Neuherberg, Germany; Institute for Biometrics and Epidemiology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany.
Clara Möser, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany; German Center for Diabetes Research (DZD e. V.), Partner Düsseldorf, Neuherberg, Germany.
Iryna Yurchenko, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany; German Center for Diabetes Research (DZD e. V.), Partner Düsseldorf, Neuherberg, Germany.
Kálmán Bódis, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany; German Center for Diabetes Research (DZD e. V.), Partner Düsseldorf, Neuherberg, Germany; Department of Endocrinology and Diabetology, Medical Faculty and University Hospital, Heinrich-Heine University, Düsseldorf, Germany.
Sofia Antoniou, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany.
Yanislava Karusheva, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany; German Center for Diabetes Research (DZD e. V.), Partner Düsseldorf, Neuherberg, Germany.
Julia Szendroedi, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany; German Center for Diabetes Research (DZD e. V.), Partner Düsseldorf, Neuherberg, Germany; Department of Endocrinology and Diabetology, Medical Faculty and University Hospital, Heinrich-Heine University, Düsseldorf, Germany.
Volker Burkart, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany; German Center for Diabetes Research (DZD e. V.), Partner Düsseldorf, Neuherberg, Germany.
Michael Roden, Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany; German Center for Diabetes Research (DZD e. V.), Partner Düsseldorf, Neuherberg, Germany; Department of Endocrinology and Diabetology, Medical Faculty and University Hospital, Heinrich-Heine University, Düsseldorf, Germany.
Funding
The GDS was initiated and financed by the German Diabetes Center (DDZ), which is funded by the German Federal Ministry of Health (Berlin, Germany) and the Ministry of Culture and Science of the state North Rhine-Westphalia (Düsseldorf, Germany) and from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.), Research Network SFB 1116/2 of the German Research Foundation, and the Schmutzler Stiftung. MR is further supported by grants from the European Funds for Regional Development (EFRE-0400191), EUREKA Eurostars-2 (E! 113230 DIA-PEP). The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author Contributions
O.P.Z. and M.S. wrote the manuscript and researched data. O.P.Z., L.L., K.B., Y.K., D.F.M., S.A., and C.M. performed the examinations and researched data. O.P.Z. and S.A. performed the measurements for inter-rater reliability. K.S. performed the statistical analyses. J.S., V.B., and M.R. contributed to the discussion and reviewed/edited the manuscript. M.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read, critically reviewed, and approved the final manuscript. Henrike Sell initiated the project, researched data, and performed preliminary analyses. Tragically, Henrike Sell died before the completion of the manuscript.
Disclosure Statement
The authors have nothing to disclose with regard to this manuscript. The German Diabetes Study is registered under NCT01055093.
Data Availability Statement
The data sets generated and/or analyzed during the current study are not publicly available, since they are subject to national data protection laws and restrictions imposed by the ethics committee to ensure data privacy of the study participants. However, they can be applied for through an individual project agreement with the principal investigator of the German Diabetes Study.
References
- 1. Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation 2012;126(6):753-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109(23 Suppl 1):III-27-III-32. [DOI] [PubMed] [Google Scholar]
- 3. Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes care. 2009;32(Suppl 2):S314-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130(5):963-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449-1457. [DOI] [PubMed] [Google Scholar]
- 6. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600. [DOI] [PubMed] [Google Scholar]
- 7. Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature 2019;576(7785):51-60. [DOI] [PubMed] [Google Scholar]
- 8. Pleiner J, Schaller G, Mittermayer F, Bayerle-Eder M, Roden M, Wolzt M. FFA-induced endothelial dysfunction can be corrected by vitamin C. J Clin Endocrinol Metab. 2002;87(6):2913-2917. [DOI] [PubMed] [Google Scholar]
- 9. Lee J-H, Lee R, Hwang M-H, Hamilton MT, Park Y. The effects of exercise on vascular endothelial function in type 2 diabetes: a systematic review and meta-analysis. Diabetol Metab Syndr. 2018;10(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawano N, Emoto M, Mori K, et al. Association of endothelial and vascular smooth muscle dysfunction with cardiovascular risk factors, vascular complications, and subclinical carotid atherosclerosis in type 2 diabetic patients. J Atheroscler Thromb. 2012;19(3):276-284. [DOI] [PubMed] [Google Scholar]
- 11. Empen K, Lorbeer R, Völzke H, et al. Do patients with type 1 and type 2 diabetes really have an impaired endothelial function? A population-based propensity score matching analysis. Cardiovasc Diabetol. 2013;12(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaharia OP, Schön M, Löffler L, et al. Metabolic factors predict changes in endothelial function during the early course of type 1 and type 2 diabetes. Repository reference. Deposited August 25, 2022. doi: 10.6084/m9.figshare.20486082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szendroedi J, Saxena A, Weber KS, et al. Cohort profile: the German Diabetes Study (GDS). Cardiovasc Diabetol. 2016;15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S13-s27. [DOI] [PubMed] [Google Scholar]
- 15. Mayer-Davis EJ, Naimi TS, Mattes RD. Proposed reductions in limits on added sugar and alcohol for the new dietary guidelines: our perspective. Am J Clin Nutr. 2021;114(2):405-406. [DOI] [PubMed] [Google Scholar]
- 16. Hijmering ML, Stroes ES, Pasterkamp G, Sierevogel M, Banga JD, Rabelink TJ. Variability of flow mediated dilation: consequences for clinical application. Atherosclerosis 2001;157(2):369-373. [DOI] [PubMed] [Google Scholar]
- 17. Ghiadoni L, Faita F, Salvetti M, et al. Assessment of flow-mediated dilation reproducibility: a nationwide multicenter study. J Hypertens. 2012;30(7):1399-1405. [DOI] [PubMed] [Google Scholar]
- 18. West SG, Wagner P, Schoemer SL, et al. Biological correlates of day-to-day variation in flow-mediated dilation in individuals with Type 2 diabetes: a study of test-retest reliability. Diabetologia. 2004;47(9):1625-1631. [DOI] [PubMed] [Google Scholar]
- 19. Saatmann N, Zaharia OP, Strassburger K, et al. Physical fitness and cardiovascular risk factors in the novel diabetes subgroups. J Clin Endocrinol Metab. 2022;107(4):1127-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aboyans V, Criqui Michael H, Abraham P, et al. Measurement and Interpretation of the Ankle-Brachial Index. Circulation 2012;126(24):2890-2909. [DOI] [PubMed] [Google Scholar]
- 21. Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio metabolism study. Diabetes. 2017;66(4):815-822. [DOI] [PubMed] [Google Scholar]
- 22. Rohling M, Strom A, Bonhof G, et al. Differential patterns of impaired cardiorespiratory fitness and cardiac autonomic dysfunction in recently diagnosed type 1 and type 2 diabetes. Diabetes Care. 2017;40(2):246-252. [DOI] [PubMed] [Google Scholar]
- 23. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 2017;66(5):1486-1501. [DOI] [PubMed] [Google Scholar]
- 24. Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34(39):3035-3087. [DOI] [PubMed] [Google Scholar]
- 25. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139(25):e1082-e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lespagnol E, Dauchet L, Pawlak-Chaouch M, et al. Early endothelial dysfunction in type 1 diabetes is accompanied by an impairment of vascular smooth muscle function: a meta-analysis. Front Endocrinol. 2020;11:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meyer MF, Lieps D, Schatz H, Pfohl M. Impaired flow-mediated vasodilation in type 2 diabetes: lack of relation to microvascular dysfunction. Microvasc Res. 2008;76(1):61-65. [DOI] [PubMed] [Google Scholar]
- 28. Henry RM, Ferreira I, Kostense PJ, et al. Type 2 diabetes is associated with impaired endothelium-dependent, flow-mediated dilation, but impaired glucose metabolism is not; the Hoorn Study. Atherosclerosis 2004;174(1):49-56. [DOI] [PubMed] [Google Scholar]
- 29. Makimattila S, Virkamaki A, Groop PH, et al. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation 1996;94(6):1276-1282. [DOI] [PubMed] [Google Scholar]
- 30. Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nature Clinical Practice Endocrinology & Metabolism 2007;3(1):46-56. [DOI] [PubMed] [Google Scholar]
- 31. Kück JL, Bönhof GJ, Strom A, et al. Impairment in baroreflex sensitivity in recent-onset type 2 diabetes without progression over 5 years. Diabetes. 2020;69(5):1011-1019. [DOI] [PubMed] [Google Scholar]
- 32. Inoguchi T, Xia P, Kunisaki M, Higashi S, Feener EP, King GL. Insulin’s effect on protein kinase C and diacylglycerol induced by diabetes and glucose in vascular tissues. Am J Physiol. 1994;267(3 Pt 1):E369-E379. [DOI] [PubMed] [Google Scholar]
- 33. Kuboki K, Jiang ZY, Takahara N, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation 2000;101(6):676-681. [DOI] [PubMed] [Google Scholar]
- 34. Kaul K, Apostolopoulou M, Roden M. Insulin resistance in type 1 diabetes mellitus. Metab Clin Exp. 2015;64(12):1629-1639. [DOI] [PubMed] [Google Scholar]
- 35. Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013;112(8):1171-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scheiber D, Zweck E, Jelenik T, et al. Reduced myocardial mitochondrial ROS production in mechanically unloaded hearts. J Cardiovasc Transl Res. 2019;12(2):107-115. [DOI] [PubMed] [Google Scholar]
- 37. Apostolova N, Iannantuoni F, Gruevska A, Muntane J, Rocha M, Victor VM. Mechanisms of action of metformin in type 2 diabetes: effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020;34:101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. El Messaoudi S, Schreuder TH, Kengen RD, et al. Impact of metformin on endothelial ischemia-reperfusion injury in humans in vivo: a prospective randomized open, blinded-endpoint study. PLoS One. 2014;9(4):e96062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jahn LA, Hartline L, Liu Z, Barrett EJ. Metformin improves skeletal muscle microvascular insulin resistance in metabolic syndrome. Am J Physiol Endocrinol Metab. 2022;322(2):E173-E180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341-1350. [DOI] [PubMed] [Google Scholar]
- 41. Li X, Zhou Z, Huang G, Su H, Yan X, Yang L. Metabolic syndrome in adult-onset latent autoimmune diabetes. Metab Syndr Relat Disord 2005;3(2):174-180. [DOI] [PubMed] [Google Scholar]
- 42. Marini MA, Fiorentino TV, Succurro E, et al. Association between hemoglobin glycation index with insulin resistance and carotid atherosclerosis in non-diabetic individuals. PLoS One. 2017;12(4):e0175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jelenik T, Flogel U, Alvarez-Hernandez E, et al. Insulin resistance and vulnerability to cardiac ischemia. Diabetes. 2018;67(12):2695-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yokoyama H, Oishi M, Takamura H, et al. Large-scale survey of rates of achieving targets for blood glucose, blood pressure, and lipids and prevalence of complications in type 2 diabetes (JDDM 40). BMJ Open Diabetes Res Care. 2016;4(1): e000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zweck E, Roden M. GLP-1 receptor agonists and cardiovascular disease: drug-specific or class effects? Lancet Diabetes Endocrin. 2019;7(2):89-90. [DOI] [PubMed] [Google Scholar]
- 46. Hirshberg B, Katz A. Cardiovascular outcome studies with novel antidiabetes agents: scientific and operational considerations. Diabetes Care. 2013;36(Suppl 2):S253-S258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akamatsu D, Sato A, Goto H, et al. Nitroglycerin-mediated vasodilatation of the brachial artery may predict long-term cardiovascular events irrespective of the presence of atherosclerotic disease. J Atheroscler Thromb. 2010;17(12): 1266-1274. [DOI] [PubMed] [Google Scholar]
- 48. Goto K, Kitazono T. Endothelium-dependent hyperpolarization (EDH) in diabetes: mechanistic insights and therapeutic implications. Int J Mol Sci. 2019;20(15) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are not publicly available, since they are subject to national data protection laws and restrictions imposed by the ethics committee to ensure data privacy of the study participants. However, they can be applied for through an individual project agreement with the principal investigator of the German Diabetes Study.