Abstract
Brucellosis is characterized by abortion in ruminants and a protracted undulant fever in humans, which often results in severe pathological manifestations. Scant information exists about the molecular mechanisms employed by Brucella abortus to combat host defenses or to persist and replicate within host cells. Transposon (Tn5) mutagenesis of B. abortus and the subsequent screening of mutants for sensitivity to killing in murine macrophages and in the mouse model led to the identification of mutants which were severely attenuated for intracellular survival. One group of mutants was interrupted in cydB, a gene that is part of the cydAB operon encoding cytochrome bd oxidase, which catalyzes an alternate terminal electron transport step in bacterial respiration. The elevated affinity for molecular oxygen of this enzyme in Escherichia coli has suggested that it is involved in the protection of sensitive enzymatic activities such as those of hydrogenases and nitrogenases from damage. B. abortus cydB::Tn5 strains exhibited heightened sensitivity to the respiratory inhibitors zinc and azide, highly reactive oxygen species such as hydrogen peroxide, low pH, and attenuated virulence in the mouse model of infection. Virulence was restored by an intact copy of cydAB or by B. abortus genes encoding the oxidative radical-scavenging enzyme Cu/Zn superoxide dismutase or catalase. These results suggest a bifunctional role for the products of the cydAB operon, both in preventing the buildup of oxidative free radicals and in detoxifying the intracellular compartment, thus indicating the importance of these products in preventing intracellular destruction. Intracellular conditions that favor expression of the cydAB operon are under investigation and may be linked to the acid sensitivity also observed in this strain.
Brucella abortus, a facultative, gram-negative, intracellular pathogen, is the etiologic agent of brucellosis, a widely distributed zoonosis (56). In agriculturally important ruminants, the brucellae invade the trophoblastic epithelium of the gravid uterus (5), where their rapid multiplication leads to abortion and infertility (50). B. abortus primarily targets organs of the reticuloendothelial system and reproductive system in primary hosts (14, 40), while diverse manifestations of brucellosis are observed in humans, a secondary host, and include fever, anorexia, and malaise. Infection leads to localized complications and is the result of survival in and dissemination by professional phagocytes in which the organism persists (13, 36).
Scant information exists regarding the strategies that Brucella uses to escape host defense mechanisms. Previous work suggests that Brucella may inhibit degranulation to evade the cytocidal effects of the myeloperoxidase-H2O2-halide system in neutrophils (10, 11). Inhibition of phagolysosomal fusion is used to protect the organism from degradative lysosomal enzymes within host macrophages (6, 22). B. abortus expresses stress response proteins under conditions observed within the host macrophage (38), including low pH and reactive oxidative agents (O2·− and H2O2). Recent ultrastructural work has shown that B. abortus avoids targeting to lysosomes of nonprofessional cells by sequestration within autophagic vacuoles (41), which subsequently deliver these microbes to the rough endoplasmic reticulum where multiplication occurs (5). Some brucellae may be found in autophagosomes in professional phagocytic cells, but the majority of bacteria remain in phagosomes and strongly affect the phagosome maturation process. At later stages, brucellae are observed in phagolysosomes but appear to be resistant to killing activities (6). Vacuole acidification in phagocytic cells to pHs between 4.0 and 4.5 has been shown to be essential for intracellular survival during early infection by Brucella suis (44) and is observed with or without lysosomal fusion. Engulfment of B. abortus is accompanied by an increased oxidative metabolism of glucose via the hexose monophosphate shunt and production of reactive oxygen intermediates (ROIs) (28, 39).
In an effort to identify gene products used by Brucella to control its intracellular fate, transposon-generated mutants were selected based on attenuated survival in J774.A1 murine macrophages. We describe here the cloning and sequence analysis of the cydAB operon of B. abortus and evaluate its role in survival and virulence using a murine infection model. Changes in cell cytochrome content consistent with a switch from cytochrome bo oxidase to cytochrome bd oxidase activity during the stationary growth phase of B. abortus were reported over 25 years ago (46). In Escherichia coli, cytochrome bd oxidase expression is activated by microaerobic and acid stress conditions (15, 16, 27). Both enzymes are ubiquinol oxidases that catalyze the oxidation of quinols, reducing molecular oxygen to water and generating the proton motive force required for metabolism (24). Cytochrome bd oxidase has a high affinity for molecular oxygen (Km = 0.02 μM in whole cells) and is implicated in scavenging oxygen under limiting conditions to protect sensitive enzymes from inactivation (30, 47). In the absence of cytochrome bd oxidase, increased cytoplasmic oxygen levels can have detrimental effects, as shown by the inhibition of the oxygen-labile nitrogenase activity in Azotobacter vinelandii (19). The phenotype of the B. abortus cydB::Tn5 strain in response to acid shock, oxidative stress, respiratory inhibitors, and growth at stationary phase was characterized. The results suggest that the diminished capacity for survival exhibited by the cydB::Tn5 strain at stationary phase reflects an inability to offset the effects of oxidative stress resulting from a buildup of oxidative radicals generated due to a disruption in the electron transport chain.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Virulent B. abortus S2308 was isolated from an aborted bovine fetus (B. L. Deyoe, National Animal Disease Center, Ames, Iowa), passaged once on potato infusion agar (PIA; Difco Laboratories, Detroit, Mich.), and stored frozen at −80°C in 50% (vol/vol) glycerol. Wild-type B. abortus as well as transposon-derived and plasmid-bearing progeny was routinely grown on tryptic soy agar (TSA; Difco) or PIA medium with appropriate antibiotics, for 48 to 72 h. Antibiotics (Sigma) used to supplement the media were 50 g of kanamycin per liter, 30 g of chloramphenicol per liter, or 100 g of ampicillin per liter. All Brucella cultures were incubated at 37°C in an atmosphere containing 5% (vol/vol) CO2, unless stated otherwise. Liquid cultures of B. abortus strains were grown in tryptic soy broth (TSB; Difco) at 37°C in an atmosphere containing 5% (vol/vol) CO2. In experiments involving phenotypic characterization, Brucella strains were grown to mid-exponential phase (optical density [OD] = 100 to 200 Klett units) in 50 ml of TSB in 300-ml Nephlo flasks with vigorous aeration. Transformed E. coli DH10B (Gibco BRL) was cultured at 37°C on Luria-Bertani (LB) plates containing antibiotic as needed. pBluescript KS II(+) (Stratagene) and its derivatives were selected on LB plates containing ampicillin. Plasmids pMEK15 and pMEK21 were kindly provided by M. R. Roop II (Louisiana State University Medical Center, Shreveport). Both plasmids are based on the broad-host-range vector pBBR1MCS (33). pBBR1MCS.cos, a low-copy-number broad-host-range cloning vector, was constructed by inserting a cos site containing a BglII fragment from pHC79 into the KasI site of pBBR1MCS (1). This plasmid was used in the subcloning of selected portions of the cydAB locus. Strains and plasmids used in mixed infections in mice are described in Table 1. Growth phase and cell density measurements of bacterial samples were made using a Klett-Summerson colorimeter fitted with a blue filter.
TABLE 1.
List of bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and relevant marker | Source |
|---|---|---|
| Strains | ||
| B. abortus | ||
| S2308 | B. abortus wild-type bovine isolate, Nalr | B. L. Deyoe |
| BA582 | S2308 cydB::Tn5 Nalr Kmr | This study |
| E. coli | ||
| DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15ΔlacX74 deoR recA1 araΔ139 Δ(ara leu)7697 galU galKI rpsL endA1 nupG | Gibco BRL |
| Plasmids | ||
| pBBR1MCS.cos | Cloning vector, modified from pBBR1MCS | C. A. Allen |
| pSEK101 | B. abortus cydAB XbaI fragment in pBBR1MCS.cos | This study |
| pSEK102 | B. abortus cydAB HindIII fragment in pBBR1MCS.cos | This study |
| pSEK103 | B. abortus ΔcydA BamHI fragment in pBBR1MCS.cos | This study |
| pMEK15 | B. abortus Cu/Zn SOD gene on pBBR1MCS | M. Roop |
| pMEK21 | B. abortus catalase gene on pBBR1MCS | M. Roop |
| pBluescript KS II | ColE1 bla | Stratagene |
Recombinant DNA techniques.
Transposon (Tn5) mutagenesis of B. abortus mutants, isolation of genomic DNA, and identification of the interrupted loci have been described previously (2). To recover an uninterrupted copy of the gene locus from the B. abortus genome, the Tn5-containing genomic fragments were radiolabeled with [32P]dATP and used as hybridization probes to screen a B. abortus S2308 genomic DNA library in λ2001 (21). Phages that hybridized with the probe were amplified, and DNA was extracted from them as described previously. The DNA fragments were digested with XbaI, and DNA fragments were subcloned into the XbaI site of pBluescript KS II(+) to create pGBS4. This construct was sequenced using primers designed based on the sequence analysis of the transposon-interrupted locus. The 7.8-kb XbaI genomic DNA fragment was subcloned into the XbaI site of pBBR1MCS.cos to generate pSEK101. The XbaI fragment was digested with HindIII to generate a 5.3-kb HindIII fragment that was introduced into the HindIII site of pBBR1MCS.cos to construct pSEK102. A recombinant plasmid (pSEK103) was generated by digesting the XbaI fragment with BamHI and subcloning a 2.5-kb BamHI fragment into the BamHI site of pBBR1MCS.cos.
Nucleotide sequence analysis.
DNA sequencing with the ABI 377 automated sequencer was performed in the DNA Technologies Laboratory at Texas A&M University. The DNA sequence was analyzed using MacVector sequence analysis and AssemblyLIGN software using the National Center for Biotechnology Information Blast server with the Swiss-Prot database (4).
Preparation and transformation of electrocompetent B. abortus
In the preparation of electrocompetent B. abortus, all steps were performed at room temperature unless stated otherwise. Strains were grown to stationary phase (OD = 300 Klett units) in Nephlo flasks as described above. The bacteria were harvested by centrifugation, and electrocompetent cells were prepared according to a published protocol (2). Electroporation was performed at 2.0 kV/cm and 246 Ù in a BTX flat-pack cuvette (Genetronics Inc.). The cell suspension was washed from the cuvette into a microcentrifuge tube using 1 ml of SOC medium (2% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgSO4, and 20 mM glucose). After incubation for 24 h at 37°C with agitation, the bacteria were pelleted by centrifugation for 5 min at 12,000 × g, resuspended in 0.2 ml of SOC medium, and plated on TSA plates containing antibiotic at an appropriate concentration. The plates were incubated at 37°C for 4 days.
Survival of B. abortus in the mouse model of infection.
Bacterial strains were grown on PIA plates and harvested from the surface of the plate in 5 ml of phosphate-buffered saline (PBS; 10 mM sodium phosphate, 0.15 M sodium chloride [pH 7.2]). The concentration of the cell suspension was estimated turbidimetrically (Klett units) and adjusted to a concentration of approximately 5 × 105 CFU/ml for infection. The infectious dose (CFU per milliliter) was confirmed after serial dilution and plating of inocula on TSA plates with appropriate antibiotics. Six- to eight-week-old female BALB/c mice (The Jackson Laboratory) in three parallel groups (five mice per treatment group) were injected intraperitoneally with 100 μl (5 × 104 CFU) of cell suspension. The mice were killed by CO2 asphyxiation at 1, 2, and 8 weeks postinoculation. Spleens were collected from each group and homogenized using a tissue homogenizer (Omni 2000; Omni International Inc.) for determining bacterial counts. The homogenates were serially diluted in PBS and plated on TSA plates, and Brucella colonies were counted after incubation for 72 h. Data are presented as a log10 value of CFU per spleen and averaged over five mice.
Competitive infections in mice.
Bacterial strains were grown as described in the previous section. Groups of four BALB/c mice (4 to 6 weeks old) were infected intraperitoneally with an inoculum (5 × 104 CFU/ml) representing a 1:1 ratio of wild-type B. abortus to mutant or mutant transformed with selected gene constructs. The titer of the inoculating doses was confirmed by serial dilution and growth on TSA plates. Spleens were harvested and homogenized from each group as described above. Serial dilutions of spleen homogenates were prepared and plated in duplicate on TSA plates with kanamycin with or without chloramphenicol. Bacterial counts associated with each spleen from a given inoculum mixture were determined at 2 and 8 weeks postinfection. Colony counts on plates containing both antibiotics represent plasmid-containing strains only, and these values were subtracted from the colony counts determined on kanamycin plates representing both the mutant and the plasmid-containing strains. Data are presented as a log10 value of CFU present per spleen averaged over four mice.
Phenotypic characterization.
MIC assays were performed using hydrogen peroxide concentrations ranging from 0.5 to 20 mM. Bacteria from mid-log-phase cultures were pelleted by centrifugation, and the cell pellets were washed twice in an equal volume of PBS and resuspended in PBS at a final concentration of approximately 4 × 104 CFU/ml. One-hundred-microliter portions of the cell suspensions were mixed with an equal volume of PBS containing hydrogen peroxide and incubated for 1 h at 37°C in the wells of microtiter plates. Following incubation, the cells were serially diluted in PBS and plated in triplicate on TSA plates with appropriate antibiotics. Alternatively, a modified disk diffusion method (Kirby-Bauer) was employed. Mid-log-phase cells were pelleted and washed as described above and resuspended in PBS at 108 CFU/ml. One-hundred-microliter portions of each culture were spread in triplicate on the surface of TSA plates (10 cm) with appropriate antibiotics. Five-microliter portions of 30% hydrogen peroxide were spotted onto 5-mm-diameter sterile Whatman paper disks placed at the center of each plate. The plates were incubated for 48 h under standard conditions, and the zone of clearance surrounding each disk was measured.
Acid tolerance was determined using Brucella cultures grown to stationary phase (OD = 250 to 300 Klett units) at 37°C. One-half-milliliter portions were removed from each culture, and the bacteria were pelleted by centrifugation at 12,000 × g for 5 min in Eppendorf tubes. The bacterial cell pellet was resuspended in TSB adjusted to various pHs (3.0 to 7.0) using concentrated HCl, and incubation was continued in the microcentrifuge tubes with agitation at 37°C for 2 h. Following incubation, portions were serially diluted, and 100 μl of each dilution was plated in triplicate. Survival (CFU per milliliter) was determined after 72 h of incubation. Brucella organisms (0.7 × 1.5 μm) are much smaller than typical members of the family Enterobacteriaceae (1.5 × 6.0 μm) (9) and grow to much higher cell concentrations (48).
Use of heavy metals to discriminate between cytochrome bd oxidase-expressing and nonexpressing strains utilized TSA plates supplemented with 0.15 mM ZnSO4 and 0.15 mM NaN3 and appropriate antibiotics. Equal numbers of bacterial cells from liquid culture were serially diluted and spread in triplicate on TSA with or without ZnSO4-NaN3 supplement. Sensitivity was determined by the reduction in the CFU appearing on the surface of the ZnSO4-NaN3 and is reported as a percentage of cells growing on the surface of TSA plates after 72 h of incubation.
Macrophage assays.
Macrophage assays were conducted with murine J774.A1 cells or bovine peripheral blood monocyte-derived macrophages that were isolated and grown as described previously (45). Intracellular survival of B. abortus strains was determined by infecting monolayers containing 104 macrophages in the wells of a microtiter plate at a multiplicity of 5:1 and incubating them at 37°C in RPMI. Following a 30-min adsorption step, the medium was replaced with fresh RPMI containing 25 μg of gentamicin/ml. Two sets of plates were prepared for incubation, one for time zero (T0) and the second for 24 h of incubation (T24). The T0 plate was incubated for 1 h, and the T24 plate was incubated for 24 h. At the end of each incubation period, the monolayers were washed four times with 10 μl of complete culture medium and subsequently lysed with 10 μl of lysis solution (0.5% [vol/vol] Tween 20). Serial dilutions were prepared from each lysate, and 100-μl portions were plated on TSA plates in triplicate to determine the number of surviving brucellae. The survival for each strain was calculated using the viable Brucella CFU remaining at T24 divided by the CFU at T0 (% survival = CFUT24/CFUT0 × 100).
Statistical analysis.
Data were analyzed using the statistics software Prism 2.0 (GraphPad, Inc.). Analysis of variance using the Tukey procedure was used to determine the level of significance of differences in recovery observed for different bacterial cultures in the mouse experiment. The Student t test was used to determine the level of significance in the ZnSO4-NaN3 sensitivity assay. The t test using the Mann-Whitney procedure was used to determine the level of significance in the hydrogen peroxide sensitivity assay.
RESULTS
B. abortus mutants with severe intracellular survival defect.
To identify B. abortus genes required for virulence and/or intracellular survival, transposon mutagenesis of B. abortus S2308 was performed as described previously (2). The mutant bank was screened for attenuated strains using an in vitro macrophage survival assay. One thousand mutants were screened for survival in at least two independent assays using the mouse macrophage-like cell line J774.A1. Mutants with the most severe growth defects were characterized in parallel in bovine peripheral blood monocyte-derived macrophages (data not shown and reference 45). Mutants that were attenuated due to a loss of O-antigen expression (rough phenotype) represented less than 1% of the mutant bank (2). In addition, auxonography revealed that 2% of the mutants were auxotrophic for growth on minimal media (25, 37). Three strains, BA234, BA411, and BA582, grew normally in vitro and retained a smooth phenotype but exhibited reduced values for survival in the J774 macrophage-like cell line of 0.24 ± 0.12, 0.28 ± 0.11, and 0.29, respectively, relative to that for the parental wild-type strain S2308 (set at 1.0) (2). (The survival ratio obtained for BA582 was from a single experiment.)
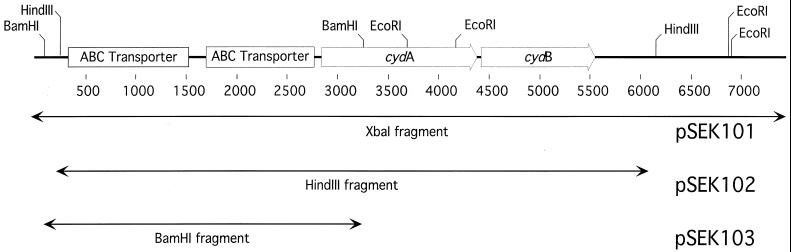
To identify the genetic loci responsible for the growth defect in Tn5 mutants, partial sequence data (∼300 bp) were obtained from BamHI-derived fragments isolated from three independently derived mutants (BA234, BA411, and BA582). In each mutant, the Tn5 interruption was traced to a single genetic locus, which shared homology with the cydB gene of the E. coli cydAB operon (27). This fragment was used to obtain an uninterrupted copy of the gene as described above. Sequencing with internal primers starting from the Tn5-derived sequence data identified a 7.8-kb XbaI fragment that contained the entire cydAB region (Fig. 1). The orientations of cydA and cydB in B. abortus are identical to those in E. coli. Interestingly, the sequences immediately upstream of the cydA region from B. abortus are homologous to the cydDC operon, which in E. coli is 3.3 min downstream from the cydAB operon.
FIG. 1.
Restriction map of the cydAB region. The restriction map was deduced from the partial sequence derived from the XbaI fragment obtained from the λ2001 recombinant. Outlined arrows represent the size and direction of transcription from the cydA and cydB structural genes. The cydA open reading frame is 526 amino acids, and the cydB open reading frame is 385 amino acids. There are two open reading frames upstream of the cydAB structural genes that share homology with the ATP binding cassette (ABC) transporters cydDC. The BamHI site within cydA is 430 nucleotides downstream from the presumed translational start site. pSEK101, pSEK102, and pSEK103 carry the corresponding XbaI, HindIII, and BamHI fragments, respectively, on pBBR1MCS, a B. abortus shuttle vector. The HindIII site downstream from cydB is 589 nucleotides from the putative stop codon of cydB.
Phenotypic characterization of cydB::Tn5 mutants.
In vitro characterization included classical biotyping in which the cyd mutants were indistinguishable from the parental strain 2308 (3). In vitro growth of the cyd mutants in rich (tryptic soy, Brucella, and potato infusion) and minimal (38) media and response to stress conditions including acid (pH ≥ 4.0) and temperatures of ≤42°C were identical to those of the parental strain. Decreased viability was observed when cyd mutants were incubated for prolonged periods (5 to 6 days) post-stationary phase. This may represent an increased sensitivity to the buildup of toxic by-products including oxidative free radicals formed during prolonged incubation (see below).
Sensitivity to respiratory inhibitors in a number of gram-negative bacteria including E. coli has been demonstrated to result from a lack of cytochrome bd oxidase activity (32, 43). Respiratory inhibitors such as sodium azide and zinc preferentially inhibit cytochrome bo oxidase from oxidizing ubiquinol, and the growth of cells lacking a functional cytochrome bd oxidase may be selectively inhibited (32). To extend the genetic analysis of the B. abortus cydB::Tn5 mutant strain 582 (BA582), a ZnSO4-NaN3 selection procedure (43) with modifications was used to verify the absence of a functional cytochrome bd oxidase in BA582. Unlike the wild-type strain, BA582 exhibited extreme sensitivity to the combined effects of 0.15 mM ZnSO4 and 0.15 mM NaN3 (Fig. 2). On TSA plates, these reagents completely inhibited the growth of BA582 but permitted the growth of S2308. Introduction of pSEK102, which contains a copy of the cydAB operon, restored resistance to zinc-azide to near-wild-type levels in the cydB::Tn5 strain. In contrast, pSEK103, which contains only a portion of the cydA gene from the cydAB operon, did not restore resistance of the cydB strain. Thus, consistent with the observed phenotype of other gram-negative bacteria, the inhibitory effects of ZnSO4-NaN3 were documented in B. abortus mutants lacking the putative cytochrome bd oxidase.
FIG. 2.
Sensitivity of S2308 and S2308 cydB::Tn5 to respiratory inhibitors. Liquid cultures were grown to mid-log phase, and portions were serially diluted and plated on TSA plates in the presence of 0.15 mM ZnSO4 and 0.15 mM NaN3 as described in Materials and Methods. Results are expressed as percentages of the cells growing on TSA plates. The limit of detection was 10 CFU/ml. Abbreviations of bacterial strains and plasmids are defined in Table 1.
In order to define the basis for the attenuated intracellular survival of BA582, we examined sensitivity to in vitro conditions that mimic those within macrophages. B. abortus mutants deficient in cytochrome bd oxidase activity exhibited heightened sensitivity to added hydrogen peroxide (Fig. 3). This sensitivity was reversed in transformants provided with pSEK102 containing the entire cydAB region of B. abortus but not with pSEK103 containing sequences upstream of the cydAB operon and only a portion of cydA (Fig. 1). These results raise the possibility that the survival defect of the B. abortus cydB::Tn5 mutants is due, in part, to oxidative stress caused by blockage of electron transport in the cyd-deficient mutants.
FIG. 3.
Hydrogen peroxide sensitivity assay. Bacteria from exponential-phase cultures grown in TSB were plated on TSA plates at 108 CFU/plate (as described in Materials and Methods). Oxidative killing was determined by measuring the diameter of the clear zone around a disk containing 5 μl of 30% hydrogen peroxide. The values are from three independent experiments and represent the averages of clearance zones from nine plates. Error bars represent the means of the three independent trials ± standard deviations. The asterisks denote values significantly different from those of the wild-type B. abortus S2308 as determined by analysis of variance using the Tukey test method. For a description of bacterial strains and plasmids, see Table 1.
The chemical susceptibilities of the cydB::Tn5 mutants transformed with either pSEK102, pMEK15, or pMEK21 (see Table 1 for plasmid descriptions) was compared with those of the cydB::Tn5 mutant and the isogenic wild-type strain 2308 (Fig. 3). In the cydB::Tn5 strain transformed with any of the three plasmids, susceptibility to hydrogen peroxide was comparable to that for wild-type strain S2308. These results indicate that the cydB::Tn5 mutant can be rescued if ROI-scavenging systems powerful enough to prevent oxygen-mediated damage are available in the mutant bacterial cell. These plasmids persist within B. abortus at 10 to 20 copies per cell, and overexpression of Cu/Zn superoxide dismutase (SOD) and catalase appears to be sufficient to alleviate the loss of cytochrome bd oxidase.
Within host macrophages, the pH of phagocytic vacuoles has been shown to decrease rapidly to 4.0 to 4.5 (44). Early acidification of vacuolar compartments has been shown to be essential for intracellular survival of Brucella (18, 44). B. abortus is resistant to pHs in this range during normal growth (34; T. A. Ficht, unpublished data). To determine whether sensitivity to reduced pH may play a role in the attenuation of the cydB::Tn5 mutants, survival at reduced pH was evaluated. Although the cydB::Tn5 strain showed diminished acid resistance at pHs of ≤3.6 relative to that of the wild type, resistance to pH within the physiologically significant range was normal (Fig. 4). Resistance to low-pH conditions was restored in trans by the cydAB region of B. abortus.
FIG. 4.
Acid tolerance response of S2308 and S2308 cydB::Tn5. Stationary-phase cells were exposed to TSB adjusted to various pHs for 2 h. After incubation, bacterial cells were serially diluted and plated in triplicate on TSA plates. The results expressed are averages of CFU per milliliter from triplicate plates, from two independent experiments. Error bars represent average values ± the standard deviations from the means. The asterisks denote values significantly different from those of wild-type S2308 (P < 0.05) as determined by analysis of variance using the Tukey test method. Abbreviations of bacterial strains and plasmids are defined in Table 1.
Survival of B. abortus cydB::Tn5 mutants in the mouse model.
In mice, Brucella establishes a self-limiting or chronic infection characterized by septicemia leading to reticuloendothelial involvement of both the liver and the spleen (23). The number of virulent organisms increases rapidly in the spleen due to their persistence within macrophages. B. abortus S2308 increased to a maximum number in the spleen by the first week postinoculation and then declined slightly by the second week postinoculation (Fig. 5). The number of organisms recovered from the spleens was maintained through the eighth week postinoculation, consistent with establishment of a chronic infection. Unlike the virulent strain 2308, the Brucella cydB::Tn5 mutant was severely compromised for survival in the spleens of inoculated mice. Recovery of the Brucella cydB::Tn5 mutant was reduced as much as 3 logs relative to that of the wild type at 1 week postinoculation (Fig. 5). The number of cydB organisms did not appear to change significantly by the second week postinoculation, but these organisms were undetectable by 8 weeks postinoculation. The limit of detection in the experiments described was ≤25 CFU/spleen. It is unclear why the cydB::Tn5 mutant did not exhibit a decline as did the wild-type strain between the first and second weeks postinoculation. The spleens of these mice had a normal gross appearance in contrast to the splenomegaly (three- to fourfold increase in spleen weights) observed in mice inoculated with S2308.
FIG. 5.
Recovery of B. abortus from the spleens of mice. Mice were inoculated with 5 × 104 CFU (solid arrow) of either the wild-type strain S2308 (open triangles); the cyd-negative mutant BA582 (open circles); or the cyd-negative mutant with either the intact cydAB locus, BA582/pSEK102 (closed triangles), or a truncated version of the cydAB locus, BA582/pSEK103 (open triangles). Recovery of inoculated organisms from the spleens of mice is presented as the log10 CFU per spleen from serial dilutions plated in duplicate and averaged over five mice. Error bars represent standard deviations from the means. The limit of detection in these experiments is ≤25 CFU per spleen.
To determine whether the growth defect observed in the cydB-deficient strain was due solely to the loss of cytochrome bd oxidase activity, a recombinant plasmid (pSEK102) harboring the intact B. abortus cydAB locus was transformed into the cydB::Tn5-containing strain. The mutant strain BA582 transformed with pSEK102 exhibited appreciable persistence within the spleen, paralleling the virulent parental strain 2308 (Fig. 5). Plasmid pSEK103, containing upstream genes and only the 5′ one-third of cydA (Fig. 1), was used as a negative control in this background. Strains harboring this plasmid were not rescued for intracellular survival (Fig. 5).
To further investigate the protective effect of Cu/Zn SOD or catalase in the context of infection, BALB/c mice were challenged with an inoculum of 105 CFU containing an equal mixture of two different strains, including (i) wild type (S2308) plus cydB::Tn5 mutant (BA582), (ii) wild type (S2308) plus cydB::Tn5 mutant complemented with pSEK102 (BA582 cyd+), (iii) wild type (S2308) plus cydB::Tn5 mutant transformed with pMEK15 (BA582 sod+), and (iv) wild type (S2308) plus cydB::Tn5 mutant transformed with pMEK21 (BA582 kat+). The ratio of wild type to cydB::Tn5 mutant reveals a 5.5-log difference in survival at 8 weeks of mixed infection. Transformation with plasmids containing cydAB, sodC, or kat genes resulted in increased persistence of the mutant in mouse spleens (Fig. 6). These data support the hypothesis that attenuation of B. abortus cydB::Tn5 mutants is the result of an increased buildup of reactive oxygen compounds. However, the levels of survival achieved suggest that resistance to other conditions may also contribute to survival.
FIG. 6.
Restoration of virulence of B. abortus cydB::Tn5 by Brucella sod and kat genes. Mice were inoculated with a 1:1 ratio of wild-type strain S2308 to cydB::Tn5 mutant strain BA582 or BA582 containing the plasmid pSEK101 (cyd+), pMEK21 (kat+), or pMEK15 (sod+). Brucellae were recovered from the spleens of mice, and the ratios of wild-type to mutant bacteria were calculated using the following formulas: CFUTSA = total number of Brucella bacteria recovered, CFUTSA/kan = the number of mutant bacteria recovered, and CFUTSA − CFUTSA/kan = the number of wild-type organisms recovered. Data are presented as the log10 of the ratios of wild-type to mutant CFU from serial dilutions plated in duplicate and averaged over four mice. Error bars represent standard deviations from the means. Asterisks indicate statistically significant differences. The limit of detection was ≤25 CFU/spleen.
DISCUSSION
Recent studies using Vero cells have provided evidence that virulent brucellae escape host defenses by sequestering themselves in autophagic vacuoles and ultimately gaining entrance to the endoplasmic reticulum where they replicate (42). However, Brucella virulence depends upon persistence in macrophages in both secondary and primary hosts (7), and in contrast to the situation using Vero cells, cellular trafficking within macrophages revealed no association with autophagosomes (6). Instead, Brucella trafficking in macrophages alters phagosomal maturation in about 50% of the Brucella-containing vacuoles and delays fusion with lysosomes. Although the remaining Brucella-containing vacuoles fused with the lysosomes, the bacteria appeared to be resistant to degradative lysosomal activities and reduced pH. B. abortus cydB::Tn5 mutants exhibit increased sensitivity to ROIs and to reduced pH in vitro and not surprisingly were attenuated for intracellular survival within macrophages, revealing the importance of cytochrome bd oxidase in intracellular persistence of B. abortus. Attenuated survival due to a loss of cydB function was confirmed by the restoration of survival when the intact cydAB locus was restored in trans (Fig. 5). It follows, therefore, that intracellular conditions may favor cytochrome bd oxidase synthesis, consistent with earlier published reports demonstrating a shift in the cytochrome content of B. abortus membranes as the organisms enter the stationary phase of growth (46). However, the reduced survival of these mutants may also be explained by the increased sensitivity of the remaining cytochrome bo oxidase to ROIs (Fig. 3) or reactive nitrogen intermediates (32).
The evidence reported here indicates that attenuated intracellular survival of the B. abortus cydB::Tn5 mutants is caused primarily by an increased sensitivity to ROIs. Although B. abortus cydB::Tn5 mutants exhibit extreme sensitivity to acidic pHs, they are below the physiologically significant range (44), and the near restoration of wild-type survival by Cu/Zn SOD in the mouse suggests that the acid sensitivity of the cyd mutants is not a major contributor to survival. The experiments performed do not rule out the possibility that acidification is essential for the activation of gene expression required for intracellular survival or proper intracellular trafficking. Replication of Brucella reportedly depends on acidification of the intracellular environment (18, 44).
Low pH also promotes the release of iron bound to transferrin receptors internalized within endocytic vacuoles (17), and pathogens having a strict requirement for iron depend on macrophage acidification for growth (55). E. coli and A. vinelandii cyd mutants have been shown to overproduce siderophores and are deficient in intracellular iron (12, 19). Growth inhibition is relieved under microaerobic conditions preventing oxygen overload or by the addition of ferrous iron sulfate, perhaps by stimulating SOD. Ferric iron exerts its lethality by catalyzing the generation of hydroxyl ions from H2O2. It is tempting to speculate that the absence of a functional cytochrome bd oxidase results in enhanced iron uptake and increased killing by generating reactive oxygen species. However, increased siderophore production by these mutants was not detected using the method described by Cook et al. (reference 12 and data not shown).
Although iron-dependent enzymes augment several essential metabolic processes in prokaryotes, iron-supplemented macrophages, whether nonactivated or activated with gamma interferon, have an enhanced capacity to kill phagocytosed brucellae (31). In such a scenario, a functional cytochrome bd oxidase might be involved in maintaining an electron flux through the electron transport chain and maintaining an overall homeostasis in the cell. The cydB::Tn5 mutants appear to be overproducing oxidative radicals, and the cell's endogenous Cu/Zn SOD and catalase are not sufficient to scavenge the ROIs in a cydB-negative background (8).
The lack of respiration is also expected to seriously affect metabolism in this strict aerobe and result in increased levels of NADH and a paucity of ATP, subverting central metabolic processes. B. abortus may require the cytochrome bd terminal oxidase to ensure NADH reoxidation, as has been suggested for E. coli (49). This may be especially lethal in the absence of fermentative pathways in B. abortus to reoxidize NADH (9). Increased production of ROIs, generated from activated H2 in the form of FADH2 or reduced iron-sulfur proteins in contact with O2 (20), may overwhelm the cell's endogenous SOD and catalase. Increased ROIs also have the effect of reducing ferric iron to ferrous iron that is used to catalyze the production of highly lethal hydroxyl radicals via the Fenton reaction (52).
Using the murine model, we have demonstrated that either Cu/Zn SOD or catalase can restore B. abortus cydB::Tn5 mutants to virulence. Sufficient levels of expression were achieved using a moderate-copy-number shuttle vector (10 to 20 copies), under the control of native promoters in both the in vitro and in vivo models. Although the in vitro experiments were not sensitive enough to distinguish differences in the survival of BA582 when complemented by the different plasmids, Cu/Zn SOD expression restored survival in the mouse model at significantly greater levels than did either catalase or cytochrome bd oxidase (Fig. 6). The increased survival of BA582 overexpressing Cu/Zn SOD is surprising given the moderate effect on virulence reported for strains deficient in the expression of this activity (35, 51, 54). The relative importance of these activities to survival must await determination of expression levels and cellular distribution. Cu/Zn SOD and catalase may protect Brucella from extracellular ROIs that damage periplasmic components or diffuse into the cytoplasm. Cytochrome bd oxidase prevents or limits the intracellular buildup of ROIs but may also participate in the inactivation of these compounds (26). Although hydrogen peroxide poses a greater threat to cytosolic components due to its ability to cross the membrane (29), the results reported here suggest that superoxide anion may pose a greater threat to viability.
The cydA and cydB genes from B. abortus share sequence similarity with those found in other intracellular pathogens, such as Haemophilus influenzae and Chlamydia spp. Of particular interest is their homology to the cydA and cydB genes from Chlamydia spp. The small genome of this obligately intracellular pathogen lacks the genes for many biosynthetic processes but has retained the genes encoding essential functions for aerobic respiration (53). The representation of the cydAB operon in Chlamydia spp. alludes to an important function of the gene in this pathogen, possibly in scavenging oxygen as well as generating a proton motive force to energize the membrane. Chlamydiae are irreversibly adapted to reside within endocytic vesicles of eukaryotic cells, and akin to brucellae, their ability to inhibit phagolysosomal fusion is their strategy to escape degradative lysosomal enzymes.
ACKNOWLEDGMENTS
We thank Renée Tsolis for helpful suggestions and discussion. We gratefully acknowledge Doris Hunter for her performance of the macrophage survival assays.
This research was supported by Research Grant Award no. 2781-96 from BARD, The United States-Israel Binational Agricultural Research and Development Fund, and from USDA Formula Animal Health (FAH-8675).
REFERENCES
- 1.Allen C A. M.S. thesis. College Station: Texas A&M University; 1996. [Google Scholar]
- 2.Allen C A, Adams L G, Ficht T A. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect Immun. 1998;66:1008–1016. doi: 10.1128/iai.66.3.1008-1016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alton G G, Jones Z M, Pietz D E. Laboratory techniques in brucellosis. Geneva, Switzerland: World Health Organization; 1975. [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Anderson T D, Cheville N F. Ultrastructural morphometric analysis of Brucella abortus-infected trophoblasts in experimental placentitis. Bacterial replication occurs in rough endoplasmic reticulum. Am J Pathol. 1986;124:226–237. [PMC free article] [PubMed] [Google Scholar]
- 6.Arenas G N, Staskevich A S, Aballay A, Mayorga L S. Intracellular trafficking of Brucella abortus in J774 macrophages. Infect Immun. 2000;68:4255–4263. doi: 10.1128/iai.68.7.4255-4263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin C L, Jiang X, Fernandes D M. Macrophage control of Brucella abortus: influence of cytokines and iron. Trends Microbiol. 1993;1:99–104. doi: 10.1016/0966-842x(93)90115-8. [DOI] [PubMed] [Google Scholar]
- 8.Battistoni A, Pacello F, Folcarelli S, Ajello M, Donnarumma G, Greco R, Ammendolia M G, Touati D, Rotilio G, Valenti P. Increased expression of periplasmic Cu,Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells. Infect Immun. 2000;68:30–37. doi: 10.1128/iai.68.1.30-37.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergey D H, Holt J G. Bergey's manual of determinative bacteriology. 9th ed. Baltimore, Md: Williams & Wilkins; 1994. [Google Scholar]
- 10.Bertram T A, Canning P C, Roth J A. Preferential inhibition of primary granule release from bovine neutrophils by a Brucella abortus extract. Infect Immun. 1986;52:285–292. doi: 10.1128/iai.52.1.285-292.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canning P C, Roth J A, Deyoe B L. Release of 5′-guanosine monophosphate and adenine by Brucella abortus and their role in the intracellular survival of the bacteria. J Infect Dis. 1986;154:464–470. doi: 10.1093/infdis/154.3.464. [DOI] [PubMed] [Google Scholar]
- 12.Cook G M, Loder C, Soballe B, Stafford G P, Membrillo-Hernandez J, Poole R K. A factor produced by Escherichia coli K-12 inhibits the growth of E. coli mutants defective in the cytochrome bd quinol oxidase complex: enterochelin rediscovered. Microbiology. 1998;144:3297–3308. doi: 10.1099/00221287-144-12-3297. [DOI] [PubMed] [Google Scholar]
- 13.Corbel M J. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corner L A, Alton G G. Persistence of Brucella abortus strain 19 infection in adult cattle vaccinated with reduced doses. Res Vet Sci. 1981;31:342–344. [PubMed] [Google Scholar]
- 15.Cotter P A, Gunsalus R P. Contribution of the fnr and arcA gene products in coordinate regulation of cytochrome o and d oxidase (cyoABCDE and cydAB) genes in Escherichia coli. FEMS Microbiol Lett. 1992;70:31–36. doi: 10.1016/0378-1097(92)90558-6. [DOI] [PubMed] [Google Scholar]
- 16.Cotter P A, Melville S B, Albrecht J A, Gunsalus R P. Aerobic regulation of cytochrome d oxidase (cydAB) operon expression in Escherichia coli: roles of Fnr and ArcA in repression and activation. Mol Microbiol. 1997;25:605–615. doi: 10.1046/j.1365-2958.1997.5031860.x. [DOI] [PubMed] [Google Scholar]
- 17.Dautry-Varsat A, Ciechanover A, Lodish H F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detilleux P G, Deyoe S L, Cheville N F. Effect of endocytic and metabolic inhibitors on the internalization and intracellular growth of Brucella abortus in Vero cells. Am J Vet Res. 1991;52:1658–1664. [PubMed] [Google Scholar]
- 19.Edwards S E, Loder C S, Wu G, Corker H, Bainbridge B W, Hill S, Poole R K. Mutation of cytochrome bd quinol oxidase results in reduced stationary phase survival, iron deprivation, metal toxicity and oxidative stress in Azotobacter vinelandii. FEMS Microbiol Lett. 2000;185:71–77. doi: 10.1111/j.1574-6968.2000.tb09042.x. [DOI] [PubMed] [Google Scholar]
- 20.Farr S B, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ficht T A, Bearden S W, Sowa B A, Adams L G. DNA sequence and expression of the 36-kilodalton outer membrane protein gene of Brucella abortus. Infect Immun. 1989;57:3281–3291. doi: 10.1128/iai.57.11.3281-3291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frenchick A J, Markham R J F, Cochrane A H. Inhibition of phagosome-lysosome fusion in macrophages by soluble extracts of virulent Brucella abortus. Am J Vet Res. 1985;46:332–335. [PubMed] [Google Scholar]
- 23.Garcia-Carillo C. Laboratory animal models for brucellosis studies. In: Neilsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. p. 423. [Google Scholar]
- 24.Gennis R B, Stewart V. Respiration. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 217–261. [Google Scholar]
- 25.Gerhardt P. The nutrition of Brucellae. Microbiol Rev. 1958;22:81–98. doi: 10.1128/br.22.2.81-98.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman B S, Gabbert K K, Kranz R G. The temperature-sensitive growth and survival phenotypes of Escherichia coli cydDC and cydAB strains are due to deficiencies in cytochrome bd and are corrected by exogenous catalase and reducing agents. J Bacteriol. 1996;178:6348–6351. doi: 10.1128/jb.178.21.6348-6351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green G N, Fang H, Lin R J, Newton G, Mather M, Georgiou C D, Gennis R B. The nucleotide sequence of the cyd locus encoding the two subunits of the cytochrome d terminal oxidase complex of Escherichia coli. J Biol Chem. 1988;263:13138–13143. [PubMed] [Google Scholar]
- 28.Harmon B G, Adams L G, Frey M. Survival of rough and smooth strains of Brucella abortus in bovine mammary gland macrophages. Am J Vet Res. 1988;49:1092–1097. [PubMed] [Google Scholar]
- 29.Hassan H M, Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979;254:10846–10852. [PubMed] [Google Scholar]
- 30.Hill S, Viollet S, Smith A T, Anthony C. Roles for enteric d-type cytochrome oxidase in N2 fixation and microaerobiosis. J Bacteriol. 1990;172:2071–2078. doi: 10.1128/jb.172.4.2071-2078.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X, Baldwin C L. Iron augments macrophage-mediated killing of Brucella abortus alone and in conjunction with interferon-gamma. Cell Immunol. 1993;148:397–407. doi: 10.1006/cimm.1993.1121. [DOI] [PubMed] [Google Scholar]
- 32.Kita K, Konishi K, Anraku A. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem. 1984;259:3375–3381. [PubMed] [Google Scholar]
- 33.Kovach E M, Phillips R W, Elzer P H, Roop II R M, Peterson K M. pBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 34.Kulakov Y K, Guigue-Talet P G, Ramuz M R, O'Callaghan D. Response of Brucella suis 1330 and B. canis RM6/66 to growth at acid pH and induction of an adaptive acid tolerance response. Res Microbiol. 1997;148:145–151. doi: 10.1016/S0923-2508(97)87645-0. [DOI] [PubMed] [Google Scholar]
- 35.Latimer E, Simmers J, Sriranganathan N, Roop II R M, Schurig G G, Boyle S M. Brucella abortus deficient in copper/zinc superoxide dismutase is virulent in BALB/c mice. Microb Pathog. 1992;12:105–113. doi: 10.1016/0882-4010(92)90113-3. [DOI] [PubMed] [Google Scholar]
- 36.Liautard J P, Gross A, Dornand J, Kohler S. Interactions between professional phagocytes and Brucella spp. Microbiologia. 1996;12:197–206. [PubMed] [Google Scholar]
- 37.Lin J. Ph.D. dissertation. College Station: Texas A&M University; 1993. [Google Scholar]
- 38.Lin J, Ficht T A. Protein synthesis in Brucella abortus induced during macrophage infection. Infect Immun. 1995;63:1409–1414. doi: 10.1128/iai.63.4.1409-1414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris J A. The interaction of “Brucella abortus” 544 and neutrophil polymorphonuclear leucocytes. Ann Sclavo. 1977;19:143–150. [PubMed] [Google Scholar]
- 40.Nicoletti P. The epidemiology of bovine brucellosis. Adv Vet Sci Comp Med. 1980;24:69–98. [PubMed] [Google Scholar]
- 41.Pizarro-Cerda J, Meresse S, Parton R G, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel J P. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pizarro-Cerda J, Moreno E, Sanguedolce V, Mege J L, Gorvel J P. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect Immun. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poole R K, Williams H D, Downie J A, Gibson F. Mutations affecting the cytochrome d-containing oxidase complex of Escherichia coli K12: identification and mapping of a fourth locus, cydD. J Gen Microbiol. 1989;135:1865–1874. doi: 10.1099/00221287-135-7-1865. [DOI] [PubMed] [Google Scholar]
- 44.Porte F, Liautard J P, Kohler S. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect Immun. 1999;67:4041–4047. doi: 10.1128/iai.67.8.4041-4047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qureshi T, Templeton J W, Adams L G. Intracellular survival of Brucella abortus, Mycobacterium bovis BCG, Salmonella dublin, and Salmonella typhimurium in macrophages from cattle genetically resistant to Brucella abortus. Vet Immunol Immunopathol. 1996;50:55–65. doi: 10.1016/0165-2427(95)05492-8. [DOI] [PubMed] [Google Scholar]
- 46.Rest R F, Robertson D C. Characterization of the electron transport system in Brucella abortus. J Bacteriol. 1975;122:139–144. doi: 10.1128/jb.122.1.139-144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice C W, Hempfling W P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978;134:115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sangari F J, Aguero J, Garcia-Lobo J M. Improvement of the Brucella abortus B19 vaccine by its preparation in a glycerol based medium. Vaccine. 1996;14:274–276. doi: 10.1016/0264-410x(95)00214-l. [DOI] [PubMed] [Google Scholar]
- 49.Siegele D A, Imlay K R, Imlay J A. The stationary-phase-exit defect of cydC (surB) mutants is due to the lack of a functional terminal cytochrome oxidase. J Bacteriol. 1996;178:6091–6096. doi: 10.1128/jb.178.21.6091-6096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith L D, Ficht T A. Pathogenesis of Brucella. Crit Rev Microbiol. 1990;17:209–230. doi: 10.3109/10408419009105726. [DOI] [PubMed] [Google Scholar]
- 51.Sriranganathan N, Boyle S M, Schurig G, Misra H. Superoxide dismutases of virulent and avirulent strains of Brucella abortus. Vet Microbiol. 1991;26:359–366. doi: 10.1016/0378-1135(91)90029-f. [DOI] [PubMed] [Google Scholar]
- 52.Stadtman E R. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 53.Stephens R S, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov R L, Zhao Q, Koonin E V, Davis R W. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 54.Tatum F M, Detilleux P G, Sacks J M, Halling S M. Construction of Cu-Zn superoxide dismutase deletion mutants of Brucella abortus: analysis of survival in vitro in epithelial and phagocytic cells and in vivo in mice. Infect Immun. 1992;60:2863–2869. doi: 10.1128/iai.60.7.2863-2869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Traub A, Mager J, Grossowicz N. Studies on the nutrition of Pasteurella tularensis. J Bacteriol. 1955;70:60–69. doi: 10.1128/jb.70.1.60-69.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zavala I, Nava A, Guerra J, Quiros C. Brucellosis. Infect Dis Clin N Am. 1994;8:225–241. [PubMed] [Google Scholar]