Abstract
Context
There is little evidence regarding the association between serum vitamin B6 status and catabolism and all-cause mortality in patients with type-2 diabetes mellitus (T2DM).
Objective
We aimed to ascertain if the serum level of vitamin B6 and catabolism, including pyridoxal 5′-phosphate (PLP) and 4-pyridoxic acid (4-PA), were associated with risk of all-cause mortality in T2DM patients.
Methods
This prospective cohort study involved 2574 patients with T2DM who participated in the National Health and Nutrition Examination Survey (NHANES) from 2005 to 2010. The serum concentrations of PLP and 4-PA were used to assess the serum level of vitamin B6. Mortality status was determined by routine follow-up using the National Death Index through December 31, 2015.
Results
Over a median follow-up of 85 months, there were 588 deaths. The fully adjusted Cox model indicated that the highest serum PLP concentrations (> 63.6 nmol/L) were associated with a decrease in all-cause mortality (hazard ratio [HR], 0.74; 95% CI, 0.55-0.99, P trend = .035). The risk for all-cause mortality was 59% higher for participants with the highest quartile of 4-PA level compared with the lowest quartile (HR, 1.62; 95% CI, 1.12-2.35; P trend = .003). The sensitivity and specificity of the combination of PLP and 4-PA levels for the prediction of all-cause mortality were 59.5% and 60.9%, respectively (area under the receiver operating characteristic curve = 0.632). The Kaplan-Meier method was used to estimate overall survival for patients based on different combinations of PLP level and 4-PA level. Patients with PLP less than 24.3 nmol/L and 4-PA greater than or equal to 25.4 nmol/L had the worst outcomes (log-rank P < .001).
Conclusion
Overall, our data suggest that a low serum level of PLP and high serum level of 4-PA, which represent the serum level of vitamin B6, increases the risk of all-cause mortality significantly in patients with T2DM.
Keywords: serum vitamin B6, pyridoxal 5′-phosphate (PLP), 4-pyridoxic acid (4-PA), all-cause mortality, type 2 diabetes mellitus, NHANES
Diabetes mellitus (DM) is a public health problem worldwide: As many as 700 million people are expected to have DM by 2045 (1). Studies have shown people with DM to have a 2- to 4-fold increased risk of all-cause mortality compared with those not suffering from DM (2). Therefore, the identification of modifiable variables is crucial for the prevention or delay of DM complications and early mortality.
Vitamin B6 is a water-soluble vitamin. Its naturally occurring forms include pyridoxine, pyridoxal (PL), pyridoxamine, and their respective phosphorylated derivatives (3). Among them, pyridoxal 5′-phosphate (PLP) is the biologically active form of vitamin B6. It operates as a cofactor in more than 160 catalytic functions, including transamination, aldol cleavage, α-decarboxylation, racemization, β- and γ-eliminations, and replacement reactions. Meanwhile, 4-pyridoxic acid (4-PA) is the major catabolite of vitamin B6 metabolism. PLP and 4-PA are used widely to determine the serum level of vitamin B6 (4).
A low serum level of PLP has been observed in cardiovascular diseases (CVDs), stroke, deep venous thrombosis, DM, and cancer (5-9). Nix and colleagues (10) showed type 2 diabetes mellitus (T2DM) to be associated with a reduced level of vitamin B6 and changes in its metabolism. Most of the research teams that have measured PLP levels have discovered an inverse relationship between the PLP concentration and DM development. Recent studies have also shown that deficiency of vitamin B6 disrupted serotonin signaling in pancreatic islets and induced gestational DM in mice (11). The Strong Heart Family Study demonstrated a higher level of vitamin B6 to be associated with DM-related outcomes (higher homeostasis model assessment 2–insulin resistance (HOMA2-IR) and increased risk for DM and metabolic syndrome) (12). Such evidence implies the possibility of a “vicious cycle” between vitamin B6 and DM. Interestingly, a recent cross-sectional study showed intake of vitamin B6 to be associated inversely with DM risk in US adults (13). According to results of a double-blind, randomized controlled trial, low-dose vitamin B6 supplementation improves cardiovascular risk in healthy elderly Chinese patients (14). Ulvik et al (15) discovered that the catabolism of vitamin B6 was a particularly powerful predictor of all-cause mortality for individuals without a history of coronary artery disease. Unlike these studies, the WAFACS study showed that daily supplementation with vitamin B6 did not reduce the risk of developing T2DM among women at high risk for CVD (16). Previous studies mainly focused on the causal relationship between DM and vitamin B6, as well as the effect of vitamin B6 supplementation on clinical outcomes in different populations. However, studies investigating the relationship between the serum level of vitamin B6 and catabolism and mortality risk in people with DM are lacking.
To address these information gaps, we used serum levels of PLP and 4-PA to represent the serum level of vitamin B6. Then, we investigated the relationship between the serum level of vitamin B6 and all-cause mortality in a nationally representative sample of individuals with DM living in the United States.
Materials and Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is designed to assess the health and nutritional status of the civilian population in the United States using a nationally representative sample. The survey protocol was approved by the research ethics review board of the National Center for Health Statistics (Hyattsville, MD, USA). NHANES obtained written informed consent from all participants. Detailed information is displayed on the website of NHANES (www.cdc.gov/nchs/nhanes/index.htm/; accessed January 12, 2022).
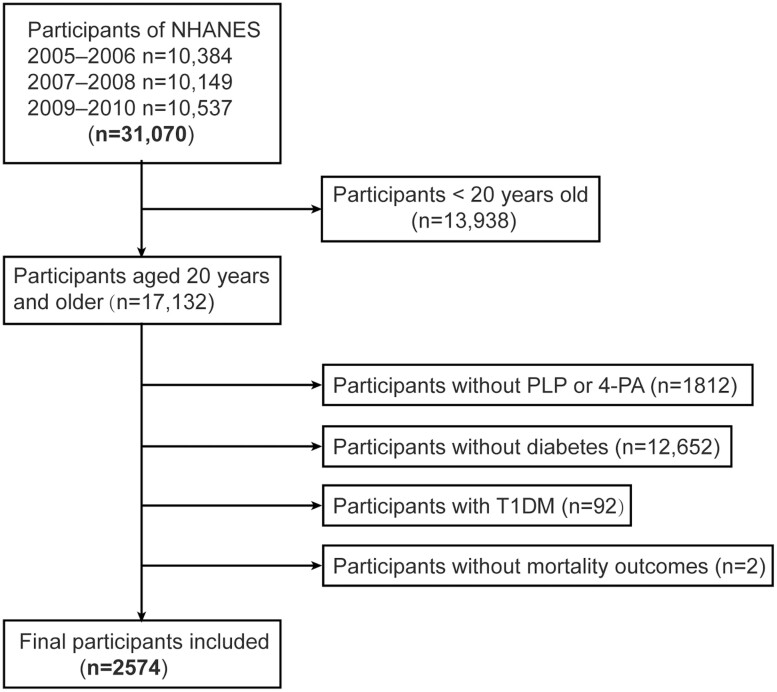
From 2003 to 2010, the concentration of vitamin B6 was measured in 4 cycles of NHANES. However, owing to the substantial variation in measurement methods between 2003 to 2004 and 2005 to 2010, and the lack of accessible adjustments to make them comparable, we used NHANES data from only 3 cycles (2005-2010) in this analysis for people aged 20 years and older (n = 17 132). We defined DM using 1 of the following 5 criteria: self-reported diagnosis of DM made by a physician; intake of anti-DM medications or insulin; glycated hemoglobin A1c (HbA1c) greater than or equal to 6.5%; fasting glucose greater than or equal to 7.0 mmol/L; and a 2-hour glucose level of 11.1 mmol/L after an oral glucose tolerance test. Among people with DM, we excluded individuals with possible type 1 DM (n = 92), which was defined by the use of insulin and age at DM onset younger than 30 years (validated as accurate in 97% of cases (17)). The remaining 2869 individuals were stated to have T2DM. Ultimately, 2574 participants with T2DM were included from NHANES (2005-2010) when the serum level of vitamin B6 was measured (Fig. 1).
Figure 1.
Flowchart of the screening process for the selection of eligible participants.
Measurement of Vitamin B6 Serum Level
The serum concentrations of PLP (nmol/L) and 4-PA (nmol/L) were used to assess the serum level of vitamin B6. Both biomarkers were measured by high-performance liquid chromatography, for which further detailed quality control steps can be found in the Mobile Examination Center laboratory procedure manual on the NHANES Internet website. PLP (active coenzyme form of vitamin B6) is transformed into PL (transport form of the vitamin) and subsequently catabolized to 4-PA, which is eliminated in urine (18).
Ascertainment of Mortality
Mortality was determined in the National Center for Health Statistics (which includes survey participants from NHANES) using a probabilistic record match between NHANES participants and death-certificate data from the National Death Index. Through December 31, 2015, NHANES-linked National Death Index publicly accessible files were used to establish mortality status and cause of death. The 10th Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) was used to code the underlying cause of death.
Assessment of Covariates
Standardized questionnaires were used to collect demographic and lifestyle characteristics (eg, age, sex, race/ethnicity, educational level, income, and physical activity) during interviews conducted at home. Physical examinations at the mobile examination center enabled information on alcohol consumption, bodyweight, and height to be documented. Body mass index (BMI) was categorized as “underweight” or “normal weight” (< 25), “overweight” (25-29.9), or “obesity” (≥ 30). Race/ethnicity was categorized as “non-Hispanic White,” “non-Hispanic Black,” “Mexican American,” and “other.” Categories for educational attainment were “less than high school,” “high school graduate/general education development,” and “college degree or more.” The family income-to-poverty ratio (PIR) is based on the ratio of the family household income to the poverty level set by the US Department of Health and Human Services, and higher PIR values indicate higher household income (19). PIR was classified as 0 to 1.0, 1.01 to 4.99, or 5.0. The serum level of cotinine was used as a marker of environmental exposure to smoking, and was categorized as “low exposed nonsmoker” (serum cotinine below the limit of detection), “high exposed nonsmoker” (serum cotinine above the limit of detection, ≤ 10.0 ng/mL), and “exposed smokers” (serum cotinine > 10.0 ng/mL). Alcohol consumption was defined as “regular drinking in the past 12 months” (yes or no). According to the questionnaire, physical activity was divided into “never,” “moderate,” and “vigorous.”
Plasma levels of glucose, insulin, HbA1c, triglycerides, total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were measured using samples at baseline after an overnight fast. Detailed descriptions of the procedures for blood collection/processing are provided in the NHANES Laboratory/Medical Technologists Procedures Manual or on the NHANES Internet website. The HOMA-IR Index was calculated as a parameter to evaluate insulin resistance. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (20). “Normal renal function” was defined as an eGFR greater than or equal to 90 mL/min/1.73 m2. “Renal impairment” was defined as an eGFR less than 90 mL/min/1.73 m2.
DM duration was divided into 3 groups (< 3 years, 3-10 years, and > 10 years). We classified the use of DM medication as “no use of DM medication,” “use of oral medication only,” “any insulin use,” or “other.” “Hypertension” was defined as “self-reported history of hypertension,” “measured systolic blood pressure ≥ 140 mm Hg,” “measured diastolic blood pressure ≥ 90 mm Hg,” or “self-reported use of blood pressure medications.” We defined “total CVD” as any self-reported physician-based diagnosis of congestive heart failure, coronary heart disease, angina pectoris, heart attack, or stroke. The total intake of vitamin B6 (mg/day) was measured by the first 24-hour and 48-hour dietary intake interviews conducted in person by trained interviewers, then averaged for final inclusion.
Statistical Analyses
All analyses incorporated sample weights to account for the complex survey design according to analytic guidelines set by NHANES. Continuous variables are presented as mean ± SD. Categorical variables are displayed as numbers and their proportion. We used the chi-square test for categorical variables, one-way analysis of variance for continuous variables with a normal distribution, and the Kruskal-Wallis test for continuous variables with a skewed distribution.
The Cox proportional hazards model was employed to estimate the hazard ratios (HRs) and 95% CIs of all-cause mortality for the second through fourth quartiles of PLP and 4-PA levels using the first quartile as the reference. Model 1 included adjustment for age and sex. Model 2 included adjustment for the variables in model 1 along with race, education, physical activity, serum level of cotinine, alcohol consumption, BMI, and PIR. Model 3 was adjusted further for a history of hypertension, CVD history, history of cancer, HbA1c level, DM duration, medication use, PLP or 4-PA level, and use of vitamin B6 supplements. Data missingness was as follows (unweighted data): education (n = 4; 0.16%), BMI (n = 74; 2.87%), PIR (n = 235; 9.13%), cotinine (n = 11; 0.43%), alcohol drinking (n = 189; 7.34%), diabetes (n = 13; 0.51%), history of CVD (n = 23; 0.89%), hypertension (n = 1; 0%), renal impairment (n = 38; 1.48%), liver condition (n = 10; 0.39%), cancer (n = 6; 0.23%), eGFR (n = 38; 1.48%), C-reactive protein (n = 3; 0.12%), high-density lipoprotein cholesterol (n = 21; 0.82%), total cholesterol (n = 21; 0.82%), low-density lipoprotein cholesterol (n = 1190; 46.23%), triglycerides (n = 1119; 43.47%), fasting glucose (n = 1107; 43%), HOMA-IR: (n = 1113; 43.24%), HbA1c (n = 10; 0.39%), day 1 vitamin B6 intake (n = 27; 1.05%), and day 2 vitamin B6 intake (n = 73; 2.84%). No assumptions were used for missing data, and the listwise deletion method was used for missing variables in multivariate models.
Log10 conversion of continuous variables with restricted cubic splines, each with 4 knots positioned at the corresponding 5th, 35th, 65th, and 95th percentiles, was used to assess possible nonlinearities. Nonlinear associations were evaluated using the likelihood ratio test. The associations of PLP and 4-PA levels with all-cause mortality were examined using the 25th percentile as the reference point based on the results of restricted cubic spline analyses.
We stratified the analyses further by age (< 60 or ≥ 60 years), sex (male or female), BMI (< 30 or ≥ 30), HbA1c level (< 7% or ≥ 7%), eGFR (< 90 or ≥ 90 mL/min/1.73 m2), hypertension (yes or no), and CVD (yes or no). The Cox proportional hazards model, with data stratified according to stratification factors, was used to estimate HRs and test for the significance of time-to-event variables. The significance of interactions was estimated using the P values for the production terms between PLP and 4-PA levels, as well as stratification factors.
A receiver operating characteristic (ROC) curve is used to evaluate the sensitivity and specificity of a prediction model. Survival analysis using Kaplan-Meier curves was carried out with a log-rank (Mantel-Cox) test for comparison between curves for overall survival, which was defined by death from any cause.
For all analyses, P less than .05 was used to define statistical significance for all analytical computations. Analyses were conducted using SPSS 24 (IBM), Stata 16.0 (Stata Corp), and R 3.6.1 (R Institute for Statistical Computing).
Results
Participant Characteristics
The demographic and clinical characteristics of participants according to serum levels of PLP and 4-PA are presented in Table 1. In total, 2574 individuals with a median follow-up of 85 months were included in our study. The sample number of all-cause mortality was 588. Among adults with DM (mean [SD] age, 62.33 (13.37) years; 1316 men [weighted, 51.13%]), the serum concentration of PLP was 35.8 nmol/L (interquartile range, 21.4-63.6 nmol/L) and of 4-PA was 28.1 nmol/L (interquartile range, 17.6-56.0 nmol/L). Participants who had the highest concentrations of PLP and 4-PA were older, more likely to be non-Hispanic White, have a higher education level, higher family income, and less likely to have a high cotinine level, or be obese.
Table 1.
Baseline characteristics of participants with diabetes by serum pyridoxal 5′-phosphate and 4-pyridoxic acid levels in National Health and Nutrition Examination Survey III (2005-2010)
| PLP | 4-PA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P |
| Participants, No. | 2574 | 641 | 646 | 644 | 643 | 640 | 643 | 64 | 644 | ||
| Age, y | 62.33 (13.37) | 62.30 (12.51) | 61.82 (13.67) | 61.18 (13.90) | 64.03 (13.19) | .001 | 56.03 (12.80) | 61.04 (12.99) | 63.75 (13.36) | 68.47 (11.09) | < .001 |
| Sex (men) | 1316 (51.13) | 312 (48.67) | 305 (47.21) | 354 (54.97) | 345 (53.66) | .012 | 283 (44.22) | 366 (56.92) | 349 (53.94) | 318 (49.38) | < .001 |
| Race/ethnicity | < .001 | < .001 | |||||||||
| Mexican American | 530 (20.59) | 105 (16.38) | 151 (23.38) | 148 (22.98) | 126 (19.60) | 156 (24.38) | 164 (25.51) | 118 (18.24) | 92 (14.29) | ||
| Non-Hispanic White | 1071 (41.61) | 264 (41.19) | 261 (40.40) | 245 (38.04) | 301 (46.81) | 165 (25.78) | 226 (35.15) | 312 (48.22) | 368 (57.14) | ||
| Non-Hispanic Black | 624 (24.24) | 200 (31.20) | 155 (23.99) | 147 (22.83) | 122 (18.97) | 228 (35.63) | 163 (25.35) | 118 (18.24) | 115 (17.86) | ||
| Other | 349 (13.56) | 72 (11.23) | 79 (12.23) | 104 (16.15) | 94 (14.62) | 91 (14.22) | 90 (14.00) | 99 (15.30) | 69 (10.71) | ||
| Education | < .001 | < .001 | |||||||||
| < High school | 1030 (40.08) | 297 (46.48) | 264 (40.93) | 262 (40.68) | 207 (32.24) | 293 (45.78) | 273 (42.59) | 250 (38.70) | 214 (33.28) | ||
| High school/GED | 625 (24.32) | 152 (23.79) | 162 (25.12) | 153 (23.76) | 158 (24.61) | 153 (23.91) | 146 (22.78) | 166 (25.70) | 160 (24.88) | ||
| > High school | 915 (35.60) | 190 (29.73) | 219 (33.95) | 229 (35.56) | 277 (43.15) | 194 (30.31) | 222 (34.63) | 230 (35.60) | 269 (41.84) | ||
| BMI | < .001 | < .001 | |||||||||
| < 25 | 345 (13.80) | 74 (12.05) | 62 (9.98) | 87 (13.77) | 122 (19.27) | 66 (10.54) | 74 (11.78) | 87 (13.90) | 118 (19.03) | ||
| 25-29.9 | 742 (29.68) | 142 (23.13) | 169 (27.21) | 208 (32.91) | 223 (35.23) | 151 (24.12) | 208 (33.12) | 188 (30.03) | 195 (31.45) | ||
| ≥ 30 | 1413 (56.52) | 398 (64.82) | 390 (62.80) | 337 (53.32) | 288 (45.50) | 409 (65.34) | 346 (55.10) | 351 (56.07) | 307 (49.52) | ||
| PIR | < .001 | < .001 | |||||||||
| 0-1.0 | 500 (21.38) | 175 (30.65) | 133 (22.50) | 110 (18.87) | 82 (13.81) | 182 (31.99) | 131 (22.24) | 97 (16.72) | 90 (14.98) | ||
| 1.01-4.99 | 1531 (65.46) | 346 (60.60) | 405 (68.53) | 375 (64.32) | 405 (68.18) | 325 (57.12) | 397 (67.40) | 393 (67.76) | 416 (69.22) | ||
| 5.0 | 308 (13.17) | 50 (8.76) | 53 (8.97) | 98 (16.81) | 107 (18.01) | 62 (10.90) | 61 (10.36) | 90 (15.52) | 95 (15.81) | ||
| Physical activity | .001 | .593 | |||||||||
| Never | 1479 (57.46) | 410 (63.96) | 369 (57.12) | 346 (53.73) | 354 (55.05) | 369 (57.66) | 353 (54.90) | 374 (57.81) | 383 (59.47) | ||
| Moderate | 541 (21.02) | 124 (19.35) | 122 (18.89) | 144 (22.36) | 151 (23.48) | 130 (20.31) | 143 (22.24) | 144 (22.26) | 124 (19.26) | ||
| Vigorous | 554 (21.52) | 107 (16.69) | 155 (23.99) | 154 (23.91) | 138 (21.46) | 141 (22.03) | 147 (22.86) | 129 (19.94) | 137 (21.27) | ||
| Cotinine, ng/mL | < .001 | < .001 | |||||||||
| > 10 | 543 (21.19) | 228 (35.74) | 143 (22.21) | 97 (15.11) | 75 (11.74) | 216 (33.96) | 146 (22.81) | 103 (15.92) | 78 (12.19) | ||
| LOD-10 | 1482 (57.82) | 312 (48.90) | 367 (56.99) | 406 (63.24) | 397 (62.13) | 332 (52.20) | 356 (55.63) | 392 (60.59) | 402 (62.81) | ||
| < LOD | 538 (20.99) | 98 (15.36) | 134 (20.81) | 139 (21.65) | 167 (26.14) | 88 (13.84) | 138 (21.56) | 152 (23.49) | 160 (25.00) | ||
| Alcohol consumption | 1479 (62.01) | 360 (61.22) | 342 (57.97) | 388 (64.03) | 389 (64.73) | .066 | 362 (61.46) | 382 (64.31) | 394 (64.59) | 341 (57.60) | .046 |
| Duration of diabetes, y | .121 | < .001 | |||||||||
| < 3 | 1249 (48.77) | 283 (44.57) | 329 (51.25) | 326 (50.70) | 311 (48.52) | 340 (53.13) | 321 (50.31) | 314 (48.83) | 274 (42.81) | ||
| 3-10 | 689 (26.90) | 172 (27.09) | 166 (25.86) | 175 (27.22) | 176 (27.46) | 177 (27.66) | 167 (26.18) | 171 (26.59) | 174 (27.19) | ||
| > 10 | 623 (24.33) | 180 (28.35) | 147 (22.90) | 142 (22.08) | 154 (24.03) | 123 (19.22) | 150 (23.51) | 158 (24.57) | 192 (30.00) | ||
| Diabetes medication | < .001 | .002 | |||||||||
| No | 397 (15.42) | 75 (11.70) | 104 (16.10) | 100 (15.53) | 118 (18.35) | 100 (15.63) | 99 (15.40) | 87 (13.45) | 111 (17.24) | ||
| Oral medication only | 1138 (44.21) | 275 (42.90) | 280 (43.34) | 290 (45.03) | 293 (45.57) | 279 (43.59) | 300 (46.66) | 281 (43.43) | 278 (43.17) | ||
| Any insulin use | 390 (15.15) | 137 (21.37) | 88 (13.62) | 90 (13.98) | 75 (11.66) | 74 (11.56) | 90 (14.00) | 105 (16.23) | 121 (18.79) | ||
| Other | 649 (25.21) | 154 (24.03) | 174 (26.94) | 164 (25.47) | 157 (24.42) | 187 (29.22) | 154 (23.95) | 174 (26.89) | 134 (20.81) | ||
| CVD | 689 (27.01) | 221 (34.75) | 172 (26.96) | 140 (22.01) | 156 (24.34) | < .001 | 127 (20.13) | 156 (24.45) | 182 (28.22) | 224 (35.17) | < .001 |
| Hypertension | 1864 (72.45) | 485 (75.66) | 469 (72.60) | 469 (72.94) | 441 (68.59) | .042 | 423 (66.09) | 457 (71.07) | 482 (74.50) | 502 (78.07) | < .001 |
| Renal impairment | 1636 (64.51) | 429 (68.20) | 406 (63.24) | 379 (59.59) | 422 (67.09) | .005 | 272 (43.45) | 387 (60.75) | 461 (71.47) | 516 (82.17) | < .001 |
| Liver impairment | 144 (5.62) | 36 (5.64) | 38 (5.90) | 35 (5.46) | 35 (5.46) | .984 | 34 (5.33) | 36 (5.63) | 38 (5.89) | 36 (5.62) | .979 |
| Cancer | 371 (14.45) | 87 (13.62) | 85 (13.22) | 87 (13.53) | 112 (17.42) | .104 | 56 (8.78) | 74 (11.53) | 105 (16.30) | 136 (21.12) | < .001 |
| eGFR, mL/min/1.73 m2 | 77.88 (24.43) | 76 (25.19) | 78.34 (24.42) | 81.01 (24.17) | 76.13 (23.58) | < .001 | 91.05 (19.69) | 82.08 (20.71) | 73.92 (23.96) | 64.58 (24.79) | < .001 |
| CRP, mg/dL | 0.31 (0.13-0.70) | 0.53 (0.23-1.18) | 0.34 (0.16-0.75) | 0.25 (0.11-0.57) | 0.20 (0.09-0.44) | < .001 | 0.41 (0.17-0.94) | 0.31 (0.12-0.69) | 0.29 (0.12-0.62) | 0.27 (0.11-0.64) | < .001 |
| HDL, mmol/L | 1.26 (0.39) | 1.22 (0.35) | 1.24 (0.39) | 1.27 (0.40) | 1.33 (0.40) | < .001 | 1.24 (0.38) | 1.23 (0.37) | 1.29 (0.39) | 1.30 (0.40) | .003 |
| TC, mmol/L | 4.93 (1.20) | 4.79 (1.12) | 5.02 (1.23) | 4.98 (1.25) | 4.93 (1.19) | .003 | 5.11 (1.21) | 5.03 (1.24) | 4.89 (1.16) | 4.69 (1.15) | < .001 |
| LDL, mmol/L | 2.69 (2.10-3.39) | 2.69 (2.07-3.23) | 2.82 (2.22-3.52) | 2.56 (1.99-3.36) | 2.64 (2.07-3.36) | .028 | 2.92 (2.35-3.54) | 2.66 (2.12-3.39) | 2.69 (2.10-3.34) | 2.46 (1.84-3.08) | < .001 |
| TGs, mmol/L | 1.57 (1.08-2.30) | 1.55 (1.14-2.34) | 1.66 (1.14-2.33) | 1.61 (1.11-2.35) | 1.43 (1.02-2.16) | .045 | 1.56 (1.13-2.25) | 1.63 (1.13-2.42) | 1.60 (1.10-2.31) | 1.47 (1.06-2.24) | .380 |
| Fasting glucose, mmol/L | 8.21 (3.26) | 8.50 (3.40) | 8.28 (3.40) | 8.30 (3.30) | 7.76 (2.86) | .019 | 8.65 (3.54) | 8.44 (3.51) | 8.09 (3.15) | 7.61 (2.61) | < .001 |
| HOMA-IR | 4.66 (2.66-7.86) | 5.02 (2.74-8.92) | 5.16 (2.93-8.15) | 4.44 (2.50-7.55) | 4.05 (2.31-6.86) | .003 | 5.41 (3.24-8.48) | 4.92 (2.82-8.50) | 4.54 (2.44-7.34) | 3.90 (2.00-6.84) | < .001 |
| HbA1c, % | 7.08 (1.65) | 7.23 (1.65) | 7.14 (1.68) | 7.10 (1.71) | 6.84 (1.54) | < .001 | 7.38 (1.84) | 7.20 (1.71) | 6.93 (1.58) | 6.81 (1.38) | < .001 |
| Vitamin B6 intake, mean, mg | 1.76 (1.24-2.66) | 1.52 (1.07-2.27) | 1.68 (1.21-2.45) | 1.84 (1.32-2.83) | 2.01 (1.41-2.93) | < .001 | 1.58 (1.14-2.40) | 1.69 (1.17-2.54) | 1.82 (1.36-2.72) | 1.93 (1.35-2.78) | < .001 |
| Vitamin B6 supplement use | 699 (27.16) | 54 (8.42) | 91 (14.09) | 184 (28.57) | 370 (57.54) | < .001 | 56 (8.75) | 85 (13.22) | 194 (29.99) | 364 (56.52) | < .001 |
Abbreviations: 4-PA, 4-pyridoxic acid; BMI, body mass index; CHD, coronary heart disease; CRP, C-reactive protein; CVD, cardiovascular diseases; eGFR, estimated glomerular filtration rate; GED, general education development; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein cholesterol; HF, heart failure; HOMA-IR, homeostatic model assessment index of insulin resistance; LDL, low-density lipoprotein cholesterol; LOD, limit of detection; PIR, poverty income ratio; PLP, pyridoxal 5′-phosphate; TC, total cholesterol; TGs, triglycerides.
Serum Level of Pyridoxal 5′-Phosphate and All-Cause Mortality
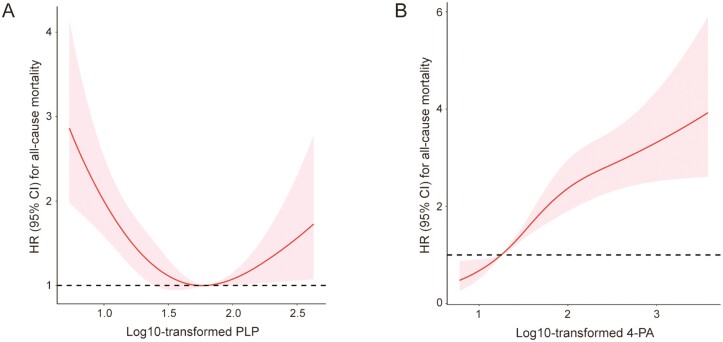
The relationship between the log-normalized serum PLP level and all-cause mortality is displayed using unadjusted restricted cubic splines. The risk of all-cause mortality dropped sharply as the serum PLP level increased, displaying a reversed J-shaped pattern (Fig. 2A). After multivariate adjustment (lifestyle factors, BMI, PIR, HbA1c level, DM duration, use of DM medication, presence of chronic diseases, 4-PA level, and use of vitamin B6 supplements), a higher serum PLP concentration was significantly associated with lower all-cause mortality (Table 2). The multivariate adjusted HRs and 95% CIs from lowest to highest serum levels of PLP (< 21.4, 21.4-35.8, 35.7-63.6, and > 63.6 nmol/L) were 1.00 (reference), 0.93 (0.71-1.23), 0.85 (0.64-1.13), and 0.74 (0.55-0.99), respectively, for all-cause mortality (P trend = .035).
Figure 2.
Association of A, pyridoxal 5′-phosphate (PLP) and B, 4-pyridoxic acid (4-PA) with all-cause mortality in unadjusted restricted cubic splines in National Health and Nutrition Examination Survey participants from 2005 to 2010.
Table 2.
Hazard ratios of all-cause mortality by pyridoxal 5′-phosphate and 4-pyridoxic acid serum levels among adults with diabetes in National Health and Nutrition Examination Survey III (2005-2010)
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P trend | HR (95% CI) | P trend | HR (95% CI) | P trend | HR (95% CI) | P trend | |
| PLP, nmol/L | .001 | < .001 | .019 | .035 | ||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| Quartile 2 | 0.78 (0.63-0.97) | 0.75 (0.61-0.94) | 0.84 (0.65-1.10) | 0.93 (0.71-1.23) | ||||
| Quartile 3 | 0.62 (0.49-0.78) | 0.60 (0.47-0.75) | 0.78 (0.60–1.03) | 0.85 (0.64-1.13) | ||||
| Quartile 4 | 0.73 (0.59-0.91) | 0.59 (0.47-0.74) | 0.73 (0.56-0.95) | 0.74 (0.55-0.99) | ||||
| 4-PA, nmol/L | <.001 | .004 | .002 | .003 | ||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| Quartile 2 | 1.46 (1.10-1.94) | 0.98 (0.73-1.30) | 1.01 (0.72-1.40) | 1.01 (0.71-1.42) | ||||
| Quartile 3 | 2.10 (1.61-2.74) | 1.17 (0.90-1.54) | 1.30 (0.94-1.80) | 1.26 (0.90-1.77) | ||||
| Quartile 4 | 3.08 (2.39-3.97) | 1.34 (1.03-1.75) | 1.49 (1.08-2.06) | 1.62 (1.12-2.35) | ||||
Model 1: adjusted for age (continuous), sex (male or female).
Model 2: further adjusted race (Mexican American, non-Hispanic White, non-Hispanic Black, or other), education (< high school, high school/GED, or > high school), physical activity (never, moderate, or vigorous), serum cotinine (> 10, LOD-10, or < LOD ng/mL), alcohol consumption (yes or no), BMI (< 25, 25-29.9, ≥ 30), and PIR (0-1.0,1.01-4.99 or 5.0).
Model 3: further adjusted for history of hypertension (yes or no), history of CVD (yes or no), history of cancer (yes or no), history of liver impairment (yes or no), renal impairment (eGFR < 90 or ≥ 90 mL/min/1.73 m2), HbA1c (continuous), duration of diabetes (< 3, 3-10, or > 10 years), medication use (no diabetes medication use, oral medication use only, any insulin use or others), 4-PA (continuous) or PLP (continuous), mean of vitamin B6 intake (continuous), and use of vitamin B6 supplement (continuous).
Abbreviations: 4-PA, 4-pyridoxic acid; BMI, body mass index; CVD, cardiovascular diseases; eGFR, estimated glomerular filtration rate; GED, general education development; HbA1c, glycated hemoglobin A1c; HR, hazard ratio; LOD, limit of detection; PLP, pyridoxal 5′-phosphate.
Serum Level of 4-Pyridoxic Acid and All-Cause Mortality
The results of the nonlinear association between the serum level of 4-PA (log10) and all-cause mortality using unadjusted restricted cubic splines are displayed in Fig. 2B. In the unadjusted Cox proportional hazards model, the highest quartiles of 4-PA level were associated with an increased risk of death (HR 3.08; 95% CI, 2.39-3.97; P < .001) (see Table 2). In the multivariate adjusted Cox regression model (model 3), the risk for all-cause mortality was 62% higher (quartile 4/quartile 1, HR, 1.62; 95% CI, 1.12-2.35; P trend = .003) for participants with the highest quartile of 4-PA concentration compared with participants with the lowest 4-PA concentration (see Table 2).
Subgroup Analyses
We found a statistically significant interaction between the serum concentration of PLP and sex with the risk of all-cause mortality (P = .01 for interaction) (Table 3). For the subgroup in men compared with the reference group (first quartile), the HR of all-cause mortality was 0.56 (95% CI, 0.38-0.83) in the fourth quartile. In the subgroup in women compared with the reference group, the HR did not differ significantly across quintiles of the PLP concentration (see Table 3). For the PLP level, a significantly decreased risk was observed only in people with a BMI greater than or equal to 30, eGFR greater than or equal to 90 mL/min/1.73 m2, HbA1c less than 7.0%, and who had history of hypertension (see Table 3).
Table 3.
Associations of pyridoxal 5′-phosphate and 4-pyridoxic acid serum levels with all-cause mortality in various subgroups among adults with diabetes in National Health and Nutrition Examination Survey III (2005-2010)
| HR (95% CIs) by quartile | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLP, nmol/L | 4-PA, nmol/L | |||||||||
| Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for interaction | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for interaction |
| (< 21.4) | (21.4-35.7) | (35.8-63.6) | (≥ 63.7) | (< 17.6) | (17.6-28.0) | (28.1-55.9) | (≥ 56.0) | |||
| Age, y | ||||||||||
| < 60 | 1 (Reference) | 0.50 (0.21-1.17) | 0.76 (0.35-1.64) | 0.77 (0.32-1.85) | .260 | 1 (Reference) | 2.27 (1.05-4.92) | 2.60 (1.09-6.23) | 2.66 (0.90-7.80) | .776 |
| ≥ 60 | 1 (Reference) | 1.06 (0.79-1.42) | 0.85 (0.62-1.16) | 0.77 (0.56-1.05) | 1 (Reference) | 0.96 (0.65-1.42) | 1.25 (0.86-1.82) | 1.78 (1.20-2.66) | ||
| Sex | ||||||||||
| Female | 1 (Reference) | 1.24 (0.80-1.94) | 1.10 (0.70-1.75) | 1.05 (0.66-1.68) | .011 | 1 (Reference) | 0.66 (0.38-1.12) | 0.99 (0.59-1.66) | 1.65 (0.95-2.86) | .391 |
| Male | 1 (Reference) | 0.75 (0.52-1.08) | 0.69 (0.47-1.00) | 0.56 (0.38-0.83) | 1 (Reference) | 1.29 (0.81-2.05) | 1.40 (0.88-2.23) | 1.62 (0.96-2.71) | ||
| BMI | ||||||||||
| < 25 | 1 (Reference) | 1.02 (0.48-2.17) | 1.56 (0.80-3.05) | 0.71 (0.35-1.44) | .722 | 1 (Reference) | 1.28 (0.47-3.46) | 1.99 (0.78-5.09) | 1.58 (0.58-4.37) | .075 |
| 25-29.9 | 1 (Reference) | 0.91 (0.55-1.50) | 0.84 (0.50-1.42) | 0.85 (0.52-1.41) | 1 (Reference) | 1.17 (0.61-2.26) | 1.75 (0.91-3.35) | 2.31 (1.16-4.59) | ||
| ≥ 30 | 1 (Reference) | 0.87 (0.59-1.28) | 0.65 (0.42-1.00) | 0.62 (0.38-0.99) | 1 (Reference) | 0.80 (0.49-1.31) | 0.95 (0.59-1.52) | 1.34 (0.79-2.27) | ||
| eGFR, mL/min/1.73 m2 | ||||||||||
| < 90 | 1 (Reference) | 0.99 (0.74-1.33) | 0.83 (0.61-1.13) | 0.78 (0.57-1.06) | .262 | 1 (Reference) | 0.98 (0.66-1.47) | 1.20 (0.81-1.77) | 1.68 (1.11-2.55) | .102 |
| ≥ 90 | 1 (Reference) | 0.44 (0.20-0.98) | 0.56 (0.26-1.24) | 0.36 (0.14-0.90) | 1 (Reference) | 1.11 (0.53-2.31) | 1.26 (0.55-2.88) | 0.59 (0.15-2.37) | ||
| Hypertension | ||||||||||
| Yes | 1 (Reference) | 0.96 (0.71-1.30) | 0.82 (0.59-1.12) | 0.69 (0.50-0.95) | .643 | 1 (Reference) | 0.98 (0.67-1.43) | 1.19 (0.82-1.73) | 1.47 (0.97-2.21) | .702 |
| No | 1 (Reference) | 0.89 (0.44-1.79) | 0.78 (0.37-1.64) | 1.01 (0.49-2.10) | 1 (Reference) | 1.35 (0.58-3.13) | 1.66 (0.71-3.88) | 2.90 (1.12-7.51) | ||
| CVD | ||||||||||
| Yes | 1 (Reference) | 1.14 (0.77-1.69) | 0.77 (0.50-1.20) | 0.73 (0.47-1.14) | .867 | 1 (Reference) | 1.70 (0.97-2.99) | 1.73 (0.99-3.02) | 2.35 (1.30-4.23) | .471 |
| No | 1 (Reference) | 0.82 (0.56-1.21) | 0.90 (0.62-1.32) | 0.77 (0.52-1.14) | 1 (Reference) | 0.70 (0.45-1.11) | 1.06 (0.69-1.63) | 1.33 (0.81-2.18) | ||
| HbA1c, % | ||||||||||
| < 7.0 | 1 (Reference) | 0.82 (0.58-1.16) | 0.65 (0.45-0.93) | 0.63 (0.44-0.90) | .448 | 1 (Reference) | 1.06 (0.67-1.67) | 1.17 (0.75-1.82) | 1.66 (1.03-2.69) | .593 |
| ≥ 7.0 | 1 (Reference) | 0.92 (0.58-1.46) | 1.10 (0.68-1.76) | 0.78 (0.47-1.29) | 1 (Reference) | 0.88 (0.51-1.50) | 1.33 (0.78-2.29) | 1.32 (0.72-2.44) | ||
Adjusted for age (continuous), sex (male or female), race (Mexican American, non-Hispanic White, non-Hispanic Black, or other), education (< high school, high school/GED, or > high school), physical activity (never, moderate, or vigorous), serum cotinine (> 10, LOD-10 or < LOD ng/mL), alcohol consumption (yes or no), BMI (< 25, 25-29.9, ≥ 30), and PIR (0-1.0, 1.01-4.99, or 5.0), history of hypertension (yes or no), history of CVD (yes or no), history of cancer (yes or no), history of liver impairment (yes or no), renal impairment (eGFR < 90 or ≥ 90 mL/min/1.73 m2), HbA1c (continuous), duration of diabetes (< 3, 3-10, or > 10 years), medication use (no diabetes medication use, oral medication use only, any insulin use, or others), 4-PA (continuous) or PLP (continuous), mean of vitamin B6 intake (continuous), and use of vitamin B6 supplement (continuous). The strata variable was not included when stratifying by itself.
Abbreviations: 4-PA, 4-pyridoxic acid; BMI, body mass index; CVD, cardiovascular diseases; eGFR, estimated glomerular filtration rate; GED, general education development; HbA1c, glycated hemoglobin A1c; HR, hazard ratio; LOD, limit of detection; PLP, pyridoxal 5′-phosphate.
Next, a stratified analysis based on 4-PA level was undertaken using fully adjusted models. Among people with T2DM diagnosed after age 60 years, the highest 4-PA level was associated with the greatest risk of all-cause mortality relative to the first quartile (HR, 1.78; 95% CI, 1.20-2.66) (see Table 3). Similar results were observed for BMI less than 30, eGFR less than 90 mL/min/1.73 m2, HbA1c less than 7.0%, and those who had a history of CVD in the fourth quartile compared with the lowest quartile (see Table 3).
Analyses of Receiver Operating Characteristic Curves and Kaplan-Meier Curves for the Benefit of using Pyridoxal 5′-Phosphate and 4-Pyridoxic Acid Levels for Predicting All-Cause Mortality
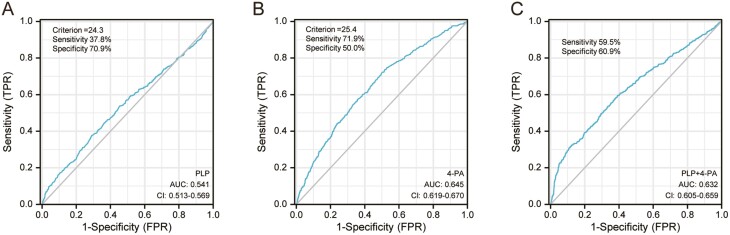
Analyses of ROC curves were undertaken to evaluate the utility of using PLP and 4-PA levels for predicting all-cause mortality (Fig. 3). The sensitivity and specificity of the PLP level for predicting all-cause mortality were 37.8% and 70.9%, respectively (area under the ROC curve (AUC) = 0.541, optimal cutoff = 24.3 nmol/L). The sensitivity and specificity of using the 4-PA level for predicting all-cause mortality were 71.9% and 50.0%, respectively (AUC = 0.761, optimal cutoff = 25.4 nmol/L). The sensitivity and specificity of a combination of PLP and 4-PA levels for predicting all-cause mortality were 59.5% and 60.9%, respectively (AUC = 0.632).
Figure 3.
Receiver operating characteristic curve analyses were used to evaluate the utility of A, pyridoxal 5′-phosphate (PLP), B, 4-pyridoxic acid (4-PA), and C, PLP combination with 4-PA in the prediction of all-cause mortality.
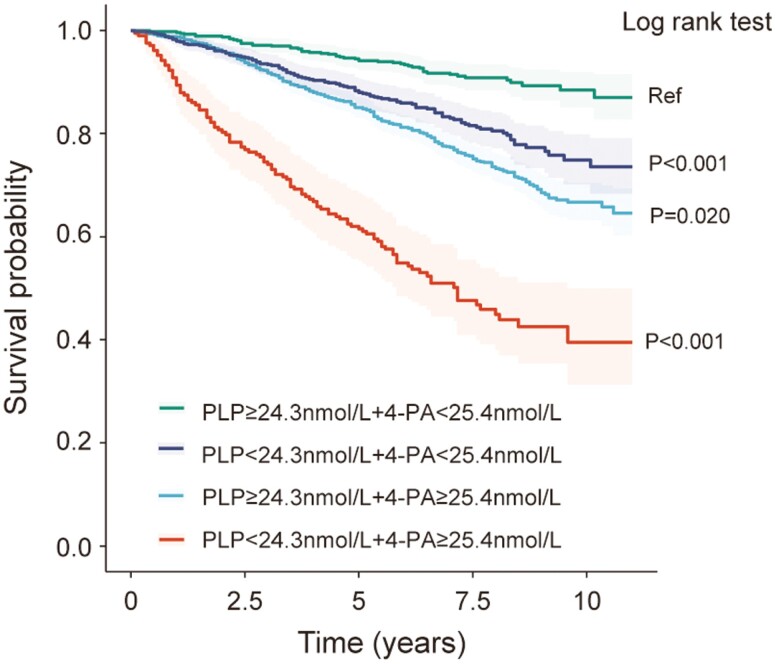
Fig. 4 shows the Kaplan-Meier plots of overall survival for patients based on different combinations of PLP level and 4-PA level. Patients with PLP greater than or equal to 24.3 nmol/L and 4-PA less than 25.4 nmol/L had a significantly better prognosis than patients with PLP less than 24.3 nmol/L and 4-PA less than 25.4 nmol/L (log-rank P < .001). Patients with 4-PA greater than or equal to 25.4 nmol/L had worse outcomes than patients with 4-PA less than 25.4 nmol/L (log-rank P = .020). Patients with PLP less than 24.3 nmol/L and 4-PA greater than or equal to 25.4 nmol/L had the worst outcomes (log-rank P < .001).
Figure 4.
Kaplan-Meier plots of overall survival for patients based on different combinations of pyridoxal 5′-phosphate (PLP) status and 4-pyridoxic acid (4-PA) status. Log-rank P values are shown.
Discussion
In this nationally representative, prospective cohort study of US adults, we discovered significant nonlinear associations between the serum vitamin B6 level and all-cause mortality. A high concentration of PLP (≥ 63.7 nmol/L) reduced the risk of all-cause mortality in DM patients. The serum 4-PA concentration was positively associated with the risk of all-cause death in DM patients.
Vitamin B6 is an important coenzyme that controls catabolic and anabolic activities (21). It functions as a cofactor in the synthesis, interconversion, and degradation of molecules (22). Serum PLP level is the most commonly used and useful marker of vitamin B6 level, and it behaves as an antioxidant molecule (23). 4-PA is a catabolite of vitamin B6 that is produced in the liver from PL and has high clearance in the kidneys (24). A large body of evidence suggests a strong relationship between vitamin B6 and DM. It is unclear if DM is the cause of decreased PLP availability or whether a low PLP level is a cause of DM (4). Several studies have found that vitamin B6 administration has a positive effect on the pathology and complications of DM (25-27). Therefore, we explored the effect of vitamin B6 level and catabolism on clinical outcomes (especially mortality) in patients with DM.
Several studies have shown that vitamin B6 deficiency is linked to an increased risk of cardiovascular events and mortality. For instance, Ulvik and colleagues (15) assessed 7796 patients with coronary artery disease, and plasma levels of PLP and 4-PA were used to predict all-cause mortality: PLP level was associated negatively, and 4-PA level was associated positively, with all-cause mortality. Those data are consistent with our findings, and other evidence also supports our findings. For example, Huang and colleagues (28) examined the relationship between B vitamins and mortality in 1747 people aged 65 years and older. They showed that a higher intake of vitamin B6 and the plasma PLP level were associated with a low risk of mortality. In a longitudinal cohort study of 687 stable renal-transplant recipients and 357 healthy controls, Minović and colleagues (29) found that the vitamin B6 concentration (as a categorical variable or continuous variable) was linked strongly with all-cause mortality. They also showed that patients with vitamin B6 deficiency had the worst prognosis and the highest prevalence of DM, leading to the highest all-cause mortality, thereby revealing that vitamin B6 deficiency may affect the prognosis of patients with DM adversely. Muller and collaborators (30) investigated the association of circulating concentrations of vitamin B6 on the diagnosis of kidney cancer with mortality risk using a case-cohort study of 630 patients with renal cell carcinoma. They discovered that patients with a high PLP level had a considerably reduced risk of all-cause mortality. Most research has been conducted on a general population or those carrying a high risk of CVD or cancer. However, among patients with DM, the evidence regarding the potential health benefits of vitamin B6 is limited, particularly with respect to all-cause mortality. We discovered that the serum level of vitamin B6 was associated strongly with all-cause mortality in patients with DM.
Most studies on the serum level of vitamin B6 have focused on PLP (31-33). Subgroup analysis in our study showed that higher PLP levels were associated with lower all-cause mortality in the subgroup of men. This may be due to higher serum levels in men than in women. This idea has been borne out by previous research (34). A previous study has shown that in 80% of the obese patients, the level of vitamin B6 was decreased to a variable degree (35). In our study, PLP level was inversely associated with all-cause mortality in patients with a BMI greater than 30.We also paid extra attention to the 4-PA level in addition to the PLP concentration. Interestingly, 4-PA (a major catabolite of vitamin B6 metabolism) was one of the markers associated most strongly with mortality in people with DM. A prospective cohort study involving 187 cases with pancreatic cancer and 258 individually matched controls investigated the association between the serum level of vitamin B6 and the risk of pancreatic cancer. Associations were not found for the serum level of 4-PA with the risk of pancreatic cancer (36). Surprisingly, we observed that a high concentration of 4-PA (≥ 56 nmol/L) increased the risk of all-cause mortality in DM patients.
This was the first study to explore the relationship between the serum vitamin B6 level and all-cause mortality in DM patients while considering many potential confounding factors. Serum levels of PLP and 4-PA were used to represent the relationship between the vitamin B6 level and catabolism and all-cause death in DM patients.
However, there are still a few limitations to this study. One is the lack of multiple interview data over periods. Another limitation is that it did not look at cause-specific mortality (eg, CVD and cancer mortality). Since our study was observational, a randomized controlled study is required to investigate the associations tested. Considering the limitations of PLP as a biomarker, the ratio 4-pPA/(pyridoxal + PLP) (PAr) has been proposed to reflect the vitamin B6 level (37, 38). Further research might look at the link between PAr and all-cause mortality and cause-specific mortality.
Conclusions
In a nationally representative sample of adults in the United States suffering from DM, we found a nonlinear association between the serum vitamin B6 level (as represented by serum levels of PLP and 4-PA) and all-cause mortality. A high serum PLP level was associated with a low risk of all-cause mortality, and a high serum 4-PA level was associated with a high risk of all-cause mortality. Our results suggest that maintaining a moderate serum level of vitamin B6 could help to reduce the risk of all-cause mortality in adult patients with T2DM.
Acknowledgments
We thank all authors for their contributions and support. We thank the Charlesworth Author Services (https://www.cwauthors.com.cn/) for editing this manuscript.
NHANES survey data are available publicly. The survey protocol has been approved by the National Center for Health Statistics Ethics Review Board. All methods were undertaken in accordance with the relevant guidelines and regulations.
Glossary
Abbreviations
- 4-PA
4-pyridoxic acid
- AUC
area under the receiver operating characteristic curve
- BMI
body mass index
- CVD
cardiovascular diseases
- DM
diabetes mellitus
- eGFR
estimated glomerular filtration rate
- HbA1c
glycated hemoglobin A1c
- HOMA-IR
homeostatic model assessment index of insulin resistance
- HR
hazard ratio
- NHANES
National Health and Nutrition Examination Survey
- PAr
4-pPA/(pyridoxal + PLP)
- PIR
income-to-poverty ratio
- PL
pyridoxal
- PLP
pyridoxal 5′-phosphate
- ROC
receiver operating characteristic
- T2DM
type 2 diabetes mellitus
Contributor Information
Dandan Zhang, Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University, Harbin, Nangang, 150001, China; Key Laboratory of Myocardial Ischemia, Ministry of Education, Harbin Medical University, Harbin, Nangang, 150001, China.
Yilan Li, Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University, Harbin, Nangang, 150001, China; Key Laboratory of Myocardial Ischemia, Ministry of Education, Harbin Medical University, Harbin, Nangang, 150001, China.
Xueyan Lang, Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University, Harbin, Nangang, 150001, China; Key Laboratory of Myocardial Ischemia, Ministry of Education, Harbin Medical University, Harbin, Nangang, 150001, China.
Yao Zhang, Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University, Harbin, Nangang, 150001, China; Key Laboratory of Myocardial Ischemia, Ministry of Education, Harbin Medical University, Harbin, Nangang, 150001, China.
Financial Support
This work was supported by the National Natural Science Foundation of China (Nos. 81770255 and 82000381) and the Open Project of Key Laboratory of Myocardial Ischemia, Ministry of Education (Nos. KF202103 and KF201906).
Disclosures
The authors have nothing to disclose.
Author Contributions
Conceptualization, D.Z. and Y.L. Methodology, D.Z. and Y.L. Software, D.Z. and X.L. Data curation, Y.L. and X.L. Visualization, D.Z. and XL. Validation, D.Z. and Y.L. Writing of original draft, D.Z. and Y.L. Writing, review, and editing, D.Z. and Y.Z. Supervision, D.Z. and Y.Z. Funding acquisition, D.Z., Y.L., and Y.Z. All authors agreed to the published version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Saeedi P, Petersohn I, Salpea P, et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2. Seshasai SRK, Kaptoge S, Thompson A, et al. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829-841. doi: 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stover PJ, Field MS. Vitamin B-6. Adv Nutr. 2015;6(1):132-133. doi: 10.3945/an.113.005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mascolo E, Verni F. Vitamin B6 and diabetes: relationship and molecular mechanisms. Int J Mol Sci . 2020;21(10):18. doi: 10.3390/ijms21103669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lotto V, Choi SW, Friso S. Vitamin B-6: a challenging link between nutrition and inflammation in CVD. Br J Nutr. 2011;106(2):183-195. doi: 10.1017/s0007114511000407 [DOI] [PubMed] [Google Scholar]
- 6. Kelly PJ, Kistler JP, Shih VE, et al. Inflammation, homocysteine, and vitamin B6 status after ischemic stroke. Stroke. 2004;35(1):12-15. doi: 10.1161/01.Str.0000106481.59944.2f [DOI] [PubMed] [Google Scholar]
- 7. Saibeni S, Cattaneo M, Vecchi M, et al. Low vitamin B-6 plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol. 2003;98(1):112-11 7. doi: 10.1016/s0002-9270(02)05828-8 [DOI] [PubMed] [Google Scholar]
- 8. Friedman AN, Hunsicker LG, Selhub J, Bostom AG. Clinical and nutritional correlates of C-reactive protein in type 2 diabetic nephropathy. Atherosclerosis. 2004;172(1):121-125. doi: 10.1016/j.atherosclerosis.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 9. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109(3):1-–9.. doi: 10.1093/jnci/djw230 [DOI] [PubMed] [Google Scholar]
- 10. Nix WA, Zirwes R, Bangert V, et al. Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res Clin Pract. 2015;107(1):157-165. doi: 10.1016/j.diabres.2014.09.058 [DOI] [PubMed] [Google Scholar]
- 11. Fields AM, Welle K, Ho ES, Mesaros C, Susiarjo M. Vitamin B6 deficiency disrupts serotonin signaling in pancreatic islets and induces gestational diabetes in mice. Commun Biol. 2021;4(1):421. doi: 10.1038/s42003-021-01900-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spratlen MJ, Grau-Perez M, Umans JG, et al. Arsenic, one carbon metabolism and diabetes-related outcomes in the Strong Heart Family Study. Environ Int. 2018;121(Pt 1):728-740. doi: 10.1016/j.envint.2018.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jin GQ, Wang JJ, Jiang XB. Association of folate, vitamin B-12 and vitamin B-6 intake with diabetes and prediabetes in adults aged 20 years and older. Asia Pac J Clin Nutr. 2021;30(1):75-86. doi: 10.6133/apjcn.202103_30(1).0010 [DOI] [PubMed] [Google Scholar]
- 14. Wang LL, Li HT, Zhou Y, Jin L, Liu JM. Low-dose B vitamins supplementation ameliorates cardiovascular risk: a double-blind randomized controlled trial in healthy Chinese elderly. Eur J Nutr. 2015;54(3):455-464. doi: 10.1007/s00394-014-0729-5 [DOI] [PubMed] [Google Scholar]
- 15. Ulvik A, Pedersen ER, Svingen GFT, et al. Vitamin B-6 catabolism and long-term mortality risk in patients with coronary artery disease. Am J Clin Nutr. 2016;103(6):1417-1425. doi: 10.3945/ajcn.115.126342 [DOI] [PubMed] [Google Scholar]
- 16. Song YQ, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effect of homocysteine-lowering treatment with folic acid and B vitamins on risk of type 2 diabetes in women a randomized, controlled trial. Diabetes. 2009;58(8):1921-1928. doi: 10.2337/db09-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gregg EW, Cheng YLJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391(10138):2430-2440. doi: 10.1016/s0140-6736(18)30314-3 [DOI] [PubMed] [Google Scholar]
- 18. Ueland PM, Ulvik A, Rios-Avila L, Midttun Ø, Gregory JF. Direct and functional biomarkers of vitamin B6 status. Annu Rev Nutr. 2015;35:33-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith J, Jain N, Normington J, Holschuh N, Zhu Y. Associations of ready-to-eat cereal consumption and income with dietary outcomes: results from the National Health and Nutrition Examination Survey 2015-2018. Front Nutr. 2022;9:816548. doi: 10.3389/fnut.2022.816548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agnoli C, Grioni S, Krogh V, et al. Plasma riboflavin and vitamin B-6, but not homocysteine, folate, or vitamin B-12, are inversely associated with breast cancer risk in the European Prospective Investigation into Cancer and Nutrition–Varese Cohort. J Nutr. 2016;146(6):1227-1234. doi: 10.3945/jn.115.225433 [DOI] [PubMed] [Google Scholar]
- 22. Percudani R, Peracchi A. The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinformatics. 2009;10:273. doi: 10.1186/1471-2105-10-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leklem JE. Vitamin-B6: a status report. J Nutr. 1990;120(11 Suppl 4): 1503-1507. doi: 10.1093/jn/120.suppl_11.1503 [DOI] [PubMed] [Google Scholar]
- 24. Zaric BL, Obradovic M, Bajic V, Haidar MA, Jovanovic M, Isenovic ER. Homocysteine and hyperhomocysteinaemia. Curr Med Chem. 2019;26(16):2948-2961. doi: 10.2174/0929867325666180313105949 [DOI] [PubMed] [Google Scholar]
- 25. Jain SK. Vitamin B-6 (pyridoxamine) supplementation and complications of diabetes. Metab Clin Exp. 2007;56(2):168-171. doi: 10.1016/j.metabol.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 26. Ellis JM, Folkers K, Minadeo M, Vanbuskirk R, Xia LJ, Tamagawa H. A deficiency of vitamin B6 is a plausible molecular-basis of the retinopathy of patients with diabetes mellitus. Biochem Biophys Res Commun. 1991;179(1):615-619. doi: 10.1016/0006-291x(91)91416-a [DOI] [PubMed] [Google Scholar]
- 27. Hayakawa M, Shibata M. The in vitro and in vivo inhibition of protein glycosylation and diabetic vascular basement-membrane thickening by pyridoxal-5′-phosphate. J Nutr Sci Vitaminol (Tokyo). 1991;37(2):149-159. doi: 10.3177/jnsv.37.149 [DOI] [PubMed] [Google Scholar]
- 28. Huang YC, Lee MS, Wahlqvist ML. Prediction of all-cause mortality by B group vitamin status in the elderly. Clin Nutr. 2012;31(2):191-198. doi: 10.1016/j.clnu.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 29. Minović I, Riphagen IJ, van den Berg E, et al. Vitamin B-6 deficiency is common and associated with poor long-term outcome in renal transplant recipients. Am J Clin Nutr. 2017;105(6):1344-1350. doi: 10.3945/ajcn.116.151431 [DOI] [PubMed] [Google Scholar]
- 30. Muller DC, Johansson M, Zaridze D, et al. Circulating concentrations of vitamin B6 and kidney cancer prognosis: a prospective case-cohort study. PLoS One. 2015;10(10):e0140677. doi: 10.1371/journal.pone.0140677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ulvik A, Midttun Ø, McCann A, et al. Tryptophan catabolites as metabolic markers of vitamin B-6 status evaluated in cohorts of healthy adults and cardiovascular patients. Am J Clin Nutr. 2020;111(1):178-186. doi: 10.1093/ajcn/nqz228 [DOI] [PubMed] [Google Scholar]
- 32. Abbenhardt C, Miller JW, Song XL, et al. Biomarkers of one-carbon metabolism are associated with biomarkers of inflammation in women. J Nutr. 2014;144(5):714-721. doi: 10.3945/jn.113.183970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clasen JL, Heath AK, Van Puyvelde H, et al. A comparison of complementary measures of vitamin B6 status, function, and metabolism in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. 2021;114(1):338-347. doi: 10.1093/ajcn/nqab045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schini M, Nicklin P, Eastell R. Establishing race-, gender- and age-specific reference intervals for pyridoxal 5′-phosphate in the NHANES population to better identify adult hypophosphatasia. Bone. 2020;141:115577. doi: 10.1016/j.bone.2020.115577 [DOI] [PubMed] [Google Scholar]
- 35. Bodunova NA, Askerkhanov RG, Khatkov IE, et al. Impact of bariatric surgery on vitamin metabolisms in obese patients [article in Russian]. Ter Arkhiv. 2015;87(2):70-76. doi: 10.17116/terarkh201587270-76 [DOI] [PubMed] [Google Scholar]
- 36. Huang JY, Butler LM, Midttun Ø, et al. Serum B6 vitamers (pyridoxal-5′-phosphate, pyridoxal, and 4-pyridoxic acid) and pancreatic cancer risk: two nested case-control studies in Asian populations. Cancer Res. 2016;76(14 Suppl):4297. doi: 10.1158/1538-7445.Am2016-4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ulvik A, Midttun Ø, Pedersen ER, Eussen SJ, Nygård O, Ueland PM. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am J Clin Nutr. 2014;100(1):250-255. doi: 10.3945/ajcn.114.083196 [DOI] [PubMed] [Google Scholar]
- 38. Ueland PM, McCann A, Midttun Ø, Ulvik A. Inflammation, vitamin B6 and related pathways. Mol Asp Med. 2017;53:10-27. doi: 10.1016/j.mam.2016.08.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in “References.”