Abstract
Context
Exaggerated postprandial incretin and insulin responses are well documented in postbariatric surgery hypoglycemia (PBH) after Roux-en-Y gastric bypass (RYGB). However, less is known about PBH after sleeve gastrectomy (SG).
Objective
We sought to compare meal-stimulated hormonal response in those with PBH after SG vs RYGB.
Methods
We enrolled 23 post-SG (12 with and 11 without PBH) and 20 post-RYGB (7 with and 13 without PBH) individuals who underwent bariatric surgery at our institution. PBH was defined as plasma glucose less than 60 mg/dL on 4-hour mixed-meal tolerance test (MTT). Islet and incretin hormones were compared across the 4 groups.
Results
Participants (N = 43) were on average 5 years post surgery, with a mean age of 48 years, mean preoperative body mass index of 48.4, 81% female, 61% White, and 53% post SG. Regardless of PBH, the SG group showed lower glucose, glucagon, and glucagon-like peptide 1 (GLP-1) responses to MTT and similar insulin and glucose-dependent insulinotropic polypeptide (GIP) responses compared to the RYGB group. Among those with PBH, the SG group following the MTT showed a lower peak glucose (P = .02), a similar peak insulin (90.3 mU/L vs 171mU/L; P = .18), lower glucagon (P < .01), early GLP-1 response (AUC0-60 min; P = .01), and slower time to peak GIP (P = .02) compared to PBH after RYGB.
Conclusion
Among individuals with PBH, those who underwent SG were significantly different compared to RYGB in meal-stimulated hormonal responses, including lower glucagon and GLP-1 responses, but similar insulin and GIP responses. Future studies are needed to better understand the differential contribution of insulin and non–insulin-mediated mechanisms behind PBH after SG vs RYGB.
Keywords: hypoglycemia, sleeve gastrectomy, gastric bypass, RYGB, GLP-1, GIP
Bariatric surgery is a highly effective treatment for obesity and related diseases such as diabetes (1). Along with its increasing utilization, there is an emerging recognition of its complications, including postbariatric surgery hypoglycemia (PBH) (2). Symptoms of PBH or late dumping syndrome usually occur 1 to 3 hours after a meal and are mainly related to hypoglycemia (HG), whereas early dumping syndrome symptoms include gastrointestinal and vasomotor symptoms (3). While the exact prevalence of PBH is unknown, it is estimated to be between 1% and 13% (4-6). Some cases of PBH can be life-threatening with reports of associated falls, seizure, car accidents, and disability (7, 8). Previously observed after upper gastrointestinal surgeries and Roux-en-Y gastric bypass (RYGB), PBH has also been reported after sleeve gastrectomy (SG) (6, 9).
The mechanism underlying PBH is thought to be multifactorial, involving insulin and noninsulin pathways triggered by food intake (10-13). In individuals with HG after RYGB, the secretion of glucagon‐like peptide 1 (GLP-1) and insulin are several-fold higher than that of post-RYGB individuals without HG (14-16). Blocking the GLP-1 pathway partially ameliorates meal-stimulated insulin secretion and HG after RYGB (11). There are few data on whether GLP-1 and insulin secretion is similarly increased postprandially in those with HG after SG. Therefore, we aimed to compare the profile of insulin and gut hormone response to a meal in individuals after SG vs RYGB, with a particular focus in this work on those with PBH.
Materials and Methods
Study Population
We recruited participants who underwent either SG or RYGB from 2009 to 2014 at the Johns Hopkins Center for Bariatric Surgery. We enrolled adults without diabetes requiring medication at time of enrollment. We screened for symptoms and/or history of PBH to ensure that at least half of each surgery group met “high suspicion for PBH” as defined by 3 or more postprandial symptoms or PBH diagnosis as described in previous publications (6). We excluded individuals with history of gastrointestinal surgery subsequent to initial bariatric surgery or history of pancreatitis, pancreatic disease, or inflammatory bowel disease. We also excluded those with a history of chronic illnesses, including uncontrolled HIV, chronic kidney disease (glomerular filtration rate < 30 mL min/1.73 m2 within the past 6 months), or decompensated liver disease. Finally, we excluded those reporting use of oral steroids within 2 months of screening or current alcohol use exceeding more than 14 alcoholic beverages per week or more than 4 per day. All participants provided written informed consent, and the study was approved by The Johns Hopkins Institutional Review Board.
Study Design
Participants arrived for an outpatient study visit at the Johns Hopkins Clinical Research Unit after an overnight fast of at least 10 hours. Participants underwent a mixed-meal tolerance test (MMT), in which they consumed one 8-ounce Ensure Plus drink (Abbott Laboratories; 350 kcal, 50 g carbohydrates, 13 g protein, and 11 g fat) within a 30-minute period. Blood was sampled at –10, 0, 15, 30, 45, 60, 90, 120, 180, and 240 minutes, with 0 minutes marking the beginning of the meal. Blood samples were collected in tubes containing potassium oxalate, sodium fluoride, and EDTA for determination of plasma glucose and in tubes containing EDTA plus dipeptidyl peptidase IV inhibitor for measurement of GLP-1, GIP, insulin, and glucagon. Samples were stored at –80 °C until further processing. PBH was defined as glucose less than 60 mg/dL at any point in time after the start of the MMT.
Assays
Plasma was used to measure glucose, insulin, glucagon, total GIP, and total GLP-1, and all assays were performed according to the kit manufacturers’ instructions. Glucose was measured using the hexokinase G-6-PDH method (Beckman Coulter). Plasma insulin (Mercodia, RRID: AB_2877672), GIP total (Millipore Corp, RRID: AB_2801401), and GLP-1 total (Alpco, RRID: AB_2801400) levels were measured by enzyme-linked immunosorbent assay. Plasma glucagon was measure by radioimmune assay (Millipore, catalog No. GL-32K, RRID: AB_2757819).
Calculations and Statistical Analysis
Glucose, insulin, glucagon, GLP-1, and GIP concentrations from 0 to 240 minutes post meal ingestion were used to compute the incremental area under the curve (AUC) using the trapezoidal rule. For insulin unit conversion we used the 1 mU/L = 6.0 pmol/L conversion.
We used chi-square, analysis of variance, or independent sample t tests to compare clinical characteristics and hormone parameters across the following 4 groups: SG with HG, SG without HG, RYGB with HG, and RYGB without HG. All variables were examined at the P less than .05 level of statistical significance. All analyses were performed using STATA version 12.1 (StataCorp).
Results
Characteristics of All Participants
Baseline characteristics of the 43 study participants are summarized in Table 1. Overall, participants were mostly female with a mean age of 48 years, and mean time since bariatric surgery was 4.8 years. Among the 2 surgery groups, the SG group was significantly younger (aged 43 vs 54 years; P < .01) with less time since surgery (4.0 vs 5.9 years; P < .1) and had nonsignificant differences in the proportions of female (87% vs 75%; P = .315), current BMI (36.5 vs 33.1; P = .949), and White individuals (52% vs 70%; P = .376) compared to the RYGB group. Baseline characteristics were similar between those with vs without HG.
Table 1.
Baseline characteristics of participants
| Statistical test P values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All participants (N = 43) | SG (n = 23) | RYGB (n = 20) | HG vs non-HG | SG vs RYGB | |||||||
| Characteristic | HG | Non-HG | HG | Non-HG | All | SG | RYGB | All | HG | Non-HG | |
| (n = 9) | (n = 14) | (n = 8) | (n = 12) | ||||||||
| Mean age, y, mean (SD) | 48 (10) | 41 (11) | 44 (11) | 50 (8) | 56 (7) | .11 | .29 | .03 | < .001 | .08 | .002 |
| % Female | 81 | 78 | 93 | 63 | 83 | .14 | .30 | .29 | .32 | .49 | .45 |
| % White | 61 | 67 | 43 | 88 | 58 | .20 | .45 | .16 | .38 | .31 | .53 |
| Current BMI, mean (SD) | 34.9 (6.8) | 38.0 (10.6) | 35.6 (5.2) | 33.0 (6.4) | 33.2 (5.0) | .70 | .76 | .46 | .95 | .87 | .88 |
| Preoperative BMI, mean (SD) | 48.4 (8.2) | 50.5 (9.2) | 47.5 (3.5) | 50.0 (11.3) | 46.9 (9.7) | .88 | .86 | .74 | .58 | .54 | .59 |
| Time since surgery, y, mean (SD) | 4.8 (1.4) | 3.9 (0.9) | 4.0 (0.9) | 6.1 (1.2) | 5.7 (1.4) | .65 | .39 | .77 | < .001 | < .001 | < .001 |
| No. of neuroglycopenic symptoms during MTT | 9 | 5 | 0 | 4 | 0 |
Abbreviations: BMI, body mass index; HG, hypoglycemia; MTT, meal tolerance test; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
All p values <0.05 are in bold
Characteristics of Participants With Hypoglycemia After Bariatric Surgery
Among those who developed HG during the MTT, the SG group reported significantly less time post surgery (3.9 vs 6.1 years; P < .01) and tended to be younger (41 vs 50 years; P = .078) with similar current BMI (38 vs 33; P = .877) compared to the RYGB group. In both surgery groups, approximately half reported neuroglycopenic symptoms such as confusion and slow thought processing during the MTT (see Table 1).
Fasting Glucose, Insulin, and Gut Hormone Data
The data discussed here are summarized in Tables 2 and 3. Regarding fasting glucose, there was a statistically significant difference across the 4 groups, but more important there was no difference between the 2 surgery groups with PBH. The remaining fasting hormone levels, including insulin, GLP-1, GIP, and glucagon, were similar across all four groups.
Table 2.
Glucose, insulin, and glucagon response to meal tolerance test in adults with postbariatric surgery hypoglycemia
| Statistical test P values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RYGB (n = 20) | SG (n = 23) | HG vs non-HG | After SG vs RYGB | |||||||
| Characteristic | Non-HG n = 12 |
HG n = 8 |
Non-HG n = 14 |
HG n = 9 |
Overall | RYGB vs SG | RYGB | SG | Non-HG | HG |
| Fasting glucose, mg/dL | 91.2 (3.4) | 79.3 (2.8) | 84.1 (2.8) | 78.6 (1.9) | .03 | .23 | .09 | .50 | .47 | ≥ .999 |
| Peak glucose, mg/dL | 195.9 (10.2) | 174.1 (8.4) | 139.7 (9.3) | 126.2 (7.4) | < .001 | < .001 | .55 | .95 | .01 | .02 |
| Time to peak glucose, min | 38.8 (3.4) | 31.9 (3.4) | 28.9 (2.9) | 28.3 (3.0) | .10 | .04 | .51 | ≥ .999 | .18 | .86 |
| Nadir glucose (mg/dL) | 70.0 (2.5) | 46.1 (2.8) | 71.0 (1.5) | 51.4 (2.4) | < .001 | .51 | .001 | < .001 | .96 | .41 |
| Time to nadir glucose, min | 167.5 (18.3) | 108.8 (11.3) | 165.0 (15.0) | 136.7 (15.1) | .06 | .35 | .15 | .55 | ≥ .999 | .24 |
| AUCglucose(0-60 min) | 9273.1 (496) | 8058.8 (302) | 6986.8 (423) | 6509.2 (360) | .001 | < .001 | .39 | .92 | .02 | .07 |
| AUCglucose(0-240 min) | 26414.4 (1551) | 19875.0 (570) | 21564.6 (654) | 18765.8 (847) | < .001 | .03 | .007 | .07 | .04 | .66 |
| Fasting insulin, mU/L | 3.1 (0.7) | 3.2 (1.2) | 3.2 (0.6) | 2.8 (0.9) | .90 | .98 | ≥ .999 | .90 | .99 | .87 |
| Peak insulin, mU/L | 90.1 (15.1) | 171.0 (19.8) | 86.4 (16.6) | 90.3 (22.2) | .03 | .1 | .03 | ≥ .999 | .99 | .18 |
| Time to peak insulin, min | 40.0 (5.3) | 37.5 (4.0) | 43.9 (4.6) | 46.7 (4.6) | .44 | .15 | ≥ .999 | .88 | .89 | .50 |
| AUCinsulin(0-60 min) | 2825.2 (470) | 6145.2 (848) | 3209.0 (586) | 3105.8 (738) | .02 | .18 | .02 | .99 | .98 | .10 |
| AUCinsulin(0-240 min) | 5048.5 (804) | 7661.3 (962) | 5207.5 (847) | 5146.7 (1233) | .12 | .18 | .16 | .98 | ≥ .999 | .31 |
| Fasting glucagon, ng/L | 40.5 (2.2) | 47.5 (5.0) | 38.1 (2.3) | 37.6 (2.9) | .23 | .06 | .83 | .99 | .80 | .31 |
| Peak glucagon, ng/L | 64.8 (3.8) | 77.0 (4.6) | 57.7 (6.3) | 49.1 (2.3) | < .001 | < .001 | .29 | .74 | .15 | .004 |
| Time to peak glucagon, min | 37.5 (3.5) | 46.9 (14.0) | 33.2 (5.7) | 58.3 (8.5) | .08 | .85 | .99 | .07 | .58 | .64 |
| AUCglucagon(0-60 min) | 3259.2 (164) | 3827.5 (215) | 2893.4 (259) | 2470.8 (138) | < .001 | < .001 | .25 | .63 | .19 | .006 |
| AUCglucagon(0-240 min) | 12166.6 (529) | 14426.3 (862) | 10553.9 (505) | 10032.7 (506) | .001 | < .001 | .13 | .92 | .19 | .008 |
Data are presented as mean ± SEM unless otherwise specified.
Abbreviations: AUC, area under the curve; BMI, body mass index; HG, hypoglycemia; MTT, meal tolerance test; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Table 3.
Incretin hormonal response: glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide
| Statistical test P values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RYGB (n = 20) | SG (n = 23) | HG vs non-HG | After RYGB vs SG | |||||||
| Characteristic | Non-HG n = 12 |
HG n = 8 |
Non-HG n = 14 |
HG n = 9 |
Overall | RYGB vs SG | RYGB | SG | Non-HG | HG |
| Fasting GLP-1, pM | 9.3 (4.2) | 13.1 (4.6) | 6.8 (2.2) | 4.9 (0.6) | .85 | .47 | .98 | ≥ .999 | .95 | .87 |
| Peak GLP-1, pM | 31.7 (5.8) | 44.4 (7.2) | 16.9 (2.8) | 12.2 (1.8) | < .001 | < .001 | .48 | .49 | .09 | .007 |
| Time to peak GLP-1, min | 30.0 (3.2) | 28.1 (1.9) | 30.0 (2.7) | 25.0 (2.5) | .57 | .61 | ≥ .999 | .61 | ≥ .999 | .76 |
| AUCGLP-1(0-60 min) | 1327.5 (287) | 1853.5 (315) | 709.7 (147) | 538.1 (62.7) | .001 | < .001 | .48 | .91 | .09 | .01 |
| AUCGLP-1(0-240 min) | 3650.3 (1170) | 4883.5 (1156) | 2308.3 (731) | 1605.6 (121) | .02 | < .001 | .43 | .98 | .29 | .06 |
| Fasting GIP, pM | 46.7 (7.9) | 57.5 (7.7) | 48.0 (6.9) | 51.1 (9.2) | .58 | .85 | .39 | .99 | .99 | .91 |
| Peak GIP, pM | 392.3 (38.9) | 511.5 (98.1) | 517.8 (45.6) | 523.9 (63.6) | .28 | .11 | .83 | .99 | .25 | .99 |
| Time to peak GIP, min | 30.0 (3.7) | 28.1 (3.4) | 41.8 (4.5) | 50.0 (4.3) | .003 | < .001 | ≥ .999 | .33 | .15 | .02 |
| AUCGIP(0-6 min) | 16 386.6 (1867) | 21 772.5 (4033) | 21 251.0 (2100) | 20 822.5 (2676) | .43 | .29 | .75 | ≥ .999 | .35 | .99 |
| AUCGIP(0-24 min) | 36 920.8 (5115) | 44 553.1 (7813) | 53 546.7 (6124) | 50 390.9 (7684) | .21 | .06 | .93 | .98 | .15 | .96 |
Data are presented as mean ± SEM unless otherwise specified.
Abbreviations: AUC, area under the curve; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon‐like peptide 1; HG, hypoglycemia; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Meal-stimulated Glucose, Insulin, and Gut Hormone Data
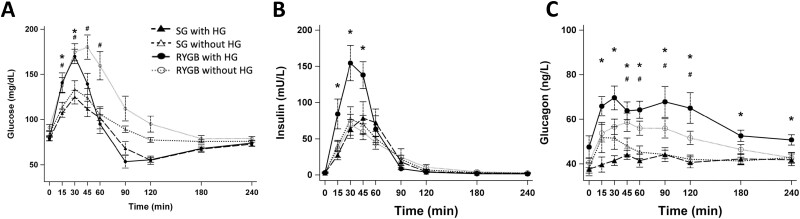
The data discussed here are also summarized in Tables 2 and 3. Regarding meal-stimulated peak glucose (Fig. 1), the SG group showed less dynamic change evidenced by a lower mean peak glucose (P < .001) and lower AUCglucose levels compared to the RYGB group (P = .03). Concerning those with vs without HG, there was no statistically significant difference in time to peak or time to nadir glucose regardless of surgery type.
Figure 1.
Plasma A, glucose; B, insulin; and C, glucagon responses to meal stimulation with hypoglycemia. *P less than .05 compared between SG with HG and RYGB with HG; #P less than .05 compared between SG without HG and RYGB without HG. HG, hypoglycemia; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Among those who developed HG on meal stimulation, the SG group reached a significantly lower peak glucose (126.2 vs 174.1 mg/dL; P = .02) while reaching a similar nadir glucose (P = .41) compared to the RYGB group. There was no significant difference in meal-stimulated insulin between the SG vs RYGB group (P = .31; AUC0-240 min for RYGB without HG: 5048.5, RYGB with HG: 7661.3, SG without HG: 5207.6, SG with HG: 5146.7 mU/L ⋅ min AUC0-240 min) (see Fig. 1). Interestingly, there was no significant difference in peak insulin between those with compared to those without HG after SG (P = .18). However, within the RYGB group, those with HG reached a peak insulin level that was nearly double that of those without HG (171.1 vs 90.1 mU/L; P = .03). Regarding the glucagon response (see Fig. 1), the SG group reached a lower early (AUC0-60 min; P < .01) and overall response (AUC0-240 min; P < .01) as well as lower peak (P < .01) compared to the RYGB group. Among those with HG, the same pattern was observed with the SG group reaching a lower early (P < .01) and overall response (P < .01) as well as lower peak (P < .01) compared to the RYGB group.
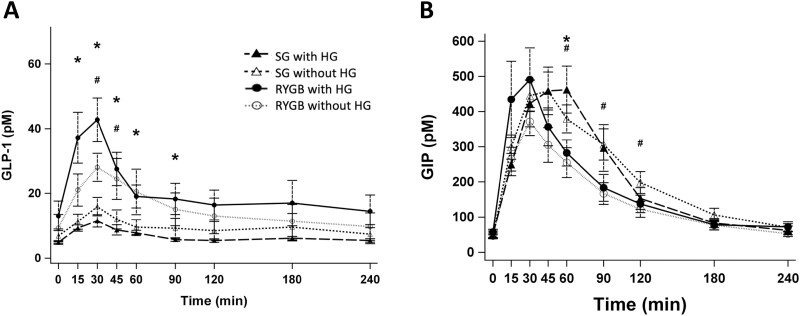
Regarding the incretin responses (Fig. 2), the GLP-1 response in the SG group was significantly lower both early (P < .01) and overall (P < .01) with lower peak (P < .01) compared to the RYGB group. Comparing those with HG, the magnitude of early and overall GLP-1 responses in the SG group were substantially lower than that of RYGB (538.1 vs 1853.5 pM ⋅ min AUC 0-60 min) and this difference reached statistical significance during peak (P < .01) and early response (P = .01) between the 2 surgical groups.
Figure 2.
Incretin responses, A, GLP-1 and B, GIP to meal stimulation with hypoglycemia. *P less than .05 compared between SG with HG and RYGB with HG; #P less than .05 compared between SG without HG and RYGB without HG. GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; HG, hypoglycemia; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Regarding the GIP response, the difference between the 2 surgical groups was less pronounced compared to the GLP-1 or insulin responses. The SG group reached the peak more slowly (P < .01) and tended to have a lower overall GIP response (P = .06) compared to the RYGB group. Comparing those with HG, time to peak GIP was significantly greater in the SG group compared to the RYGB group (P = .02), whereas the peak GIP and overall response were similar across the 2 groups.
Discussion
In this study, we showed that the glucose and hormonal responses among adults with PBH were distinctly different after SG compared to RYGB. In individuals with PBH after RYGB, we observed an exaggerated insulin peak and response as shown in other studies (14-16). In contrast, in those with PBH after SG, an exaggerated insulin response was not observed and the insulin response was similar to those without PBH after SG or after RYGB.
The reasons for this difference in postprandial insulin kinetics between the PBH after SG vs RYGB groups is not clear. We hypothesize that the unexpected lack of hyperinsulinemia in the PBH after SG group is due, in part, to the lack of high peak glucose on meal stimulation in this group compared to that of PBH after RYGB group (SG: 126 vs RYGB: 174 mg/dL mean peak glucose; P = .02), so excess insulin production is not provoked. Despite the lack of an exaggerated insulin response, the PBH after SG group reached a similar nadir glucose as the PBH after RYGB group (SG: 51.4 vs RYGB: 46.1 mg/dL; P = .41).
Given the similar postprandial glucose concentrations in those with PBH after SG vs RYGB, and relatively lower insulin response after SG vs RYGB, other mechanisms may be present besides abnormal insulin secretion that contribute to PBH after SG. In particular, abnormal glucagon response in PBH after SG compared to RYGB may be important. In healthy individuals, postprandial hyperglycemia stimulates insulin secretion, which inhibits glucagon secretion (17). As the postprandial glucose falls, the pancreas secretes glucagon to mobilize glucose into blood circulation by stimulating hepatic glycogenolysis, gluconeogenesis, and inhibiting glycogenesis (17, 18). In this study, we observed that glucagon concentrations were lower in those with PBH after SG compared to after RYGB. In fact, the glucagon response was lower after SG vs RYGB regardless of PBH status. Therefore, PBH after SG may be partly due to the attenuated glucagon response in the SG group compared to the RYGB group with PBH, though further studies are needed to confirm the role of the glucagon response in PBH after SG. Reductions in counterregulatory hormones in individuals with post-RYGB HG have been previously reported (19). In contrast, data on counterregulatory hormones in post-SG HG are limited to one preclinical study in which normal counterregulatory hormone levels were observed 4 weeks after SG in a murine model (20). To our knowledge, this is the first study in humans to examine this question.
Abnormally enhanced secretion of GLP-1 is thought to play an important role in the pathophysiology of PBH after RYGB, and the GLP-1 receptor antagonist is under investigation for treatment of PBH after RYGB (10, 11, 21). In PBH after SG, however, we found that the peak and overall GLP-1 response was reduced compared to RYGB. For example, the peak and early GLP-1 response (AUC0-60 min) in the PBH after SG group was less than a third that of the PBH after RYGB group. This notable difference may be explained by the variance in postsurgical anatomy that allows for a relatively slower food bolus transit and absorption after SG compared to RYGB, which in turn may contribute to a lower postprandial peak plasma glucose as well as slower and reduced stimulation of the hindgut containing GLP-1–secreting L-cells (22, 23). The resulting lower postprandial peak plasma glucose after SG vs RYGB seen previously and confirmed in our data likely also contributed to an attenuated GLP-1 response given that GLP-1 secretion is glucose dependent (24).
Previously, GIP response to a meal has been shown to be higher after RYGB, similar to the GLP-1 response, while GIP response to HG was lower when comparing those with, vs without, PBH after RYGB or in hypoglycemic clamp pre vs post surgery (10, 19). In this study, the GIP response to a meal was similar among those with PBH regardless of surgery type. Time to peak GIP, however, was longer in PBH after SG vs RYGB, which suggests that the expedited food transit after RYGB may stimulate the GIP-secreting K-cells in the Roux limb of RYGB faster than in the intact proximal gut after SG. Additionally, higher peak glucose after RYGB may contribute to the expedited time to peak GIP after RYGB vs SG given that GIP secretion is glucose dependent (22). There are also other factors related to incretin secretion such as autonomic neural input and insulin clearance that may contribute in those with HG after SG to a lower postprandial peak glucose and subsequently a less exaggerated (GLP-1) and slower (GIP) secretion compared to those with HG after RYGB (25, 26). Whether the slower GIP secretion observed in SG compared to RYGB plays a role in the underlying pathophysiology of PBH warrants further exploration, especially given the known role of GIP in stimulating glucagon secretion during HG and substantially diminished glucagon response in PBH after SG vs RYGB observed in this study (27).
Strengths of this study include the comparison of PBH after 2 common bariatric procedures, SG and RYGB, in terms of meal-stimulated glucose and hormonal responses. We also included control groups without PBH after either of the 2 surgery groups to provide further comparisons across all 4 groups (SG or RYGB with or without PBH). Another strength of the study is the use of the MTT to elicit PBH and postprandial hormonal response across participants. The limitations of the study include the use of postprandial glucose cutoff of less than 60 mg/dL instead of less than 54 mg/dL (28). However, the use of the higher glucose cutoff allowed us to include additional cases with neuroglycopenic symptoms supporting severe HG (eg, 3 additional cases of PBH after SG and 1 case of PBH after RYGB) that would otherwise have been excluded with the lower glucose cutoff (29). This study did not assess other counterregulatory hormones such as epinephrine or cortisol and did not include preoperative glucose and hormonal responses to meal stimulation before bariatric surgery or data from nonsurgical patients. Nevertheless, this is the largest study to date to report the hormonal response in individuals with PBH after SG and RYGB and all individuals in this study denied preexisting HG before bariatric surgery.
Conclusion
Among those with PBH, the SG group differed significantly compared to the RYGB group in terms of reaching a lower peak glucose with lower insulin and glucagon response to meal stimulation. In terms of incretin response among those with HG, the SG group had a lower GLP-1 response and similar GIP response to meal stimulation compared to the RYGB group. Combined, these findings suggest alternative mechanisms in addition to insulin and GLP-1–mediated pathways underlying PBH after SG. Consequently, the role of GLP-1 receptor antagonists in preventing PBH post SG may be limited. In contrast, the impaired glucagon response observed in PBH after SG may be an important therapeutic target to consider and strategies to restore pancreatic α-cell function in PBH may be a promising approach in PBH after SG.
Acknowledgments
We would like to thank the study participants, without whom this work would not be possible.
Glossary
Abbreviations
- AUC
area under the curve
- BMI
body mass index
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide 1
- HG
hypoglycemia
- MTT
mixed-meal tolerance test
- PBH
postbariatric surgery hypoglycemia
- RYGB
Roux-en-Y gastric bypass
- SG
sleeve gastrectomy
Contributor Information
Clare J Lee, Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, The Johns Hopkins University, Baltimore, Maryland 21287, USA.
Jeanne M Clark, Division of General Internal Medicine, Department of Medicine, The Johns Hopkins University, Baltimore, Maryland 21287, USA; Department of Epidemiology, The Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland 21287, USA.
Josephine M Egan, National Institute On Aging, National Institutes of Health, Baltimore, Maryland 21224, USA.
Olga D Carlson, National Institute On Aging, National Institutes of Health, Baltimore, Maryland 21224, USA.
Michael Schweitzer, Department of Surgery, The Johns Hopkins University, Baltimore, Maryland 21287, USA.
Susan Langan, Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, The Johns Hopkins University, Baltimore, Maryland 21287, USA.
Todd Brown, Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, The Johns Hopkins University, Baltimore, Maryland 21287, USA.
Financial Support
This work was supported by the following: C.J.L. was supported by a career development award from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (K23DK107921); and T.T.B. was supported in part by K24 AI120834. This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by grant number UL1 TR003098 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH, and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS, or NIH.
Disclosures
C.J.L. is a full employee and stock holder of Eli Lilly and Company and all work reported here was completed before transition to the present position. The remaining authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879-887. doi: 10.1001/jama.2020.12567 [DOI] [PubMed] [Google Scholar]
- 2. Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab. 2018;103(8):2815-2826. doi: 10.1210/jc.2018-00528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scarpellini E, Arts J, Karamanolis G, et al. . International consensus on the diagnosis and management of dumping syndrome. Nat Rev Endocrinol. 2020;16(8):448-466. doi: 10.1038/s41574-020-0357-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia. 2010;53(11):2307-2311. doi: 10.1007/s00125-010-1798-5 [DOI] [PubMed] [Google Scholar]
- 5. Sarwar H, Chapman WH III, Pender JR, et al. . Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014;24(7):1120-1124. doi: 10.1007/s11695-014-1260-8 [DOI] [PubMed] [Google Scholar]
- 6. Lee CJ, Clark JM, Schweitzer M, et al. . Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity (Silver Spring). 2015;23(5):1079-1084. doi: 10.1002/oby.21042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Service GJ, Thompson GB, John F, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254. [DOI] [PubMed] [Google Scholar]
- 8. Patti ME, McMahon G, Mun EC, et al. . Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48(11):2236-2240. doi: 10.1007/s00125-005-1933-x [DOI] [PubMed] [Google Scholar]
- 9. Belligoli A, Sanna M, Serra R, et al. . Incidence and predictors of hypoglycemia 1 year after laparoscopic sleeve gastrectomy. Obes Surg. 2017;27(12):3179-3186. doi: 10.1007/s11695-017-2742-2 [DOI] [PubMed] [Google Scholar]
- 10. Goldfine AB, Mun EC, Devine E, et al. . Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678-4685. doi: 10.1210/jc.2007-0918 [DOI] [PubMed] [Google Scholar]
- 11. Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669-680.e2. doi: 10.1053/j.gastro.2013.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dirksen C, Eiken A, Bojsen-Møller KN, et al. . No islet cell hyperfunction, but altered gut-islet regulation and postprandial hypoglycemia in glucose-tolerant patients 3 years after gastric bypass surgery. Obes Surg. 2016;26(9):2263-2267. doi: 10.1007/s11695-016-2197-x [DOI] [PubMed] [Google Scholar]
- 13. Mulla CM, Goldfine AB, Dreyfuss JM, et al. . Plasma FGF-19 levels are increased in patients with post-bariatric hypoglycemia. Obes Surg. 2019;29(7):2092-2099. doi: 10.1007/s11695-019-03845-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morínigo R, Moizé V, Musri M, et al. . Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91(5):1735-1740. doi: 10.1210/jc.2005-0904 [DOI] [PubMed] [Google Scholar]
- 15. Laferrère B. Effect of gastric bypass surgery on the incretins. Diabetes Metab. 2009;35(6 Part 2):513-517. doi: 10.1016/S1262-3636(09)73458-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jørgensen NB, Jacobsen SH, Dirksen C, et al. . Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303(1):E122-E131. doi: 10.1152/ajpendo.00073.2012.-Our [DOI] [PubMed] [Google Scholar]
- 17. Unger RH, Orci L. Glucagon and the A cell. N Engl J Med. 1981;304(26):1575-1580. doi: 10.1056/NEJM198106253042604 [DOI] [PubMed] [Google Scholar]
- 18. Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95(2):513-548. doi: 10.1152/physrev.00013.2014 [DOI] [PubMed] [Google Scholar]
- 19. Abrahamsson N, Börjesson JL, Sundbom M, Wiklund U, Karlsson FA, Eriksson JW. Gastric bypass reduces symptoms and hormonal responses in hypoglycemia. Diabetes. 2016;65(9):2667-2675. doi: 10.2337/db16-0341 [DOI] [PubMed] [Google Scholar]
- 20. Evers SS, Kim KS, Bozadjieva N, et al. . Continuous glucose monitoring reveals glycemic variability and hypoglycemia after vertical sleeve gastrectomy in rats. Mol Metab. 2020;32:148-159. doi: 10.1016/j.molmet.2019.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Craig CM, Lawler HM, Lee CJE, et al. . PREVENT: a randomized, placebo-controlled crossover trial of avexitide for treatment of postbariatric hypoglycemia. J Clin Endocrinol Metab. 2021;106(8):E3235-E3248. doi: 10.1210/clinem/dgab103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131-2157. doi: 10.1053/j.gastro.2007.03.054 [DOI] [PubMed] [Google Scholar]
- 23. D’Alessio R, Watanabe M, Gallo IF, Manfrini S, Tuccinardi D, Bruni V. The gastro-jejunal anastomosis site influences dumping syndrome and weight regain in patients with obesity undergoing laparoscopic Roux-en-Y gastric bypass. Eat Weight Disord. 2021;26(6):1871-1880. doi: 10.1007/s40519-020-01030-2 [DOI] [PubMed] [Google Scholar]
- 24. Herrmann C, Göke R, Richter G, Fehmann HC, Arnold R, Göke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56(2):117-126. [DOI] [PubMed] [Google Scholar]
- 25. Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140(4):1687-1694. [DOI] [PubMed] [Google Scholar]
- 26. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El K, Campbell JE. The role of GIP in α-cells and glucagon secretion. Peptides. 2020;125:170213. doi: 10.1016/j.peptides.2019.170213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40(1):155-157. doi: 10.2337/dc16-2215 [DOI] [PubMed] [Google Scholar]
- 29. Mitrakou A, Ryan C, Veneman T, et al. . Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260(1):E67-E74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.