Abstract
Programmed cell death (PCD) is integral to plant life and required for stress responses, immunity, and development. Our understanding of the regulation of PCD is incomplete, especially concerning regulators involved in multiple divergent processes. The botrytis-susceptible (bos1) mutant of Arabidopsis is highly susceptible to fungal infection by Botrytis cinerea (Botrytis). BOS1 (also known as MYB108) regulates cell death propagation during plant responses to wounding. The bos1-1 allele contains a T-DNA insertion in the 5′-untranslated region upstream of the start codon. This insertion results in elevated expression of BOS1/MYB108. We used clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated nuclease 9 (Cas9) system (CRISPR/Cas9) to create new bos1 alleles with disrupted exons, and found that these lines lacked the typical bos1-1 wounding and Botrytis phenotypes. They did exhibit reduced fertility, as was previously observed in other bos1 alleles. Resequencing of the bos1-1 genome confirmed the presence of a mannopine synthase (MAS) promoter at the T-DNA left border. Expression of the BOS1 gene under control of the MAS promoter in wild-type plants conferred the characteristic phenotypes of bos1-1: Botrytis sensitivity and response to wounding. Multiple overexpression lines demonstrated that BOS1 was involved in regulation of cell death propagation in a dosage-dependent manner. Our data indicate that bos1-1 is a gain-of-function mutant and that BOS1 function in regulation of fertility and Botrytis response can both be understood as misregulated cell death.
MYB108/BOS1 enhances cell death in the gain-of-function mutant bos1-1 after fungal infection and mechanical wounding.
IN A NUTSHELL.
Background: Programmed cell death (PCD) is essential for proper plant development and life. Following inititation of PCD in plant cells, cell death can spread to surrounding cells in a process called propagation. PCD is finely controlled and benefits plants in ways such as killing cells infected by pathogens, closing wounds, or sculpting forms during development. BOS1 (also known as MYB108) encodes a transcription factor with an important role in plant PCD. Improper function of BOS1 results in the uncontrolled spread of cell death, indicating that BOS1 regulates PCD propagation. Previous studies showed that loss of BOS1 action results in uncontrolled cell death, suggesting that BOS1 acts as a suppressor of cell death.
Questions: Here we revisit the genetics of BOS1 mutants using new experimental tools not available in previous studies and ask: How does BOS1 regulate the propagation of PCD, and how does the particular site of T-DNA insertion affect BOS1 function?
Findings: We show that BOS1 promotes, rather than inhibits, the propagation of cell death. The previous studies used a single Arabidopsis mutant (bos1-1), which contains a T-DNA insertion in the 5′-untranslated region. This T-DNA insertion did not disrupt BOS1 as expected, but instead, it altered the activation of BOS1 upon wounding and pathogen attack due to sequences in the T-DNA that regulate gene activation. T-DNAs containing similar regulatory sequences were used to construct insertion mutant collections that are widely used in plant research. Other mutants with such T-DNAs may also result in changed activation of nearby genes, as was seen in bos1-1. Thus, the regulatory sequence of a T-DNA insertion must be taken into consideration when evaluating results of studies using this type of insertion mutants.
Next steps: To better define the role of BOS1 in PCD, we aim to identify the target genes that are regulated by BOS1. We also plan to search for other MYB transcription factors that potentially act together with BOS1 to regulate PCD.
Introduction
Programmed cell death (PCD) is a finely tuned process that occurs during plant–pathogen interactions and plant development. Generally, PCD has three stages: initiation, propagation, and containment (McCabe, 2013; Van Hautegem et al., 2015). In Arabidopsis thaliana (Arabidopsis), regulators of the initiation stage have been identified primarily through analysis of lesion-mimic mutants that spontaneously develop leaf necrosis (Lorrain et al., 2003; Bruggeman et al., 2015). Regulators of cell death propagation and containment have been identified through mapping of lesion-mimic mutants (Bruggeman et al., 2015). In such mutants, initiation of PCD is followed by uncontained spread of cell death that can consume the entire leaf (Lorrain et al., 2003). These mutants have been instrumental in elucidating the mechanism of cell death, including regulation by plant hormones such as salicylic acid and jasmonates (Bruggeman et al., 2015). However, our understanding of the signals leading to the propagation and subsequent containment of cell death remains incomplete. One open question in plant PCD research is to what extent do pathogen activated and developmental PCD have overlapping regulatory mechanisms and execution (Huysmans et al., 2017).
Mechanical injury results in cell death in tissue adjacent to the wound in order to re-establish the integument (Bostock and Stermer, 1989; McCabe, 2013; Cui et al., 2013; Lakimova and Woltering, 2018). Uncontained, abscisic acid dependent, PCD propagation was found in botrytis-susceptible1-1 (bos1-1; Cui et al., 2013), a mutant allele of BOS1/MYB108 (AT3G06490; Mengiste et al., 2003). PCD propagation is enhanced in bos1-1 following PCD initiation by pathogen infection or mechanical injury (Cui et al., 2013, 2019). This makes bos1-1 a useful model to study the mechanism of PCD propagation (McCabe, 2013). BOS1 is an R2R3 MYB transcription factor that was functionally characterized in the seminal paper by Mengiste et al. (2003) through the analysis of the bos1-1 mutant, which is extremely susceptible to the necrotrophic fungal pathogen Botrytis cinerea. Results from subsequent studies utilizing the bos1-1 mutant demonstrated that BOS1 is a key regulator of cell death in plant–pathogen interactions, especially those involving necrotrophic fungi (Kraepiel et al., 2011; Cui et al., 2013, 2019). BOS1 levels are regulated by BOTRYTIS SUSCEPTIBLE1 INTERACTOR (BOI), an E3 ligase that attenuates stress-induced cell death in plants (Luo et al., 2010).The bos1-1 allele was isolated from a T-DNA mutant pool that was later released to the community as the SAIL collection (McElver et al., 2001; Sessions et al., 2002). The bos1-1 line was genetically characterized as a recessive loss-of-function mutant, although the T-DNA insertion is located in the 5′-untranslated region (5′-UTR) just upstream from the start codon and results in an increased BOS1 transcript level (Mengiste et al., 2003).
Aside from regulating stress responses, PCD is also indispensable for plant development, for example, formation and release of pollen (Mandaokar and Browse, 2009; Daneva et al., 2016; Xu et al., 2019). To study the role of BOS1/MYB108 in anther development, three mutant alleles with T-DNA insertions in the first intron were used (Mandaokar and Browse, 2009). These mutants display reduced male fertility, lower pollen viability, and delayed anther dehiscence; however, their stress responses remain untested.
Due to differences in the BOS1 transcript level observed in various bos1 alleles used in the study of pathogen induced and developmental PCD, it is difficult to assess the precise role of this transcription factor in both types of cell death. Furthermore, the T-DNA insertion sites within the existing alleles of bos1 are located either in introns (Mandaokar and Browse, 2009) or in the 5′-UTR (bos1-1; Mengiste et al., 2003), further complicating interpretation of BOS1 function in the regulation of cell death.
Here we have generated new mutant alleles of BOS1 with disrupted exons and present evidence that BOS1 is a positive regulator of cell death.
Results
Botrytis and wounding responses in new bos1 alleles made with CRISPR/Cas9
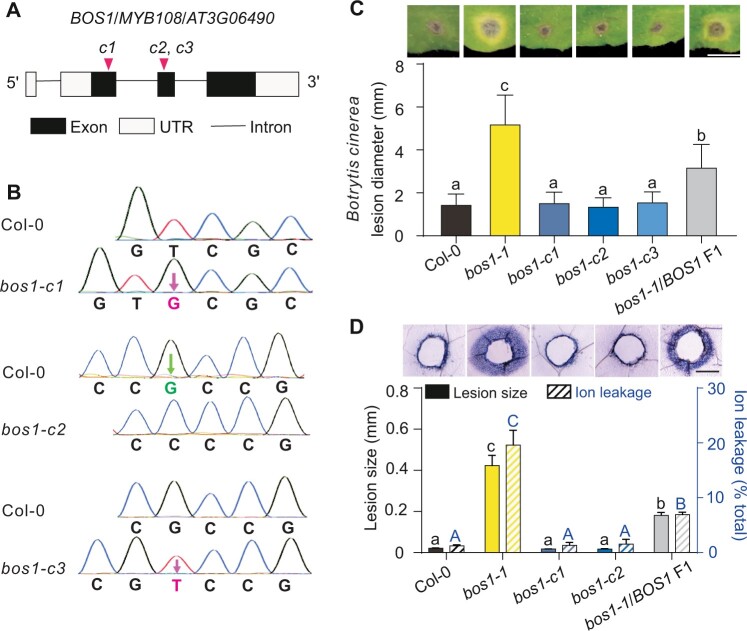
Genome editing allows the generation of precisely targeted mutations (Jiang et al., 2013; Xing et al., 2014). We used the CRISPR/Cas9 system to create three BOS1 loss-of-function alleles, targeting the first and second exons (bos1-c1 to -c3; Figure 1A). These mutations caused frame shifts resulting in the predicted truncated proteins (Figure 1B; Supplemental Figure S1). Strikingly, none of these mutants phenocopied bos1-1 when challenged by Botrytis infection or wounding. Botrytis-induced lesion size and wound-induced cell death spread in these mutants were similar to those observed in the wild-type plants, whereas the bos1-1 mutant characteristically exhibited large lesions (Figure 1, C and D). The discrepancy between the phenotypes of bos1-1 and the new mutants suggested that bos1-1 is not a loss-of-function mutant.
Figure 1.
New bos1 alleles created with CRISPR/Cas9 did not exhibit typical bos1-1 phenotypes. A, Schematic diagram of the new bos1 insertion and deletion alleles. The gRNA positions are indicated with magenta triangles, and the new bos1 alleles made with the CRISPR/Cas9 system are designated bos1-c1, bos1-c2, and bos1-c3 (abbreviated here as c1, c2, and c3). B, CRISPR/Cas9-induced changes in the indicated mutants. Single base insertions (magenta characters) and deletions (green characters) were detected with Sanger sequencing. These frame shifts resulted in predicted short missense sequences and truncated proteins with lengths approximately one-fourth the length of the wild-type BOS1 polypeptide. See Supplemental Figure S1. C, Disease symptoms and size of lesions caused by infection with B. cinerea. Droplets of a conidial suspension (3 μL, 2 × 105 spores mL−1) were inoculated onto fully expanded leaves of the indicated genotypes. Symptoms were photographed at 3 dpi. Botrytis-induced lesion size was measured from photographs using ImageJ. Bars represent means ± se (three independent biological replicates, each consisting of leaves from five individual plants; n ≥ 40 leaves total). Letters above the bars indicate significance groups (P < 0.05; one-way ANOVA, Supplemental File S1). Bar = 0.5 cm. D, Wound-induced cell-death spread. Leaves punctured with a toothpick were stained with trypan blue to visualize dead tissue at 4 dpw. Representative photos are shown to illustrate the dead tissue around the wounds. The extent of cell death spread was quantified by measuring the distance from the wound edge to the outer border of the area of cell death (black bars). Bars represent mean ± se (three biological repeats, each analyzing wounds from five individual leaves; n = 36 total). Ion leakage (blue striped bars) from wounded leaves was measured at 5 dpw and is expressed as percentage of total ions, determined after disrupting all cell membranes by freezing. Bars represent means ± se (three biological repeats; three leaves were combined as one sample; n = 12 samples in total). Letters above the bars indicate significance groups (P < 0.05; one-way ANOVA, Supplemental File S1); lower case letters, wound-induced lesion size; upper case letters, ion leakage. Bar = 0.5 mm.
To explore this hypothesis, bos1-1/BOS1 plants were generated and tested upon wounding and infection with Botrytis. Heterozygous bos1-1/BOS1 plants exhibited phenotypes that were intermediate between those observed in wild-type and the bos1-1 mutant. Both the extent of wounding-induced runaway cell death and the size of Botrytis-induced lesions observed in bos1-1/BOS1 plants were significantly larger than those in wild-type plants but smaller than those of bos1-1 (Figure 1, C and D). In addition, the distribution of Botrytis- and wounding-induced lesions was tested in an F2 population derived from multiple bos1-1/BOS1 F1 individuals. For both treatments, ∼25% of tested F2 individuals exhibited bos1-1-like symptoms; 50% had phenotypes of bos1-1/BOS1 plants; and 25% had wild-type characteristics, fitting a 1:2:1 segregation ratio. Genotyping of F2 plants revealed a correlation between genotype and phenotype. Plants exhibiting large Botrytis-induced lesions were bos1-1 homozygotes, and those with intermediate-sized lesions were heterozygotes (Figure 2). Taken together, based on the genetics in the F1 and F2 generations, we conclude that bos1-1 is a co-dominant gain-of-function mutant.
Figure 2.
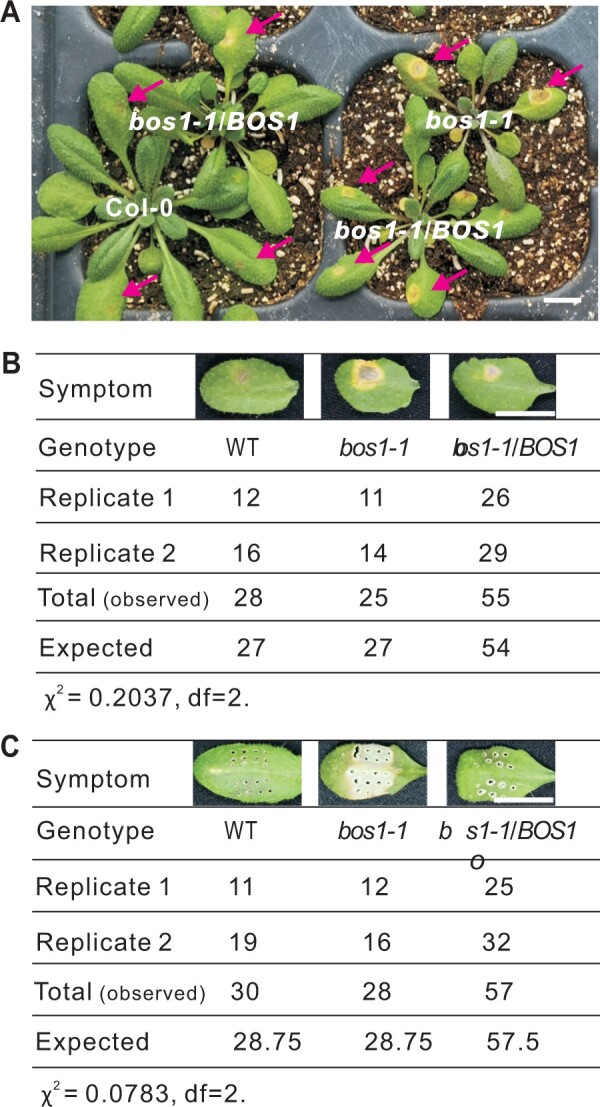
Segregation of phenotypes in F2 plants in response to Botrytis infection and wounding. A, Plant symptoms caused by Botrytis infection. Arrows point to developing lesions. Bar = 1 cm. B and C, The number of F2 individuals exhibiting the indicated symptoms upon Botrytis infection (B) or wounding (C). The genotypes of several plants were confirmed by PCR and are presented as representative symptoms. F2 individuals with similar symptoms were counted and the number of individuals in each category is listed. A model with co-dominant inheritance in bos1-1 was used as the null hypothesis for the χ2-test. For both wound and Botrytis responses χ2 < 5.99, null hypothesis cannot be rejected. Bar = 1 cm.
CRISPR/Cas9-induced bos1 alleles are impaired in fertility
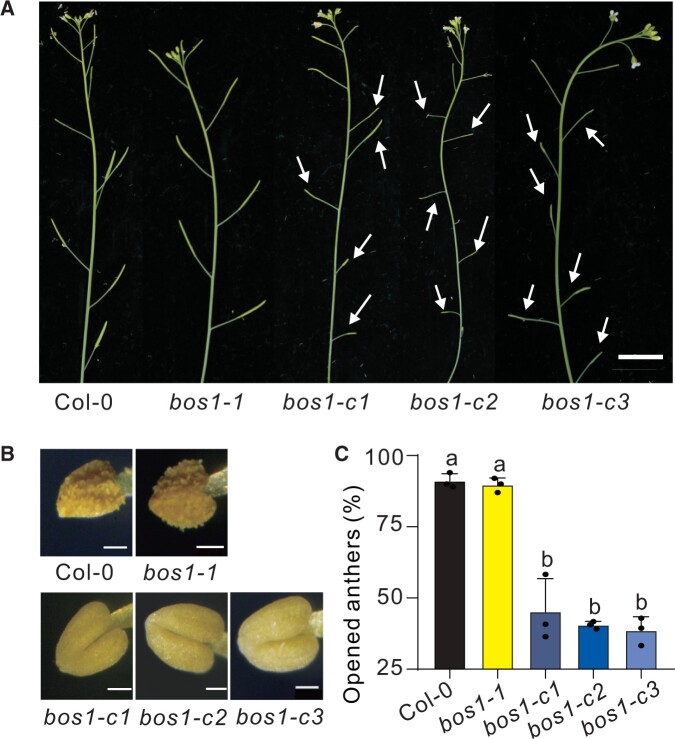
CRISPR/Cas9-induced loss-of-function alleles exhibited strong deficiencies in fertility, having siliques with reduced size and delayed flower senescence (Figure 3A). This finding is consistent with the reduced fertility phenotypes previously observed in bos1 mutants having T-DNAs insertions in introns (Xu et al., 2019; Mandaokar and Browse, 2009). In these previous studies, fewer anthers in bos1 mutants had undergone dehiscence, suggesting that the impaired fertility of bos1 is due to deficient or delayed pollen release (Xu et al., 2019). Examination of the anthers of the new CRISPR/Cas9-induced loss-of-function alleles revealed a significant reduction or delay in pollen release as compared to wild-type (Figure 3, B and C). In contrast, bos1-1 fertility and anther dehiscence were comparable to that of wild-type plants (Figure 3). These findings further suggest bos1-1 is not a loss-of-function mutant.
Figure 3.
CRISPR/Cas9-induced bos1 loss-of-function lines were impaired in pollen release. A, The bos1 alleles created with CRISPR/Cas9 (bos1-c1 to bos1-c3) resulted in impaired fertility. White arrows indicate siliques with reduced seed production. Delayed flower senescence was also apparent in the bos1 CRISPR/Cas9-induced alleles. Bar = 1 cm. B, Anthers of the CRISPR/Cas9-induced loss-of-function alleles of bos1 exhibited delayed dehiscence. Anthers were detached from flowers at the same developmental stage (floral stage 14 according to stages defined by Sanders et al. 1999). Bar = 50 μm. C, The proportion of open anthers in the indicated genotypes. Ten flowers of each genotype at floral stage 14 were measured. Bars represent mean ± se from three independent biological repeats. Letters above bars indicate significant differences between groups (P ≤ 0.05; one-way ANOVA, Supplemental File S1). Transcript levels for BOS1 in publicly available development and stress experiments are shown in Supplemental Figure S4 and further characterization of stress responses in the new bos1 CRISPR/Cas9-induced alleles in Supplemental Figures S5–S7.
The bos1-1 allele is a gain-of-function allele due to an increased BOS1 transcript level
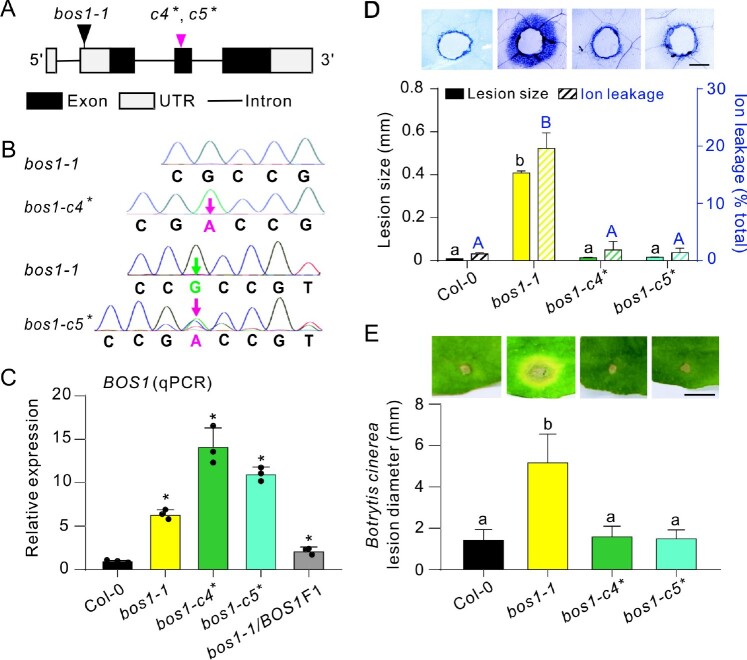
T-DNA transformation can result in genome structure changes or have epigenetic impacts, and this may contribute to phenotypes independent of the T-DNA insertion (Jupe et al., 2019). To comprehensively assess the genomic changes in bos1-1, Nanopore genome re-sequencing was performed (Brown and Clarke, 2016). This analysis identified 1,173 structural variations in bos1-1, including large rearrangements (>1,000 bp), 13 insertions, 16 deletions, 19 duplications, and 24 inversions. In contrast, no mutations were detected in the BOS1 coding sequence (Supplemental Data Set 2). Because assessing the potential influence of all these changes on bos1-1 phenotypes is not feasible, we created intragenic double mutant alleles to test the effect of additional exon-disrupting mutations in the bos1-1 background. Using CRISPR/Cas9, secondary mutations were introduced in exon 2 of BOS1 in the bos1-1 mutant background (Figure 4A) resulting in frame shifts (Figure 4B). These new alleles were named bos1-c4* and bos1-c5* (asterisk indicates that the allele is in the bos1-1 background). These secondary mutations did not attenuate the high BOS1 transcript level seen in bos1-1, as BOS1 transcript accumulation remained high in both bos1-c4* and bos1-c5* (Figure 4C). The spread of cell death and Botrytis susceptibility in bos1-c4* and bos1-c5* were similar to that of wild-type (Figure 4, D and E). The lack of characteristic bos1-1 phenotypes in these lines indicates that these secondary mutations acted as intragenic suppressors of bos1-1. Collectively, these findings demonstrate that the bos1-1 phenotypes were caused by an alteration of BOS1 function, rather than other genomic changes.
Figure 4.
Phenotypes of bos1-1 were suppressed by introduction of exon-disrupting alleles in BOS1. A, Schematic diagram of the new intragenic double mutants bos1-c4* and bos1-c5* created using the CRISPR/Cas9 system in the bos1-1 background. These alleles had both the T-DNA insertions of bos1-1 and the frame shift-inducing SNPs in the second exon of BOS1. The black triangle indicates the T-DNA insertion site in bos1-1. The magenta triangle indicates the start of the frame shifts in bos1-c4* and bos1-c5* (c4*, c5*). See Supplemental Figure S1 for the predicted amino acid sequence of the truncated proteins encoded by bos1-c4* and bos1-c5*. B, Single base insertions (magenta characters) and deletions (green characters) were detected with Sanger sequencing. The adenine insertion in bos1-c4* was homozygous, while in bos1-c5* there were two changes, the insertion of an adenine and the deletion of a guanine. C, Relative expression of BOS1 in the indicated genotypes. Fully expanded leaves of 24-day-old plants were used for qPCR. Data are shown as mean ± sd (n = 3 technical replicates, two biological replicates showed similar results). Asterisks above the bars indicate means that are significantly different from wild-type Col-0 (P < 0.05; t test, two-sided, Supplemental File S1). D, Wounding-induced cell-death spread visualized with trypan blue staining. Following puncture of leaves with a toothpick, the distance from the wound edge to the outer border of the area of cell death was measured (black bars). Representative photos illustrate the dead tissues around the toothpick-puncture wounds. Bars represent mean ± se (three biological repeats; each analyzing wounds from five individual leaves; n = 12 in total). Ion leakage (blue striped bars) from wounded leaves was measured at 5 dpw and is expressed as percentage of total ions, determined after disrupting all membranes by freezing. Bars represent mean ± se (three biological repeats; three leaves were combined as one sample; n = 12 samples in total). Letters above the bars indicated significance groups (P < 0.05; one-way ANOVA, Supplemental File S1), lower case letters, wound-induced lesion size; upper case letters, ion leakage. Bar = 0.5 mm. E, Botrytis-induced lesion size in the indicated genotype. Bars represent mean ± se (three independent biological replicates; each analyzing leaves from five individual plants; n ≥ 53 in total). Letters above the bars indicate significance groups (P < 0.05, one-way ANOVA, Supplemental File S1). Bar = 0.5 cm.
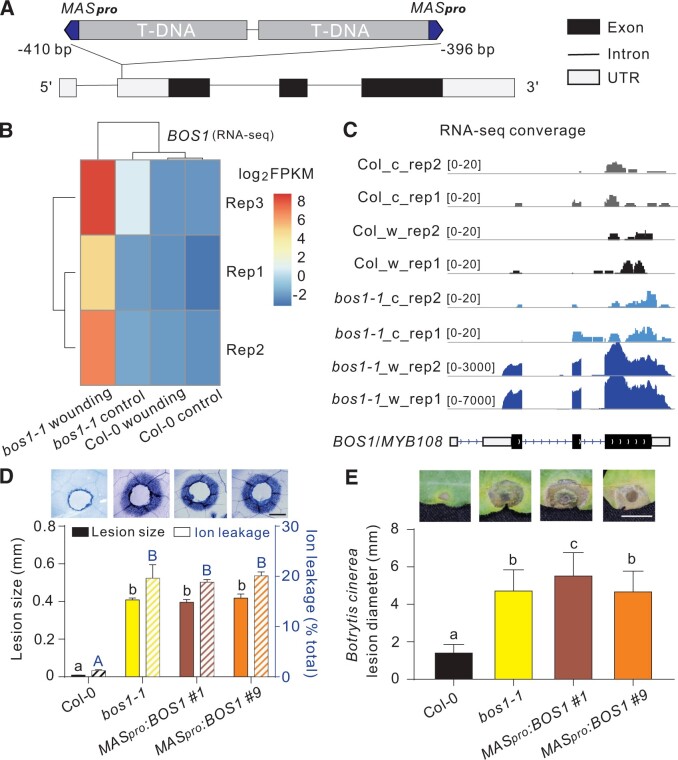
We hypothesize that the T-DNA insertion caused an elevated BOS1 transcript level, which conferred the cell death phenotype in bos1-1. The exact site of the T-DNA insertion remains unclear (Kraepiel et al., 2011). Genome resequencing and Sanger sequencing data identified two adjacent T-DNAs in opposite orientations between –410 and –396 bp in the 5′-UTR of BOS1 (Figure 5A). As expected (Sessions et al., 2002), a mannopine synthase (MAS) promoter was found adjacent to the left border of each T-DNA (Figure 5A). The MAS promoter (MASpro) is wounding-inducible and controls gene expression in a bidirectional manner (Guevara-García et al., 1999). Accordingly, the BOS1 transcript level in bos1-1 was highly responsive to wounding (Figure 5B). RNA-sequencing (RNA-seq) detected two transcripts, one on each side of the MAS promoter; the BOS1 mRNA with a truncated 5′-UTR at the right flank, and a BlpR Basta resistance gene derived from the T-DNA at the left flank (Supplemental Figure S2). The full BOS1 coding sequence was expressed with no alternative splicing or mutations detected (Figure 5C; Supplemental Figure S2).
Figure 5.
Phenotypes of bos1-1 are caused by MAS promoter-driven BOS1 expression. A, Schematic illustration of bos1-1 T-DNA structure. Two adjacent T-DNAs were inserted into the 5′-UTR of BOS1 with MAS promoters indicated in blue. The insertion position of the T-DNAs in bos1-1 is relative to the BOS1 start codon. B, Expression of BOS1 3 days after wounding. Normalized transcript abundance of BOS1 was calculated from RNAseq data as fragments per kilobase pair of exon model per million fragments mapped (FPKM). The log2 FPKM values of the indicated genotypes were used to build the heat map. C, RNA-seq reads mapped to BOS1 genomic DNA. The entire coding sequence of BOS1 was expressed in bos1-1. These data are supported by Supplemental Figures S2 and S8. The c and w indicate control and wounded, respectively. D and E, MASpro:BOS1 lines phenocopied bos1-1 upon wounding (D) and Botrytis infection (E). D, Representative photos of the spread of cell death from toothpick-puncture wounds, visualized with trypan blue staining and quantified by two methods; measurement of the distance from the wound edge to the outer border of the spreading cell death (black bars; three biological repeats; n = 12 in total) and ion leakage of leaves (blue striped bars; three biological repeats; n = 12 in total). Letters above the bars indicate significance groups (P < 0.05; one-way ANOVA, Supplemental File S1), lower case letters, wound-induced lesion size; upper case letters, ion leakage. Bar = 0.5 mm. This panel is supported by Supplemental Figure S3. E, Botrytis-induced lesion size is shown both in representative photos and as quantitative data. Bars represent means ± se (three independent biological replicates; n ≥ 45 in total). Letters above the bars indicate significance groups (P < 0.05, one-way ANOVA, Supplemental File S1). Bar = 0.5 cm.
These findings suggest that bos1-1 phenotypes result from high-level BOS1 transcript accumulation driven by the MASpro. To test this, a MASpro:BOS1 construct was transformed into wild-type plants. During generation of this tool, many lines harboring MASpro:BOS1 exhibited enhanced disease susceptibility phenotypes under standard greenhouse conditions and died after flowering (Supplemental Figure S3). This was consistent with our previous observation that bos1-1 did not survive under greenhouse conditions (Cui et al., 2019). In clean growth room experiments, MASpro:BOS1 lines exhibited spreading cell death upon wounding, and enhanced Botrytis susceptibility, similar to bos1-1 (Figure 5, D and E). Thus, both of the two key bos1-1 phenotypes were reproduced by introduction of MASpro:BOS1 into wild-type. Overall, we conclude that bos1-1 is a gain-of-function mutant caused by MASpro-driven expression of BOS1.
Multiple lines of evidence support a connection between BOS1 transcript level and bos1-1 phenotypes. Extraordinarily high BOS1 transcript levels were detected in bos1-1 upon wounding and infection with Botrytis (Figure 4C; Mengiste et al., 2003). Additionally, in genetic experiments, the extent of PCD propagation in bos1-1 was positively correlated with the BOS1 transcript level, as bos1-1/BOS1 had a lower BOS1 transcript level and proportionally less cell death than did bos1-1/bos1-1 (Figures 1, C, D and 4, C). To further test this, a series of lines overexpressing BOS1 under the control of the cauliflower mosaic virus 35S promoter (35Spro) were constructed and challenged with Botrytis, and this revealed a positive correlation between lesion size and BOS1 transcript level (Figure 6; Supplemental Data Set 1). This further supports that bos1-1 Botrytis susceptibility was conferred by enhanced BOS1 transcript accumulation.
Figure 6.
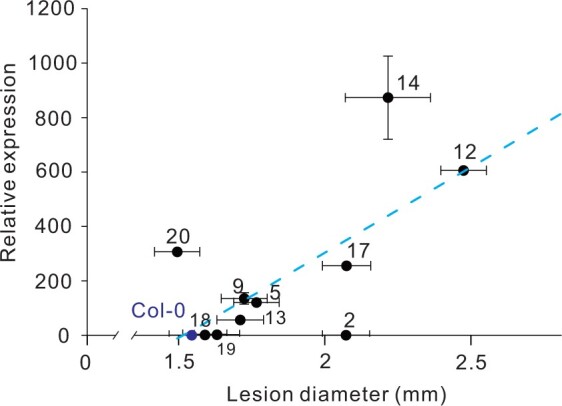
BOS1 transcript levels were positively correlated with Botrytis susceptibility. Relative BOS1 expression in 11 independent T3 35Spro:BOS1 overexpression lines was examined by qPCR. Lesion size was measured as described above. Three independent biological replicates (each replicate consists of leaves from five individual plants; n ≥ 48 in total) were combined and analyzed. The blue dashed line indicates the correlation trend. The Pearson coefficient (r) of 0.72 for these data indicates a strong correlation between BOS1 transcript levels and lesion size. The raw data for this figure are available in Supplemental Data Set 1.
BOS1 functions in hormone and abiotic stress responses
The BOS1 transcript level was elevated in response to multiple stresses (Supplemental Figure S4). In order to assess the role of BOS1 in abiotic stress and hormone responses, the responses to abscisic acid (ABA), methyl viologen, and NaCl were assayed in the new loss-of-function CRISPR/Cas9-induced alleles (Supplemental Figures S5–S7). These experiments revealed increased sensitivity to ABA in these alleles (Supplemental Figure S5). However, mutant responses were indistinguishable from wild-type under stresses induced by NaCl or methyl viologen (Supplemental Figures S6 and S7).
Discussion
Is bos1-1 a loss-of-function or gain-of-function allele?
Previous studies have noted inconsistent phenotypes of bos1-1 and other bos1 alleles. For example, bos1-1 exhibited normal fertility, whereas clearly reduced fertility was observed in alleles with T-DNA insertions in introns (Mandaokar and Browse, 2009). Conversely, bos1 intronic T-DNA alleles had no pathogen-related phenotypes (Mandaokar and Browse, 2009; Kraepiel et al., 2011). This discrepancy is perhaps related to the interpretation of bos1-1 as a loss-of-function mutant, which may have resulted from the technological limitations of the time and a lack of other bos1 alleles available for confirmation of phenotypes. We demonstrate here that cell death spread and Botrytis susceptibility in bos1-1 were the result of a mis-regulated BOS1 transcript level, rather than a loss of BOS1 function. However, it is important to note that the publication originally describing bos1-1 (Mengiste et al., 2003) has several lines of evidence that convincingly demonstrate bos1-1 was a recessive loss-of-function mutant. This included genetic segregation and genomic complementation analyses. Importantly, the differences in procedures and conditions used in different labs may have altered the phenotypes observed. Differences in growth conditions or infection protocols can significantly influence the extent of Botrytis infection (Harper et al., 1981; Ciliberti et al., 2015).
The key differences between our study and that of Mengiste et al. (2003) include the fungal cultivation medium used (2 × V8 versus potato dextrose broth), infection medium used (Sabouraud maltose broth versus potato dextrose broth), and the age of infected plants (3 weeks versus 24 days). These differences may to some extent account for the different results seen between these studies.
Mengiste et al. (2003) present transgenic mutant complementation data. Because relative lesion size in the complemented mutant (bos1-1 + BOS1) versus bos1-1 is much greater in comparison to that in the complemented mutant versus wild-type, Mengiste et al. (2003) evaluate the complementation line as wild-type. However, examination of the original figure reveals that the complemented mutant line exhibited stronger Botrytis symptoms than the wild-type, with larger lesion size and enhanced cell death around the lesion frontiers. The choice of the particular Botrytis strain may also impact lesion size. This is elegantly illustrated by a study of 96 diverse Botrytis isolates that result in contrasting symptoms (Zhang et al., 2017). This study used the Bo5.10 strain, but the exact Botrytis strain used by Mengiste et al. (2003) is not specified. We speculate that the fungal cultivation or infection method in might have increased the contrast between disease symptoms of bos1-1 and heterozygous or complementation lines. This may have obscured the intermediate phenotypes of the heterozygote or complemented line (Mengiste et al., 2003).
An increased BOS1 transcript level confers Botrytis susceptibility and uncontained cell death
Both MASpro- and 35Spro-driven expression of BOS1 conferred Botrytis susceptibility, but with some informative differences. While MASpro gave robust phenotypes, use of 35Spro resulted in outcomes that were more variable (Figure 6). MASpro conferred strong wound inducible BOS1 expression (Figure 5, B and C), and this might lead to more precise BOS1 expression in its target tissue (infection or wound sites) as compared to the general expression patterns of 35Spro. The use of 35Spro can also have unintended consequences. Multiple studies illustrate gene silencing and integration site effects with genes overexpressed using the 35S promoter (Schubert et al., 2004; Daxinger et al., 2008; Mlotshwa et al., 2010; Gelvin, 2017). To further address this, 11 35Spro:BOS1 lines were examined with Botrytis infection (Figure 6). Most, but not all, of these overexpression lines exhibited enhanced susceptibility to Botrytis. A previous study showed that overexpression of 35Spro:BOS1-GUS increased Botrytis resistance (Luo et al., 2010). It cannot be excluded that fusion to GUS may have altered BOS1 function. However, it is common to have some overexpression lines that exhibit different or even opposite phenotypes. In our study, there were two such exceptional lines, #2 and #20, among our 11 35Spro:BOS1 lines (Figure 6). Remarkably, the BOS1 transcript level in line #20 was elevated more than 300-fold, but its Botrytis-lesion size was slightly reduced (Figure 6). This demonstrates the importance of evaluating many independent overexpression lines for gene function analysis.
BOS1 also has a function in other biological processes
Under control conditions, BOS1/MYB108 is mostly expressed in the cell types responsible for anther dehiscence (Mandaokar and Browse, 2009; Xu et al., 2019), and dehiscence requires properly timed PCD for pollen release (Beals and Goldberg, 1997; Senatore et al., 2009; Wilson et al., 2011). Our CRISPR/Cas9-induced loss-of-function alleles exhibited alterations in the extent or timing of dehiscence, similar to bos1 intronic T-DNA alleles (Figure 3B; Mandaokar and Browse, 2009; Xu et al., 2019). This suggests that BOS1 could be required for cell death regulation in septum or stomium cells of the dehiscence zone.
Another important regulator of anther development is jasmonic acid (JA; Ishiguro et al., 2001). A characteristic phenotype of JA biosynthesis mutants and strong mutant alleles of the JA receptor CORONATINE INSENSITIVE 1 (COI1) is male sterility (Park et al., 2002; Mandaokar et al., 2003; Jewell and Browse, 2016). Two other MYB transcription factors, MYB21 and MYB24, act downstream from COI1 to regulate anther dehiscence (Song et al., 2011), and MYB24 acts redundantly with MYB108/BOS1 (Mandaokar and Browse, 2009). BOS1 expression is JA dependent in flowers (Mandaokar and Browse, 2009), thus it is clear that multiple MYB transcription factors coordinate anther development. JA is also crucial for defense against insect and necrotrophic pathogen attack, and a characteristic phenotype for JA deficient or insensitive mutants is of enhanced Botrytis-induced lesion size (Thomma et al., 1998; Zhang et al., 2017). Botrytis infection leads to elevated BOS1 transcript levels, and this is dependent on JA signaling via COI1 (Mengiste et al., 2003). These similarities between JA regulation of MYB transcription factors in both anthers and in Botrytis infection suggest that common components may be utilized for PCD regulation.
In addition to altered flower development, we show that the bos1 CRISPR/Cas9-induced alleles exhibited enhanced sensitivity to ABA (Supplemental Figure S5). BOS1 transcript level was elevated during multiple abiotic and biotic stresses (Supplemental Figure S4). As multiple MYB transcription factors are required for anther development (Mandaokar and Browse, 2009; Song et al., 2011), we propose that stress responses also require the function of several MYB transcription factors.
There are two possible interpretations of the role of BOS1 in stress responses in leaves. Ectopic overexpression of BOS1 driven by MASpro may result in a leaf phenotype that is purely an artifact, suggesting that BOS1 is relevant only in the context of the flower, where it is naturally highly expressed. This argues against a role of BOS1 in stress-induced cell death in leaves and is supported by the lack of leaf cell death and stress phenotypes in bos1 loss-of-function mutants. Alternatively, the ABA sensitivity phenotype of CRISPR/Cas9-induced bos1 alleles suggests a role for BOS1 in regulating hormone responses in tissues outside the flower. Support for this is that BOS1 is stress inducible in leaves (Supplemental Figure S4). Additionally, leaves in the bos1-1 mutant and MASpro:BOS1 lines with elevated BOS1 transcript levels can execute cell death (Figures 1, 4, and 5), which implies the cell death signaling pathway(s) downstream of BOS1 are active in leaves. Considering these together, the possible relevance of BOS1 to Botrytis and other stress responses in the leaf cannot be excluded, and further studies will be required to resolve this question.
Caution should be observed when using T-DNA lines containing MASpro
The original mutant pool used for bos1-1 isolation was described in McElver et al. (2001) and used the T-DNA vectors pCSA104, pDAP101, and pCSA110. Subsequently, these T-DNA mutant pools were used to create the SAIL collection of indexed T-DNA mutant lines (Sessions et al., 2002). The sequence for these vectors (download: http://seedgenes.org/FlankingSequence.html) were examined, revealing that all three vectors contain the MAS promoter (Supplemental Figure S8). MASpro is used in these constructs to drive the expression of the bialophos/phosphinothricin (BASTA) resistance gene, BlpR. However, the bi-directional promoter activity of MASpro may result in enhanced expression of genes adjacent to the T-DNA insertion site (Guevara-García et al., 1999). SAIL lines are an extensively used resource with seeds widely distributed by community stock centers. The presence of a MAS promoter in T-DNA insertions of SAIL lines, or possibly other T-DNA mutant collections, may cause unexpected changes in gene expression. Along with the unclear genetic background of the SAIL lines (Nikonorova et al., 2018), caution is advised in the interpretation of results obtained using these mutants. As other vectors for T-DNA transformation also use MASpro (Lampropoulos et al., 2013), further critical examination is warranted in the design of experiments that rely on T-DNA insertion mutants.
Summary
It appears that a re-evaluation of previous generations of genetic tools is required (Nikonorova et al., 2018) because the development of gene editing technologies allows a more accurate examination of gene function. These new tools facilitate re-evaluation of mutants and a refinement of our interpretation of the scientific literature (Westphal et al., 2008; Gao et al., 2015). Here, we have built upon the work of Mengiste et al. (2003) and demonstrated the function of BOS1 as a positive regulator of cell death. Aside from our proposed changes to some interpretations, the majority of Mengiste et al. (2003) remains valid. Based on our previous publications and results presented here, we propose that BOS1 regulates cell death propagation signals from dying cells to neighbor cells, rather than cell death initiation. This role may be of wider interest to the plant research community and warrants further investigation.
Materials and methods
Cultivation conditions
Arabidopsis seedlings (Col-0) were transplanted to a 1:1 mixture of peat and vermiculite after 1 week in vitro growth on 1/2 MS medium (Murashige and Skoog, 1962). Plant growth conditions were 23°C/18°C (day/night) temperature, 120–150 μmol m−2 s−2 light intensity, 12-h/12-h (light/dark) photoperiod, and 60% humidity. Botrytis strain BO5.10 obtained from Prof. Jean-Pierre Métraux’s Lab was cultivated on commercial potato dextrose agar (PDA) medium (P2182, Sigma-Aldrich, St. Louis, MO, USA). Botrytis plates were kept in the dark at room temperature and transferred into 4°C after conidia production.
Infection and wounding assays
Fresh Botrytis conidia were collected with mycelium into 1/3 strength PDA (P2182, Sigma-Aldrich). The mixture was vortexed and filtered to remove mycelia. Conidia were suspended at 2 × 105 spores mL−1. Fully expanded leaves of 24-day-old plants were inoculated with 3 μL of conidial suspension. Plants were covered with a transparent plastic lid to maintain 100% humidity. Symptoms were photographed at 3 days postinoculation (dpi). Wounding was conducted with a toothpick by puncturing fully expanded leaves of 23-day-old plants. Wounding-induced cell death was visualized by trypan blue staining, with wounded leaves collected at 4-day postwounding (dpw). Both Botrytis lesion size and wounding-induced cell death were measured from photographs using ImageJ (http://rsb.info.nih.gov/ij/). Procedures for cell death staining were described previously (Cui et al., 2013, 2019). For ion leakage measurements, three to four leaves were wounded with a bundle of toothpicks resulting in a matrix of wounds each 2-mm apart. At 5 dpw, leaves were submerged in ultrapure milliQ water, and resulting ion leakage was measured 4 h later with a conductivity meter as described in Cui et al. (2018). Ion leakage was expressed as the percentage of total ions that remained after disrupting all membranes by freezing the sample leaves.
Seedling growth assays
For ABA and NaCl treatments, sterilized seeds were sown on 1/2 MS media containing ABA or NaCl at the indicated concentrations. Root lengths were photographed at 9 days after sowing and were measured using ImageJ (http://rsb.info.nih.gov/ij/). For methyl viologen treatment, seeds were germinated on control plates and 4-day-old seedlings were transferred to media with the indicated concentrations of methyl viologen. Photos were taken at 15 days after transplanting.
Cloning procedures
BOS1 genomic DNA was cloned into the vector pGWB412 (Addgene plasmid # 74806) to construct the 35Spro:BOS1 plasmid. The MASpro was amplified using template DNA from the bos1-1 mutant, and then used to replace the 35S promoter of 35Spro:BOS1 to create MASpro:BOS1. To create the new CRISPR/Cas9-induced loss-of-function alleles, guide RNA (gRNA) targeting the first and second exons of BOS1 were integrated into pCBC_DT1DT2 and then into the final vector pHEC401 according to (Xing et al., 2014). Vectors were transformed into the indicated plants via Agrobacterium strain GV3101 (TSINGKE #TSC-A01, China), by the floral dip method (Clough and Bent, 1998). The primers used in this study are listed in Supplemental Table S1.
Transformation procedures
The bos1-1 mutant was found to be incompatible with Agrobacterium transformation. Test transformations of bos1-1 were performed in labs in Helsinki, Finland and Hangzhou, China. All transformed bos1-1 plants died before seed set due to spreading cell death trigged by Agrobacterium. To overcome this limitation, CRISPR/Cas9 vectors were first transformed into wild-type plants, with integration presumably into a chromosomal location not linked with BOS1. The vector was then transferred into the bos1-1 background by crossing using a CRISPR/Cas9 vector transformant as a pollen donor and bos1-1 as the pollen acceptor. The vectors functioned in the F1 generation to modify the BOS1 locus, and then were removed by segregation in the F2. Homozyotes of bos1-1 were selected by PCR and then screened for bos1-c4* and -c5* by Sanger sequencing.
Genome re-sequencing
Genomic DNA of bos1-1 was extracted and sequenced by the Biomarker Technologies Corporation (Beijing, China) following the standard procedures of Oxford Nanopore Technology sequencing (Deamer et al., 2016). Sequence depth was 129×, 99.77% of 24.37 GB clean data mapped properly to the Arabidopsis genome (TAIR10). The raw data have been deposited to NCBI (PRJNA728243). Structural variations were analyzed with Sniffles (Sedlazeck et al., 2018).
RNA-seq
Fully expanded leaves of 23-day-old plants were punctured with a bundle of toothpicks, and collected after 3 days. Unwounded plants were used as a control. RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Library construction and sequencing were carried out in LC-BIO Biotech Ltd with Illumina Hiseq 4000. Raw reads were filtered and aligned to the Arabidopsis genome (TAIR10) using hisat2 (version 2.1.0; Kim et al., 2015). To identify the transcripts adjacent to MASpro, the conjoined sequence of the T-DNA and BOS1 genome sequence were first obtained from the bos1-1 mutant resequencing analysis, and their sequences verified with Sanger sequencing. Then the combined sequence was used as a reference for read mapping. The RNA-seq raw data have been deposited to NCBI (PRJNA728243). Normalized transcript abundances of BOS1 were calculated as fragments per kilobase pair of exon model per million fragments mapped with Cufflinks (Trapnell et al., 2010). For real-time quantitative PCR (qPCR), leaves of 23-day-old plants were used for RNA extraction and reverse transcription. The raw cycle threshold values were analyzed with Qbase+ (Biogazelle; Hellemans et al., 2007) with the reference genes ACTIN2, PP2AA3, and ACTIN8.
Statistical analysis
One-way ANOVA (Analysis of Variance) and t tests were used for statistical analysis as indicated in figure legends. One-way ANOVA analysis was performed using Graphpad Prism version 7 (Mitteer et al., 2018). Briefly, all data of different biological repeats were statistically analyzed using Tukey’s multiple comparisons test with default setting. T tests were performed in Microsoft Excel 2016 using two-tailed and unpaired settings. Statistical analysis tables are presented in Supplemental File S1.
Accession numbers
Gene identifiers for Arabidopsis are BOS1/MYB108 (AT3G06490), ACTIN2 (AT3G18780), PP2AA3 (AT1G13320), and ACTIN8 (AT1G49240). New sequencing data, including bos1-1 resequencing data and RNA-seq data can be found at the NCBI SRA (PRJNA728243).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Alignments of protein sequences of BOS1/MYB108 and the truncated proteins of the new bos1 alleles.
Supplemental Figure S2. Sequences and positions of the transcripts at the BOS1 locus in the bos1-1 mutant.
Supplemental Figure S3. Col-0 lines transgenically expressing MASpro:BOS1 exhibited enhanced disease susceptibility under standard greenhouse conditions.
Supplemental Figure S4. BOS1 transcript levels in Arabidopsis RNAseq data conditions outputed from the Genevestigator (Hruz et al., 2008).
Supplemental Figure S5. The CRISPR/Cas9-induced bos1 loss-of-function alleles showed enhanced ABA sensitivity.
Supplemental Figure S6. The CRISPR/Cas9-induced bos1 loss-of-function alleles showed a wild-type methyl viologen (MV) response.
Supplemental Figure S7. The CRISPR/Cas9-induced bos1 loss-of-function alleles exhibited unaltered NaCl sensitivity.
Supplemental Figure S8. Architecture of the T-DNA insertion in bos1-1.
Supplemental Table S1. Primers used in this study.
Supplemental Data Set 1. Raw data for Figure 6, including lesion sizes and BOS1 transcript levels.
Supplemental Data Set 2. Identification of genomic changes in bos1-1 that were identified by genome re-sequencing.
Supplemental File S1. Statistical analysis tables.
Supplementary Material
Acknowledgments
We thank Cezary Waszczak and Adam Blotch for critical comments on the manuscript.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (grant no. LY22C160005); the National Natural Science Foundation of China (grant no. 31700224 and 31871233).
Conflict of interest statement. The authors have no conflicts of interest to declare. All co-authors agree with the contents of the manuscript and there is no financial interest to report.
Contributor Information
Fuqiang Cui, State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou 311300, China.
Xiaoxiao Li, State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou 311300, China.
Wenwu Wu, State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou 311300, China.
Wenbo Luo, State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou 311300, China.
Ying Wu, State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou 311300, China.
Mikael Brosché, Faculty of Biological and Environmental Sciences, Organismal and Evolutionary Biology Research Program, Viikki Plant Science Centre, University of Helsinki, Helsinki FI-00014, Finland.
Kirk Overmyer, Faculty of Biological and Environmental Sciences, Organismal and Evolutionary Biology Research Program, Viikki Plant Science Centre, University of Helsinki, Helsinki FI-00014, Finland.
F.C., K.O., and M.B. designed the project. X.L., W.L., and Y.W. performed the experiments. W.W. conduced the bioinformatic analyses. F.C. and X.L. wrote the first draft of the manuscript. All authors edited, proofread, and approved the manuscript. F.C. obtained financial support.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Fuqiang Cui (fuqiang.cui@zafu.edu.cn).
References
- Beals TP, Goldberg RB (1997) A novel cell ablation strategy blocks tobacco anther dehiscence. Plant Cell 9: 1527–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock RM, Stermer BA (1989) Perspectives on wound healing in resistance to pathogens. Annu Rev Phytopathol 27: 343–371 [Google Scholar]
- Brown CG, Clarke J (2016) Nanopore development at Oxford Nanopore. Nat Biotechnol 34: 810–811 [DOI] [PubMed] [Google Scholar]
- Bruggeman Q, Raynaud C, Benhamed M, Delarue M (2015) To die or not to die? Lessons from lesion mimic mutants. Front Plant Sci 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliberti N, Fermaud M, Roudet J, Rossi V (2015) Environmental conditions affect Botrytis cinerea infection of mature grape berries more than the strain or transposon genotype. Phytopathology 105: 1090–1096 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium -mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cui F, Brosché M, Sipari N, Tang S, Overmyer K (2013) Regulation of ABA dependent wound induced spreading cell death by MYB108. New Phytol 200: 634–640 [DOI] [PubMed] [Google Scholar]
- Cui F, Wu H, Safronov O, Zhang P, Kumar R, Kollist H, Salojärvi J, Panstruga R, Overmyer K (2018) Arabidopsis MLO2 is a negative regulator of sensitivity to extracellular reactive oxygen species. Plant Cell Environ 41: 782–796 [DOI] [PubMed] [Google Scholar]
- Cui F, Wu W, Wang K, Zhang Y, Hu Z, Brosché M, Liu S, Overmyer K (2019) Cell death regulation but not abscisic acid signaling is required for enhanced immunity to Botrytis in Arabidopsis cuticle-permeable mutants. J Ex Bot 70: 5971–5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneva A, Gao Z, Van Durme M, Nowack MK (2016) Functions and regulation of programmed cell death in plant development. Ann Rev Cell Dev Biol 32: 441–468 [DOI] [PubMed] [Google Scholar]
- Daxinger L, Hunter B, Sheikh M, Jauvion V, Gasciolli V, Vaucheret H, Matzke M, Furner I (2008) Unexpected silencing effects from T-DNA tags in Arabidopsis. Trends Plant Sci 13: 4–6 [DOI] [PubMed] [Google Scholar]
- Deamer D, Akeson M, Branton D (2016) Three decades of nanopore sequencing. Nat Biotechnol 34: 518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, Zhao Y (2015) Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci USA 112: 2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin SB (2017) Integration of agrobacterium T-DNA into the plant genome. Ann Rev Genet 51: 195–217 [DOI] [PubMed] [Google Scholar]
- Guevara-García A, López-Bucio J, Herrera-Estrella L (1999) The mannopine synthase promoter contains vectorial cis-regulatory elements that act as enhancers and silencers. Mol Gen Genet 262: 608–617 [DOI] [PubMed] [Google Scholar]
- Harper AM, Strange RN, Langcake P (1981) Characterization of the nutrients required by Botrytis cinerea to infect broad bean leaves. Physiol Plant Pathol 19: 153–167 [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysmans M, Lema A S, Coll NS, Nowack MK (2017) Dying two deaths — programmed cell death regulation in development and disease. Curr Opin Plant Biol 35: 37–44 [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13: 2191–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakimova ET, Woltering EJ (2018) The wound response in fresh-cut lettuce involves programmed cell death events. Protoplasma 255: 1225–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampropoulos A, Sutikovic Z, Wenzl C, Maegele I, Lohmann JU, Forner J (2013) GreenGate - a novel, versatile, and efficient cloning system for plant transgenesis. PLoS One 8: e83043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JB, Browse J (2016) Epidermal jasmonate perception is sufficient for all aspects of jasmonate-mediated male fertility in Arabidopsis. Plant J 85: 634–647 [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhou H, Bi H, Fromm M, Yang B, Weeks DP (2013) Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res 41: e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupe F, Rivkin AC, Michael TP, Zander M, Motley ST, Sandoval JP, Slotkin RK, Chen H, Castanon R, Nery JR, et al. (2019) The complex architecture and epigenomic impact of plant T-DNA insertions. PLoS Genet 15: e1007819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12: 357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepiel Y, Pédron J, Patrit O, Simond-Côte E, Hermand V, Van Gijsegem F (2011) Analysis of the plant bos1 mutant highlights necrosis as an efficient defence mechanism during D. dadantii/Arabidospis thaliana interaction. PLoS One 6: e18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Vailleau F, Balagué C, Roby D (2003) Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends in Plant Sci 8: 263–271 [DOI] [PubMed] [Google Scholar]
- Luo H, Laluk K, Lai Z, Veronese P, Song F, Mengiste T (2010) The Arabidopsis Botrytis susceptible1 interactor defines a subclass of RING E3 ligases that regulate pathogen and stress responses. Plant Physiol 154: 1766–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandaokar A, Browse J (2009) MYB108 acts together with MYB24 to regulate jasmonate-mediated stamen maturation in Arabidopsis. Plant Physiol 149: 851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandaokar A, Kumar VD, Amway M, Browse J (2003) Microarray and differential display identify genes involved in jasmonate-dependent anther development. Plant Mol Biol 52: 775–786 [DOI] [PubMed] [Google Scholar]
- McCabe PF (2013) Healing and closure following death: death signals from a wounded leaf. New Phytol 200: 590–591 [DOI] [PubMed] [Google Scholar]
- McElver J, Tzafrir I, Aux G, Rogers R, Ashby C, Smith K, Thomas C, Schetter A, Zhou Q, Cushman MA, et al. (2001) Insertional mutagenesis of genes required for seed development in Arabidopsis thaliana. Genetics 159: 1751–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengiste T, Chen X, Salmeron J, Dietrich R (2003) The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15: 2551–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitteer DR, Greer BD, Fisher WW, Cohrs VL (2018) Teaching behavior technicians to create publication-quality, single-case design graphs in graphpad prism 7. J Appl Behav Anal 51: 998–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlotshwa S, Pruss GJ, Gao Z, Mgutshini NL, Li J, Chen X, Bowman LH, Vance V (2010) Transcriptional silencing induced by Arabidopsis T-DNA mutants is associated with 35S promoter siRNAs and requires genes involved in siRNA-mediated chromatin silencing. Plant J 64: 699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nikonorova N, Yue K, Beeckman T, De Smet I (2018) Arabidopsis research requires a critical re-evaluation of genetic tools. J Exp Bot 69: 3541–3544 [DOI] [PubMed] [Google Scholar]
- Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31: 1–12 [DOI] [PubMed] [Google Scholar]
- Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu YC, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11: 297–322 [Google Scholar]
- Schubert D, Lechtenberg B, Forsbach A, Gils M, Bahadur S, Schmidt R (2004) Silencing in Arabidopsis T-DNA transformants: the predominant role of a gene-specific RNA sensing mechanism versus position effects. Plant Cell 16: 2561–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlazeck FJ, Rescheneder P, Smolka M, Fang H, Nattestad M, von Haeseler A, Schatz MC (2018) Accurate detection of complex structural variations using single-molecule sequencing. Nat Methods 15: 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatore A, Trobacher CP, Greenwood JS (2009) Ricinosomes predict programmed cell death leading to anther dehiscence in tomato. Plant Physiol 149: 775–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al. (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14: 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Qi T, Huang H, Ren Q, Wu D, Chang C, Peng W, Liu Y, Peng J, Xie D (2011) The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23: 1000–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and abundance estimation from RNA-Seq reveals thousands of new transcripts and switching among isoforms. Nat Biotechnol 28: 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hautegem T, Waters AJ, Goodrich J, Nowack MK (2015) Only in dying, life: programmed cell death during plant development. Trends Plant Sci 20: 102–113 [DOI] [PubMed] [Google Scholar]
- Westphal L, Scheel D, Rosahl S (2008) The coi1-16 mutant harbors a second site mutation rendering PEN2 nonfunctional. Plant Cell 20: 824–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ZA, Song J, Taylor B, Yang C (2011) The final split: the regulation of anther dehiscence. J Exp Bot 62: 1633–1649 [DOI] [PubMed] [Google Scholar]
- Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B, Wang XC, Chen QJ (2014) A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol 14: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XF, Wang B, Feng YF, Xue JS, Qian XX, Liu SQ, Zhou J, Yu YH, Yang NY, Xu P, et al. (2019) AUXIN RESPONSE FACTOR17 directly regulates MYB108 for anther dehiscence. Plant Physiol 181: 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Corwin JA, Copeland D, Feusier J, Eshbaugh R, Chen F, Atwell S, Kliebenstein DJ (2017) Plastic transcriptomes stabilize immunity to pathogen diversity: the jasmonic acid and salicylic acid networks within the Arabidopsis/botrytis pathosystem. Plant Cell 29: 2727–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.